Abstract
A patient presented with fever, generalised rash, confusion, orofacial movements and myoclonus after receiving the first dose of mRNA-1273 vaccine from Moderna. MRI was unremarkable while cerebrospinal fluid showed leucocytosis with lymphocyte predominance and hyperproteinorrachia. The skin evidenced red, non-scaly, oedematous papules coalescing into plaques with scattered non-follicular pustules. Skin biopsy was consistent with a neutrophilic dermatosis. The patient fulfilled the criteria for Sweet syndrome. A thorough evaluation ruled out alternative infectious, autoimmune or malignant aetiologies, and all manifestations resolved with glucocorticoids. While we cannot prove causality, there was a temporal correlation between the vaccination and the clinical findings.
Keywords: COVID-19, dermatology, vaccination/immunisation, neurology, unwanted effects / adverse reactions
Background
On 11 March 2020, the WHO declared COVID-19 a worldwide pandemic.1 As we write this report, COVID-19 cases have reached a total of 176 million with 3.8 million deaths worldwide.2 Due to the unprecedented nature of this pandemic, vaccines have been developed in record time using novel technologies and are expected to play a key role in preventing further spread of the disease.3 One of these vaccines, mRNA-1273, manufactured by Moderna, consists of lipid nanoparticle encapsulated mRNA which expresses the prefusion-stabilised spike glycoprotein. This vaccine demonstrated SARS-CoV-2 neutralising activity in non-human primates,4 with acceptable safety and reactogenicity profiles in healthy adults in multicentre clinical trials.5–7 Rare reactions to vaccines may be observed in the postapproval period, due to the larger number of people exposed, as compared with a clinical trial. We report an individual who developed a unique constellation of neurological and dermatological manifestations 1 day following the first dose of the mRNA-1273 vaccine.
Case presentation
A 77-year-old man with history of coronary artery disease, hyperlipidaemia and hypothyroidism was hospitalised with confusion, fever and generalised rash after receiving the first dose of the mRNA-1273 vaccine. Fever started 1 day after vaccine administration and was episodic, lasting for minutes to hours and recurring throughout the day. Over the next 48 hours, the patient developed a generalised body rash, starting from the trunk and spreading to the extremities. Thereafter, he began to experience headache, dizziness and double vision, which later progressed gradually to severe encephalopathy in the course of 5 days.
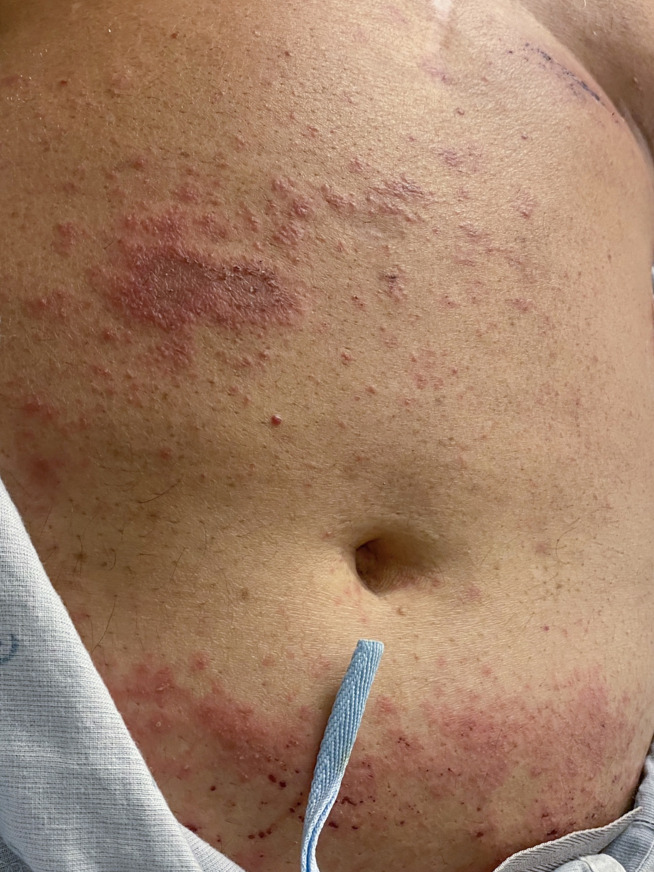
On dermatological examination, he was noted to have deep red, non-scaly, oedematous papules coalescing into plaques on the abdomen, upper chest, proximal upper extremities, bilateral upper flanks and back, with scattered non-follicular pustules (figures 1 and 2). On neurological examination, he had intermittent and irregular orofacial movements and bilateral upper extremity myoclonus. No other cranial nerve, motor or sensory deficits were noted on examination. Deep tendon reflexes were 2+ throughout, without signs of an upper motor neuron lesion. No nuchal rigidity was evident.
Figure 1.
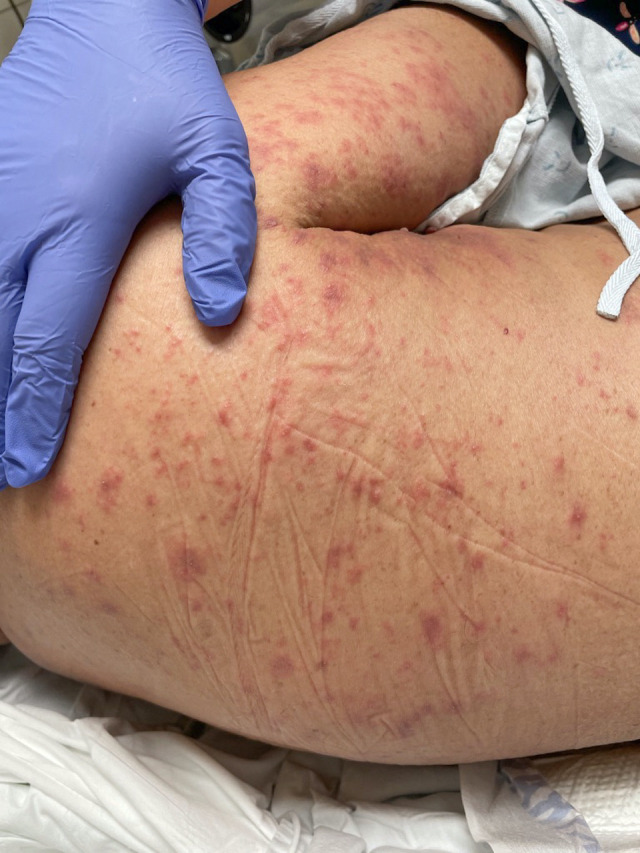
Oedematous erythematous papules and plaques with overlying pustules on the dorsum.
Figure 2.
Oedematous erythematous papules and plaques with overlying pustules on the trunkand abdomen.
Investigations
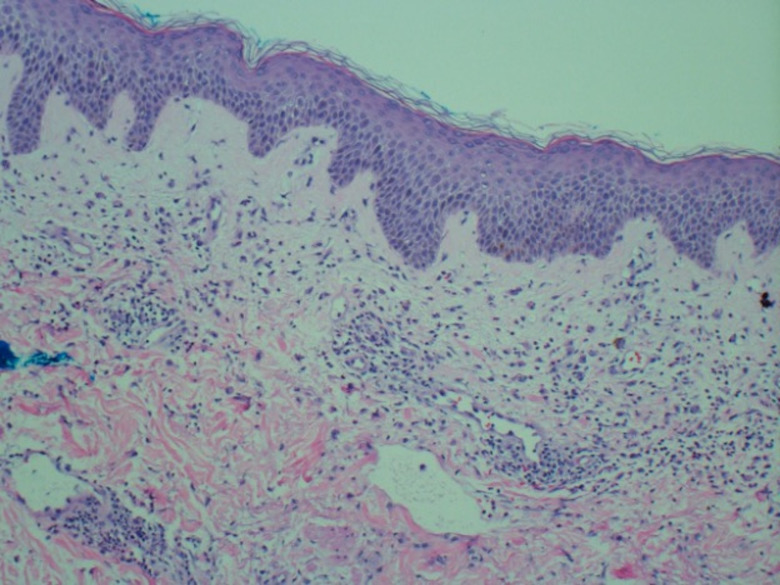
Table 1 summarises the laboratory work-up. Notably, the patient presented with leucocytosis and neutrophilia, and elevations in creatine kinase, C reactive protein and ferritin. Cerebrospinal fluid (CSF) analysis revealed 120×106/L leucocytes (77% lymphocytes), no erythrocytes, normal glucose (65 mg/dL, concomitant serum glucose of 106 mg/dL) and increased protein (124 mg/dL). All CSF cultures were negative, and the CSF meningoencephalitis panel (BioFire FilmArray System) did not detect any infectious agent. Blood and urine cultures, as well as an infectious respiratory panel, were all unremarkable. Real time-PCR (RT-PCR) for SARS-CoV-2 was negative. Antinuclear antibodies (ANA) (1:160) and rheumatoid factor (RF) (1:2) were positive. Further autoimmune testing demonstrated normal complement C3 and C4 levels, as well as negative antidouble-stranded DNA, ribonuclear protein, Smith, Ro, La and anticardiolipin antibodies. Brain and cervical spine MRI with and without contrast did not show any remarkable findings. Continuous video electroencephalogram (vEEG) monitoring revealed a generalised slow background in the theta range, with state changes and reactivity but no sleep features. Facial and upper limb movements were captured on video recording without an epileptiform correlate on electroencephalogram (EEG) (see vEEG in the video 1). Skin biopsy demonstrated intracorneal microabscesses, oedematous papillary dermis, and a band-like infiltrate of predominantly neutrophils and histiocytoid cells with nuclear debris in the superficial dermis without vasculitic changes and rare eosinophils, consistent with a neutrophilic dermatosis (figure 3). Evaluation for malignancy was unrevealing, including serum protein electrophoresis, peripheral blood smear, peripheral blood flow cytometry, and CT of chest, abdomen and pelvis.
Table 1.
Summary of laboratory evaluations
| Evaluations | Results | Reference values |
| General toxic and metabolic studies | ||
| AST, U/L | 67 | 5–34 |
| ALT, U/L | 93 | 6–55 |
| TSH, µIU/mL | 0.153 | 0.350–4.940 |
| CK, U/L | 1200 | 29–200 |
| Aldolase, U/L | 15.8 | <8.1 |
| Serological infectious studies | ||
| Leucocytes, ×109/L | 18.5 | 3.5–10.5 |
| Neutrophils, ×109/L | 15.5 | 1.78–5.38 |
| Lactic acid, mmol/L | 0.67 | 0.5–2.2 |
| Procalcitonin, ng/mL | 0.35 | <0.05 |
| RPR | Non-reactive | Non-reactive |
| HIV-1 | Non-reactive | Non-reactive |
| Cryptococcal antigen | Negative | Negative |
| Lyme disease IgG/IgM | Negative | Negative |
| Rickettsia IgG/IgM | Negative | Negative |
| Typhus IgG/IgM | Negative | Negative |
| Nasopharyngeal swab infectious studies | ||
| Human metapneumovirus, parainfluenza virus 1–4, rhinovirus, SARS-CoV-2, influenza A, influenza B, RSV, adenovirus, Bordetella pertussis, Chlamydophila pneumoniae, Mycoplasma pneumoniae, coronavirus 229E, coronavirus HKU1, coronavirus NL63, coronavirus OC43 | Not detected | Not detected |
| Immunology studies | ||
| Rheumatoid factor | Positive (1:2) | Negative |
| ANA | Positive (1:160) | Negative |
| Anti-dsDNA, RNP, Smith, Ro, La | Negative | Negative |
| Anti Jo-1 IgG | Negative | Negative |
| ANCA (Pr3, MPO) | Negative | Negative |
| Anticardiolipin IgG, IgM | Negative | Negative |
| C3 complement, mg/dL | 108 | 82–193 |
| C4 complement, mg/dL | 41 | 15–57 |
| CRP, mg/dL | 139 | 0.00–0.50 |
| Ferritin, ng/mL | 396.70 | 5–275 |
| CSF studies | ||
| White cell count (5% neutrophils, 77% lymphocytes, 18% monocytes), /×106/L | 120 | ≤5 |
| RBC, /106/L | 0 | 0–5 |
| Glucose, mg/dL | 65 | 40–70 |
| Protein, mg/dL | 124 | 15–45 |
| IgG index (CSF+blood) | Increased | Normal |
| Immunofixation electrophoresis | Normal | Normal |
| Culture/Gram stain | No organisms seen | No organisms seen |
| AFB culture+smear | No acid-fast bacilli seen | No acid-fast bacilli seen |
| Fungus | No fungi seen | No fungi seen |
| India ink | No encapsulated yeast seen | No encapsulated yeast seen |
| VDRL | Non-reactive | Non-reactive |
| Meningoencephalitis panel | ||
| CMV, Escherichia coli K1, Haemophilus influenzae, herpes simplex virus 1, 2 and 6, human parechovirus, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactiae, Streptococcus pneumoniae, Enterovirus, varicella-zoster virus, Cryptococcus neoformans/gattii, West Nile virus | Not detected | Not detected |
| Autoimmune encephalitis panel | ||
| ANNA 1 (Hu), ANNA 2 (Ri), ANNA 3, PCA1 (Yo), PCA2, PCA-Tr (DNER), AGNA/SOX1, amphiphysin, CRMP5/CV2, GAD65, Ma2/Ta, Myelin Ab, aquaporin 4, NMDAR1, AMPAR1, AMPAR2, GABABR, LGI1, CASPR2, DPPX Rec, VGKC | Negative | Negative |
Ab, antibody; AFB, acid-fast bacillus; AGNA, antiglial nuclear antibody; ALT, alanine aminotransferase; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibodies; ANNA 1, type 1 antineuronal nuclear antibody; ANNA 2, type 2 antineuronal nuclear antibody; ANNA 3, type 3 antineuronal nuclear antibody; AST, aspartate aminotransferase; CASPR2, contactin-associated protein-2; CK, creatine kinase; CMV, cytomegalovirus; CRMP5, collapsin response mediator protein-5; CRP, C-reactive protein; CSF, cerebrospinal fluid; DNER, Delta/Notch-like epidermal growth factor-related receptor; DPPX Rec, dipeptidyl-peptidase–like protein 6 receptor; dsDNA, double-stranded DNA; GABABR, γ-aminobutyric acid-B receptor; GAD, glutamic acid decarboxylase; HIV-1, human immunodeficiency virus type 1; LGI1, Leucine-rich glioma-inactivated protein 1; MPO, myeloperoxidase; NMDAR1, N-methyl-d-aspartate receptor-1; PCA, Purkinje cytoplasmic antibody; Pr3, proteinase 3; RBC, red blood cells; RNP, ribonucleoprotein; RPR, rapid plasma reagin; RSV, respiratory syncytial virus; RT-PCR, real-time PCR; TSH, thyroid stimulating hormone; VDRL, venereal disease research laboratory test; VGKC, voltage-gated potassium channel.
Video 1.
Figure 3.
Histopathology revealed oedematous papillary dermis and a band-like infiltrate of predominantly neutrophils and histiocytoid cells with nuclear debris in the superficial dermis without vasculitic changes (H&E, original magnification ×10).
Differential diagnosis
Clinical features observed in this patient were consistent with an acute encephalitis with myoclonus and neutrophilic dermatosis. We investigated infectious aetiologies that would explain the acute encephalitis, mainly viral agents (including herpes simplex virus 1 and 2, West Nile virus, and Enterovirus), non-viral agents (especially those that also cause skin rashes such as Rickettsia and Lyme disease), and bacterial and fungal causes of meningoencephalitis. Autoimmune encephalitides was another main aetiological category; therefore, we obtained both autoimmune and paraneoplastic antibody encephalitis panels from the CSF and serum. Other non-infectious inflammatory processes of the brain, including acute disseminated encephalomyelitis and collagen vascular disorders, were also assessed with serum and CSF testing alongside MRI brain neuroimaging. Finally, other rare causes of encephalitis comprising metabolic, chemical and toxins were excluded by history of exposure together with the respective laboratory work-up.8 Myoclonus and orofacial movements did not have an EEG correlate that would suggest epileptic seizures despite prolonged EEG monitoring.
The acute fever and the neutrophilic dermatosis suggested Sweet syndrome.9 Medical conditions most frequently associated with this syndrome include upper respiratory tract and gastrointestinal infections, autoimmune conditions such as inflammatory bowel disease, rheumatoid arthritis, autoimmune thyroiditis, and other connective disorders such as systemic lupus erythematosus and dermatomyositis. The patient had elevated titres of RF and ANA; however, further history, clinical examination and laboratory assays ruled out any of these diagnoses. Sweet syndrome can also be associated with malignancy, but a thorough evaluation for malignancy was negative. Lastly, drug reactions can account for a significant proportion of Sweet syndrome cases.9 However, there was no recent exposure to new medications except for the mRNA-1273 vaccine.
Treatment
The patient initially received empiric broad-spectrum antibiotics and antiviral coverage for suspected sepsis and meningitis, including vancomycin, cefepime (later switched to ceftriaxone), ampicillin, doxycycline and acyclovir (for 9 days) without any improvement. As no infectious aetiology was found, antimicrobial therapy was de-escalated. Given concerns that the clinical picture might be related to an inflammatory reaction to recent vaccination, we started a 4-day course of 1 g methylprednisolone once a day.
Outcome and follow-up
After the first day of treatment, the patient had marked improvement in his neurological examination and went from mumbling incomprehensible words to formulating full sentences and regaining orientation to self and place. He was able to follow simple commands consistently. Myoclonus also quickly resolved, as well as the orofacial movements. The patient progressively improved, achieving his baseline before the fourth dose of methylprednisolone. Moreover, the cutaneous findings significantly improved, with near complete resolution of the pustules within 36 hours of administration of steroids. At this time, he met both major and all four minor criteria for the diagnosis of Sweet syndrome. The patient was switched to prednisone 60 mg daily with a plan to taper over the course of 3 weeks. He was discharged 2 days afterwards with no evidence of neurological symptoms and normalisation of initially altered laboratory evaluations.
Discussion
This is the first report of an acute aseptic meningoencephalitis with Sweet syndrome following the mRNA-1273 vaccine. The patient met the Brighton Collaboration Encephalitis/Acute Disseminated Encephalomyelitis Working Group criteria10 for level 2 of diagnostic certainty for encephalitis after presenting with encephalopathy lasting for more than 24 hours, along with decreased to absent response to environment, decreased to absent eye contact, inconsistent response to external stimuli and decreased arousability, together with fever (>38°C), CSF pleocytosis (>5 white cell count/mm3) and EEG findings consistent with encephalitis (ie, diffuse background slowing), all of them as indicators of inflammation of the central nervous system.
After conducting a thorough infectious, malignant and autoimmune evaluation, no alternate aetiology was identified. Moreover, the patient did not improve with broad-spectrum antimicrobial therapy but showed a remarkable recovery after immunomodulation with steroids, further supporting the rationale that the clinical presentation was attributable to an inflammatory state.
While there are no published cases of isolated myoclonus post vaccination as witnessed in this patient, there are few reports of opsoclonus myoclonus presenting after receiving rubella,11 human papilloma virus12 and influenza vaccines,13 as well as opsoclonus myoclonus and ataxia with the yellow fever vaccine.14 Similarly, neutrophilic dermatoses, specifically Sweet syndrome, have been associated with several vaccines including pneumococcal, influenza, BCG and smallpox.9 The reported time frames from onset of cutaneous findings in relation to obtaining the vaccine has been 12 hours to 15 days.15 Sweet syndrome has also been described in association with COVID-1916; however, our patient tested negative for SARS-CoV-2 infection by RT-PCR, making it unlikely that this was a virus-mediated inflammatory response.
None of the clinical trials testing mRNA-1273 vaccine in humans reported adverse reactions such as those reported here. Commonly described systemic adverse events were dose-dependent7 and included fatigue, chills, headaches and myalgias, occurring in more than half of the participants, most frequently seen after the second dose of vaccination and lasting for a mean of 2.9 and 3.1 days after the first and second doses, respectively.6 In one of the phase I trials, one of the participants presented with transient urticaria after the first dose,7 while in another trial one of the participants developed paronychia 2 days after vaccination and later presented diffuse morbilliform eruption after treatment with trimethoprim-sulfamethoxazole.5 This reaction was deemed not related to vaccination and responded to systemic glucocorticoid administration.5 In the phase III trial, 1.5% of patients in the intervention arm reported hypersensitivity reactions, including a diverse array of dermatological manifestations, but none of those was described as fulfilling the diagnostic criteria for Sweet syndrome.6 Most recently, a series of 12 patients presenting with delayed large local skin reactions to the first dose of the mRNA-1273 vaccine were reported17; however, none of these patients evidenced similar dermatological features such as those described in this report.
According to the WHO ‘Causality assessment of an adverse event following immunization (AEFI)’,18 it was determined that both the encephalitis as well as the Sweet syndrome fit in the category for indeterminate B1 (where a temporal relationship is consistent but there is insufficient definitive evidence for vaccine causing the event (may be new vaccine-linked event)). We did not perform SARS-CoV-2 immunology assays in CSF or serum, which would further strengthen the hypothesis of vaccination as the cause of these adverse events.
Phases I–III clinical trials typically lack power to detect extremely rare adverse reactions and therefore postmarketing surveillance is important for the detection of these events.19 Adverse events are being actively monitored by the FDA as well as the Centers for Disease Control and Prevention, and safety monitoring from the trials are still ongoing and are expected to last for at least 2 years. Appropriate surveillance allows for establishing regular safety updates and risk characterisation while encouraging the use of vaccines as critical tools for disease control. The potential adverse event described in this case report was reported to FDA through the Vaccine Adverse Event Reporting System.
Patient’s perspective.
I would like to thank everybody for documenting and sharing the trials and tribulations of the trip down the road through the unknown that we recently took together. I am sure this document will help the health care professionals diagnose a patient with similar symptoms a bit quicker. I have all but completely recovered. Now the only unanswered question is whether I have as much protection against COVID as someone who has had both doses of the vaccine. I have tested for antibodies and the test results indicate that I have a high level of antibodies. Perhaps, I can be safely vaccinated by a different genre of vaccine in near future!
Learning points.
Prior reports of rare adverse reactions to SARS-CoV-2 vaccines include facial palsy and delayed skin reactions.
While encephalitis, opsoclonus/myoclonus syndrome and Sweet syndrome have been described after receiving other types of vaccines, they have not been reported in association with SARS-CoV-2 vaccination.
We established a temporal correlation between the mRNA-1273 vaccine along with the rash and encephalitis in this case report; skin biopsy was consistent with Sweet syndrome.
All neurological and dermatological manifestations completely resolved after methylprednisolone therapy.
Surveillance for similar presentations after COVID-19 vaccines is warranted.
Footnotes
Twitter: @DrGabrielNeuro
Contributors: All authors have contributed to the work and agree with the presented findings and that the work has not been published before nor is being considered for publication in another journal. GT-A, CRG, EMB and MIH conceptualised the report and made substantial contributions to the design, drafting and critical revision of the neurological aspects of this work. These authors approved the final version of the manuscript and assume accountability for all aspects of the work. JCM, YH and CW conceptualised the report and made substantial contributions to the design, drafting and critical revision of the dermatological aspects of this work. These authors approved the final version of the manuscript and assume accountability for all aspects of the work. MRS and SKA conceptualised the report and made substantial contributions to the design, drafting and critical revision of the infectious and immunological aspects of this work. These authors approved the final version of the manuscript and assume accountability for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Mahase E. Covid-19: WHO declares pandemic because of "alarming levels" of spread, severity, and inaction. BMJ 2020;368:m1036. 10.1136/bmj.m1036 [DOI] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawat K, Kumari P, Saha L. COVID-19 vaccine: a recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol 2021;892:173751. 10.1016/j.ejphar.2020.173751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med 2020;383:1544–55. 10.1056/NEJMoa2024671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020;383:2427–38. 10.1056/NEJMoa2028436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–16. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med Overseas Ed 2020;383:1920–31. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar R. Understanding and managing acute encephalitis. F1000Res 2020;9. 10.12688/f1000research.20634.1. [Epub ahead of print: 29 Jan 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol 2018;79:987–1006. 10.1016/j.jaad.2017.11.064 [DOI] [PubMed] [Google Scholar]
- 10.Sejvar JJ, Kohl KS, Bilynsky R, et al. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2007;25:5771–92. 10.1016/j.vaccine.2007.04.060 [DOI] [PubMed] [Google Scholar]
- 11.Lapenna F, Lochi L, Iliceto G. Post-vaccinic opsoclonus-myoclonus syndrome: a case report. Parkinsonism Relat Disord 2000;6:241–2. 10.1016/S1353-8020(00)00020-1 [DOI] [PubMed] [Google Scholar]
- 12.McCarthy JE, Filiano J. Opsoclonus myoclonus after human papilloma virus vaccine in a pediatric patient. Parkinsonism Relat Disord 2009;15:792–4. 10.1016/j.parkreldis.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 13.Piquet A, Kothari M, Ermak D, et al. Opsoclonus-Myoclonus syndrome post-vaccination and viral illness. Int J Clin Med 2012;03:304–6. 10.4236/ijcm.2012.34060 [DOI] [Google Scholar]
- 14.Martins RdeM, Pavão ALB, de Oliveira PMN, et al. Adverse events following yellow fever immunization: report and analysis of 67 neurological cases in Brazil. Vaccine 2014;32:6676–82. 10.1016/j.vaccine.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 15.Pedrosa AF, Morais P, Nogueira A, et al. Sweet's syndrome triggered by pneumococcal vaccination. Cutan Ocul Toxicol 2013;32:260–1. 10.3109/15569527.2012.759960 [DOI] [PubMed] [Google Scholar]
- 16.Taşkın B, Vural S, Altuğ E, et al. Coronavirus 19 presenting with atypical sweet's syndrome. J Eur Acad Dermatol Venereol 2020;34:e534–5. 10.1111/jdv.16662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumenthal KG, Freeman EE, Saff RR, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med 2021;384:1273–7. 10.1056/NEJMc2102131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . Causality assessment of an adverse event following immunization (AEFI): user manual for the revised who classification. 2 edn, 2019. [Google Scholar]
- 19.Umscheid CA, Margolis DJ, Grossman CE. Key concepts of clinical trials: a narrative review. Postgrad Med 2011;123:194–204. 10.3810/pgm.2011.09.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]