Despite intensive research, the pathophysiology of acute kidney injury (AKI) in critical illnesses remains poorly understood, as do the links between AKI and poor outcomes. Post–cardiac surgery AKI is not exempt. Multiple, heterogeneous mechanisms are likely in play. Hemodynamic factors, systemic inflammatory response, and cardiopulmonary bypass–induced hemolysis certainly contribute (1, 2). Other contributing factors remain unrecognized or underexplored, such as the complex role of the renin–angiotensin–aldosterone system (RAAS) (3).
In a fascinating study published in this issue of the Journal, Küllmar and colleagues (pp. 1119–1126) report the association between postoperative plasma renin level and the risk of developing AKI after cardiac surgery (4). Plasma renin levels measured 4 hours after cardiopulmonary bypass were strongly associated with AKI, whereas preoperative values were not. Patients with higher postoperative plasma renin levels and higher changes in plasma renin compared with preoperative values (Δ-renin) developed more AKI than patients with lower levels and smaller changes. Patients with AKI had a median (interquartile range) rise in plasma renin of 99.6 μU/ml (6.7, 318.0; P < 0.001). This Δ-renin was the strongest predictor of postoperative AKI in the study (area under the curve–receiver operating characteristic, 0.817) and superior to urinary AKI biomarkers DKK3 and [TIMP-2]*[IGFBP7].
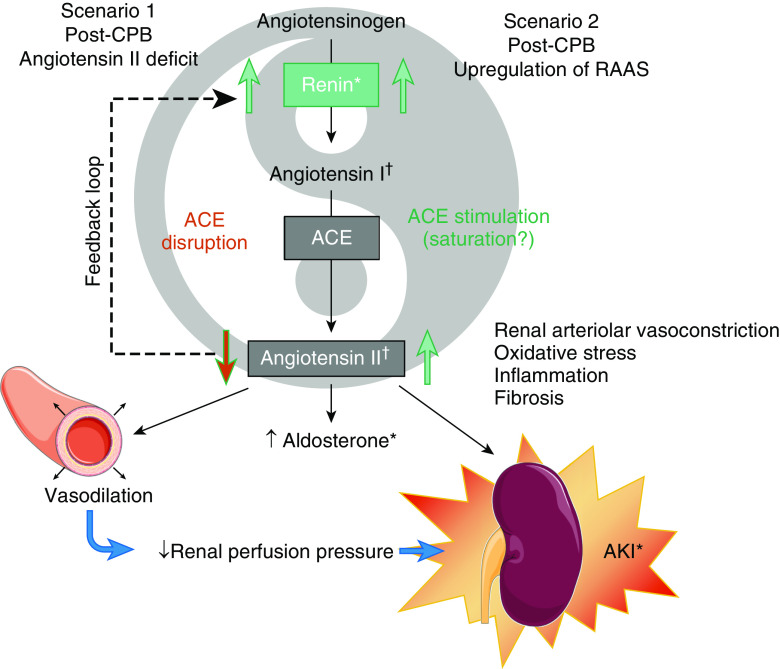
The primary hypothesis of the authors is that an angiotensin II deficit occurs after cardiac surgery to explain these findings. In a feedback loop, renin is released in response to decreased activation of the AT1R (angiotensin II type 1 receptor) by angiotensin II (Figure 1, Scenario 1). This can be caused by either impaired generation of angiotensin II or AT1R blockade. Angiotensin II is produced when the endothelial membrane–bound enzyme ACE (angiotensin-converting enzyme) cleaves angiotensin I. Conditions associated with endothelial dysfunction, such as septic shock or cardiopulmonary bypass, can reduce ACE activity, decrease angiotensin II, and increase renin levels (5, 6). Decreased expression of AT1R was also reported in sepsis-associated AKI (7). This hypothesis is supported by several findings in Küllmar and colleagues, including higher and more prolonged vasopressor requirements, high Δ-renin levels, and higher renin over aldosterone ratio in patients with AKI. Of note, although patients treated with ACE inhibitors (ACEi) or angiotensin receptor blockers (ARB) had higher plasma renin levels and overall higher risk of AKI, no interaction was found between ACEi/ARB therapy and Δ-renin for predicting AKI.
Figure 1.
Schematic of possible RAAS perturbations leading to AKI after CPB. ACE = angiotensin-converting enzyme; AKI = acute kidney injury; CPB = cardiopulmonary bypass; RAAS = renin–angiotensin–aldosterone system. *Finding of Küllmar and colleagues. †Indeterminate.
An alternative hypothesis to that of Küllmar and colleagues is that significant activation of the RAAS occurs after cardiac surgery (with consequently high angiotensin II tone), triggering intrarenal vasoconstriction, decrease in renal blood flow, and regional inflammation (Figure 1, Scenario 2). Unfortunately, plasma angiotensin II levels were not available in the study, thus making it impossible to definitively distinguish the two possibilities. The alternate hypothesis is supported by lower blood pressures (a trigger for RAAS activation) in the group with AKI and supranormal plasma levels of both renin and aldosterone after surgery (8). Furthermore, there are many reports of decline in renal blood flow associated with elevated intrarenal vascular resistance and increased angiotensin II levels after cardiac surgery (9, 10). In this line, the use of intrarenal vasodilators has long been proposed to decrease the risk of post–cardiac surgery AKI.
What Are the Implications of This Study?
This study clearly demonstrates that plasma renin elevation after cardiac surgery is associated with AKI risk and strongly implicates the RAAS. The RAAS clearly holds a pivotal pathophysiologic role in cardiovascular and renal diseases, including AKI (11). Elevated plasma renin is associated with poor outcomes both in chronic conditions such as heart failure and acute conditions such as vasodilatory shock. A post hoc analysis of the ATHOS-3 trial (angiotensin II versus placebo in catecholamine-resistant vasodilatory shock, defined as a need for norepinephrine >0.2 μg/kg/min) demonstrated that high plasma renin was associated with a risk of death and nonrecovery from AKI (6, 12). Angiotensin II has long been known as a mediator of renal injury in the subacute and chronic settings. Angiotensin II promotes inflammation, oxidative stress, and fibrosis (13). Patients with congestive heart failure, hypertension, and chronic kidney diseases have better outcomes when treated with inhibitors of the RAAS. Data from observational studies yield conflicting results regarding the impact of RAAS blockade during cardiac surgery (8, 14). In a small pilot randomized trial among patients chronically treated with ACEi or ARB, stopping the treatment 48 hours before cardiac surgery was not associated with better outcomes or lower incidence of AKI compared with the group who continued the treatment until surgery (15). RAAS blockade has been suggested to improve post-AKI survival, even though well-designed prospective interventional studies are still lacking (16).
The hypothesis in Küllmar and colleagues would provide ground for a potential use of angiotensin II infusion in post–cardiac surgery distributive shock. Although very appealing, this hypothesis needs to be balanced and refined. Although angiotensin II infusion was associated with better survival only in patients with high plasma renin levels in a post hoc analysis of ATHOS-3, the study mostly enrolled patients with sepsis, which might involve different mechanisms (6). Furthermore, administration of angiotensin II was not stratified by plasma renin level. If an angiotensin II deficit exists, it appears somehow relative with increased renin over aldosterone ration in Küllmar and colleagues. To what extent this represents a relative angiotensin deficit in the most severe patients or an activation of the RAAS with saturation of ACE activity is uncertain.
To conclude, the prognostic value and role of the RAAS in AKI is increasingly recognized. The study from Küllmar and colleagues provides important insight in the setting of cardiac surgery. We now must better understand the full profile and timing of RAAS alterations in critical illness and the impact of modulating it, both in the acute and postacute phase. Overall, the impact of short-term infusion of angiotensin II on renal injury and survival in patients with shock remains largely unknown. Although some patients might benefit from RAAS blockade, even during the acute phase of shock, others might benefit from angiotensin II infusion. Still other phenotypes may require both therapies at different times—and many others may do best with no intervention at all. Better phenotyping of patients and more well-designed interventional trials are definitely needed to better the understand the Yin and Yang of the RAAS in AKI and critical illness.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202012-4419ED on December 24, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vermeulen Windsant IC, de Wit NCJ, Sertorio JTC, van Bijnen AA, Ganushchak YM, Heijmans JH, et al. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol. 2014;5:340. doi: 10.3389/fphys.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khorashadi M, Bokoch MP, Legrand M. Is nitric oxide the forgotten nephroprotective treatment during cardiac surgery? Ann Intensive Care. 2020;10:22. doi: 10.1186/s13613-020-0631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudoignon E, Dépret F, Legrand M. Is the renin-angiotensin-aldosterone system good for the kidney in acute settings? Nephron. 2019;143:179–183. doi: 10.1159/000499940. [DOI] [PubMed] [Google Scholar]
- 4.Küllmar M, Saadat-Gilani K, Weiss R, Massoth C, Lagan A, Núñez Cortés M, et al. Kinetic changes of plasma renin concentrations predict acute kidney injury in cardiac surgery patients. Am J Respir Crit Care Med. 2021;203:1119–1126. doi: 10.1164/rccm.202005-2050OC. [DOI] [PubMed] [Google Scholar]
- 5.Vergaro G, Emdin M, Iervasi A, Zyw L, Gabutti A, Poletti R, et al. Prognostic value of plasma renin activity in heart failure. Am J Cardiol. 2011;108:246–251. doi: 10.1016/j.amjcard.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Forni LG, Busse LW, McCurdy MT, Ham KR, Boldt DW, et al. Renin and survival in patients given angiotensin II for catecholamine-resistant vasodilatory shock: a clinical trial. Am J Respir Crit Care Med. 2020;202:1253–1261. doi: 10.1164/rccm.201911-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leisman DE, Fernandes TD, Bijol V, Abraham MN, Lehman JR, Taylor MD, et al. Impaired angiotensin II type 1 receptor signaling contributes to sepsis-induced acute kidney injury Kidney Int[online ahead of print] 31 Aug 2020; DOI: 10.1016/j.kint.2020.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora P, Rajagopalam S, Ranjan R, Kolli H, Singh M, Venuto R, et al. Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol. 2008;3:1266–1273. doi: 10.2215/CJN.05271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10:500–514. doi: 10.2215/CJN.07830814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagaoka H, Innami R, Arai H. Effects of pulsatile cardiopulmonary bypass on the renin-angiotensin-aldosterone system following open heart surgery. Jpn J Surg. 1988;18:390–396. doi: 10.1007/BF02471462. [DOI] [PubMed] [Google Scholar]
- 11.Legrand M, Rossignol P. Cardiovascular consequences of acute kidney injury. N Engl J Med. 2020;382:2238–2247. doi: 10.1056/NEJMra1916393. [DOI] [PubMed] [Google Scholar]
- 12.Bellomo R, Wunderink RG, Szerlip H, English SW, Busse LW, Deane AM, et al. Angiotensin I and angiotensin II concentrations and their ratio in catecholamine-resistant vasodilatory shock. Crit Care. 2020;24:43. doi: 10.1186/s13054-020-2733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long DA, Price KL, Herrera-Acosta J, Johnson RJ. How does angiotensin II cause renal injury? Hypertension. 2004;43:722–723. doi: 10.1161/01.HYP.0000120964.22281.3e. [DOI] [PubMed] [Google Scholar]
- 14.Manning MW, Cooter M, Mathew J, Alexander J, Peterson E, Ferguson TB, Jr, et al. Angiotensin receptor blockade improves cardiac surgical outcomes in patients with metabolic syndrome. Ann Thorac Surg. 2017;104:98–105. doi: 10.1016/j.athoracsur.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Diepen S, Norris CM, Zheng Y, Nagendran J, Graham MM, Gaete Ortega D, et al. Comparison of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker management strategies before cardiac surgery: a pilot randomized controlled registry trial. J Am Heart Assoc. 2018;7:e009917. doi: 10.1161/JAHA.118.009917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legrand M, Liu K. Angiotensin-converting enzyme inhibitors and or receptor blockers after acute kidney injury: rehabilitation of the supervillains. Crit Care Med. 2020;48:1922–1923. doi: 10.1097/CCM.0000000000004641. [DOI] [PubMed] [Google Scholar]