Abstract
Background
Patients with underlying medical conditions are at increased risk for severe coronavirus disease 2019 (Covid-19). Whereas vaccine-derived immunity develops over time, neutralizing monoclonal-antibody treatment provides immediate, passive immunity and may limit disease progression and complications.
Methods
In this phase 3 trial, we randomly assigned, in a 1:1 ratio, a cohort of ambulatory patients with mild or moderate Covid-19 who were at high risk for progression to severe disease to receive a single intravenous infusion of either a neutralizing monoclonal-antibody combination agent (2800 mg of bamlanivimab and 2800 mg of etesevimab, administered together) or placebo within 3 days after a laboratory diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The primary outcome was the overall clinical status of the patients, defined as Covid-19–related hospitalization or death from any cause by day 29.
Results
A total of 1035 patients underwent randomization and received an infusion of bamlanivimab–etesevimab or placebo. The mean (±SD) age of the patients was 53.8±16.8 years, and 52.0% were adolescent girls or women. By day 29, a total of 11 of 518 patients (2.1%) in the bamlanivimab–etesevimab group had a Covid-19–related hospitalization or death from any cause, as compared with 36 of 517 patients (7.0%) in the placebo group (absolute risk difference, −4.8 percentage points; 95% confidence interval [CI], −7.4 to −2.3; relative risk difference, 70%; P<0.001). No deaths occurred in the bamlanivimab–etesevimab group; in the placebo group, 10 deaths occurred, 9 of which were designated by the trial investigators as Covid-19–related. At day 7, a greater reduction from baseline in the log viral load was observed among patients who received bamlanivimab plus etesevimab than among those who received placebo (difference from placebo in the change from baseline, −1.20; 95% CI, −1.46 to −0.94; P<0.001).
Conclusions
Among high-risk ambulatory patients, bamlanivimab plus etesevimab led to a lower incidence of Covid-19–related hospitalization and death than did placebo and accelerated the decline in the SARS-CoV-2 viral load. (Funded by Eli Lilly; BLAZE-1 ClinicalTrials.gov number, NCT04427501.)
Coronavirus disease 2019 (Covid-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread globally and poses a serious, ongoing threat to human health. Covid-19 ranges from mild to severe, and a high incidence of illness and death has been reported in a vulnerable subgroup of patients.1-7 The risk of death from Covid-19 is increased among older patients and among those with chronic medical conditions such as cardiovascular disease, cancer, diabetes, lung disease, and obesity.8-10 Symptoms of Covid-19 include dyspnea, fatigue, fever, malaise, and anosmia.11-13 These symptoms can progress to more serious complications, including viral pneumonia and the acute respiratory distress syndrome.14 Long-term sequelae of Covid-19 are not fully understood, and persistent symptoms have been reported.15-17
Various treatment options have been explored for patients with Covid-19,18-20 including convalescent plasma21 and immunomodulators,20,22,23 although results with these therapies have been mixed. In December 2020, the Food and Drug Administration (FDA) granted emergency use authorization for the messenger RNA vaccines BNT162b224 and mRNA-1273,25 which target the SARS-CoV-2 spike protein. While these vaccines are being rolled out, an ongoing need remains for therapies to treat patients who have symptomatic Covid-19 before vaccination or in whom breakthrough infection develops. Immediate passive humoral immunotherapy with neutralizing monoclonal antibodies is a potential prophylactic and therapeutic option to prevent Covid-19–related hospitalization and death.26
Two such neutralizing monoclonal antibodies, bamlanivimab and etesevimab, were isolated from convalescent plasma obtained from patients with Covid-19 in the United States and China, respectively.27,28 These potent neutralizing monoclonal antibodies target the surface spike glycoprotein of SARS-CoV-2 that mediates viral entry into host cells.29,30 Bamlanivimab was developed by Eli Lilly after its discovery by researchers at AbCellera Biologics and at the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases. Etesevimab was a result of the collaborative efforts of Eli Lilly, Junshi Biosciences, and the Institute of Microbiology of the Chinese Academy of Sciences.
The phase 2 and early portion of the phase 3 Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) clinical trial showed that both monotherapy with bamlanivimab and combination therapy with bamlanivimab and etesevimab were efficacious in reducing the risk of Covid-19–related hospitalization and progression to severe disease. As a result, the FDA granted emergency use authorization for bamlanivimab monotherapy in November 2020,31 but this authorization was later revoked. The FDA granted emergency use authorization status for bamlanivimab plus etesevimab, administered together, in February 2021.32 Here, we report the findings from the latest portion of the phase 3 BLAZE-1 trial in which a cohort of adolescent (≥12 years of age) and adult outpatients with mild or moderate Covid-19 who were at high risk for severe disease received bamlanivimab–etesevimab or placebo.
Methods
Trial Design
In this ongoing phase 2–3, randomized, double-blind, placebo-controlled, single-dose trial conducted in the United States, all the patients had recently received a diagnosis of mild or moderate Covid-19 in an outpatient setting. The patients presented with mild or moderate Covid-19 within 3 days after they had tested positive for SARS-CoV-2 by means of either direct antigen or nucleic acid identification (i.e., by reverse-transcriptase–polymerase-chain-reaction [RT-PCR] assay). Full inclusion and exclusion criteria are provided in the protocol and in the Supplementary Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org.
This multipart trial investigated a number of cohorts and treatment groups; however, here we focus on the results from the original portion of the phase 3 trial, which involved a cohort of adolescent and adult patients with at least one risk factor for severe Covid-19. These risk factors, which are specified in the inclusion criteria, are a subset of those identified by the Centers for Disease Control and Prevention (CDC)33 (i.e., an age ≥65 years; a body-mass index [BMI, the weight in kilograms divided by the square of the height in meters] ≥35; certain underlying medical conditions, including chronic kidney disease, type 1 or type 2 diabetes mellitus, an immunocompromised condition, cardiovascular disease, hypertension, and chronic respiratory disease; and receipt of immunosuppressive treatment).
In this portion of the BLAZE-1 trial, the first patient was enrolled on September 4, 2020, and the last patient was enrolled on December 8, 2020. The patients received a single intravenous infusion consisting of either a combination of 2800 mg of bamlanivimab and 2800 mg of etesevimab or placebo over a period of 1 hour. The patients were considered lost to follow-up if they repeatedly did not participate in scheduled visits and could not be contacted by the trial-site investigators or staff.
The trial was conducted in accordance with the principles of the Declaration of Helsinki, the international ethical guidelines of the Council for International Organizations of Medical Sciences, the International Council for Harmonisation Good Clinical Practice guidelines, and applicable laws and regulations. All the patients or their legally authorized representatives provided written informed consent, and patients who were 12 to 17 years of age provided assent before initiation of the trial.
Patients
Ambulatory patients who were 12 to 17 years of age and who had at least one of the following risk factors at the time of screening were included in the trial: a BMI in at least the 85th percentile for age and sex, according to CDC growth charts34; sickle cell disease; congenital or acquired heart disease; neurodevelopmental disorders such as cerebral palsy; dependence on a medical-related mechanical device or procedure such as tracheostomy, gastrostomy, or positive-pressure ventilation (not related to Covid-19); asthma, a reactive airway, or another chronic respiratory disease; type 1 or type 2 diabetes mellitus; and an immunocompromised condition or receipt of an immunosuppressive treatment. Ambulatory patients who were at least 18 years of age and who presented with at least one of the following risk factors were also included: age of at least 65 years, a BMI of at least 35, chronic kidney disease, diabetes mellitus type 1 or type 2, immunosuppressive disease or receipt of immunosuppressive treatment, and an age of at least 55 years with cardiovascular disease, hypertension, or chronic obstructive pulmonary disease or another chronic respiratory disease.
For both adolescents and adults, mild or moderate Covid-19 was defined according to FDA guidance35 and included the following eight symptoms: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, and shortness of breath with exertion. Notable exclusion criteria included a peripheral oxygen saturation of 93% or less while breathing ambient air, a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen of less than 300, a respiratory rate of at least 30 breaths per minute, and a heart rate of 125 or more beats per minute.
Outcomes
The primary outcome was the overall clinical status of the patients, defined as Covid-19–related hospitalization (acute care for ≥24 hours) or death from any cause by day 29. Key secondary outcomes were the change from baseline to day 7 in the SARS-CoV-2 viral load and a persistently high SARS-CoV-2 viral load on day 7 (defined as a log viral load >5.27, corresponding to a mean PCR cycle-threshold [Ct] value of <27.5). This threshold, which was identified in a post hoc analysis from the phase 2 part of the BLAZE-1 trial, was prespecified for the current analysis.
Other key secondary outcomes were a composite of a Covid-19–related hospitalization, a visit to an emergency department, or death from any cause by day 29 and the time to sustained patient-reported resolution of symptoms. Resolution was defined as an absence of all eight Covid-19–related symptoms except for mild cough or as an absence of fatigue for two consecutive assessments. Additional secondary outcomes were a reduction in the SARS-CoV-2 viral load from baseline to days 3 and 5, the time to viral clearance, the area under the response–time curve for the viral load through day 7, the time to a reduction and resolution of symptoms (including resolution, complete resolution [i.e., resolution that includes an absence of any cough and fatigue], and sustained complete resolution), and safety.
Statistical Analysis
We estimated that a sample size of approximately 500 patients per group would provide the trial with greater than 90% power to detect a significant difference in the percentage of patients with Covid-19–related hospitalization or death from any cause, in favor of the bamlanivimab–etesevimab group over the placebo group (defined as an odds ratio of <1). This sample-size calculation was based on an assumed event rate of 8.7% in the placebo group and a 60% lower risk in the bamlanivimab–etesevimab group than in the placebo group; these assumptions were informed by available data on Covid-19–related hospitalization or death.
The analyses included a subgroup analysis involving patients who were 12 to 17 years of age, as compared with those who were 18 years of age or older, if this analysis would be feasible given the sample size. For modeling of estimates and calculation of P values, efficacy analyses were adjusted for the baseline value of the outcome and for the randomization stratification factor (the duration from the onset of symptoms to randomization [≤8 days vs. >8 days]). The analysis of the primary outcome was conducted with the use of logistic regression with a primary success criterion of a one-sided alpha level 0.025. The SARS-CoV-2 viral load was calculated from the RT-PCR cycle-threshold value and reported in log10. A multiple testing procedure that controls the familywise error rate at the one-sided 0.025 level was applied to the primary and key secondary outcomes. Details regarding the calculation of the viral load and the multiple testing procedure are provided in the statistical analysis plan, available with the protocol at NEJM.org. Comparisons of continuous efficacy, safety, and health outcome variables with a single postbaseline time point were made with the use of analysis of covariance and included the trial group, the randomization stratification factor, and the baseline value in the model. Comparisons of continuous efficacy and pharmacodynamic variables with multiple postbaseline measurements were made with the use of repeated-measures analysis as a mixed model. The Kaplan–Meier product-limit method was used for time-to-event analyses. All the statistical analyses were performed with the use of SAS software, version 9.4 (or a higher version) (SAS Institute); FACTS software, version 6.0 (or a higher version) (Berry Consultants); R statistics software, version 3.6 (or a higher version) (R Foundation for Statistical Reporting); or all these methods.
Results
Patients
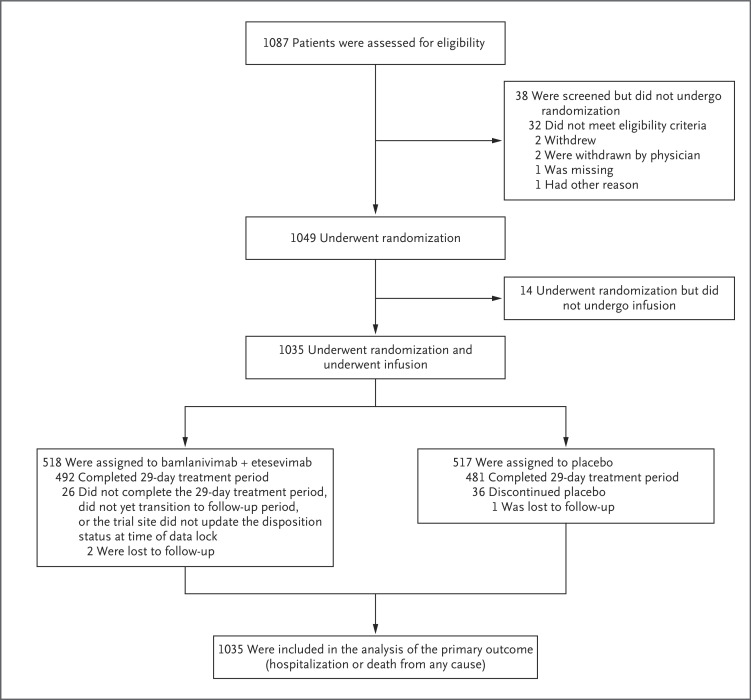
At the time of the database lock on January 20, 2021, a total of 1035 patients had been randomly assigned to receive either the combination of 2800 mg of bamlanivimab and 2800 mg of etesevimab or placebo (Figure 1). The mean (±SD) age of the patients was 53.8±16.8 years, 31.2% of the patients were 65 years of age or older, and the median BMI across the patient cohort was 34.09 (Table 1). Approximately 52.0% of all the patients were adolescent girls or women, 29.4% identified as Hispanic or Latinx, and 8.1% identified as Black. At the time of randomization, 77.3% of the patients had mild Covid-19 symptoms. The patients received an infusion of either bamlanivimab–etesevimab or placebo within a median of 4 days after the onset of symptoms. The observed mean Ct value was 23.97 on the day of infusion (Table 1).
Figure 1. Enrollment and Trial Design.
CDC denotes Centers for Disease Control and Prevention, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Table 1. Characteristics of the Patients at Baseline.*.
| Characteristic | Bamlanivimab plus Etesevimab (N=518) |
Placebo (N=517) |
Total (N=1035) |
|---|---|---|---|
| Age | |||
| Mean ±SD — yr | 54.3±17.1 | 53.3±16.4 | 53.8±16.8 |
| 65 yr or older — no. (%) | 168 (32.4) | 155 (30.0) | 323 (31.2) |
| Race or ethnic group — no./total no. (%)† | |||
| American Indian or Alaska Native | 2/512 (0.4) | 1/513 (0.2) | 3/1025 (0.3) |
| Asian | 16/512 (3.1) | 22/513 (4.3) | 38/1025 (3.7) |
| Black | 44/512 (8.6) | 39/513 (7.6) | 83/1025 (8.1) |
| Native Hawaiian or other Pacific Islander | 0 | 2/513 (0.4) | 2/1025 (0.2) |
| White | 449/512 (87.7) | 447/513 (87.1) | 896/1025 (87.4) |
| Multiple races or ethnic groups | 1/512 (0.2) | 2/513 (0.4) | 3/1025 (0.3) |
| Missing data | 6 | 4 | 10 |
| Hispanic or Latinx | 149/517 (28.8) | 155/516 (30.0) | 304/1033 (29.4) |
| Median body-mass index‡ | 34.14 | 33.90 | 34.09 |
| Peripheral oxygen saturation — no./total no. (%) | |||
| <96% | 90/516 (17.4) | 106/514 (20.6) | 196/1030 (19.0) |
| ≥96% | 426/516 (82.6) | 408/514 (79.4) | 834/1030 (81.0) |
| Risk of severe Covid-19 — no./total no. (%) | |||
| High | 493/518 (95.2) | 490/517 (94.8) | 983/1035 (95.0) |
| Low | 25/518 (4.8) | 27/517 (5.2) | 52/1035 (5.0) |
| Disease status — no. (%) | |||
| Mild Covid-19 | 397 (76.6) | 403 (77.9) | 800 (77.3) |
| Moderate Covid-19 | 121 (23.4) | 114 (22.1) | 235 (22.7) |
| Median days from symptom onset to randomization — no. (range) | 4 (0–29) | 4 (0–13) | 40 (0–29) |
| Mean viral load — Ct value§ | 23.98 | 23.97 | 23.97 |
Covid-19 denotes coronavirus disease 2019.
Race or ethnic group was reported by the patients, who could choose more than one category.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Ct denotes the cycle threshold on the reverse-transcriptase–polymerase-chain-reaction assay.
Primary Outcome
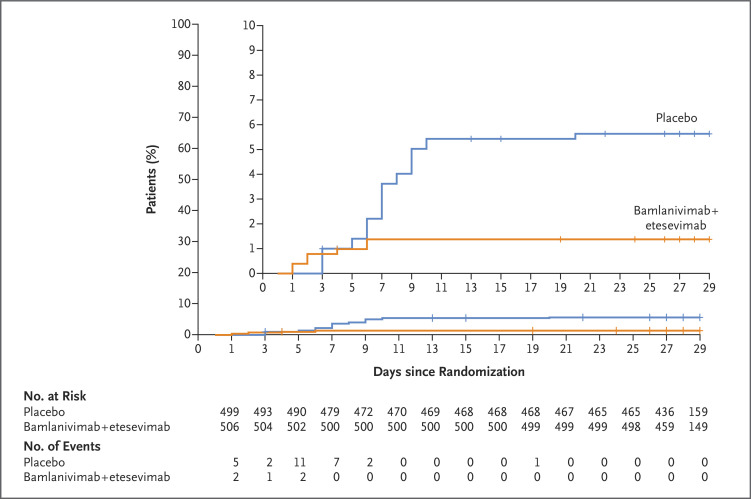
A total 11 of 518 patients (2.1%) in the bamlanivimab–etesevimab group, as compared with 36 of 517 patients (7.0%) in the placebo group, had a Covid-19–related hospitalization (defined as acute care for ≥24 hours) or death from any cause by day 29 (absolute risk difference, −4.8 percentage points; 95% confidence interval [CI], −7.4 to −2.3; relative risk difference, 70%; P<0.001) (Figure 2). By day 29, none of the patients who received bamlanivimab plus etesevimab had died, and 10 of the 517 patients who received placebo had died. Of these 10 deaths, 9 were deemed to be Covid-19–related by trial staff who were unaware of the trial-group assignments (Table S1 in the Supplementary Appendix).
Figure 2. Kaplan–Meier Estimate of the Time to Hospitalization among High-Risk Patients Who Received Bamlanivimab–Etesevimab or Placebo.
The inset shows the same data on an enlarged y axis. Tick marks indicate censored data.
Key Secondary Outcomes
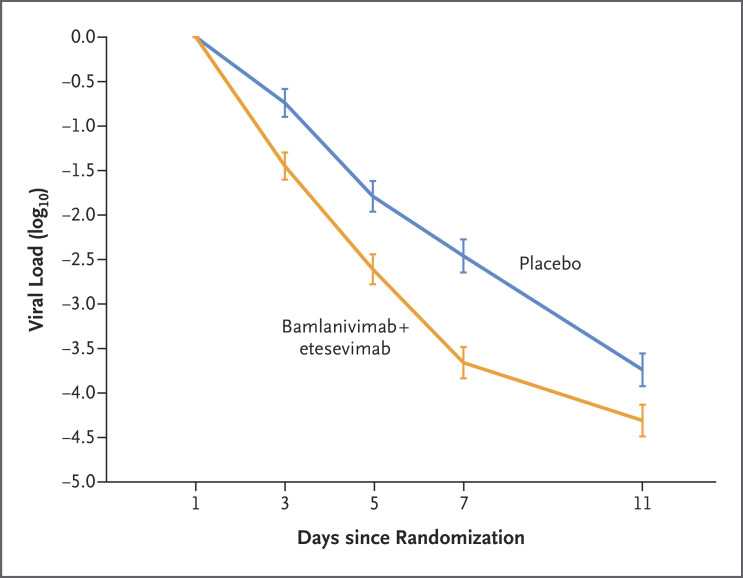
The mean reduction in the viral load from baseline to day 7 was approximately 16 times as high in patients who received bamlanivimab plus etesevimab as in those who received placebo (difference from placebo in the decrease from baseline, −1.20; 95% CI, −1.46 to −0.94; P<0.001) (Figure 3). The percentage of patients with a persistently high viral load on day 7 was 9.8% (50 of 508 patients) in the bamlanivimab–etesevimab group, as compared with 29.5% (147 of 499 patients) in the placebo group (difference, −19.6 percentage points; 95% CI, −24.4 to −14.9; P<0.001). Twelve of 518 patients (2.3%) who received bamlanivimab plus etesevimab had a Covid-19–related hospitalization, an emergency department visit, or death from any cause by day 29, as compared with 37 of 517 patients (7.2%) who received placebo (difference from placebo in the decrease from baseline, −4.8 percentage points; 95% CI, −7.4 to −2.3; P<0.001). The median time to sustained resolution of symptoms after two consecutive assessments was 1 day shorter in the bamlanivimab–etesevimab group (8 days; 95% CI, 7 to 8) than in the placebo group (9 days; 95% CI, 8 to 10) (P=0.007). Results with respect to additional secondary outcomes are provided in Figures S1 through S3, Figure S5, and Table S2.
Figure 3. Effect of Bamlanivimab–Etesevimab on Viral Load (Days 1–11).
The mean change in the viral load from baseline to day 11 after the initiation of bamlanivimab–etesevimab or placebo is shown. The viral load was calculated from the cycle-threshold value on reverse-transcriptase–polymerase-chain-reaction assay. The error bars indicate 95% confidence intervals.
Safety
Serious adverse events occurred in 7 of 518 patients (1.4%) in the bamlanivimab–etesevimab group and in 5 of 517 patients (1.0%) in the placebo group. Adverse events that occurred after the infusion was initiated were reported in 69 of 518 patients (13.3%) in the bamlanivimab–etesevimab group and in 60 of 517 patients (11.6%) in the placebo group. In both groups, the most common adverse events were nausea, rash, dizziness, diarrhea, and hypertension (Table 2).
Table 2. Adverse Events.
| Adverse Event | Bamlanivimab plus Etesevimab (N=518) |
Placebo (N=517) |
Total (N=1035) |
|---|---|---|---|
| number of patients (percent) | |||
| Serious adverse events | 7 (1.4) | 5 (1.0) | 12 (1.2) |
| Adverse events | 69 (13.3) | 60 (11.6) | 129 (12.5) |
| Mild | 37 (7.1) | 35 (6.8) | 72 (7.0) |
| Moderate | 24 (4.6) | 20 (3.9) | 44 (4.3) |
| Severe | 7 (1.4) | 5 (1.0) | 12 (1.2) |
| Missing data | 1 (0.2) | 0 | 1 (0.1) |
| Death from adverse event | 0 | 1 (0.2) | 1 (0.1) |
| Most common adverse events according to preferred term | |||
| Nausea | 5 (1.0) | 4 (0.8) | 9 (0.9) |
| Rash | 6 (1.2) | 3 (0.6) | 9 (0.9) |
| Dizziness | 4 (0.8) | 3 (0.6) | 7 (0.7) |
| Diarrhea | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Hypertension | 2 (0.4) | 2 (0.4) | 4 (0.4) |
| Vaginal infection* | 1/279 (0.4) | 1/259 (0.4) | 2/538 (0.4) |
| Gastroesophageal reflux disease | 3 (0.6) | 0 | 3 (0.3) |
| Pruritus | 3 (0.6) | 0 | 3 (0.3) |
| Urinary tract infection | 3 (0.6) | 0 | 3 (0.3) |
| Urticaria | 2 (0.4) | 1 (0.2) | 3 (0.3) |
| Vomiting | 2 (0.4) | 1 (0.2) | 3 (0.3) |
| Abdominal pain | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Increased blood level of creatine kinase | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Covid-19 | 0 | 2 (0.4) | 2 (0.2) |
| Constipation | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Dehydration | 1 (0.2) | 1 (0.2) | 2 (0.2) |
| Dysgeusia | 2 (0.4) | 0 | 2 (0.2) |
| Dyspepsia | 2 (0.4) | 0 | 2 (0.2) |
| Fungal infection | 0 | 2 (0.4) | 2 (0.2) |
| Headache | 1 (0.2) | 1 (0.2) | 2 (0.2) |
The numbers shown for this event are the numbers of adolescent girls or women who had the event and the total numbers of adolescent girls or women in the two groups in the trial.
Exploratory Outcomes
A total of 11 of 518 patients (2.1%) who received bamlanivimab plus etesevimab were hospitalized with Covid-19. The mean (±SD) duration of hospitalization in these patients was 7.3±6.4 days. More patients who received placebo (6.4% [33 of 517 patients]) were hospitalized with Covid-19, and the mean duration of hospitalization among these patients was 11.2±10.1 days.
Discussion
In this phase 3 portion of the BLAZE-1 trial, we examined the efficacy of bamlanivimab plus etesevimab in the treatment of early mild or moderate Covid-19 in a cohort of ambulatory adolescent and adult patients who were considered to be at high risk for progression of Covid-19 because of preexisting medical conditions. We assessed the effect of early intervention with antibody therapy on hospitalization, viral load, resolution of symptoms, and death in this patient cohort, and we found a greater clinical and virologic benefit with bamlanivimab plus etesevimab than with placebo across the primary and secondary outcomes.
The results with respect to the primary outcome indicated the effectiveness of early intervention with bamlanivimab plus etesevimab in reducing the incidence of hospitalization. The incidence of Covid-19–related hospitalizations or death from any cause by day 29 was 4.8 percentage points lower among the patients who received bamlanivimab plus etesevimab than among those who received placebo. These results were consistent with those reported previously in a study of bamlanivimab alone1 and in the phase 2 portion of the BLAZE-1 trial, which evaluated bamlanivimab plus etesevimab.36 No deaths were reported among the patients who received bamlanivimab plus etesevimab, whereas 10 deaths, 9 of which were considered by the investigators to be Covid-19–related, were reported among those who received placebo. The majority of these deaths occurred in male patients and in those who were 59 years of age or older, and hypertension was the most common coexisting condition in the placebo group. In addition to the lower incidences of hospitalization and death, bamlanivimab plus etesevimab resulted in more rapid resolution of symptoms within 4 days after the initiation of treatment. These results provide support for the potential of neutralizing monoclonal-antibody therapy to reduce both the risk of progression to severe disease and the severity of disease among high-risk patients with symptomatic Covid-19.
In a finding that was consistent with the clinical benefits observed, bamlanivimab plus etesevimab resulted in reductions in the SARS-CoV-2 viral load through day 7. The percentage of patients with a viral load greater than 5.27 was also lower in the bamlanivimab–etesevimab group than in the placebo group. At day 7, the reduction in viral load was approximately 16 times as high among the patients in the bamlanivimab–etesevimab group as among those in the placebo group. Our trial showed that viral load (measured by means of a nasopharyngeal swab) is a potential biomarker for Covid-19 that may correlate with clinical outcomes.
The incidence of serious adverse events was low and similar in the bamlanivimab–etesevimab group (1.4%) and the placebo group (1.0%); these findings indicate that bamlanivimab plus etesevimab had an acceptable safety profile for use in high-risk populations. None of the patients discontinued bamlanivimab–etesevimab or placebo because of adverse events, and the overall safety profile of bamlanivimab–etesevimab were consistent with those observed in previous analyses.1,36
The viral sequencing of nasopharyngeal samples obtained from patients during this trial is ongoing. Of the sequencing results available, limited (<5%) variations in the spike protein were observed in baseline or post-treatment samples at positions known to affect the in vitro neutralization capabilities of bamlanivimab plus etesevimab. Recent studies have shown that some circulating viral variants, such as the B.1.351 (or beta) variant first identified in South Africa37 and the P.1 (or gamma) variant first identified in Brazil,38 have in vitro resistance to several monoclonal antibodies in clinical development, including bamlanivimab plus etesevimab. These variants were not observed in this trial at the time of this report, but their emergence highlights the need for more extensive global viral surveillance programs that will serve an important purpose in defining the usefulness of bamlanivimab plus etesevimab.
Although the results presented here are a step forward in the fight against Covid-19, considerable logistic challenges remain for the administration of monoclonal-antibody treatment. The therapeutic delivery of bamlanivimab monotherapy has revealed the challenges that already overstretched health care facilities face, including the space, personnel, and resources necessary to administer treatment. Additional issues include postinfusion safety monitoring and reducing infusion times, although the FDA recently authorized decreased infusion times (as short as 21 minutes) for bamlanivimab plus etesevimab.39
Our trial has limitations. Only 12.6% of the patients identified as non-White, and only 1.1% of the patients were 12 to 17 years of age. The percentages of patients with common coexisting conditions such as chronic kidney disease, cardiovascular disease, and chronic obstructive pulmonary disease were 3.5%, 7.4%, and 8.2%, respectively. The percentages of patients who had a preexisting immunologic condition or who were receiving immunosuppressive agents were low, at 1.5% and 4.9%, respectively. The current emergency use authorization for bamlanivimab plus etesevimab dictates that patients must receive these drugs within 10 days after symptom onset. However, in this trial, only approximately 5% of the patients had had symptoms for more than 8 days before receiving the infusion. This trial was conducted only in the United States, and there may be differences with respect to safety and efficacy globally that cannot be determined with this limited data set. Other factors such as demographic differences and differences in health system utilization — not just the severity of Covid-19 — may have influenced the number of patients who were hospitalized as the trial progressed. The emergence of viral variants was also not accounted for in the analyses.
These data from the latest portion of the phase 3 BLAZE-1 clinical trial show the clinical benefit of early testing for SARS-CoV-2 infection coupled with prompt intervention with neutralizing antibody therapy in high-risk ambulatory patients. This trial also provides context for the recent FDA emergency use authorization granted for the use of bamlanivimab plus etesevimab in the treatment of outpatients who have mild or moderate Covid-19 and a high risk of progression to more severe disease, hospitalization, or both. While society moves toward ending the Covid-19 pandemic with widespread vaccination campaigns and efforts to achieve herd immunity, antibody therapy provides a potential treatment option to reduce the incidence of illness and death among vulnerable patients.
Acknowledgments
We thank Lynn Naughton, Ph.D., of Eli Lilly, for providing medical-writing and editorial support with an earlier version of the manuscript.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on July 14, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Eli Lilly.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021;384:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cates J, Lucero-Obusan C, Dahl RM, et al. Risk for in-hospital complications associated with COVID-19 and influenza — Veterans Health Administration, United States, October 1, 2018–May 31, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health 2020;13:1833-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piroth L, Cottenet J, Mariet A-S, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med 2021;9:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J, Tong Z, Guan X, Du B, Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open 2020;3(4):e205619-e205619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chidambaram V, Tun NL, Haque WZ, et al. Factors associated with disease severity and mortality among patients with COVID-19: a systematic review and meta-analysis. PLoS One 2020;15(11):e0241541-e0241541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest 2020;158:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and cancer: a comprehensive review. Curr Oncol Rep 2020;22:53-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim L, Whitaker M, O’Halloran A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 — COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1081-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). June 30, 2020. (https://stacks.cdc.gov/view/cdc/89980).
- 14.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med 2020;383:2451-2460. [DOI] [PubMed] [Google Scholar]
- 15.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA 2020;324:1723-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2021;93:1013-1022. [DOI] [PubMed] [Google Scholar]
- 18.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in mild-to-moderate Covid-19. N Engl J Med 2020;383:2041-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020;383:1813-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyner MJ, Senefeld JW, Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. August 12, 2020. (https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1). preprint. [Google Scholar]
- 22.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;384:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roche. Roche provides an update on the phase III COVACTA Trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. July 29, 2020. (https://www.roche.com/investors/updates/inv-update-2020-07-29.htm).
- 24.FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. News release of the Food and Drug Administration Silver Spring, MD, December 11, 2020. (https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19). [Google Scholar]
- 25.FDA takes additional action in fight against COVID-19 by issuing emergency use authorization for second COVID-19 vaccine. News release of the Food and Drug Administration Silver Spring, MD, December 18, 2020. (https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid). [Google Scholar]
- 26.Marston HD, Paules CI, Fauci AS. Monoclonal antibodies for emerging infectious diseases — borrowing from history. N Engl J Med 2018;378:1469-1472. [DOI] [PubMed] [Google Scholar]
- 27.Jones BE, Brown-Augsburger PL, Corbett KS, et al. LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection. October 1, 2020. (https://pubmed.ncbi.nlm.nih.gov/33024963/). preprint. [Google Scholar]
- 28.Shi R, Shan C, Duan X, et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020;584:120-124. [DOI] [PubMed] [Google Scholar]
- 29.Benton DJ, Wrobel AG, Xu P, et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 2020;588:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. News release of the Food and Drug Administration, Silver Spring, MD, November 9, 2020. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19). [Google Scholar]
- 32.Coronavirus (COVID-19) Update: FDA authorizes monoclonal antibodies for treatment of COVID-19. News release of the Food and Drug Administration, Silver Spring, MD, February 9, 2021. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0). [Google Scholar]
- 33.Centers for Disease Control and Prevention. People at increased risk: and other people who need to take extra precautions. April 20, 2021. (https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html).
- 34.Centers for Disease Control and Prevention. Clinical growth charts (https://www.cdc.gov/growthcharts/clinical_charts.htm).
- 35.Food and Drug Administration. COVID-19: developing drugs and biological products for treatment or prevention. May 2020. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/covid-19-developing-drugs-and-biological-products-treatment-or-prevention).
- 36.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021;325:632-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021;184(9):2348.e6-2361.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen RE, Zhang X, Case JB, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med 2021;27:717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Food and Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab and etesevimab. Indianapolis: Eli Lilly, February 2021. (https://www.fda.gov/media/145802/download). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.