Abstract
Introduction
After a vaccination, patients frequently have clinical symptoms of pain and swelling over the injection area which usually resolve 2–3 days after the injection. If the symptoms do not improve, a shoulder injury related to vaccine administration (SIRVA) will be considered, perhaps related to an improper injection technique. Herein we report our first case of a SIRVA after a Sinovac COVID-19 vaccination which occurred due to deep penetration and direction of the needle. The clinical symptoms of the patient improved after treatment with combined oral non-steroidal anti-inflammatory drugs and a short course of intravenous antibiotic.
Case presentation
A 52-year-old Thai male without prior shoulder pain had a Sinovac COVID-19 vaccination at his right shoulder. The injection was given by a nurse using a 27-gauge needle, 1.5 inches in length. The injection landmark was 3 finger breadths below the midlateral edge of the acromial process. The direction of the needle was 45° to the skin cephalad. Three days after receiving the vaccine the patient began to have right shoulder pain with limited range of motion and acute fever. He was admitted for medical treatment which his clinical symptoms gradually improved.
Conclusion
We report a case of subacromial-subcoracoid-subdeltoid bursitis following a Sinovac COVID-19 vaccine injection. This condition is rare, and usually related to an incorrect vaccination technique. To avoid this complication, nurses should identify the correct landmark, use an appropriate needle length, and point the needle in the correct direction.
Keywords: COVID-19, Pain, Shoulder, Vaccination
Highlights
-
•
After a vaccination, if the symptoms do not improve, SIRVA will be considered.
-
•
There is a risk of SIRVA if the direction of the needle was pointed to the skin cephalad.
-
•
Before diagnosis SIRVA, septic arthritis of shoulder should be ruled out.
1. Introduction
After a COVID-19 vaccination, adverse effects can occur, divided into systemic and local effects. Systemic adverse effects include headache, fever, fatigue, chills, nausea, diarrhea, unusual muscle pain and unusual joint pain, while local adverse effects include pain, redness, swelling, warmth, itching, tenderness and bruising [1]. Patients frequently have one or more of these clinical symptoms around the injection area, which usually resolve 2–3 days after the injection. If the symptoms do not improve, a shoulder injury related to vaccine administration (SIRVA) will be considered, perhaps related to an improper injection technique. SIRVA is a rare condition, usually related to an influenza vaccination [[2], [3], [4], [5], [6]]. The pathogenesis of this condition is an immune response to intracapsular inoculation after vaccination [7], which is usually treated with medications such as oral non-steroidal anti-inflammatory drugs [6] or an intrabursal steroid injection [4,5]. For the COVID-19 vaccinations, there has to date been only one report of a SIRVA following an Oxford-AstraZeneca COVID-19 injection (Serum Institute of India, India). In this study, we report the first known case of SIRVA after a Sinovac COVID-19 vaccination (Sinovac Biotech, China). This case is reported according to the SCARE criteria [8], with Ethical Committee approval from the Faculty of Medicine of Prince of Songkla University (REC 64-320-11-1).
2. Case presentation
A 52-year-old Thai male without prior shoulder pain had a Sinovac COVID-19 vaccination at his right shoulder. The injection was given by a nurse using a 27-gauge needle, 1.5 inches in length. The injection landmark was 3 finger breadths below the midlateral edge of the acromial process. The direction of the needle was 45° to the skin cephalad (Fig. 1).
Fig. 1.
Photograph of the patient receiving the Sinovac COVID-19 vaccine.
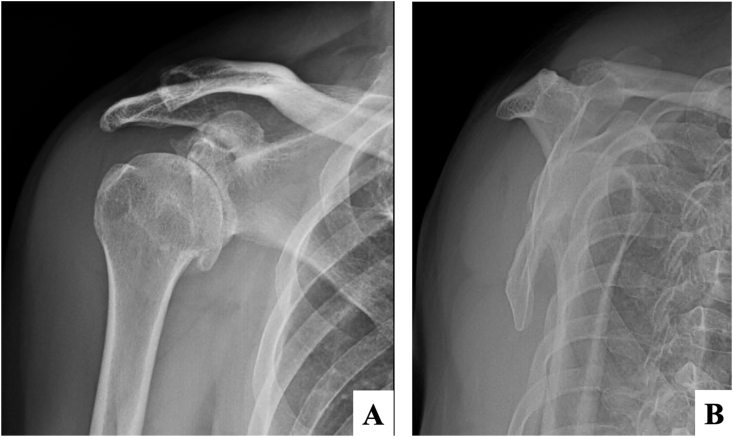
Three days after receiving the vaccine the patient began to have right shoulder pain with limited range of motion and acute fever. A physical examination showed swelling at the right deltoid area, with limited range of right shoulder motion. Anteroposterior and lateral radiographic images of the shoulder showed degenerative change without fracture (Fig. 2A and B). The initial laboratory investigations were white blood cell count 7160/μL, hematocrit 38.0%, hemoglobin 12.8/dL, polymorphonuclear neutrophils 65.2%, lymphocytes 16.9%, eosinophils 13.4%, monocytes 4.2%, platelets 273,000/μL, ESR 9 mm/hr and CRP 3.5 mg/L. To rule out septic arthritis of the right shoulder, the patient was sent for ultrasonography which showed subacromial-subcoracoid-subdeltoid bursitis. The bursa was aspirated under ultrasonography guidance and 5mL of serosanguinous fluid was removed (Fig. 3) and sent for cell analysis, gram stain and culture. The joint fluid analysis showed white blood cell count 45,500 cells/mm3, monocytes 1%, polymorphonuclear neutrophils 99%, red blood cell count 23,400 cells/mm3, and no crystal. No organisms were found in joint fluid by in the gram stain and culture evaluations. He was admitted for an intravenous cefazolin (4000 mg/day) for 3 days then changed to oral cefalexine (1000 mg/day) for 7 days. During admission, his clinical symptoms gradually improved. He had no fever after 1 day of admission. He could slowly increase movement in his right shoulder and was able to tolerate passive motion of his right shoulder after 3 days. He was sent for post-treatment ultrasonography (Fig. 4A) which showed decreased fluid in the bursas compared with the pre-treatment ultrasonography (Fig. 4B).
Fig. 2.
Initial radiographic imaging of the right shoulder in (A) anteroposterior, and (B) lateral transcapular views.
Fig. 3.
Serosanguinous fluid after aspiration from a subdeltoid bursa of right shoulder.
Fig. 4.
Ultrasonography: (A) Post-treatment ultrasonography showed decreased fluid in the bursas compared with (B) the pre-treatment ultrasonography.
3. Discussion
SIRVA is a rare condition following vaccine injection within 48 hours which is usually related to influenza vaccinations [[2], [3], [4], [5], [6]]. In this case, the patient developed right shoulder pain 72 hours following a Sinovac COVID-19 vaccine injection. The risk factors in this case were length and direction of needle during the injection.
There has to date been only one case report of a SIRVA following an Oxford-AstraZeneca COVID-19 injection, reported by Rodrigues et al. [9] This case was not a usual SIRVA case because the clinical symptoms did not appear until 8 weeks following the vaccination. The clinical symptoms of the patient in our study developed within 3 days following the vaccine injection. To reduce the risk of SIRVA, nurses are trained to follow two main steps in a shoulder vaccine injection. First, the entry point of the needle should be inferior to the extended subdeltoid bursa and higher than the axillary nerve. A study by Bodor and Montalvo showed that the subdeltoid bursa extends approximately 3.0–6.0 cm below the lateral edge of the acromial process and medial to the lateral border of the acromial process at a depth of 0.8–1.6 cm [10]. The location of the axillary nerve depends on the shoulder position. A study by Chuaychoosakoon and Suwannaphisit measured the distances from the lateral edge of the acromial process to the axillary nerve in different arm positions and found that the mean distances from the mid-lateral edge of the acromial process to the axillary nerve with the arm at the side and with 30° of arm abduction were 52.20 ± 4.21 and 49.66 ± 4.54 mm, respectively [11]. For clinical practice, there are two recommendations about an intramuscular injection at the deltoid area from two studies. Nakajima et al. [12] recommends that intramuscular injections should be given at the intersection between the anteroposterior axillary line and perpendicular line from the mid-lateral of the acromial process, with at least 5 mm needle depth perpendicular to the skin. Another study from the National Immunization Technical Advisory Groups in Ireland, New Zealand and USA recommend injections should be given into the deltoid muscle 2–3 finger breadths below the mid-lateral acromial process [13].
4. Conclusions
We report a case of subacromial-subcoracoid-subdeltoid bursitis following a Sinovac COVID-19 vaccine injection. This condition is rare, and usually related to an incorrect vaccination technique. To avoid this complication, nurses should identify the correct landmark, use an appropriate needle length, and point the needle in the correct direction.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
No funding was involved regarding this case report.
Ethical approval
The present study was approved by the Prince of Songkla University Institutional Review Board, Faculty of Medicine, Songklanagarind Hospital, Prince of Songkla University (IRB number REC 64-320-11-1).
Consent
Written informed consent was obtained from the patient for publication.
Author contribution
Chaiwat Chuaychoosakoon —Preparation of case report, Literature review, Writing the paper. Wachirapan Parinyakhup —Preparation of case report. Writing the paper. Korakot Maliwankul —Preparation of case report. Writing the paper. Prapakorn Klabklay —Preparation of case report. Writing the paper. Pramot Tanutit —Preparation of case report. Writing the paper.
Registration of research studies
This case report is not first in man.
Guarantor
Chaiwat Chuacyhoosakoon, MD.
Declaration of competing interest
No conflicts of interest.
Acknowledgements
The authors sincerely thank the patient to report this case; the orthopedic residents for providing the patient's data; Konwarat Ninlachart for assistance with drawing the illustrations and David Patterson of the International Affairs Office of the Faculty of Medicine, Prince of Songkla University for English proofreading.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102622.
Contributor Information
Chaiwat Chuaychoosakoon, Email: psu.chaiwat@gmail.com, chaiwat.c@psu.ac.th.
Wachiraphan Parinyakhup, Email: chai069-084@hotmail.com.
Pramot Tanutit, Email: ptanutit@yahoo.com.
Korakot Maliwankul, Email: koopy_medy@hotmail.com.
Prapakorn Klabklay, Email: pglabgly@yahoo.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., Hu C., Selvachandran S., Antonelli M., Murray B., Canas L.S., Molteni E., Graham M.S., Modat M., Joshi A.D., Mangino M., Hammers A., Goodman A.L., Chan A.T., Wolf J., Steves C.J., Valdes A.M., Ourselin S., Spector T.D. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/s1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenkins M., Rupp D., Goebel L.J. Post-influenza vaccine subdeltoid bursitis. Cureus. 2020;12:10–13. doi: 10.7759/cureus.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook I.F. Subdeltoid/subacromial bursitis associated with influenza vaccination. Hum. Vaccines Immunother. 2014;10:605–606. doi: 10.4161/hv.27232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright A., Patel R., Motamedi D. Influenza vaccine-related subacromial/subdeltoid bursitis: a case report. J. Radiol. Case Rep. 2019;13:24–31. doi: 10.3941/jrcr.v13i6.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Littrell L.A., Leslie D.F., Bierle D.M., Wenger D.E. Progressive monoarticular inflammatory arthritis following influenza vaccination. Mayo Clin. Proc. Innov. Qual. Outcomes. 2021;5:204–209. doi: 10.1016/j.mayocpiqo.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szari S., Belgard A., Adams K., Freiler J. Shoulder injury related to vaccine administration: a rare reaction. Fed. Pract. 2019;36:380–384. [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor B.C., Hinke D. Shoulder injury related to vaccine administration (Sirva) Appl. Radiol. 2014;43:30–31. [Google Scholar]
- 8.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Cantarelli Rodrigues T., Hidalgo P.F., Skaf A.Y., Serfaty A. Subacromial-subdeltoid bursitis following COVID-19 vaccination: a case of shoulder injury related to vaccine administration (SIRVA) Skeletal Radiol. 2021 doi: 10.1007/s00256-021-03803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodor M., Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25:585–587. doi: 10.1016/j.vaccine.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Chuaychoosakoon C., Suwannaphisit S. The relationship between arm abduction position and the risk of iatrogenic anterior branch of the axillary nerve injuries: a cadaveric study. Orthop. J. Sports Med. 2021;9 doi: 10.1177/23259671211008834. 232596712110088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima Y., Mukai K., Takaoka K., Hirose T., Morishita K., Yamamoto T., Yoshida Y., Urai T., Nakatani T. Establishing a new appropriate intramuscular injection site in the deltoid muscle. Hum. Vaccines Immunother. 2017;13:2123–2129. doi: 10.1080/21645515.2017.1334747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook I.F. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum. Vaccines Immunother. 2015;11:1184–1191. doi: 10.1080/21645515.2015.1017694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.