…consider them both, the sea and the land; and do you not find a strange analogy to something in yourself?
—Herman Melville, Moby-Dick, or the Whale
Considering the massive clinical burden caused by aspiration—ranging from foreign body asphyxiation in children to the high prevalence of aspiration pneumonia among the elderly—the precarious proximity of the trachea and esophagus in humans is evolutionarily puzzling. With every bite of food and sip of liquid, a mere sliver of tissue (and a sophisticated coordination of musculature) prevents our airways from filling with pharyngeal contents (Figure 1). Given the morbidity that arises from gastric and pharyngeal contents “going down the wrong pipe,” evolution made it remarkably easy for this to occur.
Figure 1.
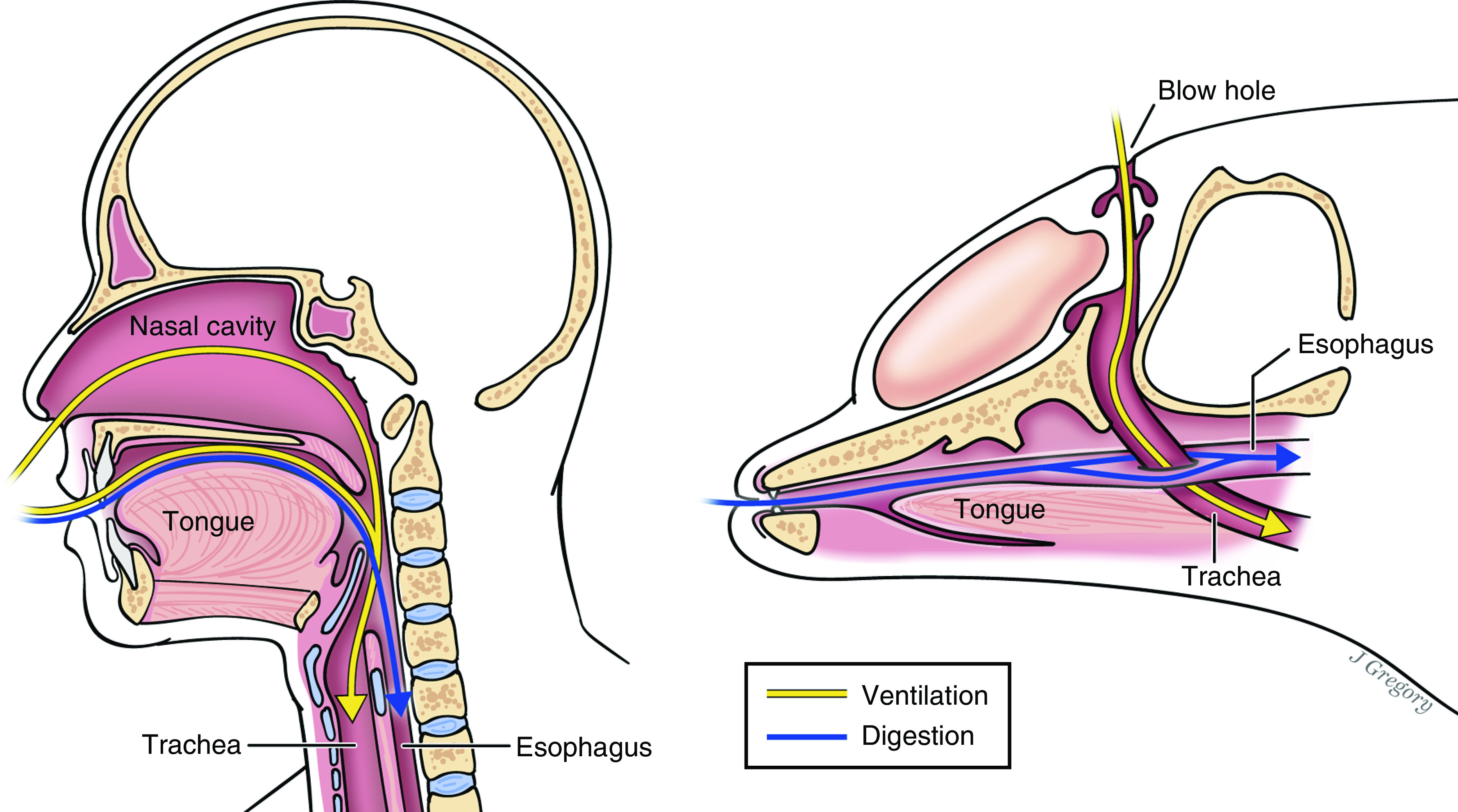
Why aren’t we built more like dolphins? Although nearly all mammals have a common “aerodigestive tract,” whales and dolphins have anatomically separate orifices for ventilation and digestion (i.e., blowholes and mouths). This occurred via the gradual posterior migration of the nasal cavity over tens of millions of years of evolution. For this reason, aspiration of pharyngeal and gastrointestinal contents is impossible in these species. For the rest of us, aspiration is a common and clinically significant problem. The current study by Wu and colleagues suggests a potential protective effect of subclinical aspiration on host susceptibility to pneumonia. Illustration by Jill Gregory.
Patients, clinicians, and investigators may thus justifiably envy the alternative design of cetaceans (e.g., whales or dolphins), who possess discrete “ports of entry” for air and food (Figure 1). Over evolutionary history, the nasal orifice of cetacean ancestors migrated posteriorly to its current dorsal position. Although this provided an immediate benefit of enabling respiration while nearly submerged, it also completely separated the trachea and esophagus, rendering modern cetaceans incapable of aspirating pharyngeal contents into their lungs. Unlike other mammals, cetaceans lack a unitary “aerodigestive tract.”
Why is this design—the anatomic segregation of ventilation and nutrition—the exception rather than the rule? Were the consequences of aspiration not severe enough for other mammals to similarly evolve distinct orifices? More provocatively, could there be an advantage to the close proximity of our airways and upper digestive tract? We have known for decades that subclinical aspiration of pharyngeal contents is a routine occurrence, even among healthy asymptomatic humans (1, 2). Recent investigations into the lung microbiome have confirmed that the lungs of healthy humans and mice are continuously exposed to pharyngeal microbiota, low in quantity but well correlated with the baseline host immune response (3–6). Although the existence of a dynamic, low-biomass lung microbiome in health is now widely accepted, the immunologic and clinical consequences of this constant microbial bombardment are unknown.
In this issue of the Journal, Wu and colleagues (pp. 1099–1111) address this question using a novel murine model of microbial aspiration using human oral commensal bacteria (7). After simulating subclinical microaspiration of nonpathogenic bacteria, the authors characterized temporal changes in lung microbiome composition, host immune response, and susceptibility to subsequent infectious challenge. As expected, aspiration of human commensals (Prevotella melaninogenica, Veillonella parvula, and Streptococcus mitis) provoked a predictable change in lung microbiota that resolved after 5 days. Remarkably, the authors found that mice were protected from subsequent S. pneumoniae infection up to 2 weeks after a single aspiration event.
Although aspiration-induced changes in lung microbiota were transient, the resulting alteration of lung inflammatory tone lasted up to 14 days and evoked sustained host transcriptional responses (including T-cell signaling, T-helper cell type 1 [Th1] and Th17 activation, and inflammasome, P38 MAPK, and PI3K/AKT signaling pathways). Further examination of induced Toll-like receptor signaling revealed upregulation of MyD88-associated genes, whereas confirmatory cellular immunology and knockout mouse experiments revealed MyD88-dependent priming of proinflammatory Th17 responses, all triggered by aspiration of oral commensals. These findings suggest the presence of a sentinel immune configuration, honed by commensal exposure to lung microbiota, that primes host-pathogen defense.
These findings represent major methodological and conceptual advances. Methodologically, Wu and colleagues demonstrate the first successful attempt to experimentally manipulate the lung microbiome in mice using commensal instillation. This is a crucial step forward in our ability to rationally modulate the lung microbiome, which will be essential as we interrogate its mechanistic importance. Conceptually, these results enhance our working model of pulmonary immune tone calibration. Whereas we once dichotomized the lung immune response (quiescent vs. activated) to mirror our incomplete understanding of lung microbiology (sterile vs. infected), the current study confirms and elaborates upon our modern, refined model: lung immunity exists along a continuum of activation, exquisitely calibrated by local microbial interactions (8). Whereas we previously knew that the lung’s immune apparatus both “reflects” its immediate microbial community (4) and “remembers” the influence of prior pulmonary infections (9), the current study reveals that specific, noninfectious microbial exposures can calibrate the lungs’ anticipatory response to subsequent microbial challenges. Like the dynamic, low-biomass pulmonary microbiome in health, local lung immunity is itself in a steady-state equilibrium, constantly surveilling and responding to aspirated microbiota, with immunologic consequences that outlive the provoking microbiota.
This revised model of lung immunity bears a strong resemblance to our existing understanding of gut immunity, in which mucosal immune tone is neither “on” nor “off” but instead dynamically calibrated to its microbial milieu. A vivid example is the Th17 response provoked by segmented filamentous bacteria, which confers protection from staphylococcal pneumonia (10). Wu and colleagues have elaborated a plausible mechanism by which immunologic gatekeeping can hone sentinel immune tone within the lungs, distinct from (and surely complementary to) the paradigm of the gut–lung axis. This distinction may shed light on the incompletely understood impact of chronic respiratory dysbiosis and antibiotics on host immunity and susceptibility to infection. If aspiration of “healthy” oral microbiota confers a protective benefit, could the opposite be the case for patients with acute and chronic respiratory dysbiosis? Antibiotics, via their effects on respiratory communities, can accentuate host immune and allergic responses, as in the case of Aspergillus fumigatus sensitization, and may underpin dysregulated host response and associated allergy observed in chronic lung disease (11, 12). Thus, Wu and colleagues may have uncovered a pivotal component of microbiome-regulated host immunity with immediate relevance to allergy and infection.
Despite the study’s convincing findings and methodological innovations, it has limitations that should motivate future work. Although mice are an invaluable model system across biologic disciplines, they differ from humans anatomically, immunologically, and microbiologically. Although it is encouraging that the murine immune response to aspiration with human-associated microbiota mirrors that of the human response, congruence across mammalian species should not be assumed. Though aspiration provided mice with a lingering protection against subsequent pneumococcal infection, it remains undetermined how pathogen specific this protection is. The same immune cascade that is protective for one pathogen may provide no benefit against others or may even potentiate off-target tissue injury via an overexuberant response (resulting in acute lung injury or sepsis). Future studies will be necessary to determine the taxonomic specificity of this protective effect.
Based on the conclusions of Wu and colleagues, we can invert our earlier speculation and ask if it is the whales who are suboptimally designed, lacking the constant immune calibration we derive from subclinical aspiration of pharyngeal bacteria. Although cetaceans may be protected from the immediate consequences of pharyngeal aspiration, they remain susceptible to pneumonia, which is a common cause of death among whales and dolphins (13, 14). Perhaps it is the whales who should aspire to be like us.
Footnotes
Supported by NHLBI grants R01HL144599 and K23HL130641 (R.P.D.).
Author Contributions: Drafting, revising, and final approval of manuscript: M.M.A., J.M.B., and R.P.D.
Originally Published in Press as DOI: 10.1164/rccm.202011-4257ED on February 17, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111:1266–1272. doi: 10.1378/chest.111.5.1266. [DOI] [PubMed] [Google Scholar]
- 2.Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64:564–568. doi: 10.1016/0002-9343(78)90574-0. [DOI] [PubMed] [Google Scholar]
- 3.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, et al. Bacterial topography of the healthy human lower respiratory tract. mBio. 2017;8:e02287-16. doi: 10.1128/mBio.02287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson RP, Erb-Downward JR, Falkowski NR, Hunter EM, Ashley SL, Huffnagle GB. The lung microbiota of healthy mice are highly variable, cluster by environment, and reflect variation in baseline lung innate immunity. Am J Respir Crit Care Med. 2018;198:497–508. doi: 10.1164/rccm.201711-2180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Gao Z, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation Microbiome 2013119[Published erratum appears in Microbiome 2:21.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol. 2016;1:16031. doi: 10.1038/nmicrobiol.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu BG, Sulaiman I, Tsay J-CJ, Perez L, Franca B, Li Y, et al. Episodic aspiration with oral commensals induces a MyD88-dependent, pulmonary T-helper cell type 17 response that mitigates susceptibility to Streptococcus pneumoniae. Am J Respir Crit Care Med. 2021;203:1099–1111. doi: 10.1164/rccm.202005-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd CM, Marsland BJ. Lung homeostasis: influence of age, microbes, and the immune system. Immunity. 2017;46:549–561. doi: 10.1016/j.immuni.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Shenoy AT, Wasserman GA, Arafa EI, Wooten AK, Smith NMS, Martin IMC, et al. Lung CD4+ resident memory T cells remodel epithelial responses to accelerate neutrophil recruitment during pneumonia. Mucosal Immunol. 2020;13:334–343. doi: 10.1038/s41385-019-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauguet S, D’Ortona S, Ahnger-Pier K, Duan B, Surana NK, Lu R, et al. Intestinal microbiota of mice influences resistance to Staphylococcus aureus pneumonia. Infect Immun. 2015;83:4003–4014. doi: 10.1128/IAI.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003. doi: 10.1128/IAI.72.9.4996-5003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mac Aogáin M, Tiew PY, Lim AYH, Low TB, Tan GL, Hassan T, et al. Distinct “Immunoallertypes” of disease and high frequencies of sensitization in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2019;199:842–853. doi: 10.1164/rccm.201807-1355OC. [DOI] [PubMed] [Google Scholar]
- 13.Di Guardo G, Agrimi U, Morelli L, Cardeti G, Terracciano G, Kennedy S. Post mortem investigations on cetaceans found stranded on the coasts of Italy between 1990 and 1993. Vet Rec. 1995;136:439–442. doi: 10.1136/vr.136.17.439. [DOI] [PubMed] [Google Scholar]
- 14.Venn-Watson S, Daniels R, Smith C. Thirty year retrospective evaluation of pneumonia in a bottlenose dolphin Tursiops truncatus population. Dis Aquat Organ. 2012;99:237–242. doi: 10.3354/dao02471. [DOI] [PubMed] [Google Scholar]


