Abstract
Background
Prognostic impact of lymph node micro-metastases (pN1mi) has been discordantly reported in the literature. The need to clarify this point for decision-making regarding adjuvant therapy, particularly for patients with endocrine receptor (ER)-positive status and HER2-negative tumors, is further reinforced by the generalization of gene expression signatures using pN status in their recommendation algorithm.
Patients and methods
We retrospectively analyzed 13 773 patients treated for ER-positive breast cancer in 13 French cancer centers from 1999 to 2014. Five categories of axillary lymph node (LN) status were defined: negative LN (pN0i−), isolated tumor cells [pN0(i+)], pN1mi, and pN1 divided into single (pN1 = 1) and multiple (pN1 > 1) macro-metastases (>2 mm). The effect of LN micro-metastases on outcomes was investigated both in the entire cohort of patients and in clinically relevant subgroups according to tumor subtypes. Propensity-score-based matching was used to balance differences in known prognostic variables associated with pN status.
Results
As determined by sentinel LN biopsy, 9427 patients were pN0 (68.4%), 546 pN0(i+) (4.0%), 1446 pN1mi (10.5%) and 2354 pN1 with macro-metastases (17.1%). With a median follow-up of 61.25 months, pN1 status, but not pN1mi, significantly impacted overall survival (OS), disease-free survival (DFS), metastasis-free survival (MFS), and breast-cancer-specific survival. In the subgroup of patients with known tumor subtype, pN1 = 1, as pN1 > 1, but not pN1mi, had a significant prognostic impact on OS. DFS and MFS were only impacted by pN1 > 1. Similar results were observed in the subgroup of patients with luminal A-like tumors (n = 7101). In the matched population analysis, pN1macro, but not pN1mi, had a statistically significant negative impact on MFS and OS.
Conclusion
LN micro-metastases have no detectable prognostic impact and should not be considered as a determining factor in indicating adjuvant chemotherapy. The evaluation of the risk of recurrence using second-generation signatures should be calculated considering micro-metastases as pN0.
Key words: breast cancer, sentinel node, micro-metastases, survival
Highlights
-
•
LN micro-metastases have no detectable prognostic impact.
-
•
pN1 status, but not pN1mi, significantly impacted overall survival, disease-free survival, metastasis-free survival.
-
•
In the subgroup of patients with known tumor subtype, pN1=1, as pN1>1, but not pN1mi, had a significant prognostic impact on OS.
-
•
LN micro-metastases should not be considered as a determining factor in indicating adjuvant chemotherapy.
Introduction
The most commonly accepted prognostic factors for proposing adjuvant therapy in early breast cancer (BC) include patient age, tumor size, axillary lymph node (LN) status, and tumor pathology, including grade, endocrine receptors (ER) status, HER2 status, lympho-vascular invasion (LVI), and proliferation assays such as the Ki67 labeling index.1, 2, 3
At the 16th St. Gallen International Breast Cancer Conference, the panel specifically acknowledged the potential impact of adjuvant therapy on the risk of BC recurrence or overall survival (OS) and highlighted the importance of prognostic factors in prescribing individualized treatments with regard to the magnitude of expected clinical benefit.1
Sentinel LN biopsy (SLNB) practice, followed by serial sectioning of the sentinel node (SN) and immunohistochemical (IHC) analysis, resulted in increased detection of occult nodes metastases compared with axillary LN dissection (ALND) that is usually associated with a single hematoxylin- and eosin-stained (HES) section. The prognostic value of micro-metastases has been discordantly reported in the literature according to the periods of inclusions, the technique used for micro-metastases identification (SLNB or ALND), the cohort sizes, and different adjustments in multivariate analysis.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The detection rates of micro-metastases were up to 8%-10%32, 33, 34 of patients with early BC and SLNB, representing 10%-25% of patients with positive SN.33, 34, 35, 36, 37 IHC analysis increased the SN involvement rate from 9% to 47% when compared with HES only.37,38 However, different rates of LN involvement according to molecular-like tumor subtypes were reported with lower rates in triple-negative BC and higher rates in HER2-positive BC.33,39, 40, 41 The lack of consensus on the importance of micro-metastasis when deciding upon adjuvant chemotherapy (AC) was further emphasized by the recent and increasing utilization of gene expression signature assay results in making adjuvant decisions, particularly for patients with ER-positive and HER2-negative tumors.42, 43, 44, 45, 46, 47, 48
To investigate the impact of LN micro-metastases on patient outcomes, we retrospectively analyzed a large, national, multicenter cohort of 13 773 patients with SLNB for their independent prognostic impact. Analyses were carried out on the entire population of patients with ER-positive BC, in subgroups of clinical interest according to tumor subtypes, and in a propensity-score-matched population to balance numeric differences in known prognostic variables.
Patients and methods
Patient selection
Medical records of early BC patients treated from January 1999 to December 2014 were retrieved from clinical databases of 13 different comprehensive cancer centers in France for retrospective analysis (ClinicalTrials.gov NCT02869607). Patient and tumor characteristics, treatments, periods, and clinical outcomes were collected. Out of an initial cohort of 23 134 patients, 13 773 were included in the present study based on histologically proven invasive BC, stage cT0, cT1, or cT2 (TNM breast cancer 8th edition) pathological tumor size ≤5 cm, clinically negative axillary lymph node (LN), positive ER status (estrogen and/or progesterone staining >10% of cells by IHC in line with the French guidelines), and evaluation of LN status determined by SLNB with or without completion of ALND. Patients with HER2-negative tumors and HER2-unknown status were included. Exclusion criteria were the use of systemic neo-adjuvant therapies, failure to identify SN, clinically positive axillary LN, and known HER2-positivity. Among 10 826 ER-positive patients with known HER2 status, 719 patients with HER2 positivity were excluded, corresponding with a HER2-positive tumor rate of 6.64% (719/10 826). Consequently, estimates of HER2-positive status among the 3666 patients with unknown HER2 status was 243.
All procedures carried out in this study involving human participants were done in accordance with the French ethical standards and with the 2008 Helsinki declaration.
All included patients provided written informed consent before surgery, including the use of their data for research.
Pathology
Involvement of SN was diagnosed by serial sections with standard HES. If all the serial sections were negative, an additional IHC analysis was carried out. Five categories of LN status were defined: negative lymph node (pN0i−), isolated tumor cells [≤0.2 mm: pN0(i+)], micro-metastases (pN1mi, >0.2-2 mm), and macro-metastases (>2 mm), divided into single (pN1 = 1) and multiple (pN1 > 1) macro-metastases. There was no central review. The method used for the detection of SN was a combined technique or isotopic only detection during the last years. Two tumor subtypes were defined as surrogates for molecular subtypes based on tumor grade and ER and HER2 status (patients with unknown HER2 status were consequently excluded): luminal A-like (ER+/HER2-/grade 1 or 2 [SBR grading]) and luminal B-like (ER+/HER2-/grade 3 [SBR grading]).49
Statistics
The associations between categorical values were evaluated via χ2 tests. Multivariate survival analyses [overall survival (OS), disease-free survival (DFS), metastasis-free survival (MFS), and breast-cancer-specific survival (BCSS)] were carried out using the Cox proportional hazard regression model adjusted for variables significantly associated with final pN status (pathologic results of SLNB and ALND when it was carried out), in the entire population and in the subgroup of patients with known tumor subtypes with distinction between pN0, pN0(i+), pN1mi, pN1 and then with distinction between pN0, pN0(i+), pN1mi, pN1 = 1, and pN1 > 1 (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100151). We conducted the same analysis according to SN status regardless of ALND when it was carried out in the sub-population of patients without AC, in all patients with positive ER status, and in the subgroup of patients with known tumor subtype with distinction between pN0, pN0(i+), and pN1mi. To balance differences in known prognostic variables associated with pN status, we generated 1 : 1 : 1 matched cohorts of the three following groups: pN0(i−), pN1mi, and pN1macro. Coefficients of a logistic regression adjusted by age, SLNB/ALND, LVI, tumor size, type of breast surgery, endocrine therapy, AC, and tumor grade were used to compute a propensity score for each pN0 and pN1macro patient first (population 1), and for matched population 1 and pN1mi second. Optimal 1 : 1 matching using Mahalanobis distance was carried out with a caliper of 0.2 for population 1, and then 2 : 1 matching for matched population 1 and pN1mi patients.50, 51, 52 Final matched pN0(i−), pN1mi, and pN1macro cohorts resulted in 409, 449, and 436 patients, respectively. The impact of pN status on DFS, MFS, OS, and BCSS was assessed on this matched population by log-rank tests stratified on the pairs.53 Statistical significance was set as P ≤ 0.05. Analyses were carried out with SPSS version 16.0 (SPSS Inc., Chicago, Illinois) and R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient population and association of LN status with other clinico-pathological features
Among 13 773 patients with pN status determined by SLNB alone or SLNB with completion ALND (cALND), 9427 (68.4%) were pN0, 546 (4.0%) pN0(i+), 1446 (10.5%) pN1mi, and 2354 (17.1%) pN1 with macro-metastases (Table 1). For 4736 patients with cALND and pathologic results of ALND known, we observed macro-metastases at cALND in 70 (7.1%) patients of 989 pN0sn, 43 (9.4%) patients of 457 pN0(i+)sn, and in 122 (9.2%) patients of 1325 pN1mi-sn (P < 0.0001).
Table 1.
Characteristics of patients according to pN status
| pN0 |
pN0(i+) |
pN1mi |
pN1macro |
Chi2 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Nb | % | Nb | % | Nb | % | Nb | % | P value | |
| 9427 | 68.4 | 546 | 4.0 | 1446 | 10.5 | 2354 | 17.1 | <0.0001 | |
| HER2 status | |||||||||
| Negative | 6967 | 73.9 | 329 | 60.3 | 970 | 67.1 | 1841 | 78.2 | |
| Unknown | 2460 | 26.1 | 217 | 39.7 | 476 | 32.9 | 513 | 21.8 | |
| Age, years | |||||||||
| ≤40 | 354 | 3.8 | 39 | 7.1 | 92 | 6.4 | 163 | 6.9 | <0.0001 |
| 40.1-50 | 1797 | 19.1 | 121 | 22.2 | 351 | 24.3 | 629 | 26.7 | |
| 50.1-74.9 | 6470 | 68.7 | 355 | 65.0 | 912 | 63.1 | 1403 | 59.6 | |
| ≥75 | 801 | 8.5 | 31 | 5.7 | 91 | 6.3 | 159 | 6.8 | |
| Histology | |||||||||
| Ductal | 7098 | 75.3 | 399 | 73.1 | 1186 | 82.0 | 1827 | 77.6 | <0.0001 |
| Lobular | 1362 | 14.4 | 103 | 18.9 | 143 | 9.9 | 378 | 16.1 | |
| Mixed | 153 | 1.6 | 20 | 3.7 | 22 | 1.5 | 76 | 3.2 | |
| Others | 814 | 8.6 | 24 | 4.4 | 95 | 6.6 | 73 | 3.1 | |
| SLNB/ALND | |||||||||
| SLNB | 8463 | 89.8 | 132 | 24.2 | 244 | 16.9 | 107 | 4.5 | <0.0001 |
| SLNB+ALND | 964 | 10.2 | 414 | 75.8 | 1202 | 83.1 | 2247 | 95.5 | |
| LVI | |||||||||
| No | 7382 | 78.3 | 354 | 64.8 | 960 | 66.4 | 1238 | 52.6 | <0.0001 |
| Yes | 827 | 8.8 | 176 | 32.2 | 372 | 25.7 | 803 | 34.1 | |
| Unknown | 1218 | 12.9 | 16 | 2.9 | 114 | 7.9 | 313 | 13.3 | |
| Periods | |||||||||
| <2005 | 3843 | 40.8 | 267 | 48.9 | 583 | 40.3 | 955 | 40.6 | 0.002 |
| ≥2005 | 5584 | 59.2 | 279 | 51.1 | 863 | 59.7 | 1398 | 59.4 | |
| Localization T | |||||||||
| Outer | 4530 | 48.1 | 305 | 55.9 | 759 | 52.5 | 1269 | 53.9 | <0.0001 |
| Inner | 2230 | 23.7 | 160 | 29.3 | 308 | 21.3 | 471 | 20.0 | |
| Unknown | 2667 | 28.3 | 81 | 14.8 | 379 | 26.2 | 614 | 26.1 | |
| RNI | |||||||||
| No | 6785 | 86.9 | 277 | 57.1 | 616 | 48.2 | 322 | 15.6 | <0.0001 |
| Yes | 1027 | 13.1 | 208 | 42.9 | 661 | 51.8 | 1738 | 84.4 | |
| T size, mm | |||||||||
| ≤5 | 871 | 9.4 | 21 | 3.9 | 48 | 3.3 | 52 | 2.2 | <0.0001 |
| 5.1-10 | 3395 | 36.5 | 123 | 22.8 | 309 | 21.5 | 304 | 13.0 | |
| 10.1-19.9 | 3590 | 38.6 | 222 | 41.1 | 663 | 46.2 | 894 | 38.4 | |
| 20-50 | 1362 | 14.7 | 159 | 29.4 | 370 | 25.8 | 944 | 40.5 | |
| >50 | 76 | 0.8 | 15 | 2.8 | 45 | 3.1 | 136 | 5.8 | |
| Surgery breast | |||||||||
| BCS | 8227 | 87.3 | 444 | 81.3 | 1216 | 84.1 | 1704 | 72.4 | <0.0001 |
| Mastectomy | 913 | 9.7 | 91 | 16.7 | 201 | 13.9 | 599 | 25.4 | |
| Unknown | 287 | 3.0 | 11 | 2.0 | 29 | 2.0 | 51 | 2.2 | |
| Subtypes | |||||||||
| Luminal A-like | 6423 | 91.8 | 291 | 83.6 | 905 | 87.6 | 1409 | 83.5 | <0.0001 |
| Lum B Her2- like | 576 | 8.2 | 57 | 16.4 | 128 | 12.4 | 279 | 16.5 | |
| Final pN | |||||||||
| pN0 | 919 | 92.9 | 0 | 0 | 0 | 0 | 0 | 0 | <0.0001 |
| pN0(i+) | 0 | 0 | 414 | 90.6 | 0 | 0 | 0 | 0 | |
| pN1mi | 0 | 0 | 0 | 0 | 1203 | 90.8 | 0 | 0 | |
| pN1macro | 70 | 7.1 | 43 | 9.4 | 122 | 9.2 | 1965 | 0 | |
| Endocrine therapy | |||||||||
| No | 1228 | 13.0 | 31 | 5.7 | 55 | 3.8 | 57 | 2.4 | <0.0001 |
| Yes | 8199 | 87.0 | 515 | 94.3 | 1391 | 96.2 | 2297 | 97.6 | |
| Chemotherapy | |||||||||
| No | 7947 | 84.3 | 352 | 64.5 | 704 | 48.7 | 382 | 16.2 | <0.0001 |
| Yes | 1459 | 15.5 | 191 | 35.0 | 721 | 49.9 | 1893 | 80.4 | |
| Neo-adjuvant | 18 | 0.2 | 2 | 0.4 | 19 | 1.3 | 75 | 3.2 | |
| Unknown | 3 | 0.0 | 1 | 0.2 | 2 | 0.1 | 4 | 0.2 | |
| Grade | |||||||||
| 1 | 4276 | 45.4 | 146 | 26.7 | 571 | 39.5 | 676 | 28.7 | <0.0001 |
| 2 | 4253 | 45.1 | 316 | 57.9 | 689 | 47.6 | 1282 | 54.5 | |
| 3 | 769 | 8.2 | 79 | 14.5 | 172 | 11.9 | 384 | 16.3 | |
| Unknown | 129 | 1.4 | 5 | 0.9 | 14 | 1.0 | 12 | 0.6 | |
| Radiotherapy and mastectomy | |||||||||
| No | 526 | 56.4 | 33 | 34.0 | 48 | 21.5 | 34 | 6.2 | <0.0001 |
| Yes | 407 | 43.6 | 64 | 66.0 | 175 | 78.5 | 510 | 93.8 | |
ALND, axillary lymph node dissection; BCS, breast conservative surgery; LVI, Lympho-vascular invasion; Nb, number; RNI, regional nodal irradiation; T, tumor; SLNB, sentinel lymph node biopsy.
In univariate analysis, axillary LN status was significantly associated with all clinical and pathological characteristics analyzed when considering the entire cohort (Table 1). No association between clinico-pathological features and axillary LN status remains statistically significant after matching (Table 2). Clinico-pathological features distributions before and after matching are represented in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100151.
Table 2.
Characteristics of patients according to pN status in the propensity-score-matched cohort
| pN0(i−) |
pN1mi |
pN1macro |
Chi2 |
||||
|---|---|---|---|---|---|---|---|
| Nb | % | Nb | % | Nb | % | P value | |
| 409 | 32 | 449 | 35 | 436 | 34 | ||
| Age, years | |||||||
| ≤40 | 18 | 4.4 | 24 | 5.3 | 20 | 4.6 | 0.982 |
| 40.1-50 | 86 | 21.0 | 96 | 21.4 | 93 | 21.3 | |
| 50.1-74.9 | 260 | 63.6 | 284 | 63.3 | 282 | 64.7 | |
| ≥75 | 45 | 11.0 | 45 | 10.0 | 41 | 9.4 | |
| SLNB/ALND | |||||||
| SLNB | 69 | 16.9 | 77 | 17.1 | 73 | 16.7 | 0.987 |
| SLNB + ALND | 340 | 83.1 | 372 | 82.9 | 363 | 83.3 | |
| LVI | |||||||
| No | 327 | 80.0 | 353 | 78.6 | 348 | 79.8 | 0.866 |
| Yes | 82 | 20.0 | 96 | 21.4 | 88 | 20.2 | |
| Tumor size, mm | |||||||
| ≤5 | 12 | 2.9 | 14 | 3.1 | 17 | 3.9 | 0.357 |
| 5.1-10 | 69 | 16.9 | 69 | 15.4 | 67 | 15.4 | |
| 10.1-19.9 | 157 | 38.4 | 165 | 36.7 | 134 | 30.7 | |
| 20-50 | 146 | 35.7 | 177 | 39.4 | 188 | 43.1 | |
| >50 | 25 | 6.1 | 24 | 5.3 | 30 | 6.9 | |
| Surgery breast | |||||||
| BCS | 311 | 76.0 | 339 | 75.5 | 323 | 74.1 | 0.791 |
| Mastectomy | 98 | 24.0 | 110 | 24.5 | 113 | 25.9 | |
| Endocrine therapy | |||||||
| No | 17 | 4.2 | 22 | 4.9 | 15 | 3.4 | 0.555 |
| Yes | 392 | 95.8 | 427 | 95.1 | 421 | 96.6 | |
| Chemotherapy | |||||||
| No | 197 | 48.2 | 219 | 48.8 | 197 | 45.2 | 0.523 |
| Yes | 212 | 51.8 | 230 | 51.2 | 239 | 54.8 | |
| Grade | |||||||
| 1 | 133 | 32.5 | 148 | 33.0 | 141 | 32.3 | 0.975 |
| 2 | 216 | 52.8 | 232 | 51.7 | 234 | 53.7 | |
| 3 | 60 | 14.7 | 69 | 15.4 | 61 | 14.0 | |
ALND, axillary lymph node dissection; BCS, breast conservative surgery; LVI, Lympho-vascular invasion; Nb, number; SLNB, sentinel lymph node biopsy.
Prognostic impact of axillary LN status on DFS, MFS, and OS in the entire population (13 773 patients): univariate analysis
Median follow-up was 61.25 months [95% confidence interval (CI) 62.1-63.3], 58.9 months (95% CI 58.5-59.8) for pN0, 66.0 months (95% CI 68.1-74.0) for pN0(i+), 66.8 months (95% CI 68.6-72.4) for pN1mi, and 67.8 months (95% CI 70.2-73.4) for pN1. OS and DFS were significantly different according to axillary LN status, age, LVI, tumor size, grade, HER2 status, type of breast surgery, tumor subtypes, endocrine therapy, chemotherapy, and regional nodal irradiation (RNI). All these factors had significant prognostic impact, as well as tumor histology and periods (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100151). Five-year OS were 97.7%, 97.9%, 97.6%, and 95.5% for pN0, pN0(i+), pN1mi, and pN1, respectively (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100151). Five-year DFS are reported in Supplementary Table S2 and Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2021.100151. The maximal potential difference that these analyses have the power to identify between pN0 and pN1mi status was 1.7% and hazard ratio (HR) of 2.0 with a power ≥85%.
Prognostic impact of axillary LN status on DFS, MFS, OS, and BCSS: multivariate analysis
In the entire population as well as in the subgroup of patients with known tumor subtype, with distinction between pN0, pN0(i+), pN1mi, pN1, only pN1, but not pN0(i+) or pN1mi, were independent prognostic factors for OS, DFS, MFS, and BCSS (Table 3). The other independent prognostic factors in the entire population are reported in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100151.
Table 3.
Survival results (overall survival, disease-free survival, metastasis-free survival and breast cancer-specific survival): Cox model adjusted on significant univariate criteria for all patients and for patients with tumor subtypes known, according to pN status [pN0, pN0(i+), pN1mi, pN1macro]
| All patients | All patients |
||
|---|---|---|---|
| HR | P value | 95% CI | |
| Overall survival | |||
| Positive ER | |||
| pN0 | 1 | ||
| pN0(i+) | 0.872 | 0.595 | 0.527-1.444 |
| pN1mi | 0.930 | 0.665 | 0.670-1.292 |
| pN1a | 1.828 | <0.0001 | 1.368-2.442 |
| Subtypes | |||
| pN0 | 1 | ||
| pN0(i+) | 1.089 | 0.788 | 0.585-2.026 |
| pN1mi | 0.999 | 0.994 | 0.674-1.479 |
| pN1a | 1.973 | <0.0001 | 1.391-2.798 |
| Disease-free survival | |||
| Positive ER | |||
| pN0 | 1 | ||
| pN0(i+) | 1.102 | 0.524 | 0.817-1.487 |
| pN1mi | 0.936 | 0.551 | 0.751-1.165 |
| pN1a | 1.408 | 0.001 | 1.148-1.726 |
| Subtypes | |||
| pN0 | 1 | ||
| pN0(i+) | 1.328 | 0.133 | 0.918-1.921 |
| pN1mi | 0.960 | 0.759 | 0.739-1.246 |
| pN1a | 1.505 | 0.001 | 1.178-1.921 |
| Metastasis-free survival | |||
| Positive ER | |||
| pN0 | 1 | ||
| pN0(i+) | 0.728 | 0.250 | 0.423-1.251 |
| pN1mi | 0.851 | 0.371 | 0.598-1.212 |
| pN1a | 1.443 | 0.016 | 1.071-1.943 |
| Subtypes | |||
| pN0 | 1 | ||
| pN0(i+) | 0.847 | 0.635 | 0.426-1.685 |
| pN1mi | 0.905 | 0.633 | 0.601-1.363 |
| pN1a | 1.509 | 0.021 | 1.064-2.140 |
| Breast-cancer-specific survival | |||
| Positive ER | |||
| pN0 | 1 | ||
| pN0(i+) | 0.848 | 0.663 | 0.404-1.779 |
| pN1mi | 1.122 | 0.372 | 0.787-1.897 |
| pN1a | 1.960 | 0.001 | 1.328-2.893 |
| Subtypes | |||
| pN0 | 1 | ||
| pN0(i+) | 0.893 | 0.829 | 0.321-2.489 |
| pN1mi | 1.355 | 0.255 | 0.803-2.288 |
| pN1a | 2.216 | 0.001 | 1.382-3.554 |
Significant values are indicated in bold.
In the entire population, with distinction between pN0, pN0(i+), pN1mi, pN1 = 1 (1008 patients), and pN1 > 1 (1173 patients), both pN1 = 1 and pN1 > 1 status were independent prognostic factors for OS and DFS. Only pN1 > 1 status significantly impacted MFS (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100151). In the subgroup of patients with known tumor subtype, an independent adverse prognostic effect was observed in OS in patients with pN1 = 1 and pN1 > 1. Only pN1 > 1 status significantly impacted DFS and MFS (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100151, Figure 1).
Figure 1.
Survival results adjusted on tumor subtypes with distinction between pN0, pN0(i+), pN1mi, 1pN1, and more than 1pN1.
(A) Overall survival. (B) Disease-free survival. (C) Metastasis-free survival.
In the population with luminal A-like tumors (grade 1-2, n = 8547), significant results were observed for OS for patients with pN1 = 1 and pN1 > 1 status, for DFS in patients with pN1 > 1 status, and also in patients with pN0(i+) status and for MFS and BCSS in patients with pN1 > 1 status (Supplementary Table S5 and Figure S4, available at https://doi.org/10.1016/j.esmoop.2021.100151). Other independent prognostic factors in the population with luminal A-like tumors are reported in Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2021.100151. For 1040 luminal B-like tumors, including 128 pN1mi, significant results were observed for OS, DFS, and MFS for patients with pN1 status, but not for pN1mi status (Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2021.100151).
Results of analysis according to SN status whatever ALND pathologic results were similar without significant survival impact of pN1mi(sn) (data not shown).
Results in the population without AC with pN0, pN0(i+), and pN1mi adjusted on ER status (n = 7305) and adjusted on tumor subtypes (n = 5154) did not show any significant survival impact (OS, DFS, MFS) for pN1mi status in comparison with pN0 (Supplementary Table S8, available at https://doi.org/10.1016/j.esmoop.2021.100151). A significant BCSS difference was observed for pN1mi, only for the model applied on all patients according to ER status, without difference in the model adjusted on tumor subtypes.
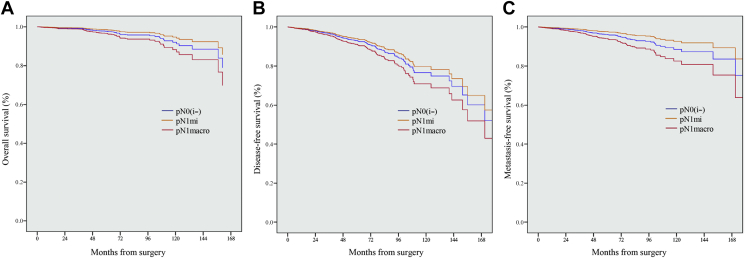
Prognostic impact of axillary LN status on OS, DFS, MFS, and BCSS in the matched population
In the final pN0(i−) (n = 409), pN1mi (n = 449), and pN1macro (n = 436) matched cohorts, log-rank tests stratified on the pairs revealed the statistically significant impact of pN1macro compared with pN1mi on OS, MFS, and BCSS, but not DFS (Figure 2 and Supplementary Table S9, available at https://doi.org/10.1016/j.esmoop.2021.100151). When considering pN0(i−) as the reference category, the trends in the HRs of pN1mi versus pN1macro were strictly opposite: 0.62 (95% CI 0.32-1.18) versus 1.48 (95% CI 0.87-2.52), 0.97 (95% CI 0.61-1.53) versus 1.45 (95% CI 0.94-2.23), 0.64 (95% CI 0.38-1.08) versus 1.65 (95% CI 1.07-2.54), and 0.59 (95% CI 0.24-1.44) versus 1.93 (95% CI 0.95-3.91) for OS, DFS, MFS, and BCSS, respectively.
Figure 2.
Kaplan Meier estimates for propensity-score-matched population with distinction between pN0(i−), pN1mi, and pN1macro.
(A) Overall survival. (B) Disease-free survival. (C) Metastasis-free survival.
Discussion
In this large retrospective cohort of patients with SLNB and ER-positive tumors, only macro-metastatic LN involvement, including pN1 = 1 and pN1 > 1, but not pN1mi, had a significant and independent pejorative prognostic impact on survival outcomes. However, the maximal potential difference that these analyses have the power to identify between pN0 and pN1mi status was 1.7% and HR 2.0.
These results differ from previous studies that have reported different survival rates between micro-metastases and pN0.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23,31,32 In contrast, our results are consistent with recent studies with no20,24,26, 27, 28,54 or only little22,29,30 significant survival impact of pN1mi. In the recent study reported by Andersson et al.,30 including 123 patients with SN micro-metastases with distinction of tumor subtypes, a negative BCSS impact of pN1mi was shown. In agreement with Andersson et al.,30 we share the point of view that a follow-up >7 years or 10 years is contributive since ER-positive BC recurrences can be observed many years after treatment.55 In our study, the number of pN1mi patients still at risk after 7 years and 10 years of follow-up were 404 and 121, respectively, in comparison with 111 and 93 patients at risk at 6 years and 10 years, respectively in the Andersson et al. study.30 We identified six main reasons that could potentially explain the discordant results with Andersson et al.: (i) more AC in our study, 51.25% (740/1444) versus 24.4% (30/123), (ii) more endocrine therapy in our study, 96.2% (1391/1446) versus 81.3% (100/123), (iii) more radiotherapy in our study, 91.9% (11 825/12 864) versus 71.5% (88/123), (iv) lower rate of mastectomies in our study, 10.0% (913/9140) versus 24.4% (30/123), (v) a multivariate model adjusted on more criteria which was possible due to a large number of patients in our study, and (vi) serial sectionings of SN were not carried out in the Andersson et al. study, and only 3.7% of the patients had micro-metastases (with a probably higher rate of large micro-metastases) versus 10.5% in our study. Interestingly, we did not observe any significant survival impact of pN1mi in comparison with pN0 status for patients without AC. The numerically worse outcome of pN0(i+) may be in relation to the short follow-up.
Contrary to the present work, no analysis of SN micro-metastases impact according to tumor subtypes was carried out in other series. In a previous research article, we reported the negative survival impact of occult axillary LN metastases for triple-negative tumors.56 Nevertheless, axillary micro-metastases do not impact the indication of adjuvant treatment with chemotherapy +/− trastuzumab in HER2-positive and triple-negative tumors with pathologic size >2 cm, and even >5 mm or 10 mm.57,58 For patients with HER2-positive tumors, the benefit of AC with trastuzumab has been reported, even for patients with pT1b node-negative tumors. Consequently, SN micro-metastases have no impact on AC indication in this situation.58 In contrast, the impact of pN1mi on adjuvant chemotherapy indication may be of particular interest for ER+ HER2- tumors with grade 1-2 and/or with high proliferation index (Ki67 > 20%). We did not capture proliferation index such as Ki67 in our study, which may be of interest for the distinction of ER-positive, HER2-negative, grade-2 tumors between luminal A-like and luminal B-like HER2-negative tumor subtypes.
Our study is also the first one generating a 1 : 1 : 1 matched pN0(i−), pN1mi, and pN1macro cohort. This statistical technique attempts to control for confounding factors by matching individual patients from a pN status group with a patient with the same clinico-pathological features in the other two groups. Thus, patients in the 1 : 1 : 1 matching cohort no longer differed for clinical and pathological characteristics (as described in Table 2 and illustrated by Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100151), except for pN status, allowing an estimate of its independent impact. Finding corresponding matches in each cohort for all features included in the model is not always possible and results in the exclusion of many unmatched patients from the analysis, underlining the need to start with a large number of patients. In our case, the matching process resulted in a cohort of 1294 patients with consequent loss in statistical power compared with the analyses carried out in the entire population. Nevertheless, pN1macro, but not pN1mi, was significantly associated with worse MFS. This point is critical since most of the benefit expected from AC is the prevention of ultimately lethal metastases.
St. Gallen consensus confirmed the important role of adjuvant therapy in reducing recurrence and improving survival. Subsequently, there is a need for precise prognostic factors to further improve the specificity of treatment with regard to the magnitude of clinical benefit.1 In the present study, patients were treated at 13 centers and adjuvant treatments may have differed. However, this multicenter cohort reflects clinical reality out of clinical trials. The decision to offer AC is most often made for patients with node-positive BC, while prognostic factors such as tumor size, tumor grade, proliferation factors (Ki-67 in particular), ER and HER2 status are used to identify a subset of node-negative patients who are at lower risk of recurrence without additional therapy.4 For ER-positive HER2-negative tumors, detection of occult metastases could lead to a discussion about AC indication. SN isolated tumor cells (SN-ITC) are usually not considered of negative prognostic impact and treated as pN0(i−). Our result support that micro-metastases do not negatively impact patient outcomes. Therefore, the relevance of pN1mi on AC indication should be the same as pN0(i−) or pN0(i+). Interestingly, we did not observe a significant prognostic difference between pN0(i−) and pN0(i+) in contrast with Anderson et al. study.31 This observation was also suggested in a recent review from Tsuda12 and results of de Boer et al. study for patients with ITC and micro-metastases in the absence of AC.9,10
Moreover, the results of our national, multicenter study demonstrated that the presence of micro-metastases was not an independent significant prognostic factor on OS, DFS, and MFS in the entire cohort, in patients according to tumor subtypes, in luminal A-like tumors, and in a 1 : 1 : 1 matched population compared with N0(i−) on OS, DFS, and BCSS. The apparent and counterintuitive positive effect of pN1mi on MFS compared with pN0 patients, while supporting the difference in these patients compared with those with pN1macro involvement, should be interpreted with caution, and may be, as mentioned, in relation to a short follow-up.
In the predictive tool Oncotype DX, a first-generation genomic test, pN1mi patients were classified at the same level of pN0. However, second generation of gene expression signatures, such as EndoPredict or Prosigna, includes tumor size and nodal status in the final recurrence risk score for ER-positive HER2-negative tumors.47,48 These signatures consider micro-metastases and macro-metastases as equivalent factors and may consequently overestimate the risk of recurrence for patients with micro-metastases. Considering our results, which show no significant independent impact of SN micro-metastases for ER-positive tumors and ER-positive HER2-negative tumors, including luminal A-like tumors, we strongly believe that the recurrence risk assessment for pN1mi tumors should no longer be calculated as equivalent to pN1, but as pN0.
Finally, neo-adjuvant treatment was excluded and residual LN tumor as ypN1mi for patients initially cN1 probably have a negative prognosis impact.
Conclusions
The results of our study, based on a large retrospective multicenter cohort, demonstrated that the presence of LN micro-metastases has no detectable prognostic impact on ER-positive early BC. Consequently, LN micro-metastases should not be considered a determining factor in indicating AC, and the recurrence risk assessment using second-generation signatures should be calculated considering micro-metastases as pN0. However, this study adds to the knowledge in this field but there will always some debate and further prospective studies should continue to accrue data with longer follow-up and with analysis of cohorts within genomic testing.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
The data sets generated and/or analyzed during the current study are not publicly available, as the study has used clinical databases of 13 different comprehensive cancer centers in France (ClinicalTrials.gov NCT02869607).
Footnotes
☆Note: This work was approved by our institutional review board (IPC – Comité d'Orientation Stratégique).
Supplementary data
Supplementary Figure S1.
Supplementary Figure S2.
Supplementary Figure S3.
Supplementary Figure S4.
References
- 1.Burstein H.J., Curigliano G., Loibl S. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541–1557. doi: 10.1093/annonc/mdz235. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz A.M., Henson D.E., Chen D., Rajamarthandan S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161 708 cases of breast cancer from the SEER program. Arch Pathol Lab Med. 2014;138(8):1048–1052. doi: 10.5858/arpa.2013-0435-OA. [DOI] [PubMed] [Google Scholar]
- 3.Mohammed R.A.A., Martin S.G., Gill M.S., Green A.R., Paish E.C., Ellis I.O. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol. 2007;31(12):1825–1833. doi: 10.1097/PAS.0b013e31806841f6. [DOI] [PubMed] [Google Scholar]
- 4.Cianfrocca M. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9(6):606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 5.Jafferbhoy S., McWilliams B. Clinical significance and management of sentinel node micrometastasis in invasive breast cancer. Clin Breast Cancer. 2012;12(5):308–312. doi: 10.1016/j.clbc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Salhab M., Patani N., Mokbel K. Sentinel lymph node micrometastasis in human breast cancer: an update. Surg Oncol. 2011;20(4):e195–e206. doi: 10.1016/j.suronc.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Chen S.L., Hoehne F.M., Giuliano A.E. The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Ann Surg Oncol. 2007;14(12):3378–3384. doi: 10.1245/s10434-007-9513-6. [DOI] [PubMed] [Google Scholar]
- 8.Colleoni M., Rotmensz N., Peruzzotti G. Size of breast cancer metastases in axillary lymph nodes: clinical relevance of minimal lymph node involvement. J Clin Oncol. 2005;23(7):1379–1389. doi: 10.1200/JCO.2005.07.094. [DOI] [PubMed] [Google Scholar]
- 9.de Boer M., van Dijck J.A., Bult P., Borm G.F., Tjan-Heijnen V.C. Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. J Natl Cancer Inst. 2010;102(6):410–425. doi: 10.1093/jnci/djq008. [DOI] [PubMed] [Google Scholar]
- 10.de Boer M., van Deurzen C.H., van Dijck J.A. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361(7):653–663. doi: 10.1056/NEJMoa0904832. [DOI] [PubMed] [Google Scholar]
- 11.Wada N., Imoto S. Clinical evidence of breast cancer micrometastasis in the era of sentinel node biopsy. Int J Clin Oncol. 2008;13(1):24–32. doi: 10.1007/s10147-007-0736-0. [DOI] [PubMed] [Google Scholar]
- 12.Tsuda H. Histological examination of sentinel lymph nodes: significance of macrometastasis, micrometastasis, and isolated tumor cells. Breast Cancer. 2015;22(3):221–229. doi: 10.1007/s12282-015-0588-9. [DOI] [PubMed] [Google Scholar]
- 13.Andersson Y., Frisell J., Sylvan M., de Boniface J., Bergkvist L. Breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. J Clin Oncol. 2010;28(17):2868–2873. doi: 10.1200/JCO.2009.24.5001. [DOI] [PubMed] [Google Scholar]
- 14.Cox C.E., Kiluk J.V., Riker A.I. Significance of sentinel lymph node micrometastases in human breast cancer. J Am Coll Surg. 2008;206(2):261–268. doi: 10.1016/j.jamcollsurg.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Grabau D., Jensen M.B., Rank F., Blichert-Toft M. Axillary lymph node micrometastases in invasive breast cancer: national figures on incidence and overall survival. APMIS. 2007;115(7):828–837. doi: 10.1111/j.1600-0463.2007.apm_442.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuijt G.P., Voogd A.C., van de Poll-Franse L.V., Scheijmans L.J., van Beek M.W., Roumen R.M. The prognostic significance of axillary lymph-node micrometastases in breast cancer patients. Eur J Surg Oncol. 2005;31(5):500–505. doi: 10.1016/j.ejso.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Tan L.K., Giri D., Hummer A.J. Occult axillary node metastases in breast cancer are prognostically significant: results in 368 node-negative patients with 20-year follow-up. J Clin Oncol. 2008;26(11):1803–1809. doi: 10.1200/JCO.2007.12.6425. [DOI] [PubMed] [Google Scholar]
- 18.Truong P.T., Vinh-Hung V., Cserni G., Woodward W.A., Tai P., Vlastos G. The number of positive nodes and the ratio of positive to excised nodes are significant predictors of survival in women with micrometastatic node-positive breast cancer. Eur J Cancer. 2008;44(12):1670–1677. doi: 10.1016/j.ejca.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Iqbal J., Ginsburg O., Giannakeas V., Rochon P.A., Semple J.L., Narod S.A. The impact of nodal micrometastasis on mortality among women with early-stage breast cancer. Breast Cancer Res Treat. 2017;161(1):103–115. doi: 10.1007/s10549-016-4015-5. [DOI] [PubMed] [Google Scholar]
- 20.Maaskant-Braat A.J., van de Poll-Franse L.V., Voogd A.C. Sentinel node micrometastases in breast cancer do not affect prognosis: a population-based study. Breast Cancer Res Treat. 2011;127(1):195e203. doi: 10.1007/s10549-010-1086-6. [DOI] [PubMed] [Google Scholar]
- 21.Hansen N.M., Grube B., Ye X. Impact of micrometastases in the sentinel node of patients with invasive breast cancer. J Clin Oncol. 2009;27(28):4679e84. doi: 10.1200/JCO.2008.19.0686. [DOI] [PubMed] [Google Scholar]
- 22.Weaver D.L., Ashikaga T., Krag D.N. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364(5):412–421. doi: 10.1056/NEJMoa1008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z.J., Yu Y., Hou X.W. The prognostic value of node status in different breast cancer sub-types. OncoTarget. 2017;8(3):4563–4571. doi: 10.18632/oncotarget.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gobardhan P.D., Elias S.G., Madsen E.V. Prognostic value of lymph node micrometastases in breast cancer: a multicenter cohort study. Ann Surg Oncol. 2011;18(6):1657–1664. doi: 10.1245/s10434-010-1451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giuliano A.E., Hawes D., Ballman K.V. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA. 2011;306(4):385–393. doi: 10.1001/jama.2011.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meattini I., Desideri I., Saieva C. Impact of sentinel node tumor burden on outcome of invasive breast cancer patients. Eur J Surg Oncol. 2014;40(10):1195–1202. doi: 10.1016/j.ejso.2014.08.471. [DOI] [PubMed] [Google Scholar]
- 27.Rovera F., Fachinetti A., Rausei S. Prognostic role of micrometastases in sentinel lymph node in patients with invasive breast cancer. Int J Surg. 2013;11(1):60022–60029. doi: 10.1016/S1743-9191(13)60022-9. [DOI] [PubMed] [Google Scholar]
- 28.Maibenco D.C., Dombi G.W., Kau T.Y., Severson R.K. Significance of micrometastases on the survival of women with T1 breast cancer. Cancer. 2006;107(6):1234–1239. doi: 10.1002/cncr.22112. [DOI] [PubMed] [Google Scholar]
- 29.van Roozendaal L.M., Schipper R.J., Van de Vijver K.K. The impact of the pathological lymph node status on adjuvant systemic treatment recommendations in clinically node negative breast cancer patients. Breast Cancer Res Treat. 2014;143(3):469–476. doi: 10.1007/s10549-013-2822-5. [DOI] [PubMed] [Google Scholar]
- 30.Andersson Y., Bergkvist L., Frisell J., de Boniface J. Long-term breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. Breast Cancer Res Treat. 2018;171(2):359–369. doi: 10.1007/s10549-018-4820-0. [DOI] [PubMed] [Google Scholar]
- 31.Houvenaeghel G., Classe J.M., Garbay J.R. Prognostic value of isolated tumor cells and micrometastases of lymph nodes in early-stage breast cancer: a French sentinel node multicenter cohort study. Breast. 2014;23(5):561–566. doi: 10.1016/j.breast.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Madsen E.V., Elias S.G., van Dalen T. Predictive factors of isolated tumor cells and micrometastases in axillary lymph nodes in breast cancer. Breast. 2013;22(5):748–752. doi: 10.1016/j.breast.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Houvenaeghel G., Lambaudie E., Classe J.-M. Lymph node positivity in different early breast carcinoma phenotypes: a predictive model. BMC Cancer. 2019;19(1):45. doi: 10.1186/s12885-018-5227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi M., Giordano S.H., Meric-Bernstam F. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. 2010;17(suppl 3):343–351. doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bilimoria K.Y., Bentrem D.J., Hansen N.M. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27(18):2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 36.Meretoja T.J., Leidenius M.H., Heikkilä P.S. International multicenter tool to predict the risk of non-sentinel node metastases in breast cancer. J Natl Cancer Inst. 2012;104(24):1888–1896. doi: 10.1093/jnci/djs455. [DOI] [PubMed] [Google Scholar]
- 37.Dowlatshahi K., Fan M., Anderson J.M., Bloom K.J. Occult metastases in sentinel nodes of 200 patients with operable breast cancer. Ann Surg Oncol. 2001;8(8):675–681. doi: 10.1007/s10434-001-0675-3. [DOI] [PubMed] [Google Scholar]
- 38.Cserni G. Metastases in axillary sentinel lymph nodes in breast cancer as detected by intensive histopathological work up. J Clin Pathol. 1999;52(12):922–924. doi: 10.1136/jcp.52.12.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheang M.C., Voduc D., Bajdik C. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 40.Perou C.M., Sørlie T., Eisen M.B. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 41.Reyal F., Rouzier R., Depont-Hazelzet B. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS One. 2011;6(5):e20297. doi: 10.1371/journal.pone.0020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen T.O., Jensen M.-B., Burugu S. High-risk premenopausal luminal a breast cancer patients derive no benefit from adjuvant cyclophosphamide-based chemotherapy: results from the DBCG77B clinical trial. Clin Cancer Res. 2017;23(4):946–953. doi: 10.1158/1078-0432.CCR-16-1278. [DOI] [PubMed] [Google Scholar]
- 43.Sparano J.A., Gray R.J., Makower D.F. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sparano J.A., Gray R.J., Makower D.F. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cardoso F., van’t Veer L.J., Bogaerts J. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 46.Sparano J.A., Gray R.J., Ravdin P.M. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395–2405. doi: 10.1056/NEJMoa1904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gnant M., Filipits M., Dubsky P. Predicting risk for late metastasis: the PAM50 risk of recurrence (ROR) score after 5 years of endocrine therapy in postmenopausal women with HR+ early breast cancer: a study on 1478 patients from the ABCSG-8 trial. Ann Oncol. 2013;24(suppl 3):iii29. [Google Scholar]
- 48.Dubsky P., Filipits M., Jakesz R. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013;24(3):640–647. doi: 10.1093/annonc/mds334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Minckwitz G., Untch M., Blohmer J.U. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 50.Rosenbaum P.R., Rubin D.B. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 51.Little R.J., Rubin D.B. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health. 2000;21:121–145. doi: 10.1146/annurev.publhealth.21.1.121. [DOI] [PubMed] [Google Scholar]
- 52.Rosenbaum P.R. Modern algorithms for matching in observational studies. Annu Rev Stat Appl. 2020;7(1):143–176. [Google Scholar]
- 53.Klein J., Moeschberger M., Hawkins K. Survival analysis: techniques for censored and truncated data. Pharm Stat. 2004;3:303–304. [Google Scholar]
- 54.Bae H.W., Yoon K.H., Kim J.H. Impact of micrometastatic axillary nodes on survival of breast cancer patients with tumors ≤2 cm. World J Surg. 2018;42(12):3969–3978. doi: 10.1007/s00268-018-4725-4. [DOI] [PubMed] [Google Scholar]
- 55.Pan H., Gray R., Braybrooke J. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houvenaeghel G., Sabatier R., Reyal F. Axillary lymph node micro-metastases decrease triple-negative early breast cancer survival. Br J Cancer. 2016;115(9):1024–1031. doi: 10.1038/bjc.2016.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Nonneville A., Gonçalves A., Zemmour C. Benefit of adjuvant chemotherapy with or without trastuzumab in pT1ab node-negative human epidermal growth factor receptor 2-positive breast carcinomas: results of a national multi-institutional study. Breast Cancer Res Treat. 2017;162(2):307–316. doi: 10.1007/s10549-017-4136-5. [DOI] [PubMed] [Google Scholar]
- 58.de Nonneville A., Gonçalves A., Zemmour C. Adjuvant chemotherapy in pT1ab node-negative triple-negative breast carcinomas: results of a national multi-institutional retrospective study. Eur J Cancer. 2017;84:34–43. doi: 10.1016/j.ejca.2017.06.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.