Abstract
Background
Subacute thyroiditis (SAT) is a rare inflammatory disease of the thyroid gland. It has been noticed that patients with a diagnosis of SAT visit more other clinics and receive antibiotics unnecessarily. Therefore, the aim of this study was to reveal the degree of delay in the diagnosis of SAT, prediagnosis antibiotic use rates, and the awareness of clinics for the diagnosis of SAT.
Methods
A total of 121 patients with SAT were enrolled in the study. A retrospective analysis was made of the history of patient symptoms during the diagnosis, which physicians they visited, antibiotic use, laboratory test results, and ultrasonographic findings.
Results
The median age of the patients was 41 years. Neck pain radiating to the jaw/ear was seen in most patients (71.1%). The median time from symptom onset to a diagnosis of SAT was 23 days (range, 6–70 days). Antibiotics were erroneously prescribed to 71 patients (58.7%) before the diagnosis. The median time to diagnosis was 28 days in patients using antibiotics and 20 days in the group not using antibiotics (p < 0.001). Two or more physicians had been visited before SAT diagnosis by 89 (73.6%) patients, and more antibiotics were prescribed to these patients than the group who visited fewer physicians (p < 0.05). The frequency of prescribing antibiotics by physicians was 73.7% by emergency physicians, 53.1% by family doctors, 51.1% by ENT specialists, and 35.4% by internal medicine specialists.
Conclusion
The diagnosis of SAT is often delayed, and misdiagnosis leads to erroneous antibiotic overuse. Physicians should increase their awareness of the diagnosis of SAT in patients with neck pain.
Keywords: Antibiotic overuse, Late diagnosis, Neck pain, Subacute thyroiditis
Introduction
Subacute thyroiditis (SAT) is a self-limiting, inflammatory process of the thyroid gland that often presents with painful swelling of the thyroid. SAT affects females more often than males (female to male ratio of 4:1) [1, 2, 3]. The peak age of incidence is 40–50 years [1]. Although viral infections are thought to trigger the disease, the exact etiology has not been elucidated [4]. Some studies have suggested that certain types of human leukocyte antigens may play a role in the development of SAT [5, 6].
Common initial clinical presentation includes anterior neck pain that can spread up to the jaw or ear and/or down to the sternum, mild to moderate fever, fatigue, malaise, and mild to moderate thyrotoxic symptoms [1, 2, 3, 4]. The affected side of the thyroid gland (unilateral or bilateral) becomes tender to palpation. Atypical cases of SAT without neck pain or tenderness have occasionally been reported in the literature [2, 7, 8]. Some authors have claimed that there is a seasonal peak in SAT cases during the spring and/or early autumn [1, 3]. Laboratory findings usually include high C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), suppressed thyroid-stimulating hormone (TSH), and elevated free thyroxin (fT4) levels. Characteristic ultrasound features of SAT include ill-defined heterogeneous, hypoechoic areas and a lack of flow color Doppler in those areas, and in some patients, diffuse enlargement in the thyroid gland may be seen [9, 10]. These symptoms and laboratory findings are characteristic for the initial (thyrotoxic) phase of SAT.
Despite recent advances in diagnostic methods, the diagnosis of SAT is still often delayed, and patients are unable to continue normal daily activities for weeks or even months. It has been realized that primary care physicians and some specialists do not consider the possibility of SAT in the differential diagnosis of neck pain. Therefore, physicians frequently prescribe antibiotics unnecessarily as the symptoms and laboratory results of the patients can be easily confused with different infective processes. The purpose of this study was to demonstrate the time lag between symptom onset and diagnosis of SAT and to determine the number of doctors who were visited and the frequency of antibiotic use during the diagnosis process. It was also aimed to characterize the frequency of symptoms and laboratory findings in SAT patients.
Materials and Methods
Study Population and Design
This retrospective study was designed in the Diskapi Yildirim Beyazit Training and Research Hospital Endocrinology and Metabolism Department. A scan of the database identified 138 patients who were diagnosed with SAT between November 2017 and July 2020. A total of 17 of these patients were excluded due to deficiencies in the laboratory and/or ultrasound examinations, insufficient clinical history before the diagnosis, or no history of neck pain. Thus, a total of 121 patients were enrolled in the study. The data of the patients, including how many and which physician(s) they visited before the diagnosis, antibiotic use, symptoms at the time of diagnosis, the severity of neck pain according to pain scales, laboratory results, ultrasonographic features at the time of diagnosis, and the treatment they received, were obtained retrospectively from the system records.
The diagnosis of SAT was made based on clinical presentation, physical examination, laboratory test results, and ultrasonographic findings following the recommendations of 2016 American Thyroid Association guidelines [11]. The diagnosis was established with the presence of painful, tender, and hard goiter, elevation of ESR or CRP, elevation of fT4 and suppression of serum TSH, and the presence of hypoechoic areas with blurred margins and decreased vascularization appearance on ultrasonography of painful thyroid regions. Cytological verification was performed in patients with a suspected diagnosis to rule out possible malignancy/painful hashitoxicosis. Besides, improvements in ultrasonographic findings in the posttreatment period were confirmed in all patients. Neck pain was accepted as the initial symptom of the patients to determine the time from symptom onset to diagnosis of SAT.
Laboratory Evaluation
The laboratory examinations were performed in our institute using a biochemical analyzer. Serum levels of TSH, fT4, free tri-iodothyronine (fT3), ESR, CRP, and WBC were examined at the time of diagnosis. Thyroid function tests were measured with direct chemiluminescent immunoassay (Beckman Coulter, CA, USA). Reference ranges were defined as TSH: 0.38–5.33 mIU/L, fT4: 0.60–1.25 ng/dL, fT3: 2.28–4 ng/L, ESR: 0–20 mL/h, CRP: 0–5 mg/L, and WBC: 3,570–11,010 103/μL. US examinations were obtained using a Hitachi HI Vision Preirus unit (EUB 7000 HV; Hitachi, Tokyo, Japan) with a 13-MHz linear array transducer.
Statistical Analysis
Statistical analysis was made using SPSS software (version 23.0, SPSS; IBM Corporation, New York, NY, USA). Categorical variables were summarized with frequencies and percentages (%). Data distribution was assessed with the Kolmogorov-Smirnov test. Data with abnormal distribution were expressed as median (range) and those with normal distribution as mean ± standard deviation values. Categorical variables were compared using the χ2 test. Continuous variables with normal distribution were compared using the independent samples t test and those not showing normal distribution with the Mann-Whitney U test. The statistical significance level was determined as p < 0.05.
Results
A total of 121 patients diagnosed with SAT were included in the final analyses. The median age was 41 years (range, 26–72 years). The female-male ratio was approximately 3:1. The mean pain intensity score, which measures the severity of neck pain in the last 24 h, was high (7.74 ± 1.56). Neck pain radiating to the jaw/ear was seen in most patients (71.1%). The vast majority (93.4%) of patients complained of fatigue. Fever, which was documented at ≥38°C, and palpitations were observed in approximately half of the patients (46.3 and 50.4%, respectively), while weight loss, defined as the loss of ≥5% of body weight in the last month, was present in 17.4%. The median time from symptom onset to diagnosis of SAT was 23 days (range, 6–70 days). There were 22 patients who were diagnosed within 2 weeks of symptom onset (18.2%). Most of the patients (86.0%) were in the hyperthyroid phase at the time of diagnosis. The mean ESR value was 57.9 ± 22.2 mm/h, and the median CRP value was 69.4 mg/L. The baseline characteristics of the patients are summarized in Table 1.
Table 1.
Baseline characteristics of the patients (n = 121)
| Variables | Patients |
|---|---|
| N (%) | 121 (100) |
| Age, median (range), years | 41 (26–72) |
| Female gender, n (%) | 91 (75.2) |
| Hypoecogenic areas at US, n (%) | |
| Bilateral | 62 (51.2) |
| Unilateral | 59 (48.8) |
| Symptoms | |
| Severity of pain, mean ± SD | 7.74±1.56 |
| Spread of pain to jaw/ear, n (%) | 86 (71.1) |
| Fatigue, n (%) | 113 (93.4) |
| Fever,* n (%) | 56 (46.3) |
| Palpitation, n (%) | 61 (50.4) |
| Weight loss,¶ n (%) | 21 (17.4) |
| Time to diagnosis, median (range), days | 23 (6–70) |
| Diagnosed at ≥15 days, n (%) | 99 (81.8) |
| Presence of antibiotic use, n (%) | 71 (58.7) |
| Beta-lactam | 48 (67.6) |
| Quinolone | 14 (19.7) |
| Macrolide | 9 (12.7) |
| Visiting 2 or more physicians, n (%) | 89 (73.6) |
| Thyroid hormone status, n (%) | |
| Hyperthyroid | 104 (86.0) |
| Euthyroid | 17 (14.0) |
| TSH, median (range), mIU/L | 0.01 (0.001–3.60) |
| fT4, median (range), ng/dL | 2.17 (0.86–7.77) |
| fT3, median (range), ng/L | 5.69 (2.83–14.9) |
| WBC, median (range), 103/mm3 | 8.9 (4.8–16.8) |
| ESR, mean±SD, mm/h | 57.9±22.2 |
| CRP, median (range), mg/L | 69.4 (10.1–208.0) |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; fT3, free tri-iodothyronine; fT4, free thyroxin; TSH, thyroid-stimulating hormone; US, thyroid ultrasonography; WBC, white blood cells.
≥38°C documented fever.
Loss of 5% or more of body weight in the last month.
Antibiotics were erroneously prescribed to 71 patients (58.7%) by physicians before the diagnosis of SAT. Two or more antibiotics were prescribed to 25 of these 71 patients (35.2%) at different times. Beta-lactam group antibiotics had been used by 67.6% of these patients. The most prescribed antibiotic was amoxicillin plus clavulonic acid. The patients were separated into 2 groups as those who received antibiotics and those who did not. The groups were comparable in terms of age, gender, and pain severity (p > 0.05). The TSH, fT4, fT3, and ESR levels of both groups were similar (p > 0.05). While high CRP levels and frequency of fever and weight loss tended to be seen more in the antibiotic group, these factors did not reach statistical significance (p = 0.16, p = 0.12, and p = 0.07). Patients receiving antibiotics visited more doctors than the no-antibiotic group (p < 0.001). The median time of diagnosis in the antibiotic group was 28 days, and it was 20 days in the no-antibiotic group, with a statistically significant difference determined between the diagnosis times of the groups (p < 0.001). The comparisons of these 2 groups are summarized in Table 2.
Table 2.
Patient characteristics according to antibiotic use
| Variables | Antibiotic group (n = 71) | No-antibiotic group (n = 50) | p value |
|---|---|---|---|
| N (%) | 71 (58.7) | 50 (41.3) | − |
| Age, median (range), years | 42.0 (30–72) | 41.0 (26–71) | 0.55 |
| Female gender, n (%) | 54 (76.0) | 37 (74.0) | 0.79 |
| Symptoms | |||
| Severity of neck pain, mean±SD | 7.80±1.69 | 7.64±1.35 | 0.57 |
| Fatigue, n (%) | 68 (95.7) | 45 (90.0) | 0.20 |
| Fever, n (%) | 37 (52.1) | 19 (38.0) | 0.12 |
| Palpitation, n (%) | 38 (53.5) | 23 (46.0) | 0.41 |
| Weight loss, n (%) | 16 (22.5) | 5 (10.0) | 0.07 |
| Time to diagnosis, median (range), days | 28 (6–65) | 20 (6–70) | 0.02* |
| Physician visits, n (%) | |||
| Diagnosed at first time or one visit | 6 (8.4) | 26 (52.0) | <0.001* |
| Two or more visits | 65 (91.6) | 24 (48.0) | <0.001* |
| TSH, median (range), mIU/L | 0.01 (0.001–3.60) | 0.01 (0.003–2.45) | 0.98 |
| fT4, median (range), ng/dL | 2.17 (0.9–7.8) | 2.24 (0.9–7.6) | 0.91 |
| fT3, median (range), ng/L | 6.00 (2.9–14.9) | 5.46 (2.8–14.2) | 0.92 |
| WBC, median (range), 103/mm3 | 8.7 (4.8–16.8) | 9.0 (5.3–16.8) | 0.48 |
| ESR, mean±SD, mm/h | 58.0±22.8 | 57.6±21.7 | 0.92 |
| CRP, median (range), mg/L | 74.1 (10.1–208.0) | 67.6 (12.0–193.9) | 0.16 |
CRP, C-reactive protein; ESR: erythrocyte sedimentation rate; fT3, free tri-iodothyronine; fT4, free thyroxin; TSH, thyroid-stimulating hormone; WBC, white blood cells.
Statistically significant.
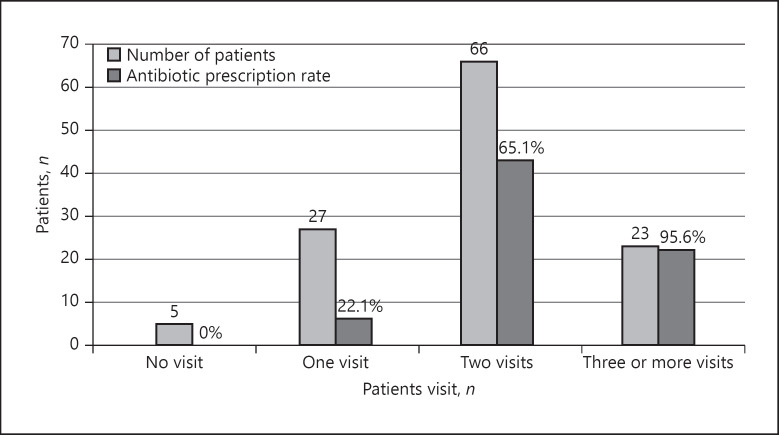
Before the diagnosis of SAT, 89 patients had visited 2 or more different physicians (73.6%). This group that visited more physicians (≥2) (MVG) had higher mean neck pain scores (p = 0.014) and more fever and palpitation symptoms compared to the group that visited fewer physicians (FVG) (p < 0.05). The frequency of antibiotic prescription in the MVG group was 73.0%, which was significantly higher than that of the other group (p < 0.001). Five patients had not been treated by another physician before the diagnosis. Antibiotics were prescribed to 6 (22.2%) of 27 patients examined by only one physician before diagnosis, to 43 (65.1%) of 66 patients examined by 2 physicians, and to 22 (95.6%) of 23 patients examined by 3 or more physicians (Fig. 1). Statistically significant differences were determined between the 2 groups in terms of the levels of TSH with the median value in MVG of 0.01 (0.001–3.6) and in FVG of 0.03 (0.003–3.02) mIU/L (p = 0.006), median fT4 in MVG of 2.32 (0.86–7.77) and in FVG of 2.00 (0.92–5.37) ng/dL (p = 0.05), mean ESR value in MVG of 60.2 ± 22.5 and in FVG of 51.3 (±20.4) mm/h (p = 0.05), and median CRP value in MVG of 76.5 (10.1–208.0) and in FVG of 45.9 (12.1–132.0) mg/L (p = 0.015) (Table 3).
Fig. 1.
The number of patients relative to how many physicians they visited before the diagnosis and the antibiotic prescription rates for each group (n = 121). The figure demonstrates that the frequency of prescribing antibiotics increased in correlation with the number of patients' visits.
Table 3.
Patient characteristics according to number of physician visits
| Variables | Fewer visit group (n = 32) | More (≥2) visit group (n = 89) | p value |
|---|---|---|---|
| N (%) | 32 (26.4) | 89 (73.6) | − |
| Age, median (range), years | 42.5 (29–66) | 41.0 (26–72) | 0.82 |
| Female gender, n (%) | 23 (71.9) | 68 (76.4) | 0.61 |
| Symptoms | |||
| Severity of neck pain, mean±SD | 7.16±1.55 | 7.94±1.52 | 0.014* |
| Fatigue, n (%) | 29 (90.6) | 84 (94.4) | 0.46 |
| Fever, n (%) | 7 (21.9) | 49 (55.1) | 0.001* |
| Palpitation, n (%) | 9 (28.1) | 52 (58.4) | 0.003* |
| Weight loss, n (%) | 2 (6.2) | 19 (21.3) | 0.05 |
| Time to diagnosis, median (range), days | 20.0 (7–70) | 23.5 (6–65) | 0.15 |
| Presence of antibiotic use, n (%) | 6 (18.7) | 65 (73.0) | <0.001* |
| Overt hyperthyroidism, n (%) | 25 (78.1) | 79 (88.8) | 0.14 |
| TSH, median (range), mlU/L | 0.03 (0.003–3.02) | 0.01 (0.001–3.6) | 0.006* |
| fT4, median (range), ng/dL | 2.00 (0.92–5.37) | 2.32 (0.86–7.77) | 0.052 |
| fT3, median (range), ng/L | 4.48 (2.8–10.1) | 6.07 (3.0–14.9) | 0.11 |
| WBC, median (range), 103/mm3 | 8.5 (5.6–14.0) | 8.9 (4.8–16.8) | 0.14 |
| ESR, mean±SD, mm/h | 51.3±20.4 | 60.2±22.5 | 0.05 |
| CRP, median (range), mg/L | 45.9 (12.1–132.0) | 76.5 (10.1–208.0) | 0.015* |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; fT3, free tri-iodothyronine; fT4, free thyroxin; TSH, thyroid-stimulating hormone; WBC, white blood cells.
Statistically significant.
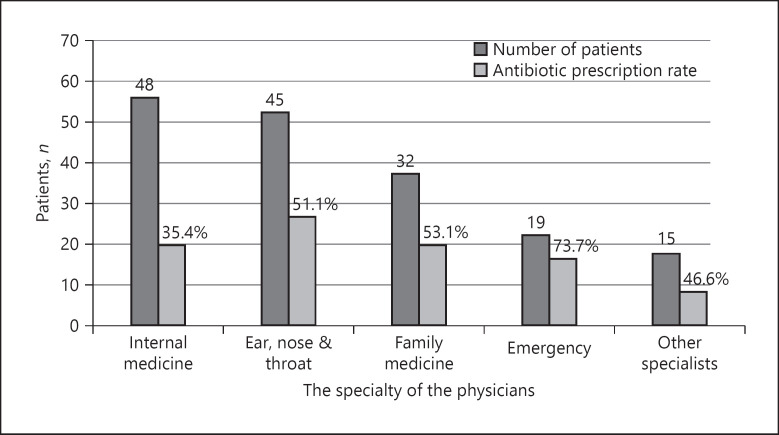
The data on which physicians based the prescription or not of antibiotics in 100 of the 121 patients were available in the hospital system records. Before the SAT diagnosis, 48 of 100 patients were examined by an internal medicine specialist, 45 by an ear-nose-throat (ENT) specialist, 33 by the family doctor, 17 by an emergency doctor, and 15 by other specialists (e.g., infectious diseases specialist, pulmonologist, and general surgery specialist) at different times. The frequency of prescribing antibiotics by physicians was 73.7% by emergency physicians, 53.1% by family doctors, 51.1% by ENT specialists, and 35.4% by internal medicine specialists (Fig. 2). When the internal medicine physicians and other physicians were compared, ENT, family medicine, and emergency physicians had prescribed more antibiotics significantly (35.4 vs. 56.2%, p = 0.02).
Fig. 2.
The number of patients who applied to different specialist groups and the antibiotic prescription rates for each group (n = 100).
Discussion
The diagnosis of SAT is frequently missed as it is a rare disease, and SAT cases tend to occur at the seasonal peak time of upper respiratory tract infections (URI), and similar symptoms may cause confusion [1, 2, 3]. In the literature, recent studies have focused on the diagnosis and treatment modalities of SAT. The delays in the prediagnosis period of the disease and its consequences have been presented in only one recent study [12].
The results of this retrospective study showed that the time to diagnosis in 81.8% of the patients was 2 weeks or more, and the diagnosis of SAT is often delayed. The prediagnosis period was prolonged up to 70 days in some patients. Also, Stasiak et al. [12] reported a delay of >2 weeks in SAT diagnosis in 73% of 64 patients. The pain radiating up to the ear/jaw was observed in 71% of the patients. Fever and palpitations were present in approximately half of the patients in parallel with findings in the literature, while weight loss was present in a small number of patients [3]. ESR and CRP levels were above the upper limit of normal, similar to data reported in the literature [1, 3, 4, 13]. In addition, some authors have suggested that there may be a 1- to 6-week delay between the onset of symptoms and the appearance of laboratory findings [3, 14]. These results indicate that there are some factors contributing to delayed SAT diagnosis. The absence of thyrotoxic symptoms in half of the patients and the normal thyroid function tests in the early stages and the presence of nonspecific symptoms that are confused with other infectious processes seem to be the factors that lead to delays in the diagnosis of SAT. Therefore, in patients presenting with neck pain and who have high acute-phase reactants in the laboratory tests, questioning the character and history of the pain (e.g., the presence of pain radiating up to the jaw/ear), performing thyroid function tests, and palpating the thyroid gland in the physical examination are important steps to prevent a delayed diagnosis of SAT.
Delays in the diagnosis of SAT also lead to inappropriate treatment practices. In the current study, 58.7% of the patients had used antibiotics at least once before the diagnosis of SAT. The most commonly used antibiotics were seen to be from the beta-lactam group, and amoxicillin-clavulonate was the most prescribed antibiotic. It was observed that patients who received antibiotics were diagnosed later and visited clinicians more often than those who did not (p < 0.05). The misdiagnosis on the first presentation seems to lead to inappropriate antibiotic use and late diagnosis. Patients whose symptoms did not improve despite receiving antibiotics applied to more physicians, and this, therefore, results in both an increase in healthcare expenditure and a decrease in the functional capacity of the patients.
Stasiak et al. [12] reported that unnecessary antibiotic use was seen in 46.7% of SAT patients, and patients who received antibiotics had more fever symptoms, more suppressed TSH, and elevated fT4, fT3, WBC, and CRP levels. However, in the current study, there was no significant difference between the groups in terms of symptoms and laboratory findings, which could be attributed to the larger sample size of the current study. But, the main reason for this discrepancy between the 2 studies seems to depend on the factor of the physician. Because of the differences in antibiotic prescription between physicians in our study, heterogeneous distribution of physician's attitudes seems to influence the subgroup analyses. The results of the present study demonstrated that the tendency of the clinician to prescribe erroneously antibiotics in patients with SAT lies in the lack of awareness of the diagnosis of SAT in the differential diagnosis rather than high acute-phase reactants or severity of the clinical features. Also, Stasiak et al. [12] claimed that there was no difference in the diagnosis delay between the groups that received and did not receive antibiotics (p = 0.57). However, in the current study, it was shown that unnecessary use of antibiotics causes a delay in the diagnosis of SAT patients as expected.
Unnecessary overuse of antibiotics may cause antibiotic resistance by disrupting the microbiota of the patients and so threaten the individual and public health [15, 16]. Of the OECD countries with the highest antibiotic resistance, Turkey together with Korea and Greece has a rate of 38.8% [17]. A recent study demonstrated that antibiotics are most often prescribed in primary care hospitals for uncomplicated respiratory tract infections, and beta-lactam group antibiotics were prescribed most frequently [18]. Antibiotics are not recommended as first-line therapy unless there are complicated respiratory infections (such as bronchitis complicated by pneumonia and culture-proven streptococcal pharyngitis) [19]. Therefore, antibiotics should be given only when necessary, especially in patients presenting with URI symptoms. Inflammatory conditions of the thyroid gland should definitely be included in the differential diagnosis in patients with URI-like symptoms and pain in the anterior neck region.
It was seen in the current study that three-quarters of the patients diagnosed with SAT had consulted at least 2 other physicians before applying to our clinic. Patients in the MVG group had higher neck pain scores and thyrotoxicosis symptoms such as fever, palpitations, and weight loss (p < 0.05). ESR and CRP values were significantly higher in the MVG group than in the FVG group. Patients in the MVG group were also more likely to be prescribed antibiotics (p < 0.05). While the rate of prescribing antibiotics was 22.1% in patients who had visited 1 physician before the diagnosis, this rate increased up to 95.6% in patients who had visited 3 or more physicians. It seems that misdiagnosis on the first presentation resulted in the unnecessary use of antibiotics, and the fact that the symptoms of the patients do not improve leads to repeated visits to different specialists. Moreover, 27.3% of the patients in the MVG group had used antibiotics 2 or more times. This result indicates that new antibiotics are prescribed for symptomatic patients, and this vicious cycle continues until the correct diagnosis is made.
The specialists most frequently visited by patients before the diagnosis were internal medicine, ENT, family, and emergency physicians, respectively. While emergency physicians stand out as the group that prescribed erroneously antibiotics most frequently (73.7%), ENT and family physicians prescribed antibiotics for approximately half of the patients (51.1 and 53.1%, respectively), and internal medicine specialists prescribed antibiotics for approximately 35.4% of the patients. These results demonstrate that the diagnosis of SAT is not suspected in most patients on the first presentation. Therefore, internal medicine, ENT, family, and emergency physicians, to whom patients frequently present first, should increase their awareness of this issue. Thus, delays in the diagnosis of SAT and the unnecessary use of antibiotics can be prevented.
There were some limitations to the study. Due to the retrospective nature of the study, there may have been some deficiencies in the patient files, and the results may have been affected by patient-related factors (such as problems remembering symptoms and treatment).
In conclusion, the diagnosis of SAT is often delayed, thereby limiting the functional capacity of patients for a long time, and misdiagnosis leads to inappropriate use of antibiotics. It seems that the factor underlying this inappropriate approach is the low awareness of physicians. Therefore, clinicians who may encounter these patients frequently should increase their awareness of the diagnosis of SAT in patients with neck pain.
Statement of Ethics
The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Approval for this study was granted by the Ethics Committee of the University of Health Sciences, Diskapi Yildirim Beyazit Training and Research Hospital (Approval No. 95/09). Written consent was obtained from all participants before the study procedures.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive any funding for the current study.
Author Contributions
H.B. and M.E.S. designed the study; all authors contributed to intellectual content; H.B., M.Ç., S.H., and H.D. collected data; H.B. and M.E.S. wrote the manuscript; and all authors reviewed the manuscript.
References
- 1.Samuels MH. Subacute, silent, and postpartum thyroiditis. Med Clin North Am. 2012 Mar;96((2)):223–33. doi: 10.1016/j.mcna.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Fatourechi V, Aniszewski JP, Fatourechi GZE, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, Study. J Clin Endocrinol Metab. 2003 May;88((5)):2100–5. doi: 10.1210/jc.2002-021799. [DOI] [PubMed] [Google Scholar]
- 3.Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T, et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med. 2008;47((8)):725–9. doi: 10.2169/internalmedicine.47.0740. [DOI] [PubMed] [Google Scholar]
- 4.Alfadda AA, Sallam RM, Elawad GE, Aldhukair H, Alyahya MM. Subacute thyroiditis: clinical presentation and long term outcome. Int J Endocrinol. 2014;2014:794943. doi: 10.1155/2014/794943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyulassy S, Hnilica P, Buc M, Guman M, Hirschova V, Stefanovic J. Subacute (de Quervain's) thyroiditis: association with HLA-Bw35 antigen and abnormalities of the complement system, immunoglobulins and other serum proteins. J Clin Endocrinol Metab. 1977 Aug;45((2)):270–4. doi: 10.1210/jcem-45-2-270. [DOI] [PubMed] [Google Scholar]
- 6.Stasiak M, Tymoniuk B, Michalak R, Stasiak B, Kowalski ML, Lewiński A. Subacute thyroiditis is associated with HLA-B* 18: 01,-DRB1* 01 and-C* 04: 01: the significance of the new molecular background. J Clin Med. 2020 Feb;9((2)):534. doi: 10.3390/jcm9020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels GH. Atypical subacute thyroiditis: preliminary observations. Thyroid. 2001 Jul;11((7)):691–5. doi: 10.1089/105072501750362772. [DOI] [PubMed] [Google Scholar]
- 8.Tsai CH, Lee JJ, Liu CL, Tzen CY, Cheng SP. Atypical subacute thyroiditis. Surgery. 2010 Mar;147((3)):461–2. doi: 10.1016/j.surg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Cappelli C, Pirola I, Gandossi E, Formenti AM, Agosti B, Castellano M. Ultrasound findings of subacute thyroiditis: a single institution retrospective review. Acta Radiol. 2014 May;55((4)):429–33. doi: 10.1177/0284185113498721. [DOI] [PubMed] [Google Scholar]
- 10.Sencar ME, Calapkulu M, Sakiz D, Akhanli P, Hepsen S, Duger H, et al. The contribution of ultrasonographic findings to the prognosis of subacute thyroiditis. Arch Endocrinol Metab. 2020 May-Jun;64((3)):306–11. doi: 10.20945/2359-3997000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016 Oct;26((10)):1343–421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 12.Stasiak M, Michalak R, Stasiak B, Lewiński A. Time-lag between symptom onset and diagnosis of subacute thyroiditis: how to avoid the delay of diagnosis and unnecessary overuse of antibiotics. Horm Metab Res. 2020 Jan;52((1)):32–8. doi: 10.1055/a-1033-7524. [DOI] [PubMed] [Google Scholar]
- 13.Sencar ME, Calapkulu M, Sakiz D, Hepsen S, Kus A, Akhanli P, et al. An evaluation of the results of the steroid and non-steroidal anti-inflammatory drug treatments in subacute thyroiditis in relation to persistent hypothyroidism and recurrence. Sci Rep. 2019 Nov;9((1)):16899–8. doi: 10.1038/s41598-019-53475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tachibana T, Orita Y, Ogawara Y, Matsuyama Y, Abe I, Nakada M, et al. Time-lag between symptom onset and laboratory findings in patients with subacute thyroiditis. Auris Nasus Larynx. 2014 Aug;41((4)):369–72. doi: 10.1016/j.anl.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016 Apr;8((1)):39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna S, Tosh PK. A clinician's primer on the role of the microbiome in human health and disease. Mayo Clin Proc. 2014 Jan;89((1)):107–14. doi: 10.1016/j.mayocp.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Isler B, Keske Ş, Aksoy M, Azap ÖK, Yilmaz M, Yavuz SŞ, et al. Antibiotic overconsumption and resistance in Turkey. Clin Microbiol Infect. 2019 Jun;25((6)):651–3. doi: 10.1016/j.cmi.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Chang Y, Chusri S, Sangthong R, McNeil E, Hu J, Du W, et al. Clinical pattern of antibiotic overuse and misuse in primary healthcare hospitals in the southwest of China. PLoS One. 2019 Jun;14((6)):e0214779. doi: 10.1371/journal.pone.0214779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016 Mar;164((6)):425–34. doi: 10.7326/M15-1840. [DOI] [PubMed] [Google Scholar]