Abstract
Spontaneous preterm labor is one of the “great obstetrical syndromes” which may lead to preterm delivery, a leading cause for neonatal and infant death. Prostaglandins are considered universal mediators for the onset of spontaneous labor at term. This concept is largely based on the observations that amniotic fluid concentrations of prostaglandins are elevated prior to and during the onset of labor; however, these studies have largely been performed using immunoassays. Distinguishing prostaglandins from similarly structured molecules (i.e. prostamides) is difficult given the cross-reactivity of available antibodies and the chemical similarity of this family of compounds. Herein, this limitation was overcome by utilizing mass spectrometry to determine prostaglandin and prostamide concentrations in the amniotic fluid of women who had an episode of preterm labor with intact membranes. Patients were classified into the following groups: 1 subsequent delivery at term (n=23); 2) preterm delivery in the absence of intra-amniotic inflammation (n=51); 3) preterm delivery with sterile intra-amniotic inflammation (amniotic fluid interleukin (IL)-6 >2.6 ng/mL without detectable microorganisms) (n=35); and 4) preterm delivery with intra-amniotic infection [amniotic fluid IL-6 > 2.6 ng/mL with detectable microorganisms] (n=16). Amniotic fluid samples were analyzed by liquid chromatography-tandem mass spectrometry. We found that 1) both prostaglandins and prostamides were detectable and distinguishable in the amniotic fluid of women with preterm labor; 2) amniotic fluid concentrations of PGE2, PGF2α, and PGFM were higher in patients with intra-amniotic infection than in those without intra-amniotic inflammation; 3) amniotic fluid concentrations of PGE2 and PGF2α were also greater in patients with intra-amniotic infection than in those with sterile intra-amniotic inflammation; 4) patients with sterile intra-amniotic inflammation had higher amniotic fluid concentrations of PGE2 and PGFM than those without intra-amniotic inflammation who delivered at term; 5) amniotic fluid concentrations of PGFM were also greater in women with sterile intra-amniotic inflammation than in those without intra-amniotic inflammation who delivered preterm; 5) amniotic fluid concentrations of prostamides (PGE2-EA and PGF2α-EA) were not different among patients with preterm labor; 6) amniotic fluid concentrations of prostaglandins, but no prostamides, were higher in cases with intra-amniotic inflammation (interleukin-6 >2.6 ng/mL); and 7) the PGE2:PGE2-EA and PGF2α:PGF2α-EA ratios were higher in patients with intra-amniotic infection compared to those without inflammation. Mass spectrometric analysis of amniotic fluid indicated that amniotic fluid concentrations of PGE2, PGF2α, and PGFM, but no prostamides, were significantly higher in women with preterm labor and intra-amniotic infection than in other patients with an episode of preterm labor. Yet, women with intra-amniotic infection had greater amniotic fluid concentrations of PGE2, and PGF2α than those with sterile intra-amniotic inflammation, suggesting that these two clinical conditions may be differentiated by using mass spectrometric analysis of amniotic fluid.
Keywords: chorioamnionitis, eicosanoids, mass spectrometry, parturition, prostamides
Introduction
Preterm birth (delivery before 37 weeks of gestation) and its complications are responsible for 35% of the 3.1 million neonatal deaths per year [1,2]. Each year, about 15 million preterm neonates are born worldwide [3]. The risk factors for preterm birth include prior preterm birth, maternal nutritional status, a very low maternal body mass index or maternal obesity, ethnicity, socioeconomic status, smoking during pregnancy, maternal age, parity, use of assisted reproductive technologies, a short cervix, and multi-fetal gestations [4–21]. Preterm birth is associated with severe short- and long-term complications, including bronchopulmonary dysplasia, cerebral palsy, blindness, and deafness [22–26].
Preterm labor is a syndrome caused by multiple pathologic processes [27,28]. Intra-amniotic infection/inflammation affects one in four preterm births and is largely subclinical in nature [29–35]. However, there is compelling evidence that the relationship between intra-amniotic infection/inflammation and spontaneous preterm labor is causal [31,35–48]. Intra-amniotic inflammation can result from microbial invasion of the amniotic cavity, referred to as intra-amniotic infection (IAI), or it can occur in the absence of detectable microorganisms using both culture and molecular microbiological techniques (i.e. “sterile intra-amniotic inflammation” or SIAI) [49–54]. The latter is an inflammatory process thought to be induced by danger signals or alarmins (endogenous molecules derived from cellular stress or necrosis [55–57]) found in the amniotic fluid [58–65]. Sterile intra-amniotic inflammation is more commonly observed than intra-amniotic infection in women with preterm labor and intact membranes [49,50]; however, to date, there are no biomarkers that can assist in the differential diagnosis of these two clinical conditions.
Prostaglandins are central mediators in the process of preterm and term parturition [66–78]. This family of molecules can induce uterine contractility and cervical ripening and participate in the mechanisms of extracellular matrix remodeling in the chorioamniotic membranes [27,71,79]. Both term and preterm labor require a combination of endocrine and mechanical stimuli from both mother and infant [27,79–85], including products of prostaglandin endoperoxide synthase-2 (PGHS, also known as PTGS-2 or cyclooxygenase-2 [COX-2]) [86,87]. Spontaneous parturition is preceded by an increased concentration of prostaglandins in the amniotic fluid, which is a cause—and not a result—of labor at term [88], given that it occurs before onset [89]. Therefore, the determination of prostaglandin concentrations could be of potential clinical value.
The accurate identification of endocannabinoids and eicosanoids by immunoassay has been a major challenge in parturition research. The lack of specific antibodies and the similarity of the molecular structures of these substances have led to difficulty in distinguishing among these moieties [90]. In recent years, the use of high-resolution mass spectrometry has enabled the more specific identification of prostaglandins and prostamides in biological fluids and placental explant culture media [74,76,77,91,92]. Therefore, herein, we used mass spectrometry to determine the prostaglandin and prostamide concentrations of the amniotic fluid of women presenting with an episode of preterm labor with intact membranes. Since prostaglandins are inflammatory mediators, we also determined whether the concentrations varied between patients with intra-amniotic infection and those with sterile intra-amniotic inflammation.
Methods
Sample collection
A retrospective cross-sectional study was conducted by searching the clinical database and Bank of Biologic Samples of the Detroit Medical Center, Wayne State University, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS; Bethesda, MD, and Detroit, MI, USA). The inclusion criteria required 1) a singleton gestation; 2) a transabdominal amniocentesis performed between 20 and 36 weeks of gestation, before the rupture of the chorioamniotic membranes; and 3) sufficient amniotic fluid available for mass spectrometry studies.
All patients provided written informed consent before the collection of amniotic fluid samples. The collection and utilization of the samples were approved by the Institutional Review Boards of the participating institutions where the patients received care. Many of these samples had been used in previous studies of the biology of cytokines and inflammatory mediators in intra-amniotic infection/inflammation [49,50,54,64,76,93–96].
Clinical definitions
Preterm labor was diagnosed by the presence of at least two regular uterine contractions every 10 min associated with cervical changes in patients with a gestational age between 20 and 36/37 weeks. Microbial invasion of the amniotic cavity was diagnosed based on the results of amniotic fluid cultures for microorganisms [36,97–100] and broad-range polymerase chain reaction (PCR) assays, coupled with electrospray ionization mass spectrometry (PCR/ESI-MS; Ibis Technology; Athogen, Carlsbad, CA, USA) [49–51,53,54,64,77,101–108]. Intra-amniotic inflammation was defined as an amniotic fluid interleukin (IL)-6 concentration ≥ 2.6 ng/mL [32,62,93–96,109–115]. Intra-amniotic infection was defined as the presence of microbial invasion of the amniotic cavity with intra-amniotic inflammation [49–51,53,96,102–106,116–131]. Sterile intra-amniotic inflammation was defined as intra-amniotic inflammation without microorganisms detected by culture or PCR/ESI-MS [49–51,53,54].
Women who had an episode of preterm labor (PTL) were divided into four groups: 1) subsequent delivery at term (PTL-TD); 2) preterm delivery without intra-amniotic inflammation (PTL-PTD NI); 3) preterm delivery with sterile intra-amniotic inflammation (PTL-PTD SIAI); and 4) preterm delivery with intra-amniotic infection (PTL-PTD IAI).
Sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis performed for evaluation of the microbial and inflammatory status (i.e. Gram stain [132], white blood cell count [133], and amniotic fluid glucose concentration [134]) of the amniotic cavity in patients diagnosed with an episode of preterm labor. The results of microbiology and the inflammatory status of the amniotic fluid were used for clinical management. Samples of amniotic fluid were transported to the laboratory in a sterile, capped syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas. Amniotic fluid IL-6 concentrations were used only for research purposes.
Detection of microorganisms with molecular methods
In addition to standard cultivation techniques, which determined aerobic and anaerobic bacteria as well as genital mycoplasmas, the amniotic fluid was analyzed by using broad-range real-time PCR/ESI-MS (Ibis Technology; Athogen) [49]. In brief, DNA was extracted from 300 μl of amniotic fluid by using a method that combines bead-beating cell lysis with a magnetic bead-based extraction method [135,136]. The extracted DNA was amplified by the previously described broad bacteria and Candida (BAC) detection assay, according to the manufacturer’s instructions. PCR/ESI-MS can identify 3400 bacteria and 40 Candida spp., which are represented in the platform’s signature database [137,138]. After PCR amplification, 30 μl aliquots of each PCR product were desalted and analyzed via ESI-MS. The presence of microorganisms was determined by signal processing and triangulation analysis of all base composition signatures obtained from each sample and compared to a database. The sensitivity (limit of detection) of the assay for the detection of bacteria in blood, on average, is 100 CFU/ml (95% CI, 6–600 CFU/ml). A comparison of detection limits between the blood and amniotic fluid showed that the assays have comparable detection limits (100 CFU/ml).
Determination of IL-6 in AF
The concentrations of IL-6 in amniotic fluid were determined by a sensitive and specific enzyme immunoassay obtained from R&D Systems (Minneapolis, MN, USA). The initial assay validation was performed in our laboratory before this study was conducted. The immunoassay uses the quantitative sandwich enzyme immunoassay technique, and the concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for IL-6 were 8.7 and 4.6%, respectively. The sensitivity of the assay for IL-6 was 0.09 pg/ml.
Mass spectrometry sample preparation
Standards and samples were subjected to an extraction protocol in organic solution. Extraction solution was prepared containing internal standards (250fmol each). Internal standards include the deuterated standards, namely, for prostaglandin E2-d4 (PGE2-d4), prostaglandin F2α-d4 (PGF2α-d4), 13,14-dihydro-15-keto-PGF(2alpha) -d4 (PGFM-d4), prostaglandin E2-ethanolamide-d4 (PGE2-EA-d4), and prostaglandin F2α-ethanolamide-d4 (PGF2α-EA-d4) in an extraction solution of methanol/formic acid (99:1). The extraction solution (250 μL) was added to a 96-plate well and vortexed (900 rpm, 5 min) followed by the addition of 850μL of chilled water, placed on a Teflon mat, and vortexed (900 rpm, 5 min). Using a vacuum manifold, the solid phase extraction (SPE) plate was pre-equilibrated consecutively using 500μL of methanol and 2 × 500μL of water. The lipid/eicosanoid extract was loaded onto the plate on low flow (< 5 mm Hg or dropwise and the gauge was slowly closed until <5mmHg was reached) onto the SPE plate and left to bind for 1 min. The plate was then washed twice with chilled water. The clean and bound lipid extract preparation was eluted and dried. The dried lipids were then reconstituted in an appropriate solvent (20% Methanol in water) and prepared in triplicate (sample replicates).
Mass Spectrometry Analyses
Ultra-performance liquid chromatography (UPLC; Shimadzu Ltd) was used for separation of the sample analytes coupled with a Kinetek C8 column (Phenomenex Australia Pty Ltd) attached to a guard column (Phenomenex Australia Pty Ltd). Oven temperature was set to 60°C and a 15-min gradient was set up using aqueous and organic mobile phase solvent preparations. A scheduled multiple reaction monitoring (sMRM) method was carried out for the analyses and quantitation of 5 analytes (PGE2, PGF2α, PGFM, PGE2-EA, and PGF2α-EA) and their respective deuterated versions (PGE2-d4, PGF2α-d4, PGFM-d4, PGE2-EA-d4, and PGF2α-EA-d4) by using both negative and positive modes. Each sample was injected in triplicate (technical replicates). A list of mass spectrometry conditions for both negative and positive modes is provided in Supplementary Material 1 (including limits of detection).
MultiQuant Analyses
Individual peaks for each analyte were selected using the retention time window of +/− 0.5 min. A signal-to–noise ratio of below 10 was excluded from the list. The multiquant data were used to generate area and peak area ratio (PAR) information for both standards and samples. The standard curve was plotted with individual concentrations against the PARs. The equation of the line was used to extrapolate the concentrations (pmol/L) for the samples.
Statistical analyses
The linear mixed model method was applied to analyze differences in amniotic fluid concentration among the study groups. Linear mixed modeling offers flexibility to account for dependency between observations from the same individual. Additionally, the linear mixed model method allows each sample to contribute to the variance estimation, suitable for analyzing repeated measurements with a missing value, contrary to the traditional analysis of variance approach. The amniotic fluid concentration for each analyte was log-transformed to better fit the normal distribution of the data. The Fisher’s exact test for categorical variables and the Kruskal-Wallis test for continuous variables were applied to compare maternal characteristics among the groups, with the P values for multiple testing corrected for the false discovery rate in post-hoc analysis. All the analyses were conducted using R language and environment for statistical computing (www.r-project.org).
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of patients in the four study groups are presented in Table 1. A total of 125 patients, comprising the PTL-TD NI (n=23), PTL-PTD NI (n=51), PTL-PTD SIAI (n=35), and PTL-PTD IAI (n=16) groups, was involved in the study. Gestational ages at amniocentesis/delivery and birthweights were significantly different among the groups (Table 1). Lower gestational age at delivery and lower birthweight were observed in women with PTL-PTD IAI and PTL-PTD SIAI compared to those delivering at term (PTL-TD NI) and delivering preterm (PTL-PTD NI) without infection. No significant differences were identified in maternal age or body mass index among the study groups.
Table 1.
Maternal and clinical characteristics compared among the groups.
| Clinical characteristics | PTL-TD NI (n=23) |
PTL-PTD NI (n=51) |
PTL-PTD SIAI (n=35) |
PTL-PTD IAI (n=16) |
p-value |
|---|---|---|---|---|---|
| Age (years; median [IQR]) | 25 (21–29) | 23 (20–26) | 23 (20–26.5) | 25 (20.75–30.25) | 0.47 |
| Height (cm; median [IQR]) | 160 (154–165) | 162 (157–166) | 162 (157–167) | 166 (160–170) | 0.31 |
| Weight (kg; median [IQR]) | 60 (54–76) | 61 (54–74) | 72 (52–86) | 70 (63–77) | 0.18 |
| Body mass index (kg/m2; median [IQR]) | 23.9 (21–30.8) | 23.6 (21.1–28.9) | 26.2 (22.1–32.4) | 27 (24.9–33.5) | 0.27 |
| Parity (parous; n[%]) | 17 (73.9) | 33 (64.7) | 20 (57.1) | 7 (43.8) | 0.25 |
| Race (African-American; n[%]) | 20 (87) | 45 (88.2) | 31 (88.6) | 12 (75) | 0.55 |
| Smoking status (smoker; n[%]) | 5 (21.7) | 13 (25.5) | 8 (22.9) | 3 (18.8) | 0.98 |
| Gestational age at amniocentesis (weeks; median [IQR]) | 31.9 (31–32.2) | 31.4 (28.9–32.7) | 24.35 (23.3–28.1) | 26.5 (24.8–32.1) | <.001 |
| Gestational age at delivery (weeks; median [IQR]) | 39 (37.5–39.9) | 34.9 (33.2–36) | 26.4 (24.2–30.5) | 26.7 (25.6–32.6) | <.001 |
| Birthweight (grams; median [IQR]) | 3080 (2772–3340) | 2312 (1990–2588) | 890 (558–1415) | 1048 (747–1970) | <.001 |
| Sex (male; n[%]) | 9 (39.1) | 24 (48) | 15 (42.9) | 8 (50) | 0.86 |
IQR = interquartile range.
Mass spectrometric detection of prostaglandins in the amniotic fluid of women with preterm labor and intact membranes
Figure 1 shows the linear calibration range of prostaglandins and prostamides. Each molecule was positively identified by UPLC retention time with an authentic standard and unique MRM transition.
Figure 1. Linear calibration range of prostaglandins and prostamides.
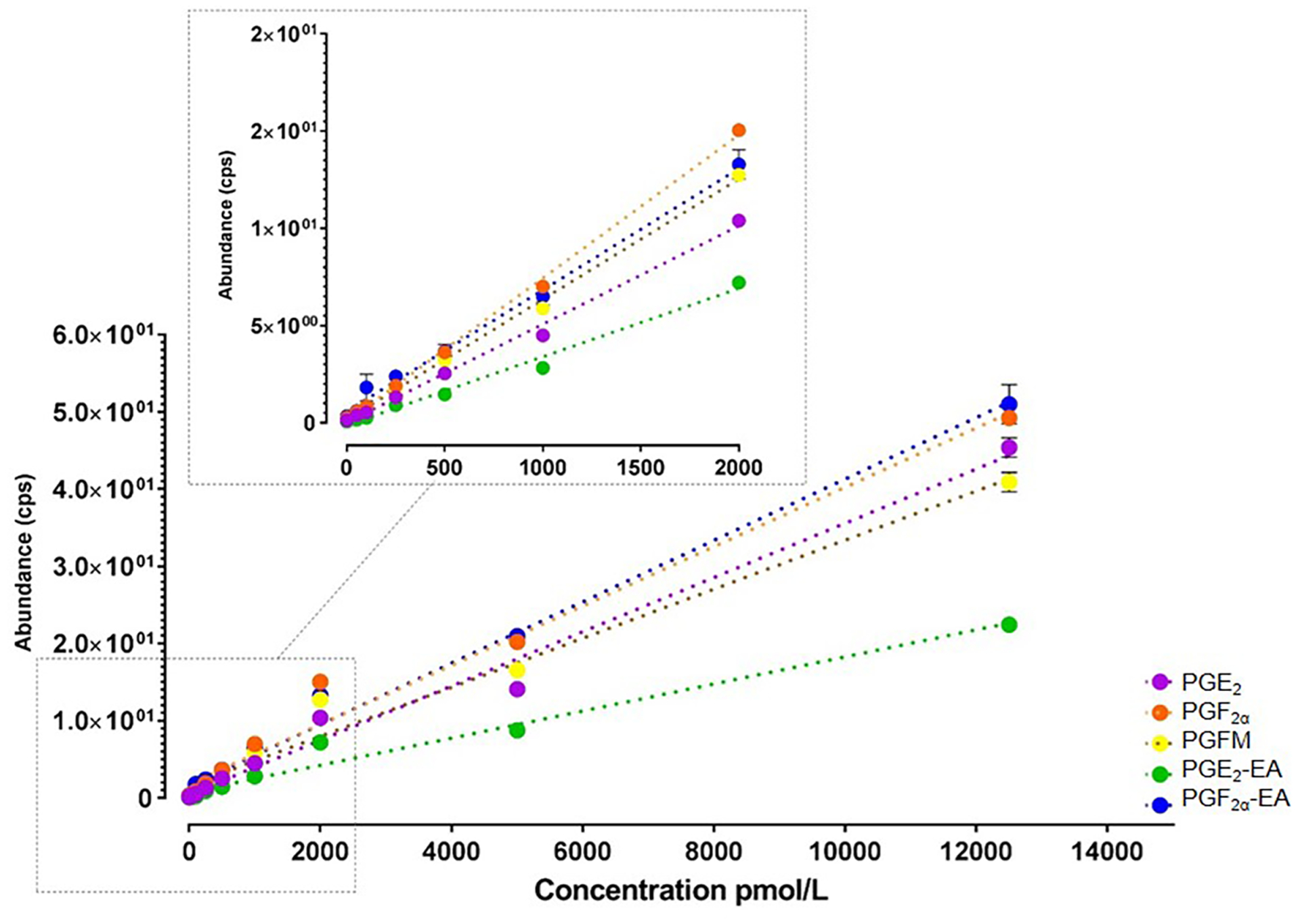
LC-MS/MS multiple reaction monitoring (MRM) transitions for PGE2, PGF2a, PGFM, PGE2-EA, and PGF2a-EA. Each detectable molecule was positively identified by UPLC retention time with an authentic standard and unique MRM transition.
The amniotic fluid concentrations of prostaglandins and prostamides among the four study groups are described in Figure 2, and statistics are provided in Table 2 and Table 3. Patients with intra-amniotic infection had significantly higher median concentrations of PGE2 (p<0.001), PGF2α (p<0.001), and PGFM (p<0.001) than those who delivered either at term or preterm without infection or inflammation (Figure 2A–C). In addition, patients with intra-amniotic infection had greater median concentrations of PGE2 (p<0.001) and PGF2α (p<0.001) than those with sterile intra-amniotic inflammation (Figure 2A&B).
Figure 2. Amniotic fluid concentrations of prostaglandins and prostamides in patients with preterm labor.

Amniotic fluid concentrations of PGE2 (A), PGF2a (B), PGFM (C), PGE2-EA (D), and PGF2a-EA (E) were determined using LC-MS/MS. Women who had an episode of preterm labor (PTL) were divided into four groups: (1) term delivery (PTL-TD); (2) preterm delivery without intra-amniotic inflammation (PTL-PTD NI); (3) preterm delivery with sterile intra-amniotic inflammation (PTL-PTD SIAI); and (4) preterm delivery with intra-amniotic infection (PTL-PTD IAI).
Table 2.
Amniotic fluid analyte concentration differences among study groups.
| Analyte | Log2 fold change (SD) with PTL-TD NI as reference group | p-value | ||
|---|---|---|---|---|
| PTL-PTD NI | PTL-PTD SIAI | PTL-PTD IAI | ||
| PGE2 | 0.318 (0.412) | 1.142 (0.445) | 4.166 (0.541) | <.001 |
| PGF2α | 0.102 (0.261) | 0.28 (0.278) | 2.202 (0.344) | <.001 |
| PGFM | 0.618 (0.323) | 2.913 (0.345) | 3.616 (0.427) | <.001 |
| PGE2.EA | 0.13 (0.199) | −0.083 (0.206) | 0.267 (0.258) | 0.378 |
| PGF2α.EA | 0.029 (0.266) | −0.007 (0.279) | −0.219 (0.361) | 0.893 |
| PGE2:PGE2.EA | 0.071 (0.457) | 0.858 (0.5) | 3.793 (0.599) | <.001 |
| PGF2α:PGF2α.EA | 0.088 (0.361) | 0.156 (0.378) | 1.798 (0.494) | 0.001 |
SD = standard deviation.
Table 3.
Post-hoc analysis of amniotic fluid concentration differences among study groups per analyte.
| Analyte | Group pair | Fold change | Log2 fold change | Standard error | Adjusted p-value |
|---|---|---|---|---|---|
| PGE2 | PTL–TD NI vs PTL–PTD NI | 1.247 | 0.318 | 0.412 | 0.64 |
| PTL–TD NI vs PTL–PTD SIAI | 2.207 | 1.142 | 0.445 | 0.03 | |
| PTL–TD NI vs PTL–PTD IAI | 17.951 | 4.166 | 0.541 | <.001 | |
| PTL–PTD NI vs PTL–PTD SIAI | 1.77 | 0.824 | 0.37 | 0.065 | |
| PTL–PTD NI vs PTL–PTD IAI | 14.41 | 3.849 | 0.481 | <.001 | |
| PTL–PTD SIAI vs PTL–PTD IAI | 8.134 | 3.024 | 0.51 | <.001 | |
| PGF2α | PTL–TD NI vs PTL–PTD NI | 1.073 | 0.102 | 0.261 | 0.813 |
| PTL–TD NI vs PTL–PTD SIAI | 1.214 | 0.28 | 0.278 | 0.512 | |
| PTL–TD NI vs PTL–PTD IAI | 4.601 | 2.202 | 0.344 | <.001 | |
| PTL–PTD NI vs PTL–PTD SIAI | 1.131 | 0.178 | 0.228 | 0.64 | |
| PTL–PTD NI vs PTL–PTD IAI | 4.287 | 2.1 | 0.305 | <.001 | |
| PTL–PTD SIAI vs PTL–PTD IAI | 3.789 | 1.922 | 0.32 | <.001 | |
| PGFM | PTL–TD NI vs PTL–PTD NI | 1.535 | 0.618 | 0.323 | 0.122 |
| PTL–TD NI vs PTL–PTD SIAI | 7.532 | 2.913 | 0.345 | <.001 | |
| PTL–TD NI vs PTL–PTD IAI | 12.261 | 3.616 | 0.427 | <.001 | |
| PTL–PTD NI vs PTL–PTD SIAI | 4.908 | 2.295 | 0.282 | <.001 | |
| PTL–PTD NI vs PTL–PTD IAI | 7.994 | 2.999 | 0.378 | <.001 | |
| PTL–PTD SIAI vs PTL–PTD IAI | 1.629 | 0.704 | 0.397 | 0.158 | |
| PGE2.EA | PTL–TD NI vs PTL–PTD NI | 1.094 | 0.13 | 0.199 | 0.692 |
| PTL–TD NI vs PTL–PTD SIAI | 0.944 | −0.083 | 0.206 | 0.813 | |
| PTL–TD NI vs PTL–PTD IAI | 1.203 | 0.267 | 0.258 | 0.507 | |
| PTL–PTD NI vs PTL–PTD SIAI | 0.863 | −0.213 | 0.16 | 0.324 | |
| PTL–PTD NI vs PTL–PTD IAI | 1.1 | 0.138 | 0.223 | 0.692 | |
| PTL–PTD SIAI vs PTL–PTD IAI | 1.275 | 0.351 | 0.229 | 0.234 | |
| PGF2α.EA | PTL–TD NI vs PTL–PTD NI | 1.02 | 0.029 | 0.266 | 0.934 |
| PTL–TD NI vs PTL–PTD SIAI | 0.995 | −0.007 | 0.279 | 0.981 | |
| PTL–TD NI vs PTL–PTD IAI | 0.859 | −0.219 | 0.361 | 0.692 | |
| PTL–PTD NI vs PTL–PTD SIAI | 0.975 | −0.036 | 0.223 | 0.921 | |
| PTL–PTD NI vs PTL–PTD IAI | 0.842 | −0.248 | 0.319 | 0.64 | |
| PTL–PTD SIAI vs PTL–PTD IAI | 0.863 | −0.212 | 0.331 | 0.692 | |
| PGE2:PGE2.EA | PTL–TD NI vs PTL–PTD NI | 1.05 | 0.071 | 0.457 | 0.921 |
| PTL–TD NI vs PTL–PTD SIAI | 1.813 | 0.858 | 0.5 | 0.17 | |
| PTL–TD NI vs PTL–PTD IAI | 13.861 | 3.793 | 0.599 | <.001 | |
| PTL–PTD NI vs PTL–PTD SIAI | 1.725 | 0.787 | 0.397 | 0.111 | |
| PTL–PTD NI vs PTL–PTD IAI | 13.196 | 3.722 | 0.517 | <.001 | |
| PTL–PTD SIAI vs PTL–PTD IAI | 7.642 | 2.934 | 0.556 | <.001 | |
| PGF2α:PGF2α.EA | PTL–TD NI vs PTL–PTD NI | 1.063 | 0.088 | 0.361 | 0.909 |
| PTL–TD NI vs PTL–PTD SIAI | 1.114 | 0.156 | 0.378 | 0.813 | |
| PTL–TD NI vs PTL–PTD IAI | 3.477 | 1.798 | 0.494 | <.001 | |
| PTL–PTD NI vs PTL–PTD SIAI | 1.048 | 0.068 | 0.301 | 0.909 | |
| PTL–PTD NI vs PTL–PTD IAI | 3.272 | 1.71 | 0.438 | <.001 | |
| PTL–PTD SIAI vs PTL–PTD IAI | 3.119 | 1.641 | 0.453 | <.001 |
Patients with sterile intra-amniotic inflammation had higher median concentrations of PGE2 (p=0.03) and PGFM (p<0.0001) than those who delivered at term without intra-amniotic inflammation (Figure 2A&C). These patients also had a higher median concentration of PGFM than those with preterm labor who delivered preterm without intra-amniotic inflammation (p<0.001; Figure 2C).
No significant differences were observed in the concentrations of PGE2-EA and PGF2α-EA among the study groups (Figure 2D&E).
Amniotic fluid concentrations of PGE2 (p<0.001), PGF2α (p<0.001), and PGFM (p<0.001) were significantly higher in cases with intra-amniotic inflammation (IL-6 >2.6 n/ml) (Figure 3A–C). However, amniotic fluid concentrations of prostamides were not significantly higher in cases with intra-amniotic inflammation (IL-6 >2.6 n/ml) (Figure 3D&E).
Figure 3. Correlations between amniotic fluid concentrations of prostaglandins-prostamides and intra-amniotic inflammation in patients with preterm labor.
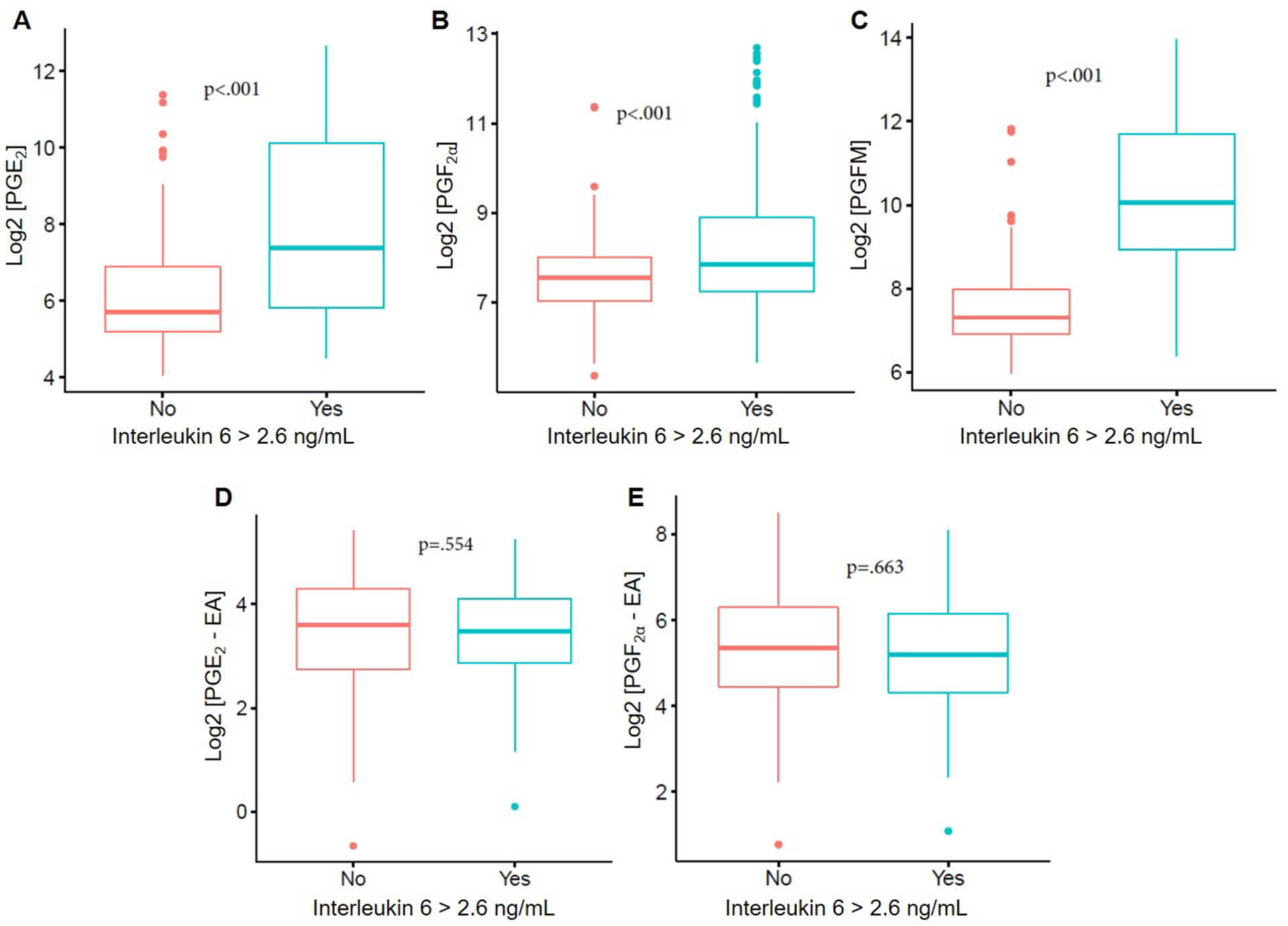
Correlations between amniotic fluid concentrations of PGE2 (A), PGF2a (B), PGFM (C), PGE2-EA (D), and PGF2a-EA (E) and elevated concentrations of IL-6 (2.6 ng/mL).
When the ratio of prostaglandin-to-prostamide was compared (Figure 4), significant differences were observed between patients with intra-amniotic infection or those with sterile intra-amniotic inflammation compared to the other study groups. Patients with either intra-amniotic infection or sterile intra-amniotic inflammation had higher ratios of PGE2: PGE2-EA (p<0.001) and PGF2α: PGF2α-EA (p<0.001) compared to patients without intra-amniotic inflammation (Figure 4A&B).
Figure 4. Amniotic fluid prostaglandin and prostamides ratios in patients with preterm labor.
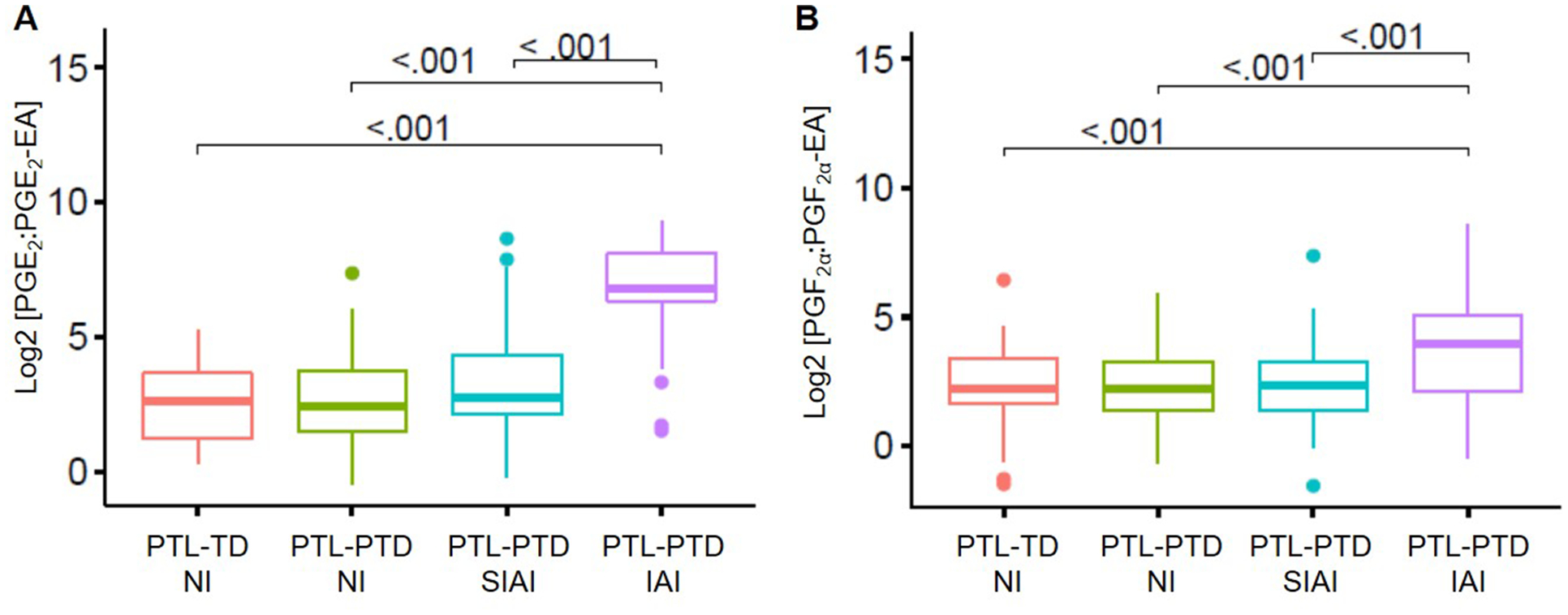
Ratio of PGE2 to PGE2-EA and PGF2a to PGF2a-EA in the study groups. Women who had an episode of preterm labor (PTL) were divided into four groups: (1) term delivery (PTL-TD); (2) preterm delivery without intra-amniotic inflammation (PTL-PTD NI); (3) preterm delivery with sterile intra-amniotic inflammation (PTL-PTD SIAI); and (4) preterm delivery with intra-amniotic infection (PTL-PTD IAI).
Discussion
Principal findings of the study:
1) The amniotic fluid concentrations of PGE2, PGF2α, and PGFM were significantly higher in patients with intra-amniotic infection than in those without inflammation who delivered preterm or at term; 2) patients with intra-amniotic infection had significantly higher median amniotic fluid concentrations of PGE2 and PGF2α than those with sterile intra-amniotic inflammation; 3) the amniotic fluid concentrations of PGE2 and PGFM were higher in women with sterile intra-amniotic inflammation than in those without inflammation; and 4) the amniotic fluid concentrations of prostamides did not differ according to the presence or absence of sterile intra-amniotic inflammation or intra-amniotic infection in patients with preterm labor.
Prostaglandins in term labor
Eicosanoids, the essential lipid molecules, play a major role in inflammation [139] as well as in several key reproductive processes (e.g. ovulation [140,141], implantation [142], maintenance of pregnancy [143], and parturition [66–78]). Eicosanoids are synthesized via the metabolism of arachidonic acid through the action of several enzymes, giving rise to three major groups of molecules: prostanoids (derived from arachidonic acid), prostamides (derived from anandamide), and prostaglandin glycerol esters (derived from 2-acyl glycerol) [139]. During late pregnancy, the amniotic fluid contains factors that trigger the production of PGF2α by the fetal membranes [144–146], and concentrations of amniotic fluid PGF2α and PGE2 are elevated during the course of labor [70,73,74]. Thus, the evidence indicating that eicosanoids are involved in the physiological process of term parturition is solid.
Inflammation and infection in preterm labor
Preterm labor is a syndrome caused by multiple pathologic processes [28]. Intra-amniotic infection is an important cause of preterm labor [27,28]. Most cases of intra-amniotic infection follow an ascending pathway from the lower genital tract [147]. Microbial invasion of the amniotic cavity has been identified in 20% of preterm births [98] and 40–50% of preterm births with premature rupture of the membranes [148,149]. Indeed, with the application of molecular microbiologic techniques (detection of microbial DNA), the rate of intra-amniotic infection has become higher than with the use of cultivation techniques only [49,50,53,150–155]. Microorganisms elicit a response via the activation of the immune system of the mother and fetus [48,108,117,118,120,156–158], resulting in the release of inflammatory cytokines [156,158] as well as the stimulation of prostaglandin production [159–161].
Sterile intra-amniotic inflammation also contributes to spontaneous preterm parturition [49,50]. This condition is associated with elevated concentrations of endogenous danger signals or alarmins in the amniotic fluid [58–62,64,162]. The mechanisms whereby alarmins induce sterile intra-amniotic inflammation and preterm birth involve the activation of the inflammasome [54,65,163–166], a multi-protein complex responsible for the processing of mature IL-1β [167,168].
Intra-amniotic infection is the most important identifiable cause of preterm labor [169]. Infection may induce the secretion of cross-reacting prostamides as seen by the anandamide release that has been observed in response to hemorrhagic shock [170] and a challenge of bacterial endotoxin in human peripheral lymphocytes [171]. Moreover, COX-2, which can be induced by several inflammatory stimuli such as IL-1β and bacterial endotoxin [87,172–176], may be present at the site of inflammation or infection, and the increases in both anandamide and COX-2 may synergistically induce secretion of PGE2-ethanolamide.
Prostaglandins in preterm labor
Previous studies have reported an association between changes in the eicosanoid concentration and preterm parturition [68,76,78,91,177–179]. Using radioimmunoassays, higher amniotic fluid concentrations of PGE2 and PGF2α have been reported in patients with premature rupture of the membranes (PROM) who delivered preterm and had an identifiable infection compared to those without infection [178]. Moreover, women with preterm labor and intact membranes with intra-amniotic infection had increased amniotic fluid concentrations of PGE2, PGFM, and PGF2α [68,78,177]. Our findings are in agreement with the latter report, given the identification that amniotic fluid concentrations of PGE2, PGF2α, and PGFM are increased in pregnancies complicated by preterm labor with intra-amniotic infection compared to those without inflammation.
Using gas chromatography, investigators have measured concentrations of PGE2, PGD2, and PGF2α, among others, in the amniotic fluid of patients who delivered at preterm or term [91]. Higher concentrations of PGE2 and PGD2 were found in the amniotic fluid of term compared to preterm deliveries [91]. However, PGF2α concentrations were higher in preterm births compared to term deliveries [91]. The authors also reported that the presence of microorganisms in the amniotic cavity was not associated with increased concentrations of PGE2, PGD2, and PGF2α [91]. This finding contrasts with our previous report using radioimmunoassays, in which the amniotic fluid concentrations of PGE2, PGF2α, PGFM, 14-dihydro-15-keto-prostaglandin F2α, and 11-deoxy-13 were significantly higher in the context of intra-amniotic infection [68]. In our study, PGE2 and PGFM were higher in preterm pregnancies with intra-amniotic infection compared to those without inflammation. Moreover, in comparison to sterile intra-amniotic inflammation, significantly higher concentrations of PGE2 and PGF2α were observed in the amniotic fluid of women with intra-amniotic infection. This finding is consistent with previous reports showing that intra-amniotic inflammation, whether sterile or microbial-associated, resulted in higher amniotic fluid concentrations of PGE2 compared to those without inflammation [180]. Moreover, PGF2α concentrations were higher in the amniotic fluid of pregnancies with preterm premature rupture of the membranes and intra-amniotic inflammation, compared to those without this clinical condition (as determined by MMP-8 >23ng/mL and negative culture) [179]. Higher concentrations of PGF2α, regardless of their inflammatory status (and adjusted for gestational age), correlated with earlier delivery; therefore, it was proposed that PGF2α could be a predictor of early delivery [179]. Our observation was that higher concentrations of PGE2 and PGFM (but no significant difference in PGF2α) were found in the amniotic fluid of women with sterile intra-amniotic inflammation compared to those without inflammation delivering preterm or at term. Therefore, amniotic fluid PGF2α may serve as a predictor of early delivery in women with preterm premature rupture of the membranes and intra-amniotic inflammation but not in those with preterm labor and intact membranes.
It is worth mentioning that patients with an episode of preterm labor who delivered preterm without intra-amniotic inflammation did not have increased concentrations of prostaglandins compared to those who delivered at term. These data suggest that the pathophysiology of idiopathic preterm labor and birth (without intra-amniotic inflammation) involves mechanisms different from those implicated in intra-amniotic inflammation-associated preterm labor and birth. It would be relevant to investigate whether the concentrations of prostaglandins or other inflammatory/labor mediators are different between episodes of preterm labor; yet, serial amniotic fluid sampling would be required to conduct such a study.
The use of mass spectrometry for the detection of intra-amniotic inflammation and/or infection
The use of mass spectrometry enabled us to determine that the ratio of PGE2-EA and PGF2α-EA in pregnancies complicated with preterm labor and preterm birth with intra-amniotic infection was higher than that of the other study groups. The use of mass spectrometry helped to overcome one of the major obstacles of accurate identification of endocannabinoids and eicosanoids by immunoassays, resulting from the structural similarities of these compounds. Our findings suggest that a targeted mass spectrometric approach (as used herein) could prove to be more reliable in a clinical setting [90]. Indeed, other lipid mediators related to eicosanoid pathways have also been recently studied in amniotic fluids using mass spectrometry. In a recent study, it was found that leukotriene B4 is elevated in cases of microbial invasion of the amniotic cavity [76], while several eicosanoids of the epoxygenase pathway have also been measured in women undergoing spontaneous labor at term [74] and in patients with clinical chorioamnionitis at term [77].
Conclusions
In this study, we successfully employed a high-resolution mass spectrometric approach to characterize the relative changes in the amniotic fluid concentrations of five eicosanoids in pregnancies complicated with preterm labor. The profile of eicosanoids differs among the study groups. The highest concentrations of PGE2, PGF2α, and PGFM were found in patients with intra-amniotic infection. Further development of mass spectrometry for the separation of these products, thus reducing the non-specific identification of these compounds, may have potential utility in the clinical context.
Supplementary Material
Acknowledgements
This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government. NG-L is supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. HNP is funded by The Lalor Foundation, Boston, MA, USA.
Abbreviations:
- AF
Amniotic fluid
- PGHS or PTGS-2
prostaglandin endoperoxide synthase-2
- COX-2
Cyclooxygenase-2
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2α
- PGFM
13,14-dihydro-15-keto-PGF(2alpha)
- PGE2-EA
prostaglandin E2-ethanolamide
- PGF2α-EA
prostaglandin F2α-ethanolamide
- IL-6
interleukin 6
- IL-1β
interleukin 1β
- LPS
Lipopolysaccharides
- MRM
scheduled multiple reaction monitoring
- PAR
peak area ratio
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Blencowe H, Cousens S, Chou D, et al. Born Too Soon: The global epidemiology of 15 million preterm births. Reproductive Health. 2013. 11/15;10(Suppl 1):S2–S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Linsingen R, Bicalho MD, de Carvalho NS. Baby born too soon: an overview and the impact beyond the infection. J Matern Fetal Neonatal Med. 2016. August 2:1–5. [DOI] [PubMed] [Google Scholar]
- 3.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010. January;88(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrady GA, Sung JF, Rowley DL, et al. Preterm delivery and low birth weight among first-born infants of black and white college graduates. Am J Epidemiol. 1992. August 1;136(3):266–76. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008. January 5;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013. June 12;309(22):2362–70. [DOI] [PubMed] [Google Scholar]
- 7.Johansson S, Villamor E, Altman M, et al. Maternal overweight and obesity in early pregnancy and risk of infant mortality: a population based cohort study in Sweden. Bmj. 2014. December 02;349:g6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph KS, Fahey J, Shankardass K, et al. Effects of socioeconomic position and clinical risk factors on spontaneous and iatrogenic preterm birth. BMC Pregnancy and Childbirth. 2014. 2014//;14(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P, Xu L, Wang Y, et al. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes Rev. 2016. November;17(11):1091–1102. [DOI] [PubMed] [Google Scholar]
- 10.Koullali B, Oudijk MA, Nijman TAJ, et al. Risk assessment and management to prevent preterm birth. Semin Fetal Neonatal Med 2016. 4//;21(2):80–88. [DOI] [PubMed] [Google Scholar]
- 11.Lee HJ, Park TC, Norwitz ER. Management of pregnancies with cervical shortening: a very short cervix is a very big problem. Rev Obstet Gynecol. 2009. Spring;2(2):107–15. [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Andrade E, Garcia M, Ahn H, et al. Strain at the internal cervical os assessed with quasi-static elastography is associated with the risk of spontaneous preterm delivery at </=34 weeks of gestation. J Perinat Med. 2015. November;43(6):657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Nicolaides KH, Conde-Agudelo A, et al. Vaginal progesterone decreases preterm birth </= 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol. 2016. September;48(3):308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018. February;218(2):161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018. July;219(1):10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez-Andrade E, Maymon E, Luewan S, et al. A soft cervix, categorized by shear-wave elastography, in women with short or with normal cervical length at 18–24 weeks is associated with a higher prevalence of spontaneous preterm delivery. J Perinat Med. 2018. July 26;46(5):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conde-Agudelo A, Romero R. Prediction of preterm birth in twin gestations using biophysical and biochemical tests. Am J Obstet Gynecol. 2014. December;211(6):583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melamed N, Pittini A, Hiersch L, et al. Serial cervical length determination in twin pregnancies reveals 4 distinct patterns with prognostic significance for preterm birth. Am J Obstet Gynecol. 2016. October;215(4):476.e1–476.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R, Conde-Agudelo A, El-Refaie W, et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017. March;49(3):303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh KJ, Hong JS, Romero R, et al. The frequency and clinical significance of intra-amniotic inflammation in twin pregnancies with preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2019. February;32(4):527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarca AL, Romero R, Gudicha DW, et al. A new customized fetal growth standard for African American women: the PRB/NICHD Detroit study. Am J Obstet Gynecol. 2018. February;218(2s):S679–S691.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012. June 9;379(9832):2162–72. [DOI] [PubMed] [Google Scholar]
- 23.Monier I, Ancel PY, Ego A, et al. Fetal and neonatal outcomes of preterm infants born before 32 weeks of gestation according to antenatal vs postnatal assessments of restricted growth. Am J Obstet Gynecol. 2017. May;216(5):516.e1–516.e10. [DOI] [PubMed] [Google Scholar]
- 24.Joseph RM, Korzeniewski SJ, Allred EN, et al. Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a large cohort study of 10-year-old children born at 23–27 weeks’ gestation. Am J Obstet Gynecol. 2017. March;216(3):304 e1–304 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Travers CP, Carlo WA, McDonald SA, et al. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol. 2018. January;218(1):130.e1–130.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell T, MacDonald JW, Srinouanpranchanh S, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol. 2018. April;218(4):438 e1–438 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. Bjog. 2006. December;113 Suppl 3:17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014. August 15;345(6198):760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duff P, Kopelman JN. Subclinical intra-amniotic infection in asymptomatic patients with refractory preterm labor. Obstet Gynecol. 1987. May;69(5):756–9. [PubMed] [Google Scholar]
- 30.Gibbs RS, Romero R, Hillier SL, et al. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992. May;166(5):1515–28. [DOI] [PubMed] [Google Scholar]
- 31.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8(1):3–13. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Kadar N, Miranda J, et al. The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med. 2014. May;27(8):757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SM, Romero R, Lee J, et al. About one-half of early spontaneous preterm deliveries can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. J Matern Fetal Neonatal Med. 2016;29(15):2414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adanir I, Ozyuncu O, Gokmen Karasu AF, et al. Amniotic fluid “sludge”; prevalence and clinical significance of it in asymptomatic patients at high risk for spontaneous preterm delivery. J Matern Fetal Neonatal Med. 2018. January;31(2):135–140. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Lopez N, Romero R, Arenas-Hernandez M, et al. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med. 2018. February;31(4):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988. October;12(4):262–79. [PubMed] [Google Scholar]
- 37.Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol. 1995. May;172(5):1598–603. [DOI] [PubMed] [Google Scholar]
- 38.Baggia S, Gravett MG, Witkin SS, et al. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. J Soc Gynecol Investig. 1996. May-Jun;3(3):121–6. [DOI] [PubMed] [Google Scholar]
- 39.Gomez R, Romero R, Edwin SS, et al. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997. March;11(1):135–76. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch E, Blanchard R, Mehta SP. Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am J Obstet Gynecol. 1999. February;180(2 Pt 1):429–34. [DOI] [PubMed] [Google Scholar]
- 41.Hirsch E, Muhle R. Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biol Reprod. 2002. October;67(4):1337–41. [DOI] [PubMed] [Google Scholar]
- 42.Vadillo-Ortega F, Sadowsky DW, Haluska GJ, et al. Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. Am J Obstet Gynecol. 2002. January;186(1):128–38. [DOI] [PubMed] [Google Scholar]
- 43.Sadowsky DW, Novy MJ, Witkin SS, et al. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003. January;188(1):252–63. [DOI] [PubMed] [Google Scholar]
- 44.Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006. May;194(5):1334–40. [DOI] [PubMed] [Google Scholar]
- 45.Kim MJ, Romero R, Gervasi MT, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest. 2009. August;89(8):924–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snegovskikh VV, Schatz F, Arcuri F, et al. Intra-amniotic infection upregulates decidual cell vascular endothelial growth factor (VEGF) and neuropilin-1 and −2 expression: implications for infection-related preterm birth. Reprod Sci. 2009. August;16(8):767–80. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Flores V, Romero R, Miller D, et al. Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Front Immunol. 2018;9:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faro J, Romero R, Schwenkel G, et al. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasomedagger. Biol Reprod. 2019. May 1;100(5):1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014. April;71(4):330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014. November;72(5):458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2015. July;28(11):1343–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014. February;210(2):125 e1–125 e15. [DOI] [PubMed] [Google Scholar]
- 53.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015. August;28(12):1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez-Lopez N, Romero R, Panaitescu B, et al. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol. 2018. November;80(5):e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005. August;17(4):359–65. [DOI] [PubMed] [Google Scholar]
- 56.Lotze MT, Zeh HJ, Rubartelli A, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007. December;220:60–81. [DOI] [PubMed] [Google Scholar]
- 57.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007. October;28(10):429–36. [DOI] [PubMed] [Google Scholar]
- 58.Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989. May;160(5 Pt 1):1117–23. [DOI] [PubMed] [Google Scholar]
- 59.Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992. Apr-May;27(3–4):117–23. [DOI] [PubMed] [Google Scholar]
- 60.Friel LA, Romero R, Edwin S, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra-amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35(5):385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaiworapongsa T, Erez O, Kusanovic JP, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. J Matern Fetal Neonatal Med. 2008. July;21(7):449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011. December;24(12):1444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero R, Chaiworapongsa T, Savasan ZA, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012. June;25(6):558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015. December;213(6):836.e1–836.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plazyo O, Romero R, Unkel R, et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod. 2016. December;95(6):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell MD, Patrick JE, Robinson JS, et al. Prostaglandins in the plasma and amniotic fluid of rhesus monkeys during pregnancy and after intra-uterine foetal death. J Endocrinol. 1976. October;71(1):67–76. [DOI] [PubMed] [Google Scholar]
- 67.Romero R, Emamian M, Quintero R, et al. Amniotic fluid prostaglandin levels and intra-amniotic infections. Lancet. 1986. June 14;1(8494):1380. [DOI] [PubMed] [Google Scholar]
- 68.Romero R, Wu YK, Sirtori M, et al. Amniotic fluid concentrations of prostaglandin F2 alpha, 13,14-dihydro-15-keto-prostaglandin F2 alpha (PGFM) and 11-deoxy-13,14-dihydro-15-keto-11, 16-cyclo-prostaglandin E2 (PGEM-LL) in preterm labor. Prostaglandins. 1989. January;37(1):149–61. [DOI] [PubMed] [Google Scholar]
- 69.Romero R, Baumann P, Gomez R, et al. The relationship between spontaneous rupture of membranes, labor, and microbial invasion of the amniotic cavity and amniotic fluid concentrations of prostaglandins and thromboxane B2 in term pregnancy. Am J Obstet Gynecol. 1993. June;168(6 Pt 1):1654–64; discussion 1664–8. [DOI] [PubMed] [Google Scholar]
- 70.Romero R, Baumann P, Gonzalez R, et al. Amniotic fluid prostanoid concentrations increase early during the course of spontaneous labor at term. Am J Obstet Gynecol. 1994. December;171(6):1613–20. [DOI] [PubMed] [Google Scholar]
- 71.Mitchell MD, Romero RJ, Edwin SS, et al. Prostaglandins and parturition. Reprod Fertil Dev. 1995;7(3):623–32. [DOI] [PubMed] [Google Scholar]
- 72.Edwin SS, Romero RJ, Munoz H, et al. 5-Hydroxyeicosatetraenoic acid and human parturition. Prostaglandins. 1996. June;51(6):403–12. [DOI] [PubMed] [Google Scholar]
- 73.Lee SE, Romero R, Park IS, et al. Amniotic fluid prostaglandin concentrations increase before the onset of spontaneous labor at term. J Matern Fetal Neonatal Med. 2008. February;21(2):89–94. [DOI] [PubMed] [Google Scholar]
- 74.Maddipati KR, Romero R, Chaiworapongsa T, et al. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. Faseb j. 2014. November;28(11):4835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong JS, Romero R, Lee DC, et al. Umbilical cord prostaglandins in term and preterm parturition. J Matern Fetal Neonatal Med. 2016;29(4):523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maddipati KR, Romero R, Chaiworapongsa T, et al. Lipidomic analysis of patients with microbial invasion of the amniotic cavity reveals up-regulation of leukotriene B4. Faseb j. 2016. October;30(10):3296–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maddipati KR, Romero R, Chaiworapongsa T, et al. Clinical chorioamnionitis at term: the amniotic fluid fatty acyl lipidome. J Lipid Res. 2016. October;57(10):1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park JY, Romero R, Lee J, et al. An elevated amniotic fluid prostaglandin F2alpha concentration is associated with intra-amniotic inflammation/infection, and clinical and histologic chorioamnionitis, as well as impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2016;29(16):2563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Challis JR, Sloboda DM, Alfaidy N, et al. Prostaglandins and mechanisms of preterm birth. Reproduction. 2002. July;124(1):1–17. [DOI] [PubMed] [Google Scholar]
- 80.Challis JR. Molecular aspects of preterm labor. Bull Mem Acad R Med Belg. 1998;153(5–6):263–70; discussion 270–3. [PubMed] [Google Scholar]
- 81.McLean M, Smith R. Corticotropin-releasing Hormone in Human Pregnancy and Parturition. Trends Endocrinol Metab. 1999. July;10(5):174–178. [DOI] [PubMed] [Google Scholar]
- 82.Snegovskikh V, Park JS, Norwitz ER. Endocrinology of parturition. Endocrinol Metab Clin North Am. 2006. March;35(1):173–91, viii. [DOI] [PubMed] [Google Scholar]
- 83.Smith R. Parturition. N Engl J Med. 2007. January 18;356(3):271–83. [DOI] [PubMed] [Google Scholar]
- 84.Challis JR, Lockwood CJ, Myatt L, et al. Inflammation and pregnancy. Reprod Sci. 2009. February;16(2):206–15. [DOI] [PubMed] [Google Scholar]
- 85.Norwitz ER, Bonney EA, Snegovskikh VV, et al. Molecular Regulation of Parturition: The Role of the Decidual Clock. Cold Spring Harb Perspect Med. 2015. April 27;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tornblom SA, Patel FA, Bystrom B, et al. 15-hydroxyprostaglandin dehydrogenase and cyclooxygenase 2 messenger ribonucleic acid expression and immunohistochemical localization in human cervical tissue during term and preterm labor. J Clin Endocrinol Metab. 2004. June;89(6):2909–15. [DOI] [PubMed] [Google Scholar]
- 87.Premyslova M, Li W, Alfaidy N, et al. Differential expression and regulation of microsomal prostaglandin E(2) synthase in human fetal membranes and placenta with infection and in cultured trophoblast cells. J Clin Endocrinol Metab. 2003. December;88(12):6040–7. [DOI] [PubMed] [Google Scholar]
- 88.Romero R, Gonzalez R, Baumann P, et al. Topographic differences in amniotic fluid concentrations of prostanoids in women in spontaneous labor at term. Prostaglandins Leukot Essent Fatty Acids. 1994. February;50(2):97–104. [DOI] [PubMed] [Google Scholar]
- 89.Romero R, Munoz H, Gomez R, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids. 1996. March;54(3):187–91. [DOI] [PubMed] [Google Scholar]
- 90.Glass M, Hong JW, Sato TA, et al. Misidentification of prostamides as prostaglandins. J Lipid Res. 2005. July;46(7):1364–1368. [DOI] [PubMed] [Google Scholar]
- 91.Menon R, Fortunato SJ, Milne GL, et al. Amniotic fluid eicosanoids in preterm and term births: effects of risk factors for spontaneous preterm labor. Obstet Gynecol. 2011. July;118(1):121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mitchell MD, Rice GE, Vaswani K, et al. Differential regulation of eicosanoid and endocannabinoid production by inflammatory mediators in human choriodecidua,. PLoS One. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J Matern Fetal Neonatal Med. 2015. September;28(13):1510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med. 2016;29(3):349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Romero R, Chaemsaithong P, Chaiyasit N, et al. CXCL10 and IL-6: Markers of two different forms of intra-amniotic inflammation in preterm labor. Am J Reprod Immunol. 2017. July;78(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chaemsaithong P, Romero R, Docheva N, et al. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes() [Comparative Study]. J Matern Fetal Neonatal Med. 2018. January;31(2):228–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988. September;31(3):553–84. [DOI] [PubMed] [Google Scholar]
- 98.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989. September;161(3):817–24. [DOI] [PubMed] [Google Scholar]
- 99.Romero R, Shamma F, Avila C, et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol. 1990. September;163(3):757–61. [DOI] [PubMed] [Google Scholar]
- 100.Romero R, Ghidini A, Mazor M, et al. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991. December;34(4):769–78. [DOI] [PubMed] [Google Scholar]
- 101.Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015. January;43(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016. January;44(1):5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med. 2016. January;44(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med. 2016. January;44(1):77–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med. 2016. January;44(1):53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med. 2016. January;44(1):33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaiyasit N, Romero R, Chaemsaithong P, et al. Clinical chorioamnionitis at term VIII: a rapid MMP-8 test for the identification of intra-amniotic inflammation. J Perinat Med. 2017. July 26;45(5):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gomez-Lopez N, Romero R, Maymon E, et al. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. J Perinat Med. 2019. April 24;47(3):276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001. November;185(5):1130–6. [DOI] [PubMed] [Google Scholar]
- 110.Madan I, Romero R, Kusanovic JP, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. J Perinat Med. 2010. May;38(3):275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012. June;40(4):329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29(3):360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Musilova I, Andrys C, Holeckova M, et al. Interleukin-6 measured using the automated electrochemiluminescence immunoassay method for the identification of intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2018. October 8:1–131. [DOI] [PubMed] [Google Scholar]
- 114.Oh KJ, Romero R, Park JY, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol. 2019. March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoon BH, Romero R, Park JY, et al. Antibiotic administration can eradicate intra-amniotic infection or inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2019. March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Musilova I, Andrys C, Drahosova M, et al. Amniotic fluid clusterin in pregnancies complicated by the preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2017. November;30(21):2529–2537. [DOI] [PubMed] [Google Scholar]
- 117.Gomez-Lopez N, Romero R, Xu Y, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol. 2017. December;217(6):693.e1–693.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gomez-Lopez N, Romero R, Garcia-Flores V, et al. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol. 2017. October;78(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Musilova I, Andrys C, Drahosova M, et al. Intraamniotic inflammation and umbilical cord blood interleukin-6 concentrations in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2017. April;30(8):900–910. [DOI] [PubMed] [Google Scholar]
- 120.Gomez-Lopez N, Romero R, Xu Y, et al. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod Sci. 2017. August;24(8):1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oh KJ, Kim SM, Hong JS, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol. 2017. June;216(6):604.e1–604.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Musilova I, Andrys C, Drahosova M, et al. Amniotic fluid cathepsin-G in pregnancies complicated by the preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2017. September;30(17):2097–2104. [DOI] [PubMed] [Google Scholar]
- 123.Gomez-Lopez N, Romero R, Xu Y, et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol. 2018. April;79(4):e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kusanovic JP, Romero R, Martinovic C, et al. Transabdominal collection of amniotic fluid “sludge” and identification of Candida albicans intra-amniotic infection [Case Reports]. J Matern Fetal Neonatal Med. 2018. May;31(10):1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Varrey A, Romero R, Panaitescu B, et al. Human beta-defensin-1: A natural antimicrobial peptide present in amniotic fluid that is increased in spontaneous preterm labor with intra-amniotic infection. Am J Reprod Immunol. 2018. October;80(4):e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee SM, Kim BJ, Park JS, et al. Risk of intra-amniotic infection/inflammation and respiratory distress syndrome according to the birth order in twin preterm neonates. J Matern Fetal Neonatal Med. 2018. October 29:1–6. [DOI] [PubMed] [Google Scholar]
- 127.Revello R, Alcaide MJ, Abehsera D, et al. Prediction of chorioamnionitis in cases of intraamniotic infection by ureaplasma urealyticum in women with very preterm premature rupture of membranes or preterm labour. J Matern Fetal Neonatal Med. 2018. July;31(14):1839–1844. [DOI] [PubMed] [Google Scholar]
- 128.Pacora P, Romero R, Erez O, et al. The diagnostic performance of the beta-glucan assay in the detection of intra-amniotic infection with Candida species. J Matern Fetal Neonatal Med. 2019. May;32(10):1703–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oh KJ, Romero R, Park JY, et al. A high concentration of fetal fibronectin in cervical secretions increases the risk of intra-amniotic infection and inflammation in patients with preterm labor and intact membranes. J Perinat Med. 2019. April 24;47(3):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kusanovic JP, Vargas P, Ferrer F, et al. Comparison of two identification and susceptibility test kits for Ureaplasma spp and Mycoplasma hominis in amniotic fluid of patients at high risk for intra-amniotic infection. J Matern Fetal Neonatal Med. 2019. February 20:1–9. [DOI] [PubMed] [Google Scholar]
- 131.Para R, Romero R, Miller D, et al. Human beta-defensin-3 participates in intra-amniotic host defense in women with labor at term, spontaneous preterm labor and intact membranes, and preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2019. April 18:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Romero R, Emamian M, Quintero R, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988. July;159(1):114–9. [DOI] [PubMed] [Google Scholar]
- 133.Romero R, Quintero R, Nores J, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991. October;165(4 Pt 1):821–30. [DOI] [PubMed] [Google Scholar]
- 134.Romero R, Jimenez C, Lohda AK, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990. September;163(3):968–74. [DOI] [PubMed] [Google Scholar]
- 135.Eshoo MW, Crowder CC, Rebman AW, et al. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One. 2012;7(5):e36825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shin JH, Ranken R, Sefers SE, et al. Detection, identification, and distribution of fungi in bronchoalveolar lavage specimens by use of multilocus PCR coupled with electrospray ionization/mass spectrometry. J Clin Microbiol. 2013. January;51(1):136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ecker DJ, Sampath R, Li H, et al. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn. 2010. May;10(4):399–415. [DOI] [PubMed] [Google Scholar]
- 138.Metzgar D, Frinder M, Lovari R, et al. Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J Clin Microbiol. 2013. August;51(8):2670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chan HW, McKirdy NC, Peiris HN, et al. The role of endocannabinoids in pregnancy. Reproduction. 2013. September;146(3):R101–9. [DOI] [PubMed] [Google Scholar]
- 140.Espey LL. Ovulation as an inflammatory reaction--a hypothesis. Biol Reprod. 1980. February;22(1):73–106. [DOI] [PubMed] [Google Scholar]
- 141.Priddy AR, Killick SR. Eicosanoids and ovulation. Prostaglandins Leukot Essent Fatty Acids. 1993. November;49(5):827–31. [DOI] [PubMed] [Google Scholar]
- 142.Griffith OW, Chavan AR, Protopapas S, et al. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci U S A. 2017. August 8;114(32):E6566–E6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kelly RW. Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocr Rev. 1994. October;15(5):684–706. [DOI] [PubMed] [Google Scholar]
- 144.Skinner KA, Challis JR. Changes in the synthesis and metabolism of prostaglandins by human fetal membranes and decidua at labor. Am J Obstet Gynecol. 1985. February 15;151(4):519–23. [DOI] [PubMed] [Google Scholar]
- 145.Orlov VI, Pogorelova TN, Melkonova KY. The role of fetal myoglobin in the initiation of human parturition. J Perinat Med. 1996;24(5):495–500. [DOI] [PubMed] [Google Scholar]
- 146.Brennand JE, Leask R, Kelly RW, et al. Mechanisms involved in the stimulatory effect of amniotic fluid on prostaglandin production by human fetal membranes. Prostaglandins Leukot Essent Fatty Acids. 1998. May;58(5):369–75. [DOI] [PubMed] [Google Scholar]
- 147.Romero R, Espinoza J, Chaiworapongsa T, et al. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002. August;7(4):259–74. [DOI] [PubMed] [Google Scholar]
- 148.Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988. September;159(3):661–6. [DOI] [PubMed] [Google Scholar]
- 149.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993. October;169(4):839–51. [DOI] [PubMed] [Google Scholar]
- 150.Jalava J, Mantymaa ML, Ekblad U, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103(7):664–669. [DOI] [PubMed] [Google Scholar]
- 151.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189(4):919–924. [DOI] [PubMed] [Google Scholar]
- 152.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008. August 26;3(8):e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.DiGiulio DB, Gervasi M, Romero R, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med. 2010. September;38(5):503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.DiGiulio DB, Gervasi MT, Romero R, et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med. 2010. September;38(5):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010. July 1;64(1):38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martinez-Varea A, Romero R, Xu Y, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med. 2017. July 26;45(5):523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miller D, Motomura K, Garcia-Flores V, et al. Innate Lymphoid Cells in the Maternal and Fetal Compartments. Front Immunol. 2018;9:2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gomez-Lopez N, Roberto R, Galaz J, et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am J Reprod Immunol. 2019. July 19:e13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jongh RD, Jorens P, Student I, et al. The contribution of the immune system to parturition. Mediators Inflamm. 1996;5(3):173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pomini F, Caruso A, Challis JR. Interleukin-10 modifies the effects of interleukin-1beta and tumor necrosis factor-alpha on the activity and expression of prostaglandin H synthase-2 and the NAD+-dependent 15-hydroxyprostaglandin dehydrogenase in cultured term human villous trophoblast and chorion trophoblast cells. J Clin Endocrinol Metab. 1999. December;84(12):4645–51. [DOI] [PubMed] [Google Scholar]
- 161.Rogers LM, Anders AP, Doster RS, et al. Decidual stromal cell-derived PGE2 regulates macrophage responses to microbial threat. Am J Reprod Immunol. 2018. October;80(4):e13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gomez-Lopez N, Romero R, Plazyo O, et al. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol. 2016. January;75(1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008. September;21(9):605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gomez-Lopez N, Romero R, Xu Y, et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod Sci. 2017. October;24(10):1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gomez-Lopez N, Romero R, Garcia-Flores V, et al. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth and adverse neonatal outcomes. Biol Reprod. 2018. December 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Strauss JF 3rd, Romero R, Gomez-Lopez N, et al. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol. 2018. March;218(3):294–314.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015. July;21(7):677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.He Y, Hara H, Nunez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016. December;41(12):1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Romero R, Munoz H, Gomez R, et al. Does Infection Cause Premature Labor and Delivery. Semin Reprod Endocrinol. 1994. November;12(4):227–239. [Google Scholar]
- 170.Wagner JA, Varga K, Ellis EF, et al. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997. December 4;390(6659):518–21. [DOI] [PubMed] [Google Scholar]
- 171.Maccarrone M, De Petrocellis L, Bari M, et al. Lipopolysaccharide downregulates fatty acid amide hydrolase expression and increases anandamide levels in human peripheral lymphocytes. Arch Biochem Biophys. 2001. September 15;393(2):321–8. [DOI] [PubMed] [Google Scholar]
- 172.Silver RM, Edwin SS, Trautman MS, et al. Bacterial lipopolysaccharide-mediated fetal death. Production of a newly recognized form of inducible cyclooxygenase (COX-2) in murine decidua in response to lipopolysaccharide. J Clin Invest. 1995. February;95(2):725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brown NL, Alvi SA, Elder MG, et al. Regulation of prostaglandin production in intact fetal membranes by interleukin-1 and its receptor antagonist. J Endocrinol. 1998. December;159(3):519–26. [DOI] [PubMed] [Google Scholar]
- 174.Brown NL, Alvi SA, Elder MG, et al. The regulation of prostaglandin output from term intact fetal membranes by anti-inflammatory cytokines. Immunology. 2000. January;99(1):124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gross G, Imamura T, Vogt SK, et al. Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am J Physiol Regul Integr Comp Physiol. 2000. June;278(6):R1415–23. [DOI] [PubMed] [Google Scholar]
- 176.Yan X, Wu Xiao C, Sun M, et al. Nuclear factor kappa B activation and regulation of cyclooxygenase type-2 expression in human amnion mesenchymal cells by interleukin-1beta. Biol Reprod. 2002. June;66(6):1667–71. [DOI] [PubMed] [Google Scholar]
- 177.Romero R, Wu YK, Mazor M, et al. Amniotic fluid prostaglandin E2 in preterm labor. Prostaglandins Leukot Essent Fatty Acids. 1988. December;34(3):141–5. [DOI] [PubMed] [Google Scholar]
- 178.Romero R, Emamian M, Wan M, et al. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987. December;157(6):1461–7. [DOI] [PubMed] [Google Scholar]
- 179.Lee SE, Park IS, Romero R, et al. Amniotic fluid prostaglandin F2 increases even in sterile amniotic fluid and is an independent predictor of impending delivery in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2009. October;22(10):880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Musilova I, Andrys C, Drahosova M, et al. Amniotic fluid prostaglandin E2 in pregnancies complicated by preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2016. September;29(18):2915–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


