Abstract
Objective
This systematic review aimed to describe the connection between the inspired oxygen fraction and pulmonary complications in adult patients, with the objective of determining a safe upper limit of oxygen supplementation.
Methods
MEDLINE and Embase were systematically searched in August 2019 (updated July 2020) for studies fulfilling the following criteria: intubated adult patients (Population); high fractions of oxygen (Intervention) versus low fractions of (Comparison); atelectasis, acute respiratory distress syndrome (ARDS), pneumonia and/or duration of mechanical ventilation (Outcome); original studies both observational and interventional (Studies). Screening, data extraction and risk of bias assessment was done by two independent reviewers.
Results
Out of 6120 records assessed for eligibility, 12 were included. Seven studies were conducted in the emergency setting, and five studies included patients undergoing elective surgery. Eight studies reported data on atelectasis, two on ARDS, four on pneumonia and two on duration of mechanical ventilation. There was a non-significant increased risk of atelectasis if an oxygen fraction of 0.8 or above was used, relative risk (RR): 1.37 (95% CI 0.95 to 1.96). One study showed an almost threefold higher risk of pneumonia in the high oxygen fraction group (RR: 2.83 (95% CI 2.25 to 3.56)). The two studies reporting ARDS and the two studies with data on mechanical ventilation showed no association with oxygen fraction. Four studies had a high risk of bias in one domain.
Conclusions
In this systematic review, we found inadequate evidence to identify a safe upper dosage of oxygen, but the identified studies suggest a benefit of keeping inspiratory oxygen fraction below 0.8 with regard to formation of atelectases.
PROSPERO registration number
CRD42020154242.
Keywords: adult anaesthesia, adult intensive & critical care, respiratory medicine (see thoracic medicine), respiratory physiology, adult intensive & critical care
Strengths and limitations of this study.
The use of predefined Population, Intervention, Comparison, Outcome and Study design to asses studies for eligibility.
The use of a wide search string in two databases.
Two independent reviewers screening and including studies, assessing risk of bias and extracting data.
There is a risk of publication bias that arises due to the possibility of missing unpublished studies.
It is possible that our search did not identify all relevant studies.
Introduction
Oxygen is a molecule vital for life, as it is the cornerstone in cellular respiration in all aerobic organisms. In trauma care, during anaesthesia and in the management of respiratory failure, an oxygen fraction (FiO2) of 0.21 may not be sufficient to maintain an acceptable oxygen concentration in arterial blood and oxygen supplementation is therefore often part of standard care.1 2
Supplementary oxygen may result in hyperoxaemia, with the risk of tissue hyperoxia. An increasing amount of evidence has connected hyperoxia and hyperoxaemia with increased mortality3–6 possibly as a consequence of a variety of factors associated with hyperoxia: atelectasis in the lungs,7 8 formation of reactive oxygen species,9 impairment of the innate immune system,10 as well as vasoconstriction with paradox tissue hypoxia to follow.11
All in all, hypoxia should be avoided, but at the same time it seems that exposure to high concentrations of oxygen may have serious consequences. Therefore, it is relevant to investigate if a safe upper dosage of oxygen can be identified.
This systematic review aimed to describe the connection between the FiO2 and pulmonary complications in intubated adult patients, with the objective of determining a safe upper limit of oxygen supplementation. We defined pulmonary complications as atelectasis, pneumonia and acute respiratory distress syndrome (ARDS).
Methods
Protocol and registration
Methods of the analysis and inclusion criteria were prespecified and documented in a protocol. The protocol was completed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines for protocols.12 a13
Eligibility criteria
Studies were selected according to following predefined Population, Intervention, Comparison, Outcome and Study design (PICOS).
Inclusion criteria
Population: intubated patients ≥18 years.
Intervention and Comparison: low inspiratory FiO2 (as defined by author) versus high FiO2 (as defined by authors).
Outcome: atelectasis, pneumonia, ARDS and duration of mechanical ventilation (as defined by authors).
Study design: original studies both interventional and observational.
Exclusion criteria
Hyperbaric oxygen treatment.
Case reports, review articles and editorials.
We had no restrictions on year of publication. The search was restricted to studies published in French, English or Danish.
Information sources and search
We searched MEDLINE and Embase using the following predefined search string (presented search strategy is from MEDLINE).
(((((((oxygen [Title/Abstract]) OR oxygen[MeSH Terms]) OR hyperoxia[Title/Abstract]) OR “supplemental oxygen”[Title/Abstract]) OR “oxygen supplementation”[Title/Abstract]) OR fio2[Title/Abstract])))
((((((((((((((atelectasis[Title/Abstract]) OR pulmonary atelectasis[MeSH Terms]) OR pneumonia[Title/Abstract]) OR pneumonia[MeSH Terms]) OR “lung collapse”[Title/Abstract]) OR “collapsed lung”[Title/Abstract]) OR “acute lung injury”[Title/Abstract]) OR acute lung injury[MeSH Terms]) OR ARDS[Title/Abstract]) OR “acute respiratory distress syndrome”[Title/Abstract]) OR respiratory distress syndrome, adult[MeSH Terms])))
(intub*) OR ”mechanical ventilation”
#1 AND #2 AND #3.
The search was done the 6 August 2019. The search was updated the 6 July 2020. Modifications were made to fit Embase.
We identified one additional record14 by obtaining the full-text article of an abstract identified through the search string. Another record15 was identified by screening the reference list of an article.
Selection process
Two independent reviewers (MLL and BR) screened all titles and abstracts yielded by the search against the inclusion criteria using Covidence (an online programme facilitating the production of systematic reviews developed by the Cochrane group).16 A Cohen’s Kappa for inter-rater reliability was calculated. The same reviewers obtained full text articles for all titles that appeared to meet the inclusion criteria or where there was any uncertainty. Disagreements were resolved through discussion until consensus. All full-text articles were assessed by the same two independent reviewers and those not meeting the inclusion criteria were excluded.
Data collection and data items
Data extraction was done by two authors (MLL and BR), and was facilitated by the data-extraction tool Covidence and by using predefined forms. We collected study characteristics including trial design, trial size, country, period and year of publication. From the included studies we extracted the dosage of oxygen, type of control used, duration of treatment, patient characteristics (gender, age, patient type) as well as data on the predefined outcomes (atelectasis, pneumonia, ARDS) as defined by the authors.
Risk of bias
Risk of bias for non-randomised studies were assessed by using the Newcastle-Ottawa Scale.17 Here each study can be awarded from zero to nine stars, with zero stars representing a high risk of bias, and nine stars a low risk. Each study can be judged and awarded stars on eight items, categorised into three domains: selection of the study group, comparability of cohorts, and evaluation of the outcome of interest.
For randomised studies we used the Cochrane Collaboration tool for assessing risk of bias (Table 8.5.a in the Cochrane Handbook for Systematic Reviews of Intervention) in Covidence, which covers: sequence generation, allocation concealment, blinding, incomplete data and selective outcome reporting. A judgement as to the possible risk of bias on each domain were made from the extracted information, rated as ‘high risk’, ‘low risk’ or ‘unclear’ risk of bias. These judgements were made based on the criteria for judging the risk of bias (Table 8.5.d in the Cochrane Handbook Higgins 2011).
Summary measures and synthesis of results
This systematic review was expected to be a descriptive summary of the current evidence on oxygen supplementation and pulmonary complications. Relative risk (RR) was calculated where possible and a forest plot was used to illustrate the results. RRs with 95% CIs, was calculated in studies where this information was missing and the calculation was possible. The forest plot was made with a random-effects model.
Patient and public involvement
No patient involved.
Results
Study selection
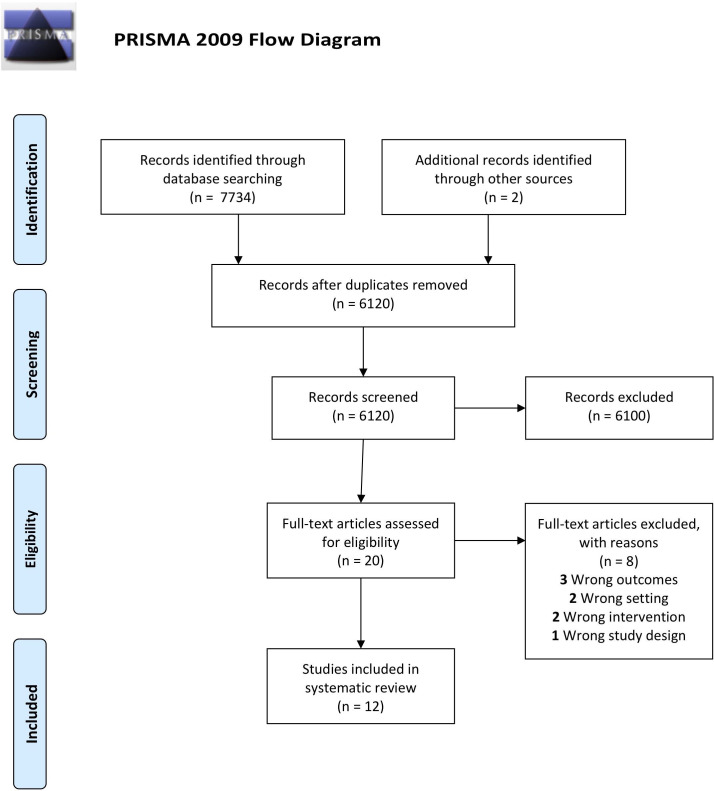
Our initial search strategy identified 7734 records. After duplicates were removed and two additional records from other sources were added, 6120 records were screened. Of these, 6100 were excluded as they did not fulfil eligibility criteria leaving 20 records for full-text screening. Cohen’s kappa for inter-rater reliability of 0.43 (95% CI 0.26 to 0.60) was calculated, which is judged to be moderate agreement. After full-text review, 12 records fulfilled the inclusion criteria (figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram of identification, screening, eligibility and inclusion process28 on a search for studies comparing low dose oxygen supplementation with high dose oxygen supplementation with pulmonary complications as an outcome.37
Study characteristics
Study characteristics are summarised in table 1. Eight of the 12 included studies were randomised controlled trials. Among the four remaining there were two retrospective observational studies18 19 and two prospective observational studies.20 21 About half of the studies were conducted in Europe. Seven studies were conducted in the acute care setting. Of these seven, one study22 included patients with septic shock, four studies14 18 21 23 recruited surgical, medical and trauma patients that were mechanically ventilated in the intensive care unit, one study20 included patients with acute lung injury and the last study24 recruited patients with traumatic brain injury. The remaining five studies included patients undergoing different types of elective surgery.
Table 1.
Characteristics of studies comparing low FiO2 with high FiO2, with lung complications as an outcome.
| Reference and year of publication | Country | Setting | Study design | Sample size | Low-dose oxygen | High-dose oxygen | Primary outcome |
| Akca et al (1999)15 | Austria | Elective surgery | Randomised controlled trial | 30 | 0.3 | 0.8 | Atelectasis |
| Asfar et al (2017)22 | France | Septic shock | Randomised controlled trial | 434 | SpO2 between 88% and 95% | 1.0 | Mortality day-28 |
| Barrot et al (2020)23 | France | Critical care | Randomised controlled trial | 205 | SpO2 between 88% and 92% | SpO2 ≥96% | Mortality day-28 |
| Benoît et al (2002)26 | Switzerland | Elective surgery | Randomised controlled trial | 20 | 0.4 | 1.0 | Atelectasis |
| Ishii et al (2015)18 | Japan | Trauma | Retrospective cohort study | 911 | <0.6 | >0.6 | Atelectasis |
| Lång et al (2018)24 | Finland | Critical care | Randomised controlled trial | 65 | 0.4 | 0.7 | Levels of ROS, IL-6 and NSE |
| Panwar et al (2015)14 | Australia, New Zealand and France | Critical care | Randomised controlled trial | 104 | Mean=0.26 | Mean=0.36 | Mean AUC for SpO2, SaO2, PaO2, and FIO2 on days 0–7 |
| Rachmale et al (2012)20 | USA | Critical care | Prospective, observational study | 210 | Mean=0.4 | Mean=0.6 | Duration of exposure to excessive FiO2 during the first 48 hours of mechanical ventilation |
| Rothen et al (1995)8 | Sweden | Elective surgery | Randomised controlled trial | 24 | 0.3 | 1.0 | Atelectasis |
| Staehr et al (2012)25 | Denmark | Laparotomy for ovarian cancer | Randomised controlled trial | 35 | 0.3 | 0.8 | Change in PaO2/FiO2 |
| Staehr-Rye et al (2017)19 | USA | Non-cardiothoracic surgery | Register study | 26 841 | 0.31 | 0.79 | Major respiratory complications |
| Suzuki et al (2015)21 | Australia | Critical care | Prospective before-and-after study | 105 | 0.27 | 0.40 | Changes in atelectasis score |
Lung complications were atelectasis, ARDS, pneumonia and duration of mechanical ventilation.
ARDS, acute respiratory distress syndrome; AUC, area under the curve; FiO2, oxygen fraction; IL-6, interleukin-6; NSE, neuron-specific enolase; PaO2, arterial oxygen tension; ROS, reactive oxygen species; SaO2, oxygen saturation as measured by blood analysis.
The administered FiO2 varied substantially among the studies, with FiO2 ranging from 0.26 to 0.60 in the low FiO2 group and from 0.36 to 1.0 in the high FiO2 group.
Table 2 presents the outcomes of interest reported in the included studies. Eight studies reported on the incidence of atelectasis, two studies reported on ARDS, four studies reported on pneumonia and two studies reported on the duration of mechanical ventilation.
Table 2.
Patient outcomes comparing low doses of oxygen supplementation with high doses of oxygen supplementation
| Reference | Low-dose oxygen | High-dose oxygen | RR (95% CI) |
| Atelectasis | |||
| Akca et al15 | 9 (64%) | 15 (94%) | 1.46 (0.97 to 2.2) |
| Asfar et al22 | 13 (6%) | 26 (12%) | 2.0 (1.06 to 3.79) |
| Benoît et al26 | 2.5% of total surface | 7% of total surface | – |
| Ishii et al18 | 64% of patients | 76.8% of patients | – |
| Lång et al24 | 14 (52%) | 18 (47%) | 0.914 (0.56 to 1.5) |
| Rothen et al8 | 0.25 cm2 ±0.4 | 4.2 cm2 ±5.6 | – |
| Staehr et al25 | 2 (13.3%) | 5 (25%) | 1.88 (0.42 to 8.37) |
| Suzuki et al21 | TWA AS=1.5 (0.7–2) | TWA AS=2 (1.2–2.2) | – |
| ARDS | |||
| Lång et al24 | 3 (11%) | 0 (0%) | – |
| Panwar et al14 | 11 (32%) | 11 (28%) | 0.87 (0.43 to 1.75) |
| Pneumonia | |||
| Asfar et al22 | 32 (15%) | 30 (14%) | 0.94 (0.59 to 1.49) |
| Barrot et al23 | 17 (17.2%) | 22 (21.6%) | 1.26 (0.71 to 2.22) |
| Lång et al24 | 6 (22.2%) | 6 (15.8%) | 0.71 (0.26 to 1.97) |
| Staehr-Rye et al19 | 104 (0.7%) | 227 (1.9%) | 2.83 (2.25 to 3.56) |
| Duration of mechanical ventilation | |||
| Lång et al24 | 6.3 days (4.7–10) | 5 days (2.5–7.5) | – |
| Rachmale et al20 | 2.8 days1–6 | 6 days (3–10.5) | – |
Continuous data are presented as mean (SD) or median (IQR). RR is presented with high-dose oxygen in the numerator.
ARDS, acute respiratory distress syndrome; RR, relative risk; TWA AS, time-weighted average atelectasis.
Atelectasis
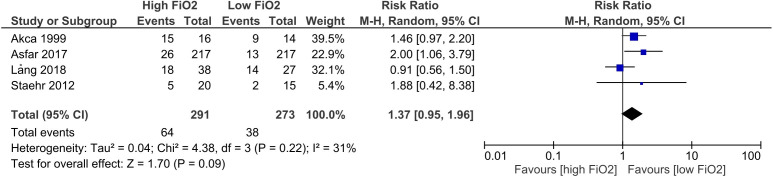
The eight studies reporting on atelectasis, generally showed better outcomes for patients in the low FiO2 group, as two studies22 25 showed almost two-fold higher risk of atelectasis in the high FiO2, with RR: 1.875 (95% CI 0.42 to 8.37) and RR: 2.0 (95% CI 1.06 to 3.79), respectively. One study15 suggested a minor benefit of treatment with low FiO2, but this was not statistically significant, RR: 1.46 (95% CI 0.97 to 2.20). Another study24 found RR: 0.91 (95% CI 0.56 to 1.50) suggesting a benefit of treatment with high FiO2, but this was not statistically significant. These studies are illustrated in the forest plot (figure 2), which shows that in general treatment with high FiO2 was associated with higher risk of atelectasis formation, RR:1.37 (95% CI 0.95 to 1.96). The heterogeneity (I2) of the meta-analysis presented in figure 2 is 31%, which corresponds to a moderate heterogeneity (Cochrane Handbook for Systematic Reviews of Intervention, section 9.5.2 Identifying and measuring heterogeneity).
Figure 2.
Forest plot of formation of atelectasis in studies comparing low FiO2 with high FiO2. FiO2, oxygen fraction; M.H, Maentel-Haentzel.
Rothen et al8 found a 16.8 times greater area of atelectasis in the high FiO2 group and similarly, the study by Benoît et al26 found a threefold larger atelectatic surface in the high FiO2 group. Suzuki et al21 estimated atelectasis as time-weighted averages, and also found a beneficial effect of a low FiO2. In the study by Ishii et al18 additional information on intubated patients were found in an abstract27 from the same study. They found a higher incidence of atelectasis in the high FiO2 group, but the total number of patients was not reported.
Acute respiratory distress syndrome
Panwar et al14 showed an increase of new-onset ARDS in the low FiO2 group, RR: 0.87 (95% CI 0.43 to 1.75), but this was not statistically significant. The study by Lång et al24 found three patients with ARDS in the low FiO2 group, while no patients with ARDS were identified in the group receiving high FiO2.
Pneumonia
The study by Staehr-Rye et al19 showed a significant increase in the incidence of pneumonia, RR: 2.83 (95% CI 2.25 to 3.56) in the high FiO2 group. Similarly, Barrot et al23 showed a small, but non-significant, tendency to ventilator-associated pneumonias in the high FiO2 group, RR: 1.26 (95% CI 0.71 to 2.22). The two other studies, Asfar et al22 and Lång et al,24 found a non-significant tendency for pneumonia in the low FiO2 group with RR: 0.94 (95% CI 0.59 to 1.49) and RR: 0.71 (95% CI 0.26 to 1.97), respectively. These studies are illustrated in the forest plot (figure 3), which shows a non-significant tendency that treatment with high FiO2 was associated with higher risk of pneumonia, RR: 1.32 (95% CI 0.65 to 2.70).
Figure 3.
Forest plot of risk of pneumonia in studies comparing low FiO2 with high FiO2. FiO2, oxygen fraction.; M.H. Random, Maentel-Haentzel.
Duration of mechanical ventilation
The two studies reporting the duration of mechanical ventilation pointed in opposite direction. Lång et al24 reported slightly more time spent on mechanical ventilation in the low FiO2 group, while Rachmale et al20 reported a twofold increase in time in the high FiO2 group.
Risk of bias assessment
Risk of bias for randomised studies are illustrated in table 3. Three studies had no blinding of participants, personal or outcome assessment, leaving them with a high risk of bias on these domains.8 14 22 In the study by Rothen et al8 it was unclear if a randomisation was performed between the low FiO2 group and the high FiO2 group, indicating a high risk of bias.
Table 3.
Risk of bias assessment for randomised controlled trials comparing low dose oxygen supplementation with high dose oxygen supplementation
| Akca et al15 | Asfar et al22 | Barrot et al23 | Benoit et al26 | Lång et al24 | Panwar et al14 | Rothen et al8 | Staehr et al25 | |
| Random sequence generation |  |
 |
 |
 |
 |
 |
 |
 |
| Allocation concealment |  |
 |
 |
 |
 |
 |
 |
 |
| Blinding of participants and personal |  |
 |
 |
 |
 |
 |
 |
 |
| Blinding of outcome assessment |  |
 |
 |
 |
 |
 |
 |
 |
| Incomplete outcome data |  |
 |
 |
 |
 |
 |
 |
 |
| Selective reporting |  |
 |
 |
 |
 |
 |
 |
 |
| Other bias |  |
 |
 |
 |
Risk of bias was assessed using Cochrane Collaboration tool for assessing risk of bias (Table 8.5.a in the Cochrane Handbook for Systematic Reviews of Intervention).
Green, low risk of bias; Red, high risk of bias; Yellow, unclear risk of bias.
Lång et al24 was an open-label trial, and was therefore awarded a high risk of bias on the domain of blinding of participants and personnel, however, the outcome assessor was blinded.
The four non-randomised studies were assessed using the New-Castle Ottawa Scale.17 One study20 scored six stars, two studies18 19 scored seven stars and one study21 scored eight stars, indicating an overall high quality of the studies.
Discussion
Summary of findings
In this study, we were not able to determine a safe upper limit of oxygen supplementation, due to inadequate evidence and heterogeneity as the included studies had different endpoints with varying definitions, and also different ways of defining low and high FiO2. In some studies the FiO2 in the low FiO2 group was higher than in the high FiO2 group in other studies.
Regarding atelectasis, seven of the eight studies favoured a conservative oxygen strategy with low FiO2 and an FiO2 above 0.8 seemed to be associated with higher risk of atelectasis formation. Looking at figure 2, there is an RR of 1.37, which suggests a clinically relevant difference with less atelectasis with a lower FiO2. However, the CI is wide (0.95–1.96), indicating that more information is needed before any firm conclusions can be made.
Strengths and limitations
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines,28 ensuring a systematic and broadly acknowledged approach to the present literature. The strengths of this approach include predefined PICOS criteria to assess study eligibility, use of a wide search string in two databases and two independent reviewers screened and assessed studies, including risk of bias.
Our study is limited by general weaknesses of systematic reviews. This includes risk of publication bias that arises due to the possibility of missing non-published studies. Despite the systematic search with predefined search string, and screening of reference lists of included studies, there is always a possibility that our search did not identify all relevant studies. However, the heterogeneity of the 12 studies reviewed makes us believe that potentially missed studies would not change the conclusion substantially. It is possible that more studies could have been found by searching in a wider set of databases. However, we chose the most commonly used databases MEDLINE and EMBASE, where the quality is known to be best and where most studies are found.
The patient population was determined in very broad terms (intubated adult patients), resulting in more heterogeneity among the included studies.
The trials varied in patient groups, associated clinical care and disease severity. Furthermore, in some studies it is unclear when exactly the outcome of interest was measured (early or late onset of ARDS and timing of CT/X-ray for measuring the presence of atelectasis). It is also unclear how pneumonia was defined in the four studies reporting this outcome. Therefore, conclusions should be drawn with caution.
Half of the randomised controlled trials were not blinded to personnel and participants, increasing the risk of performance bias. Three of these were not blinded to outcome assessors which increase the risk of detection bias. In general, many of the studies are relatively small, increasing the risk of other bias such as publication bias (table 3).
Atelectasis was defined in different ways complicating the pooling of data and the possibility to undertake a meta-analysis. Three studies8 15 25 used CT-scans and they all considered densities between −100 and +100 Hounsfield as atelectasis. Of these three, one8 measured areas of atelectasis in cm2 whereas the two others15 25 measured if atelectases were present or not. Ishii et al18 also used CT-scans, but defined atelectases as areas with formation of more than 10 mm thick atelectasis from the first to the second scan. The study by Staehr et al25 did not define specific criteria on when densities were judged as atelectasis or not.
Asfar et al22 and Suzuki et al21 used chest X-rays, without defining atelectasis specifically, as this was decided by the individual physician. Lång et al24 used chest X-rays in the same manner, however they allowed the appliance of positive end-expiratory pressure to minimise atelectasis, which makes it hard to directly compare results with other studies. Only Suzuki et al21 used more than one radiologist to perform the outcome assessment.
In Panwar et al,14 new-onset ARDS was defined as subsequent occurrence of ARDS in those patients who did not have ARDS on day 0, and where ARDS was present according to the Berlin definition.29 Lång et al24 did not report their definition of ARDS.
Regarding pneumonia, the database study of 26 841 patients performed by Staehr-Rye et al19 found a significant, almost threefold higher risk of pneumonia in the liberal oxygen group, indicating that excess levels of oxygen may be harmful. However, this is an analysis of administrative data, with risk of misclassification bias, and therefore, direct conclusions should be drawn with caution.
Other reviews
The evidence for the use of supplemental oxygen has been investigated in recently published systematic reviews. A systematic review and meta-analysis by Damiani et al30 from 2014 suggests an association between hyperoxia and mortality in patients with stroke, traumatic brain injury and those resuscitated from cardiac arrest. However, they concluded that their results were limited by the heterogeneity of the included studies. The same conclusion was drawn in another meta-analysis from 2015 by Helmerhorst et al.31 No definite conclusions could be made due to heterogeneity in the included studies; however, the meta-analysis suggested a benefit of conservative oxygen therapy. In a Cochrane review from 2015 by Wetterslev et al,32 comparing low (FiO2 0.30–0.40) vs high (FiO2 0.60–0.90) perioperative inspiratory FiO2, they found no association between perioperative FiO2 and postoperative surgical site infection and mortality. In another Cochrane review from 2016 performed by Cabello et al,33, they focused on patients with acute myocardial infarctions. They included five studies and found no clear recommendations on the use of oxygen supplementation.
In a recent meta-analysis performed in 2018 by Chu et al,4 they included 25 randomised controlled trials on acutely ill patients and found a significant association between liberal oxygenation strategies and increased mortality in-hospital, at 30 days and at longest follow-up. Nevertheless, morbidity outcomes were similar between groups.
The available reviews are limited because of heterogeneity, including different outcome measures, overall indicate that excess oxygen is harmful, stressing the need for further investigation on this subject.
Oxygen supplementation is obviously a vital part of trauma care, practice of anaesthesia, the management of respiratory distress and treatment of a variety of other conditions. However, supplemental oxygen should be carefully considered a drug and prescribed adequately. There is a general lack of strong evidence for supplemental oxygen, and an upper limit of oxygen supplementation is not included in many guidelines.1 34–36 Our study contributes to the current evidence in a different way, by looking at the association between FiO2 and pulmonary complications, which is a highly relevant indicator in the search for a safe upper limit of oxygen supplementation.
As oxygen supplementation is so widely used, it is crucial that better evidence-based guidelines are developed. Future research is required to precisely define the oxygen therapy strategies to maximise benefits and minimise harms.
Conclusion
In this systematic review, we found that there was inadequate evidence to identify a safer upper dosage of oxygen, but the identified studies suggest a benefit of conservative oxygen therapy, defined as FiO2 ≤0.8 with regard to formation of atelectasis.
Supplementary Material
Footnotes
Contributors: The idea for the article was formed by LSR. Literature search was performed by MLL and BR. MLL wrote the article. JSB contributed with high subject knowledge and all authors gave substantial contributions to the work. Every author revised the work critically for important intellectual content. Every author made a final approval of the version to be published. Every author agreed to be accountable for all aspects of the work.
Funding: Departmental funding. Award/Grant number is not applicable.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. This was a systematic review and researchers can contact the authors to access the material.
Ethics statements
Patient consent for publication
Not required.
References
- 1.O’Driscoll BR, Howard LS, Earis J. British thoracic society guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Respir Res 2017;4:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thim T, Krarup NHV, Grove EL, et al. Initial assessment and treatment with the airway, breathing, circulation, disability, exposure (ABCDE) approach. Int J Gen Med 2012;5:117–21. 10.2147/IJGM.S28478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner M, Stein D, Hu P, et al. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg 2012;147:1042–6. 10.1001/archsurg.2012.1560 [DOI] [PubMed] [Google Scholar]
- 4.Chu DK, Kim LH-Y, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet 2018;391:1693–705. 10.1016/S0140-6736(18)30479-3 [DOI] [PubMed] [Google Scholar]
- 5.Wang C-H, Chang W-T, Huang C-H, et al. The effect of hyperoxia on survival following adult cardiac arrest: a systematic review and meta-analysis of observational studies. Resuscitation 2014;85:1142–8. 10.1016/j.resuscitation.2014.05.021 [DOI] [PubMed] [Google Scholar]
- 6.Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA 2016;316:1583–9. 10.1001/jama.2016.11993 [DOI] [PubMed] [Google Scholar]
- 7.Aboab J, Jonson B, Kouatchet A, et al. Effect of inspired oxygen fraction on alveolar derecruitment in acute respiratory distress syndrome. Intensive Care Med 2006;32:1979–86. 10.1007/s00134-006-0382-4 [DOI] [PubMed] [Google Scholar]
- 8.Rothen HU, Sporre B, Engberg G, et al. Prevention of atelectasis during general anaesthesia. Lancet 1995;345:1387–91. 10.1016/S0140-6736(95)92595-3 [DOI] [PubMed] [Google Scholar]
- 9.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 2003;552:335–44. 10.1113/jphysiol.2003.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baleeiro CEO, Wilcoxen SE, Morris SB, et al. Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol 2003;171:955–63. 10.4049/jimmunol.171.2.955 [DOI] [PubMed] [Google Scholar]
- 11.Ariyaratnam P, Loubani M, Bennett R. Hyperoxic vasoconstriction of human pulmonary arteries: a novel insight into acute ventricular septal defects. ISRN Cardiol 2013;2013:1–4. 10.1155/2013/685735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 13.PROSPERO . International prospective register of systematic reviews. Available: https://www.crd.york.ac.uk/prospero/
- 14.Panwar R, Hardie M, Bellomo R, et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med 2016;193:43–51. 10.1164/rccm.201505-1019OC [DOI] [PubMed] [Google Scholar]
- 15.Akça O, Podolsky A, Eisenhuber E, et al. Comparable postoperative pulmonary atelectasis in patients given 30% or 80% oxygen during and 2 hours after colon resection. Anesthesiology 1999;91:991–8. 10.1097/00000542-199910000-00019 [DOI] [PubMed] [Google Scholar]
- 16.Covidence systematic review software. Veritas health innovation. Melbourne. Available: www.covidence.org
- 17.Wells GA, Shea B, O’Conell D. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Evidence-based public health, 2012. Available: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf
- 18.Ishii K, Morimatsu H, Ono K, et al. Relationship between a high-inspired oxygen concentration and dorsal atelectasis in high-energy trauma patients. Acta Med Okayama 2020;74:17–26. 10.18926/AMO/57948 [DOI] [PubMed] [Google Scholar]
- 19.Staehr-Rye AK, Meyhoff CS, Scheffenbichler FT, et al. High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. Br J Anaesth 2017;119:140–9. 10.1093/bja/aex128 [DOI] [PubMed] [Google Scholar]
- 20.Rachmale S, Li G, Wilson G. Practice of excessive FIO2 and effect on pulmonary outcomes in mechanically ventilated patients with acute lung injury. Respir Care 2012;57:1887–93. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, Eastwood GM, Goodwin MD, et al. Atelectasis and mechanical ventilation mode during conservative oxygen therapy: a before-and-after study. J Crit Care 2015;30:1232–7. 10.1016/j.jcrc.2015.07.033 [DOI] [PubMed] [Google Scholar]
- 22.Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med 2017;5:180–90. 10.1016/S2213-2600(17)30046-2 [DOI] [PubMed] [Google Scholar]
- 23.Barrot L, Asfar P, Mauny F, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med 2020;382:999–1008. 10.1056/NEJMoa1916431 [DOI] [PubMed] [Google Scholar]
- 24.Lång M, Skrifvars MB, Siironen J, et al. A pilot study of hyperoxemia on neurological injury, inflammation and oxidative stress. Acta Anaesthesiol Scand 2018;62:801–10. 10.1111/aas.13093 [DOI] [PubMed] [Google Scholar]
- 25.Staehr AK, Meyhoff CS, Henneberg SW, et al. Influence of perioperative oxygen fraction on pulmonary function after abdominal surgery: a randomized controlled trial. BMC Res Notes 2012;5:383. 10.1186/1756-0500-5-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benoît Z, Wicky S, Fischer J-F, et al. The effect of increased Fio2 before tracheal extubation on postoperative atelectasis. Anesthesia & Analgesia 2002;95:1777–81. 10.1097/00000539-200212000-00058 [DOI] [PubMed] [Google Scholar]
- 27.Ishii K, Morimatsu H, Ono K. Relationship between a high inspired oxygen concentration and a gravity dependent atelectasis in trauma patients: a subgroup analysis. 2015 Annu Meet Int Anesth Res Soc IARS 2015 Honolulu, HI United States 2015;120:S109. [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:658. [DOI] [PubMed] [Google Scholar]
- 29.Rawal G, Yadav S, Kumar R. Acute respiratory distress syndrome: an update and review. J Transl Intern Med 2018;6:74–7. 10.1515/jtim-2016-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damiani E, Adrario E, Girardis M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care 2014;18. 10.1186/s13054-014-0711-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helmerhorst HJF, Roos-Blom MJ, Van Westerloo DJ. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med 2015;43:1508–19. [DOI] [PubMed] [Google Scholar]
- 32.Wetterslev J, Meyhoff CS, Jørgensen LN, et al. The effects of high perioperative inspiratory oxygen fraction for adult surgical patients. Cochrane Database Syst Rev 2015;6:CD008884. 10.1002/14651858.CD008884.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabello JB, Burls A, Emparanza JI, et al. Oxygen therapy for acute myocardial infarction. Cochrane Database Syst Rev 2016;12:CD007160. 10.1002/14651858.CD007160.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casaubon LK, Boulanger J-M, Blacquiere D, et al. Canadian stroke best practice recommendations : Hyperacute Stroke Care Guidelines, Update 2015. Int J Stroke 2015;10:924–40. 10.1111/ijs.12551 [DOI] [PubMed] [Google Scholar]
- 35.Nikolaou NI, Welsford M, Beygui F, et al. Part 5: acute coronary syndromes: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2015;95:e121–46. 10.1016/j.resuscitation.2015.07.043 [DOI] [PubMed] [Google Scholar]
- 36.Moga C, Chojecki D. Oxygen therapy in acute care settings. Institute of health economics, 2016. Available: https://www.ihe.ca/publications/oxygen-therapy-in-acute-care-settings [PubMed]
- 37.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. This was a systematic review and researchers can contact the authors to access the material.