Abstract
Objectives
NutriCoviD30 is a longitudinal multicenter cohort study that aimed to provide nutritional objective data of inpatients during COVID-19 infection. The aims of this study were to describe the nutritional effects of COVID-19 infection on adult inpatients on the short- to mid-term (≤30 d after hospital discharge), using food intake and weight measurements and to identify factors associated with a decrease in food intake and weight.
Methods
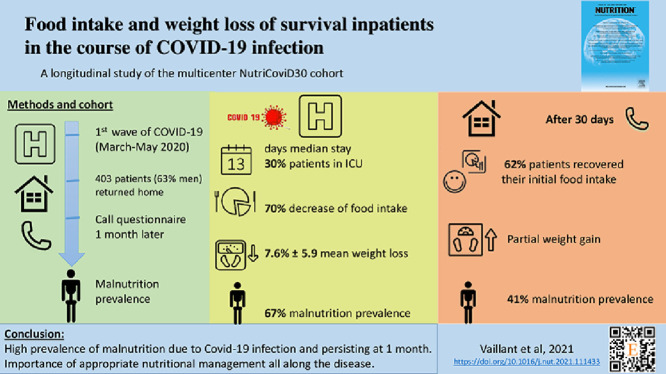
Food intake and weight trajectories, as well as clinical signs of the disease, preexisting chronic diseases, and nutritional strategies were collected and analyzed during the course of the disease. Their association was estimated using mixed-effect regression modeling. Patients were recruited from French university hospitals from May to July 2020. For the 403 included patients (mean 62.2 ± 14.2 y of age; 63% men), median (interquartile range [IQR]) hospital length of stay was 13 d (IQR = 8, 20), and 30% of patients were admitted to the intensive care unit.
Results
Patients declared a median 70% food intake decrease in the acute phase, and the disease resulted in an average loss of 8% of predisease weight (corresponding to –6.5 kg). Although most patients recovered their usual food intake 1 month after hospital discharge, they only regained half of their weight loss, such that malnutrition, which affected 67% of patients during hospitalization, persisted in 41%. Patients with overweight, obesity, and diabetes reported an additional weight loss of >1.5% of their initial bodyweight during hospitalization and recovery phase.
Conclusions
To prevent malnutrition and its long-term effects, mainly combined with a rapid weight loss predominantly affecting lean body mass, implementation of nutritional support is needed for COVID-19 inpatients. It should be started early in the course of the infection, and be extended up to the recovery phase.
Keywords: COVID-19, Pneumonia, Malnutrition, Weight loss, Food intake
Graphic abstract
Introduction
The sudden onset of the coronavirus SARS-CoV-2 has caused organizational upheavals and challenges in managing the current COVID-19 epidemics worldwide. Nutritional care is one of the key aspects of patient management in situations of serious infection. In community-acquired pneumonia for example, malnutrition is known to affect long-term recovery and to increase 1- and 2-y mortality in surviving patients, especially for older patients [1]. Given the effect of SARS-CoV-2 on patient taste and appetite [2], as well as on anosmia, ageusia, diarrhea, odynophagia, and anorexia [3,4], patients with COVID-19 are particularly at risk for undernutrition. This risk is often heightened by hospitalization [5, 6].
Reports of dramatic weight loss during this epidemic have led the European [7], American [8], and French-speaking [9] learned societies of nutrition to issue expert opinions and recommendations for the nutritional management of patients. Despite this advice, and in the face of the short-term respiratory emergency, nutritional care has received limited attention from clinicians during the initial pandemic wave of infection.
The aim of this study was to describe the nutritional effects of COVID-19 infection on adult inpatients on the short- to mid-term (≤30 d after hospital discharge), using food intake and weight measurements. It also aimed to identify factors associated with a decrease in food intake and weight. Although SARS-CoV-2 is an emerging and developing disease, this study is expected to improve the understanding of malnutrition in COVID-19 inpatients, and to help clinicians preventing the occurrence and long-term effects of this side effect of the disease.
Methods
Study design
The NutriCoviD30 study was designed as a prospective multicenter cohort of adult inpatients who were hospitalized with a confirmed COVID-19 infection, and who returned home after hospitalization. Patients were recruited from 11 French university hospitals from May 7 to July 10, 2020, after ethical clearance from the French Committee for the Protection of Persons North West IV.
Patients were called by a nutritionist, medical doctor, or dietician, 30 d after hospital discharge. Data was collected from the medical records or during the phone interview, regarding the following:
-
•
Prior chronic disease, lifestyle, and eating habits before the disease (referred to as t0);
-
•
COVID-19 symptoms, hospital length of stay (LOS), and nutritional care characteristics during hospitalization (referred to as t1); and
-
•
World Health Organization performance status score, persistence of symptoms, and nutritional outcomes 1 mo after hospital discharge (referred to as t2). Appetite assessment can be evaluated with a 10-point visual analog scale [10,11].
Food intake was assessed at t1 and t2 using the verbal form of the Self-Evaluation of Food Intake scale (SEFI) scored from 0 (I eat nothing) to 10 (I eat as usual). This scale has been validated and showed good reliability for the assessment of food and energy intake, and malnutrition among adults [12], [13], [14].
Weight assessment was based on patient declaration at t0 (considered as the reference weight) and t2. The lowest weight during the acute phase of the disease (reported by the patient or during hospitalization) was used as weight at t1. Weight loss at t1 and t2 were defined in terms of proportion of the reference weight at t0.
Statistical analysis
Quantitative parameters were described by their mean ± SD, or by their median [25th; 75th] percentiles, depending on the normality of the data. Qualitative parameters were expressed in numbers and percentages. A two-sided P ≤ 0.05 was considered statistically significant.
SEFI and weight were compared between two time points, between two groups, and between more than two groups
-
1.
If the variable was normally distributed using Student's paired test, Student's t test, and analysis of variance, respectively; and
-
2.
If the variable was not normally distributed using Wilcoxon matched-pairs signed-rank tests, Wilcoxon signed-rank tests, and Kruskall–Wallis tests, respectively.
Mixed-effect regression modeling was used to study the evolution of SEFI at t1 and t2, with subjects nested within centers being modeled as random effects. Data from the Parisian hospital centers of Saint-Antoine, Tenon, and Paul Brousse were aggregated owing to their small population sizes. Measures at t0 were not included in the model because they were considered constant among patients and equal to 10 (i.e., “I eat as usual”).
Weight loss at t1 and t2 was analyzed similarly (weight loss at t0 was null for all patients), with the following adjustment factors being additionally included in the model: the number of days in the intensive care unit (ICU) (coded as “0 d,” “1–7 d,” “8–15 d,” and “>15 d”) and the admission in post-acute rehabilitation (PAR) unit (yes/no), which are expected to efficiently reflect disease severity; and edema status (coded as “appeared,” “disappeared,” or “did not change” since the previous period) which has a direct effect on weight. Adjustment factors were implemented with independent fixed effects at t1 and t2.
Mixed modeling can cope with partly missing data at the individual level. The analyses were ran on 402 (99.7%) patients for SEFI (including 2 patients with one missing data at t1 or t2); and on 386 (95.8%) patients for weight loss (including 86 patients with one missing data).
The following COVID-19 potentially influential factors were further tested one by one for their association with SEFI and with weight loss evolution, preexisting chronic conditions, clinical signs of the disease, and implementation of nutritional strategies. Alike adjustment factors, they were modeled with an independent fixed effect at t1 and t2. If the factor only referred to one period (e.g., to the hospitalization period), the model was fitted only over this period, without a subject random effect.
All data were processed and analyzed using Stata version 15.1 (StataCorp, College Station, TX, USA) and R version 3.3.3 (https://cran.r-project.org/).
Results
Population characteristics
In all, 1584 adult inpatients were screened in COVID units. Of these, 945 were eligible and 403 were finally recruited in the present study. Of those not included, half did not answer our call or message. Other reasons are listed in Supplementary Figure 1.
t0: Before the COVID-19 infection
On average, patients were 62.2 ±14.2 y of age; 63% were men (Table 1 ). Eighty percent of the patients presented with one or more chronic conditions (Table 2 ).
Table 1.
Descriptive statistics of NutriCoviD30 population (N = 403)
| Missing data, n (%) | Non-missing data,*n (%), mean (SD), or median [IQR] | |
|---|---|---|
| Population characteristics | ||
| Men | 0 (0) | 255 (63%) |
| Age (y) | 0 (0) | 62.2 (14.2) Min: 22; Max: 97 |
| Height (m) | 0 (0) | 1.7 (0.09) Min: 1.45; Max: 1.96 |
| ≥1 chronic conditions | 0 (0) | 323 (80%) |
| Lifestyle | 0 (0) | |
| Lives with ≥1 relatives | 312 (78%) | |
| Lives in a nursing home | 9 (2%) | |
| Lives alone | 82 (20%) | |
| Weight before COVID-19 infection | ||
| Weight (kg) | 10 (2) | 83.4 (17.3) Min: 43; Max: 144 |
| Weight considered stable (patient declaration) | 14 (3) | 311 (80%) |
| Pays attention to own weight (patient declaration) | 2 (0) | 230 (57%) |
| Reason for paying attention to own weight (several possible answers)† | 2 (1) | |
| To lose weight | 91 (40%) | |
| To be in good health | 56 (25%) | |
| To gain weight | 9 (4%) | |
| To stabilize/maintain weight | 102 (45%) | |
| BMI (kg/m2) | 10 (2) | 28.8 (5.3) Min: 17; Max: 49 |
| At risk for malnutrition (BMI <18.5 kg/m2 for patients <70 y, or BMI <21 kg/m2 for patients >70 y) | 10 (2) | 11 (2.8%) |
| BMI <18.5 kg/m2 for patients <70 y‡ | 7 (7) | 1 (0.3%) |
| BMI <21 kg/m2 for patients ≥70 y§ | 3 (3) | 10 (8.1%) |
| Diet before COVID-19 infection | ||
| On diet | 1 (0) | 168 (42%) |
| Type of diet (several possible answers)║ | 0 (0) | |
| Healthy/Organic foods | 67 (40%) | |
| Vegetarian/Flexitarian | 8 (5%) | |
| Restrictive (low-calorie, sugar-free, lactose, fat, salt, fiber, or gluten) | 82 (49%) | |
| Hospital stay characteristics | ||
| University hospital center | 0 (0) | 403 (100%) |
| Grenoble | 95 (23%) | |
| Lyon | 51 (13%) | |
| Paris AP-HP Pitié-Salpêtrière | 51 (13%) | |
| Paris AP-HP Bichat | 33 (8%) | |
| Paris AP-HP Paul Brousse/St Antoine/Tenon | 31 (8%) | |
| Paris AP-HP Beaujon | 29 (7%) | |
| Rennes | 28 (7%) | |
| Rouen | 25 (6%) | |
| Toulouse | 60 (15%) | |
| Hospital LOS (d) | 0 (0) | 138;20 Min: 1; Max: 97 |
| Admission to ICU | 1 (0) | 122 (30%) |
| ICU LOS (d)¶ | 3 (2) | 106;20 Min: 1; Max: 65 |
| Admission to PAR unit | 13 (3) | 102 (25%) |
| Maximum ventilatory support level | 12 (3) | |
| No oxygen | 78 (20%) | |
| Oxygen ≤3l/min | 134 (34%) | |
| Oxygen >3l/min | 106 (27%) | |
| Intubation | 73 (19%) | |
| Food intake and weight during hospitalization (t1) and 1 mo after hospital discharge (t2) | ||
| SEFI score at t1 | 3 (1) | 31;5 Min: 0; Max: 10 |
| SEFI score at t2 | 1 (0) | 108;10 Min: 1; Max: 10 |
| Weight at t1 vs. t0 (%) | 22 (5) | –7.6 (5.9) Min: –32; Max: +8 |
| Weight at t1 vs. t0 (kg) | 22 (5) | –6.5 (5.4) Min: –30; Max: +7 |
| Weight at t1 vs. t0 <5% | 22 (5) | 256 (67%) |
| Weight at t2 vs. t0 (%) | 98 (24) | –4.2 (5.1) Min: –23; Max: +15 |
| Weight at t2 with t0 (kg) | 98 (24) | –3.8 (4.7) Min: –25; Max: +10 |
| Weight at t2 vs. t0 <5% | 98 (24) | 125 (41%) |
BMI, body mass index; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; PAR, post-acute and rehabilitation; SD, standard deviation; SEFI, Self-Evaluation of Food Intake
n (%) used for qualitative data; mean (SD) for normally distributed quantitative data (based on data visualization); and median [interquartile range] for non-normal quantitative data
Among the 230 patients who paid attention to their weight
Among the 277 patients <70 y of age
Among the 126 patients aged ≥70 y of age
||Among the 168 patients who paid attention to their diet
Among the 122 patients admitted to the ICU
Table 2.
Association between COVID-19 potentially influential factors and the evolution of SEFI at t1 and t2, estimated independently for each symptom using linear mixed-effect regression modeling
| SEFI at t1* |
SEFI at t2* |
||||
|---|---|---|---|---|---|
| N/nb of non-missing data (%) | Coefficient [95% CI] | P value | Coefficient [95% CI] | P value | |
| COVID-19 symptoms | |||||
| Anorexia/Early feeling of fullness/Long satiation | 314/401 (78) | –3.6 [–4.2 to –3.1] | < 0.001† | –0.7 [–1.2 to –0.2] | 0.010‡ |
| Anosmia/Ageusia or dysgeusia/Change in taste | 225/387 (58) | –1.4 [–1.9 to –0.9] | < 0.001† | 0.1 [–0.4 to 0.5] | 0.801 |
| Nausea/Vomiting | 133/401 (33) | –1.1 [–1.6 to –0.6] | < 0.001† | –0.5 [–0.9 to 0] | 0.067 |
| Difficulties swallowing/Pharyngeal or esophageal pain | 88/398 (22) | –0.7 [–1.2 to –0.1] | 0.021‡ | –0.3 [–0.9 to 0.3] | 0.307 |
| Painful mouth/White, pasty tongue | 146/387 (38) | –0.9 [–1.4 to –0.4] | < 0.001† | –0.7 [–1.1 to –0.2] | 0.007† |
| Difficulties drinking | 76/396 (19) | –1.6 [–2.2 to –1] | < 0.001† | –0.8 [–1.4 to –0.2] | 0.006† |
| Food disgust | 187/397 (47) | –1.4 [–1.9 to –1] | < 0.001† | –0.5 [–1 to –0.1] | 0.023‡ |
| Fever | 309/395 (78) | –1.5 [–2 to –0.9] | < 0.001† | 0.5 [–0.1 to 1] | 0.090 |
| Dyspnea/Coughing | 314/401 (78) | –0.9 [–1.4 to –0.3] | 0.002† | 0.1 [–0.5 to 0.6] | 0.850 |
| Pain (muscular, cranial, headaches, etc.) | 249/394 (63) | –0.8 [–1.2 to –0.3] | 0.002† | –0.1 [–0.5 to 0.4] | 0.850 |
| Fatigue | 352/402 (88) | –2.1 [–2.8 to –1.4] | < 0.001† | –0.1 [–0.8 to 0.6] | 0.830 |
| Digestive or transit disorders | 287/400 (72) | –0.5 [–1.1 to 0.0] | 0.044‡ | –0.2 [–0.7 to 0.3] | 0.425 |
| Preexisting chronic diseases | |||||
| Cognitive disorders | 13/403 (3) | –0.3 [–1.6 to 1.0] | 0.609 | –0.8 [–2.1 to 0.5] | 0.212 |
| Chronic respiratory disease (with chronic medication/home oxygen therapy/sleep apnea) | 72/403 (18) | 0.1 [–0.5 to 0.7] | 0.648 | –0.7 [–1.3 to –0.1] | 0.026‡ |
| Immunodepression/cancer (presently treated) | 51/403 (13) | 0.1 [0.6–0.8] | 0.869 | 0.0 [–0.8 to 0.7] | 0.894 |
| Hypertension | 169/403 (42) | 0.4 [–0.1 to 0.9] | 0.091 | 0 [–0.5 to 0.4] | 0.836 |
| Heart failure | 19/403 (5) | 0.7 [–0.4 to 1.9] | 0.191 | –1.1 [–2.2 to 0] | 0.054 |
| Diabetes (all types) | 92/403 (23) | 0.2 [–0.3 to 0.8] | 0.464 | –0.1 [–0.6 to 0.5] | 0.821 |
| Chronic inflammatory bowel diseases (Crohn's disease, etc.) | 5/403 (1) | 0.9 [–1 to 1.3] | 0.378 | –0.2 [–2.3 to 1.9] | 0.829 |
| Inflammatory rheumatic diseases (lupus, rheumatoid arthritis, etc.) | 17/403 (4) | 0.2 [–0.9 to 1.4] | 0.694 | –0.4 [–1.6 to 0.7] | 0.486 |
| BMI before COVID-19 infection, kg/m2 | |||||
| <18.5 | 3/393 (1) | 1.6 [–1.1 to 4.2] | 0.256 | 0.4 [–2.3 to 3.1] | 0.761 |
| ≥18.5–25 | 92/393 (23) | (ref) | (ref) | (ref) | (ref) |
| ≥25–30 | 147/393 (37) | –0.0 [–0.6 to 0.6] | 0.933 | 0.4 [–0.2 to 1] | 0.237 |
| ≥30 kg/m2 | 151/393 (38) | –0.3 [–0.9 to 0.4] | 0.412 | 0.2 [–0.5 to 0.8] | 0.621 |
| Nutritional strategies and difficulties | |||||
| Food supply difficulties related to home confinement | 16/401 (4) | –1.2 [–2.3 to 0] | 0.055 | –0.4 [–1.6 to 0.8] | 0.512 |
| Incentives to eat, and if needed, help given by caregivers or a relative | 132/379 (35) at t1, 196/398 (49) at t2 | –0.2 [–0.7 to 0.3] | 0.415 | –0.1 [–0.6 to 0.4] | 0.640 |
| Advice given by a nutritionist | 105/366 (29) at t1, 93/395 (24) at t2 | –0.2 [–0.8 to 0.3] | 0.378 | 0.2 [–0.3 to 0.8] | 0.406 |
| Adapted meals during hospitalization§ | 235/387 (61) at t1 | –0.5 [–1.1 to 0.2] | 0.139 | / | / |
| Snacking during hospitalization§ | 189/386 (49) at t1 | 0.4 [–0.2 to 1] | 0.197 | / | / |
| ONS (yes/no, patient declaration) | 196/385 (51) taken at t1, 120/400 (30) prescribed at t2 | –0.4 [–0.9 to 0.1] | 0.104 | –0.2 [–0.8 to 0.3] | 0.336 |
| Total ONS (units, patient declaration)|| | 14 [4.5–22.5] taken at t1, 60 [15-60] prescribed at t2 | –0.00 [–0.01 to 0.02] | 0.921 | 0.00 [–0.01 to 0.00] | 0.248 |
BMI, body mass index; ONS, oral nutrition supplement; SEFI, Self-Evaluation of Food Intake
SEFI analyses were performed using a mixed-effect regression model, with an individual random effect nested in a center random effect, and an independent fixed effect at t1 and at t2 for the variable of interest
P < 0.01
P < 0.05
Variable affecting only the hospitalization period: Analysis was performed at t1 only
60 (15%) missing data at t1, 18 (4%) missing data at t2
The study population had an average reference weight (i.e., before the disease) of 83.4 ±17.3 kg, and an average body mass index (BMI) of 28.8 ±5.3 kg/m2.
Of the patients, 311 (80%) considered their weight stable before the disease. Less than 3% of patients were at risk for malnutrition, based on BMI <18.5 kg/m2 for patients <70 y of age, and BMI<21 kg/m2 for patients >70 y.
t1: During hospitalization for COVID-19 infection
Patients were hospitalized for a median duration of 13 d. One-third were admitted to the ICU (median duration: 10 d).
Regarding oxygen requirement, 20% of patients did not receive oxygen therapy; 34% had oxygen therapy ≤3l/min, 27% had oxygen therapy >3l/min, and 19% were intubated ( Table 1). As expected, patients who received oxygen during their hospitalization had longer LOS (median duration: 14 versus 7 d, Wilcoxon test P < 0.0001; Supplementary Table 1).
Patients massively reported COVID-19 symptoms ( Table 2).
t2: 1 mo after returning home
Only 5% of patients were still on oxygen therapy 1 mo after hospital discharge. Fatigue persisted in 39% of the patients; other symptoms persisted in 10% to 27% of affected patients.
Changes in diet were reported by one-third of patients, mainly toward a balanced diet or a diet adapted to COVID-19 symptoms (i.e., split, enriched, or adapted meals to the patient taste modifications).
General recovery was assessed using WHO performance status score. The median score was 1(IQR = 0.25; 2), corresponding to patients who were restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature ( Table 3 ).
Table 3.
Patients characteristics 1 mo after hospital discharge (t2)
| Missing data (%) | Non-missing data,* n (%), mean (SD), or median [IQR] | |
|---|---|---|
| Oxygen supply | 5 (1) | 19 (5%) |
| WHO performance status | 1 (0) | 1 [0.25; 2] Min: 0; Max: 4 |
| Partial persistence of symptoms of COVID-19 infection | ||
| Anorexia/Early feeling of fullness/Long satiation† | 0 (0) | 32 (12%) |
| Anosmia/Ageusia or dysgeusia/Change in taste† | 0 (0) | 35 (18%) |
| Nausea/Vomiting† | 0 (0) | 16 (12%) |
| Difficulties swallowing/Pharyngeal or esophageal pain† | 0 (0) | 16 (22%) |
| Painful mouth/White tongue, pasty† | 0 (0) | 25 (16%) |
| Difficulties drinking† | 0 (0) | 8 (10%) |
| Fever† | 0 (0) | 3 (1%) |
| Dyspnea/Coughing† | 0 (0) | 86 (27%) |
| Pain (muscular, cranial, headaches)† | 0 (0) | 61 (24%) |
| Fatigue† | 0 (0) | 136 (39%) |
| Diet and appetite | ||
| Adaptation of diet after COVID-19 infection | 4 (1) | 148 (37%) |
| Type of adaptation‡ | 0 (0) | |
| Diet adaptation owing to COVID-19 infection | 59 (40%) | |
| Diet alteration owing to COVID-19 infection | 18 (12%) | |
| Balanced diet | 71 (48%) | |
| Feeling hungrier than before the disease | 7 (2) | 77 (19%) |
| Patient quickly satiated, no appetite | 6 (1) | 102 (26%) |
| SEFI score of 10 | 1 (0) | 248 (62%) |
IQR, interquartile range; SD, standard deviation; SEFI, Self-Evaluation of Food Intake; WHO, World Health Organization
n (%) used for qualitative data; mean (SD) for normally distributed quantitative data (based on data visualization); and median [IQR] for non-normal quantitative data.
Among patients with this symptom at t1.
Among the 148 patients who adapted their diet after COVID-19 infection. Diet adaptation is a strategy to fight or a consequence of COVID-19 infection. It consists of split meals, enriched food, or increased protein intake. Diet alteration consists of a decreased food intake due to a loss of appetite, or a loss/change in taste.
Food intake and weight effects of COVID-19
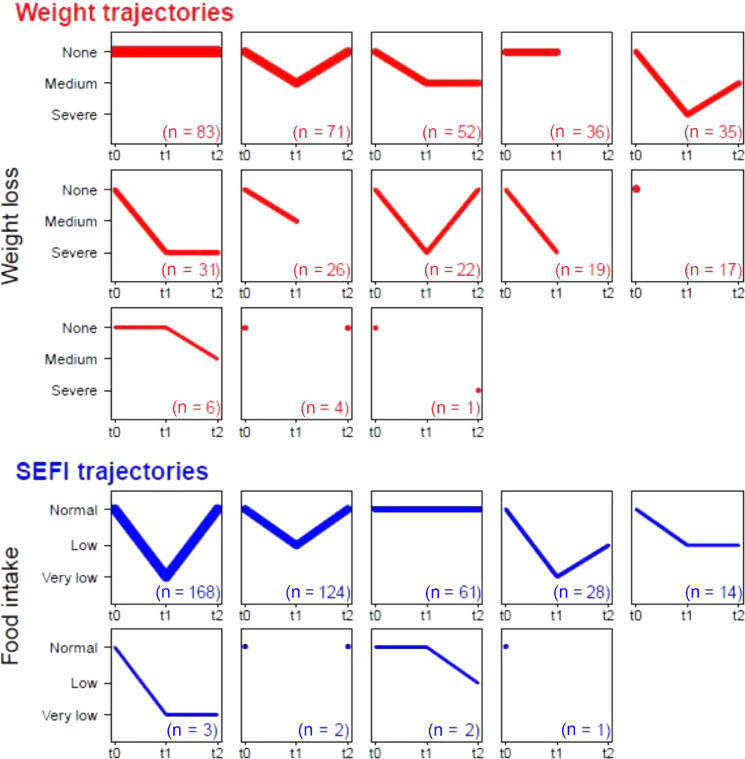
SEFI and weight trajectories are illustrated in Figure 1 . Five percent of patients reported neither a weight loss ≥5% of their reference weight before the disease, nor a food intake decrease >30% of usual food intake during the course of the disease.
Fig. 1.
Weight loss and SEFI trajectories during the course of COVID-19 infection and recovery period (N = 403 patients). Weight loss and SEFI trajectories at t0 (before disease), t1 (in the course of COVID-19), and t2 (recovery period at 1 mo); uncomplete trajectories (i.e., with missing values) are displayed as shorter segments or as dots. Weight loss values (as a percentage of weight at t0) were grouped into classes: none: <5%, medium: [5%; 10%], and severe weight loss: ≥10%. Weight loss at t0 was 0 for all patients. Food intake values were grouped into classes: normal: [0, 3], low:3,7, and very low food intake:7,10. SEFI at t0 was assumed to be 10 for all patients. SEFI, Self-Evaluation of Food Intake.
At t1, the median (IQR) SEFI was 3 (1; 5) (representing a decrease of 70% of patients’ usual food intake), and the average weight decreased to 77 ± 16 kg. This represents a weight loss of 6.5 ± 5.4 kg (maximum of 30 kg), and a 7.6% ± 5.9% decrease compared with the reference weight (maximum of 32%). Based on etiologic and phenotypic diagnosis criteria (i.e., acute disease and weight loss) from the International Global Leadership Initiative on Malnutrition (GLIM)15 and French Health Authorities guidelines, 67% of patients were malnourished. Of these patients, 42% were severely malnourished.
During the recovery phase (t2), 62% of patients recovered their initial SEFI (i.e., a score of 10), and 19% reported better appetite than before the disease. Yet 25% of patients were not hungry when lunch or dinnertime came ( Table 3).
Although the majority of patients regained weight during recovery, the study population still recorded an average weight loss of 4.2% ± 5% of their reference weight 1 mo after hospital discharge (i.e., –3.8 ± 4.7 kg; Table 1), indicating that 41% of patients remained malnourished. Of these patients, 25% were severely malnourished.
Among the 49% patients who did not recover their initial weight, 62% declared they did so voluntarily.
Association between potentially influential factors and food intake or weight
Mixed models confirmed a significant difference between t1 and t2 in SEFI, and in weight loss, after adjusting for patients and recruitment center variability, and the selected set of adjustment factors: SEFI regression coefficient was 3.4 at t1 and 8.9 at t2; weight loss coefficient was 6.1% at t1 and 3.4% at t2. As per the adjustment factors, ICU LOS displayed a significant association with a greater weight loss (6.9% at t1 and of 4.3% at t2 for patients who spent >15 d in the ICU compared with those who were not admitted to the ICU; both P < 0.001); while PAR stay and edema status were in global not further associated with weight loss at t1 and t2 (Supplementary Table 2). In the present study population, age was not associated with differences in weight loss (Supplementary Table 3).
We further screened for an additional effect of COVID-19 potentially influential factors, one by one ( Tables 2 and 4 ). Regarding SEFI, chronic diseases had no effect at t1 and t2, except for chronic respiratory diseases, which were associated with a 0.7-point decrease in SEFI at t2 (P = 0.026).
Table 4.
Association between COVID-19 potentially influential factors and the evolution of weight at t1 and t2, estimated independently for each symptom using linear mixed-effect regression modeling
| Weight at t1 vs. t0 (%)* |
Weight at t2 vs. t0 (%)* |
||||
|---|---|---|---|---|---|
| N/nb of non-missing data (%) | Coefficient [95% CI] | P value | Coefficient [95% CI] | P value | |
| COVID-19 SYMPTOMS symptom | |||||
| Anorexia/Early feeling of fullness/Long satiation | 314/401 (78) | –1.8 [–3.1 to –0.5] | 0.006† | –1.2 [–2.6 to 0.2] | 0.082 |
| Anosmia/Ageusia or dysgeusia/Change in taste | 225/387 (58) | 0.2 [–0.9 to 1.3] | 0.678 | 0.2 [–1 to 1.3] | 0.768 |
| Nausea/Vomiting | 133/401 (33) | –0.4 [–1.6 to 0.7] | 0.431 | 0.2 [–0.9 to 1.4] | 0.683 |
| Difficulties swallowing/Pharyngeal or esophageal pain | 88/398 (22) | –0.8 [–2.1 to 0.5] | 0.221 | –0.2 [–1.5 to 1.2] | 0.809 |
| Painful mouth/White, pasty tongue | 146/387 (38) | –0.5 [–1.6 to 0.6] | 0.376 | 0.4 [–0.8 to 1.6] | 0.511 |
| Difficulties drinking | 76/396 (19) | –0.4 [–1.7 to 0.9] | 0.578 | –0.1 [–1.5 to 1.3] | 0.930 |
| Food disgust | 187/397 (47) | –1.3 [–2.4 to –0.3] | 0.013‡ | –0.7 [–1.8 to 0.4] | 0.196 |
| Fever | 309/395 (78) | –0.2 [–1.5 to 1.1] | 0.769 | –0.1 [–1.5 to 1.2] | 0.863 |
| Dyspnea/Coughing | 314/401 (78) | –0.2 [–1.5 to 1] | 0.721 | 0.0 [–1.3 to 1.4] | 0.948 |
| Pain (muscular, cranial, headaches, etc.) | 249/394 (63) | –0.7 [–1.8 to 0.4] | 0.195 | –0.3 [–1.5 to 0.9] | 0.608 |
| Fatigue | 352/402 (88) | –2.3 [–3.9 to –0.8] | 0.003† | –1 [–2.7 to 0.6] | 0.227 |
| Digestive or transit disorders | 287/400 (72) | 0.3 [–0.9 to 1.4] | 0.661 | –0.7 [–2 to 0.5] | 0.238 |
| Preexisting chronic diseases | |||||
| Cognitive disorders | 13/403 (3) | –1.8 [–4.7 to 1.1] | 0.223 | 0.0 [–3.1 to 3.2] | 0.980 |
| Chronic respiratory disease (with chronic medication/home oxygen therapy/sleep apnea) | 72/403 (18) | 0.8 [–0.6 to 2.1] | 0.275 | 0.7 [–0.8 to 2.1] | 0.384 |
| Immunodepression/Cancer (presently treated) | 51/403 (13) | –0.9 [–2.5 to 0.6] | 0.235 | 0.7 [–0.9 to 2.3] | 0.390 |
| Hypertension | 169/403 (42) | –0.4 [–1.5 to 0.6] | 0.446 | –1.4 [–2.5 to –0.2] | 0.018‡ |
| Heart failure | 19/403 (5) | 1 [–1.4 to 3.4] | 0.405 | 0.2 [–2.5 to 2.9] | 0.900 |
| Diabetes (all types) | 92/403 (23) | –1.8 [–3 to –0.6] | 0.004† | –1.5 [–2.8 to –0.2] | 0.026‡ |
| Chronic inflammatory bowel diseases (Crohn's disease, etc.) | 5/403 (1) | 2.6 [–2.2 to 7.5] | 0.291 | 3.4 [–1.5 to 8.3] | 0.175 |
| Inflammatory rheumatic diseases (lupus, rheumatoid arthritis, etc.) | 17/403 (4) | 0 [–2.7 to 2.7] | 0.994 | 1.6 [–1.3 to 4.4] | 0.275 |
| BMI, kg/m2, before COVID-19 infection (in categories) | |||||
| <18.5 | 3/393 (1) | –4.8 [–10.6 to 1] | 0.105 | –0.5 [–6.3 to 5.4] | 0.879 |
| 18.5–25 | 92/393(23) | (ref) | (ref) | (ref) | (ref) |
| 25–30 | 147/393 (37) | –1.6 [–3 to –0.3] | 0.018‡ | –1.7 [–3.1 to –0.3] | 0.018‡ |
| ≥30 kg/m2 | 151/393 (38) | –2.2 [–3.5 to –0.9] | 0.001 † | –3.7 [–5.1 to –2.3] | < 0.001† |
| Nutritional strategies and difficulties | |||||
| Food supply difficulties related to home confinement | 16/401 (4) | –1.8 [–4.6 to 1] | 0.216 | –1.4 [–4.3 to 1.4] | 0.327 |
| Incentives to eat, and if needed, help given by caregivers or a relative | 132/379 (35) at t1, | ||||
| 196/398 (49) at t2 | –0.5 [–1.3 to 0.4] | 0.270 | –0.1 [–0.9 to 0.7] | 0.839 | |
| Advice given by a nutritionist | 105/366 (29) at t1, 93/395 (24) at t2 | –1.2 [–2.1 to –0.3] | 0.007 † | –0.5 [–1.5 to 0.5] | 0.307 |
| Adapted meals during hospitalization§ | 235/387 (61) at t1 | –0.7 [–1.9 to 0.5] | 0.239 | / | / |
| Snacking during hospitalization§ | 189/386 (49) at t1 | –1 [–2.1 to 0.1] | 0.087 | / | / |
| ONS (yes/no, patient declaration) | 196/385 (51) taken at t1, 120/400 (30) prescribed at t2 | -–0.6 [–1.5 to 0.2] | 0.150 | 1.6 [0.6–2.5] | 0.001† |
| Total ONS|| (units, patient declaration) | 14 [4.5; 22.5] taken at t1, 6015;60 prescribed at t2 | –0.01 [–0.04 to 0.01] | 0.325 | 0.02 [0.01–0.04] | 0.001† |
BMI, body mass index; ONS, Oral nutritional supplements
Weight analyses were performed using a mixed-effect regression model, with an individual random effect nested in a center random effect, and an independent fixed effect at t1 and at t2 for the variable of interest and for the adjustment factors (the number of days in intensive care, admission to post-acute and rehabilitation and edema status).
P < 0.01.
P < 0.05.
Variable affecting only the hospitalization period; analysis was performed at t1 only.
60 (15%) missing data at t1, 18 (4%) missing data at t2.
All the symptoms of COVID-19 were associated with a statistically significant decrease in SEFI at t1 (0.5–3.6 points, all P < 0.045). The strongest associations were observed for anorexia and fatigue ( Table 2). Difficulties drinking, food disgust, anorexia, and painful mouth remained associated with a significant decrease in SEFI at t2, but of lower magnitude compared to t1 (0.5–0.8 points, all P < 0.025).
Regarding weight loss, only fatigue (P = 0.003), anorexia (P = 0.006), and food disgust (P = 0.013) were significantly associated with a weight loss at t1 (with, respectively, a 2.3%, 1.8%, and 1.3% greater weight loss compared with patients who did not display such symptoms). None were significantly associated with weight loss at t2.
Among the chronic diseases, diabetes was significantly associated with a 1.8% (P = 0.004) and 1.5% (P = 0.026) increased weight loss at t1 and t2, respectively; hypertension was associated with a 1.4% increased weight loss at t2 (P = 0.018). Overweight (BMI between 25 and 30 kg/m2) and obesity (BMI >30 kg/m2) were associated with a greater weight loss compared with patients with a normal BMI (18.5–25 kg/m2), of respectively 1.6% and 2.2% at t1, and 1.7% and 3.7% at t2 (all P < 0.020).
Nutritional strategies during COVID-19 infection and recovery phases
In terms of nutritional strategies, half of the population took oral nutritional supplements (ONS) during their hospitalization, for a median of 8 d, and 14 units in total; and 30% of the patients were prescribed ONS after returning home, for a median 60 units in total. During their hospitalization, 61% of patients received adapted meals (i.e., different from the standard meal, corresponding to enriched meals or texture-modified food) and 49% received snacks.
Nutritional care data during hospitalization and the recovery phase does not include artificial nutrition owing to their non-exhaustive collection in several centers.
Using mixed models ( Tables 3 and 4), none of these strategies were found to be significantly associated with SEFI or weight loss, except for advice given by a nutritionist that were associated at t1 with a 1.2% increased weight loss (P = 0.007), and ONS that were associated with a 1.6% weight gain at t2 for patients who were prescribed ONS at home (P = 0.001).
Despite the fact that there was no interventional group, we compared patients who did or did not receive ONS during their hospitalization ( Table 5 ). The 196 patients who were prescribed ONS lost more weight at t1 than the 189 patients who did not receive ONS (P < 0.001). However, they recovered better at hospital discharge and at 1 mo after hospital discharge (P = 0.0005 and 0.009, respectively). Note that the two groups did not differ in terms of sex ratio, predisease weight, or SEFI at t1, whereas the ONS group was older.
Table 5.
Comparison between patients who did and did not receive or no ONS during hospitalization
| Patients who received ONS at t1, (n = 196): n (%), mean (SD), or median [IQR]* | Patients who did not received ONS at t1, (n = 189): n (%), mean (SD), or median [IQR]* | P value of equality test | |
|---|---|---|---|
| Age (y) | 63.6 (1) | 60.4 (1) | 0.023† (Student's t test) |
| Sex (% men) | 122 (62%) | 124 (66%) | 0.492 (Proportion test) |
| Weight before COVID-19 (kg) | 83.1 (1.2) | 84.3 (1.3) | 0.510 (Student's t test) |
| Weight at t1 vs. t0 (%) | –8.6 (0.4) | –6.5 (0.4) | < 0.001‡ (Student's t test) |
| Weight recovery between hospitalization and hospital discharge (kg) | 0.6 (0.2) | –0.6 (0.3) | 0.005‡ (Student's t test) |
| Weight recovery between t1 and t2, vs. t0 (%) | 4.3 (0.4) | 3.1 (0.3) | 0.009‡ (Student's t test) |
| SEFI at t1 | 2.51;5 | 31;5 | 0.303 (Wilcoxon test) |
IQR, interquartile range; OS, oral nutritional supplement; SD, standard deviation SEFI, Self-Evaluation of Food Intake
Among non-missing data, n (%) used for qualitative data; mean (SD) for normally distributed quantitative data (based on data visualization); and median [IQR] for non-normal quantitative data.
P < 0.05.
P < 0.01.
Discussion
In the present population of 403 inpatients who survived the COVID-19 infection, 67% had malnutrition (defined by a weight loss >5% within the past 6 mo in a population of infected patients). Other studies [11,16] using the same malnutrition definition reported an overall prevalence of malnutrition of 31% and 42.1%, respectively. Another study [6] reported 52.7% malnutrition based on Mini Nutritional Assessment score. These differences result in great part from differences in the study populations, typically in terms of gravity of the disease. For example, 3% of the 156 inpatients were admitted to the ICU in the Di Filippo et al. study [11], as were 16% of the 114 inpatients in the Bedock et al. study [16], whereas it affected 30% of the 403 inpatients in the present study (data not available for Li et al. [6]).
As previously reported [17,18], we found that anorexia, fever, dyspnea, and fatigue were the most prevalent clinical features of COVID-19. Fatigue, anorexia, and food disgust were major aggravating factors for weight loss in the acute phase of the disease, along with chronic diabetes, overweight, and obesity. Not surprisingly, we found that the more severe the disease (as reflected by the number of days spent in the ICU), the greater the weight loss.
Distinguishing our work from previous studies, we aimed to understand the recovery process, to investigate breaks on regaining weight and on recovering a normal food intake, as well as to assess the effects of nutritional strategies. The observed decrease in food intake is likely an immediate consequence of the infection-related anorexia and food disgust but it was transient; unlike the COVID-19 effects on weight.
One month after returning home, 41% of the present study patients still suffered from malnutrition. This suggests that rapid weight loss is a major side effect that likely affects muscle mass. Interestingly, a substantial proportion of our patients voluntarily did not aim to recover their initial weight. These were predominantly patients with overweight or obesity, who were happy with their weight loss. This observation highlights the risk for patients with obesity and sarcopenia, as warned by Barazzoni et al. [19], to be more conscious of public health messages regarding the benefits of losing weight, than of the importance of preserving their muscle mass and function. In line with the European Society for Clinical Nutrition and Metabolism and the European Association for the Study of Obesity [19], and the European Society of Endocrinology [20], this leads us to call for particular attention to this population. Adults with diabetes should also benefit from an acute surveillance, as they tend to lose more weight than other patients [21].
The benefit of nutritional strategies during hospitalization (encouragement to eat, fortified food, etc.) is likely biased by the fact that nutritional supports were predominantly given to the patients most affected by the disease (i.e., those who had the greatest drop in appetite and weight). For example, patients receiving ONS had lost more weight than patients without ONS.
Disentangling this confounding effect from the effect of a nutritional strategy would have required an intensive follow-up of patients during the acute phase (i.e., routine measures of weight and energy intake), which was not possible during the first pandemic wave when health professionals were overloaded. Consequently, the results at t1 should be interpreted with great caution.
On the contrary, the results obtained during the recovery phase are probably not affected by this confounding effect (all patients were in recovery during this period). Interestingly, ONS were significantly associated with a weight gain at t2. This observation is in favor of guidelines for COVID-19 [22] stating that meals and snacks should be adapted to patients’ disgusts and capabilities [23] and combined with resistance physical exercise [24]. This is also in agreement with Caccialanza et al. [25] who recommended two or three bottles of ONS for non-critically ill patients as a systematic prescription for patients with nutritional risk. Note, however, that this study was not randomized. Consequently, further interventional studies are needed to confirm our results.
Strengths and limitations
The strength of the NutriCoviD30 study design lies in collecting repeated SEFI and body weight measures during the course of the COVID-19 infection (i.e., before the disease, during hospitalization, and 1 mo after hospital discharge). When analyzing food intake and weight loss trajectories using linear mixed models, individual characteristics affecting the measure (e.g., educational status) are accounted for by the individual random effect, as long as they do not differentially affect the three periods.
There were, however, several limitations to the present study. First, the study population consisted only of inpatient survivors. This excludes the most severe inpatients who were still hospitalized or in PAR units at the time of the study, and the less severe patients who did not require hospitalization.
A second limitation relates to weight measurements. Its assessment was not complete. At the highest, 98 patients did not provide a weight measurement at t2 because they did not have a bathroom scale at home, or because they refused to weigh themselves. Additionally, weight accuracy depends on the scale's tuning, which is expectedly equally affected by positive and negative calibration fluctuations. Hence, weights are measured with a between-person random error, resulting in unbiased estimates of the average weight [26]. Yet, the variance is inflated, which can lower statistical power in mixed models. Finally, the consistency observed between weight measurements (Supplementary Table 4) lends confidence in the data and analyses we reported.
The use of SEFI relies on declarative assessment, which is less precise than a professional evaluation or measuring energy intake. Yet, our interest lies in the SEFI trajectories, and was investigated using models that account for interindividual differences and focuses on intraindividual variations between measures. For individuals with moderate cognitive decline (3; Table 2), a family member helped answer the questionnaire during the interview.
Conclusion
To our knowledge, NutriCoviD30 was the largest multicenter longitudinal cohort studying the nutrition of hospitalized patients with COVID-19 infection to date. Its main interest lies in assessing malnutrition on the mid-term (i.e., including the recovery phase up to 1 mo after hospital discharge). This study provided a description of COVID-19 symptoms and preexisting chronic conditions, as well as their potential effects on patients’ food intake and weight loss. It also describes and investigates the nutritional interventions implemented during the infection and recovery phase.
COVID-19 resulted in a substantial weight loss in inpatients, which infers that it affected the muscle mass much more than the fat mass. Patients only regained half of their weight loss 1 mo after hospital discharge, despite their food intake returning to normal. Malnutrition affected 67% of patients during hospitalization and persisted for 41% of them 1 mo after hospital discharge. This is partly due to patients not being aware of the severity of muscle wasting during rapid weight loss. The mid- to long-term effects of COVID-19 infection have been widely demonstrated to date; yet, avoiding important weight loss during the acute phase would help to limit these effects. On this goal, nutritional support is needed for COVID-19 inpatients. It should start early in the course of the infection, and should be extended up to the recovery phase. Prevention messages should be delivered regarding the importance of maintaining muscle mass and function.
Author contributions
MF Vaillant had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Vaillant, Agier, Albaladejo, Lathière, Fontaine. Acquisition of data : Vaillant, Martineau, Philipponneau, Romand, Masdoua, Behar, Nesseler, Achamrah, Laubé, Lambert, Duquesnoy. Analysis, or interpretation of data: Vaillant, Agier, Bosson, Fontaine. Drafting of the manuscript: Vaillant, Agier, Lathière, Fontaine. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Vaillant, Agier, Fontaine. Administrative, technical, or material support: Vaillant, Albaladejo, Lathière. Supervision: Bosson, Fontaine.
Declaration of interests
M.F.V. received support from Fresenius, Nestlé; D.R. from Nutricia, Nestlé, and Fresenius; and V.M. from Nutricia for congress participations. V.M. was an employee of Lactalis International (Medical Nutrition) 2017-2019. E.F. received personal fees for teaching from Baxter, B Braun, Nutricia, and Périmètre. No other disclosures were reported.
Acknowledgments
The authors acknowledge the NutriCoviD30 participants for their time and commitment to the study and the following members of participating centers: Reine-Marie Nunzi, Carole Neveu, Coline Quehen, Lise Joly, Clara Meyer, all of the dietitian team, Stéphanie Schmitt, Aida Mandzo, Cyrielle Clapé (Grenoble), Caroline Gagliotta, Catherine Le Saux, all of the dietitian team, Monelle Bertrand, Patrick Ritz (Toulouse), Aline Auzeric, Estelle Bovier, Marie Dragon, Karin Montagut, Maxime Paturel, Brigitte Boniteau, Mireille Philibert, Didier Barnoud, Cécile Chambrier (Lyon), Coralie Prebet, Edith Marchesi-Samedi, all of the dietitian teams of Pitié-Sâlpétrière, Tenon, Saint-Antoine, Marion Thompson, Manuel Sanchez, Agathe Raynaud-Simon, Francisca Joly, Nicole Cournède, Jean-Claude Melchior (Paris), Mathilde Gâté, Pierre Déchelotte (Rouen), Ronan Thibault (Rennes). The authors also acknowledge Didier Quilliot and the French-speaking Society for Clinical Nutrition and Metabolism (SFNCM) for their scientific contributions. We obtained permission to name them.
Footnotes
This study was funded by Nutricia nutrition Clinique. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. All authors received a donation from Nutricia Nutrition clinique for the study.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.nut.2021.111433.
Appendix. Supplementary materials
References
- 1.Yeo HJ, Byun KS, Han J, Kim JH, Lee SE, Yoon SH, et al. Prognostic significance of malnutrition for long-term mortality in community-acquired pneumonia: a propensity score matched analysis. Korean J Intern Med. 2019;34:841–849. doi: 10.3904/kjim.2018.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glezer I, Bruni-Cardoso A, Schechtman D, Malnic B. Viral infection and smell loss: the case of COVID-19. J Neurochem. 2021;157:930–943. doi: 10.1111/jnc.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaira LA, Salzano G, Fois AG, Piombino P, De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol. 2020;10:1103–1104. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SL, Ong KCB, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr Edinb Scotl. 2012;31:345–350. doi: 10.1016/j.clnu.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Li T, Zhang Y, Gong C, Wang J, Liu B, Shi L, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74:871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martindale R, Patel JJ, Taylor B, Arabi YM, Warren M, McClave SA. Nutrition therapy in critically ill patients with coronavirus disease 2019. JPEN J Parenter Enteral Nutr. 2020;44:1174–1184. doi: 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thibault R, Quilliot D, Seguin P, Tamion F, Schneider S, Déchelotte P. Stratégie de prise en charge nutritionnelle à l'hôpital au cours de l’épidémie virale Covid-19: avis d'experts de la Société Francophone de Nutrition Clinique et Métabolisme (SFNCM) Nutr Clin Métabolisme. 2020;34:97–104. [Google Scholar]
- 10.Flint A, Raben A, Blundell JE, Reproducibility Astrup A. power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 11.Di Filippo L, De Lorenzo R, D'Amico M, et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin Nutr. 2021;40:2420–2426. doi: 10.1016/j.clnu.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thibault R, Goujon N, Le Gallic E, Clairand R, Sébille V, Vibert J, et al. Use of 10-point analogue scales to estimate dietary intake: a prospective study in patients nutritionally at-risk. Clin Nutr. 2009;28:134–140. doi: 10.1016/j.clnu.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Bouëtté G, Esvan M, Apel K, Thibault R. A visual analogue scale for food intake as a screening test for malnutrition in the primary care setting: prospective non-interventional study. Clin Nutr. 2021;40:174–180. doi: 10.1016/j.clnu.2020.04.042. [DOI] [PubMed] [Google Scholar]
- 14.Mesbah S, Mesbah H, Haumont L-A, Thibault R. The self evaluation of food intake SEFI® is feasible and diagnoses malnutrition in the older people living in a nursing home. Clin Nutr. 2018;37:S6. [Google Scholar]
- 15.Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Bedock D, Bel Lassen P, Mathian A, Moreau P, Couffignal J, Ciangura C, et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN. 2020;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395:e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, et al. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13:667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barazzoni R, Bischoff S, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: time to meet the challenge. Obes Facts. 2018;11:294–305. doi: 10.1159/000490361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khadra D, Itani L, Tannir H, Kreidieh D, El Masri D, El Ghoch M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: a systematic review and meta-analysis. World J Diabetes. 2019;10:311–323. doi: 10.4239/wjd.v10.i5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handu D, Moloney L, Rozga M, Cheng FW. Malnutrition care during the COVID-19 pandemic: considerations for registered dietitian nutritionists. J Acad Nutr Diet. 2021;121:979–987. doi: 10.1016/j.jand.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson L. Providing nutritional support for the patient with COVID-19. Br J Nurs. 2020;29:458–459. doi: 10.12968/bjon.2020.29.8.458. [DOI] [PubMed] [Google Scholar]
- 24.Liao CD, Tsauo J-Y, Wu Y, Cheng CP, Chen HC, Huang YC, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106:1078–1091. doi: 10.3945/ajcn.116.143594. [DOI] [PubMed] [Google Scholar]
- 25.Caccialanza R, Laviano A, Lobascio F, Montagna E, Bruno R, Ludovisi S, et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett DA, Landry D, Little J, Minelli C. Systematic review of statistical approaches to quantify, or correct for, measurement error in a continuous exposure in nutritional epidemiology. BMC Med Res Methodol. 2017;17:146. doi: 10.1186/s12874-017-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.