To the Editor: Because of concerns about thrombotic events after vaccination with ChAdOx1 nCoV-19 (Oxford–AstraZeneca),1 several European countries have recommended heterologous messenger RNA (mRNA) boost strategies for persons younger than 60 or 65 years of age who have received one dose of ChAdOx1 nCoV-19.2 To date, data on the safety and immunogenicity of these regimens are limited.
Through an ongoing clinical study of the longitudinal immunogenicity of coronavirus disease 2019 (Covid-19) vaccines (EudraCT number, 2021-000683-30; the protocol is available with the full text of this letter at NEJM.org), we were able to assess 88 health care workers who had received one dose of ChAdOx1 nCoV-19 vaccine 9 to 12 weeks earlier. Among these participants, 37 chose a homologous boost with ChAdOx1 nCoV-19 and 51 chose a heterologous boost with mRNA-1273 (Moderna). The median age of the participants was 46 years (range, 28 to 62) and 40 years (range, 23 to 59), respectively. Blood specimens were obtained at the time of boost, 7 to 10 days after the boost, and 30 days after the boost. Levels of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein (S)–specific and receptor-binding domain (RBD)–specific IgG were assessed with the use of an enzyme-linked immunosorbent assay and expressed as the area under the curve. Serum neutralization of the original SARS-CoV-2 isolate from Sweden (SARS-CoV-2/01/human/2020/SWE; GenBank accession number, MT093571.1) was measured in an immunofluorescence assay, with results expressed as the reciprocal of the 50% inhibitory dilution (ID50); serum neutralization of the original SARS-CoV-2 isolate from Sweden and the B.1.351 (or beta) variant was also measured in a cytopathic effect assay. Information on reactogenicity before and after administration of the booster injection was reported by the study participants. Demographic characteristics of the participants and full details of the methods are provided in the Supplementary Appendix, available at NEJM.org.
On the day of the boost, the two groups had similar levels of SARS-CoV-2 S-specific and RBD-specific IgG and neutralizing antibodies. Levels of S-specific and RBD-specific IgG at 7 to 10 days after a ChAdOx1 nCoV-19 boost were 5 times as high as on the day of the boost (P<0.001); at 7 to 10 days after an mRNA-1273 boost, levels of S-specific IgG were 115 times as high and levels of RBD-specific IgG were 125 times as high as on the day of the boost (P<0.001) (Fig. S1 in the Supplementary Appendix). After 30 days, levels of S-specific IgG remained similar to those at the 7-to-10-day time point in both groups.
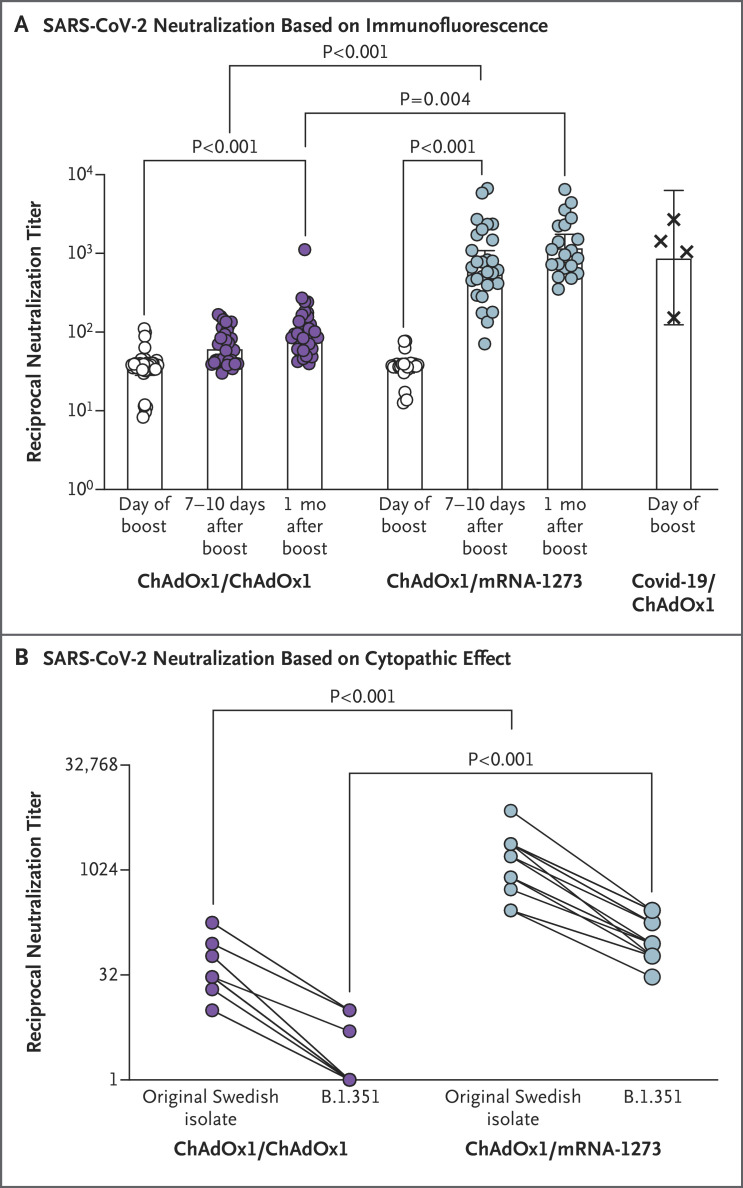
The potent induction of SARS-CoV-2 S-specific antibodies after a heterologous boost with mRNA-1273 was reflected by an increase in the in vitro reciprocal serum neutralization titer, with a reciprocal ID50 at 7 to 10 days after the boost that was 20 times as high as that on the day of the boost (P<0.001) (Figure 1A). In contrast, a homologous ChAdOx1 nCoV-19 boost led to a near doubling of the reciprocal ID50 within 7 to 10 days (P=0.09). At 1 month after the boost, an additional increase in neutralizing antibodies (to levels 1.6 to 1.7 times as high as the levels at 7 to 10 days) occurred in both groups, but the increase was not significant. We verified our results for neutralization of the original SARS-CoV-2 isolate from Sweden in another laboratory (Figure 1B). In addition, we found that an mRNA-1273 boost had induced antibodies that could neutralize the B.1.351 variant of SARS-CoV-2 (Figure 1B); however, a ChAdOx1 nCoV-19 boost did not induce potent neutralizing antibodies against this variant, a finding consistent with findings from a previous study.3
Figure 1. In Vitro Neutralization of Original SARS-CoV-2 Isolate from Sweden and the B.1.351 Variant.
Panel A shows serum neutralization of the original severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) isolate from Sweden (SARS-CoV-2/01/human/2020/SWE) on the day of the boost, 7 to 10 days later, and 1 month later. Data points are the reciprocals of the individual serum dilutions that achieved a 50% reduction in infection (reciprocal 50% inhibitory dilution) in an assay in which infection of Vero E6 cells was measured by virus-specific immunofluorescence. Bars indicate geometric means, and 𝙸 bars indicate 95% confidence intervals. In the group that received a ChAdOx1 nCoV-19 boost, the numbers of participants with specimens analyzed were 35 for the day of the boost, 34 for days 7 to 10, and 34 for 1 month; the corresponding numbers in the group that received an mRNA-1273 boost were 26, 28, and 20. As a reference, neutralizing antibody responses to SARS-CoV-2 in 4 persons who had had coronavirus disease 2019 (Covid-19) and had received one dose of ChAdOx1 nCoV-19 vaccine 9 to 12 weeks before sampling were also evaluated. Panel B shows serum neutralization of the original SARS-CoV-2 isolate from Sweden and the B.1.351 variant at the 7-to-10-day time point, with neutralization evaluated as the lowest reciprocal serum dilution at which the cytopathic effect of SARS-CoV-2 on Vero E6 cells was reduced by 50% or more (50% cytopathic effect). Specimens from 18 participants in the group that received a ChAdOx1 nCoV-19 boost and from 16 participants in the group that received an mRNA-1273 boost were analyzed. All assays were performed under biosafety level 3 conditions at Umeå University (Panel A) or the Karolinska Institutet (Panel B).
In this relatively small cohort, the mRNA-1273 boost led to more frequent reports of fever, headache, chills, and muscle aches than the ChAdOx1 nCoV-19 boost. However, we found no significant difference between the groups when the events were graded according to intensity level (Fig. S2). The reported adverse events are in line with what has been published previously for homologous ChAdOx1 nCoV-19 or mRNA-127 vaccination regimens.4,5
We conclude that the mRNA-1273 vaccine can efficiently stimulate the SARS-CoV-2–specific B-cell memory that has been generated by a prime dose of ChAdOx1 nCoV-19 vaccine 9 to 12 weeks earlier and that it may provide better protection against the B.1.351 variant than a ChAdOx1 nCoV-19 boost. These data also suggest that mRNA vaccines (here in the form of mRNA-1273) may be useful for vaccination strategies in which a third dose is to be administered to persons who have previously received two doses of ChAdOx1 nCoV-19.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This letter was published on July 14, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this letter at NEJM.org.
Footnotes
Supported by grants from Vetenskapsrådet (2020-06235, to Dr. Forsell, and 2020-05782, to Dr. Klingström), SciLife Laboratories (VC-2020-0015, to Dr. Forsell), Region Västerbotten and Umeå University (RV-938855, to Dr. Ahlm), and the Center for Innovative Medicine (CIMED) (20200141, to Dr. Klingström). Dr. Normark is a Wallenberg Center for Molecular Medicine Associated Researcher.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control. Overview of EU/EEA country recommendations on COVID-19 vaccination with Vaxzevria, and a scoping review of evidence to guide decision-making. May 18, 2021. (https://www.ecdc.europa.eu/en/publications-data/overview-eueea-country-recommendations-covid-19-vaccination-vaxzevria-and-scoping).
- 3.Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021;384:1885-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396:467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.