To the Editor:
ACE2 (angiotensin-converting enzyme 2) serves as the entry receptor for the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease (COVID-19). ACE2 is also the key enzyme of the alternative renin–angiotensin system (RAS) and counterbalances angiotensin II activity by enzymatically converting angiotensin II to angiotensin 1–7. Anchored in the membrane of type II alveolar and lung epithelial cells, ACE2 can be cleaved by the metalloproteinase ADAM17 (1).
In an earlier study, infection of mice with SARS-CoV-1, the coronavirus causing the 2002–2004 severe acute respiratory syndrome outbreak, resulted in lung injury, which appeared to be mediated by ACE2 downregulation and impaired degradation of angiotensin II (2). In line, the genetic deletion of ACE2 in mice led to a more severe disease course in an influenza model (3). However, gene expression data from BAL fluid cells suggest that, in humans, ACE2 becomes upregulated by IFN during SARS-CoV-2 infection (4). Moreover, significantly greater numbers of ACE2-positive cells were counted in the lungs from patients who died of COVID-19 or influenza compared with uninfected control subjects (5). Hypothesizing that ACE2 may play an essential role in the human response to viral lung injury, we aimed at assessing plasma ACE2 in patients with COVID-19 of variable severity over the time course of their disease in comparison with a retrospective cohort of patients with influenza.
Methods
We measured enzymatically active plasma ACE2 concentration (subsequently referred to as “ACE2”) and key RAS metabolites in 532 blood samples obtained from 126 patients with SARS-CoV-2 infection admitted to the primary COVID-19 care facility in the metropolitan area of Vienna (Klinik Favoriten) between March 15 and June 30, 2020. The study was approved by the Ethics Committee of the Medical University of Vienna (EK#1315/2020). Severity of disease was graded by the maximum requirement for respiratory support during hospitalization as severe (requiring invasive, mechanical ventilation at least once during the disease course) versus nonsevere (ranging from asymptomatic [patients who were hospitalized for non–SARS-CoV-2–related disease on top of SARS-CoV-2 infection] to noninvasive ventilation). As a comparator group, we included 27 patients who had been critically ill with influenza pneumonia, all requiring invasive, mechanical ventilation (years 2015–2019), and measured ACE2 and RAS metabolites from their previously stored serum samples. The equilibrium concentrations of the RAS metabolites angiotensin II and angiotensin 1–7 were determined by liquid chromatography–tandem mass spectrometry (Attoquant Diagnostics), as previously described (6). ACE2 was quantified using a natural substrate conversion assay (by quantifying angiotensin 1–7 formation from angiotensin II with commercially available recombinant human ACE2 as calibrator) (7). The starting point for all analyses was the day of hospitalization or transfer to our dedicated COVID-19 care facility after testing positive for SARS-CoV-2 at another hospital (Day 0). For statistical between-group comparisons of baseline characteristics and clinical variables, we used Student’s t test, χ2, and Fisher’s exact tests when appropriate. Laboratory values, ACE2 and RAS metabolite concentrations were compared between groups and by disease severity using Mann-Whitney U test. Multiple measurements per individual were averaged over given time intervals (all time points, Days 0–3 [early], and Days 9–11 [late]). A mixed-effects model was used to predict ACE2, as further described below.
Results
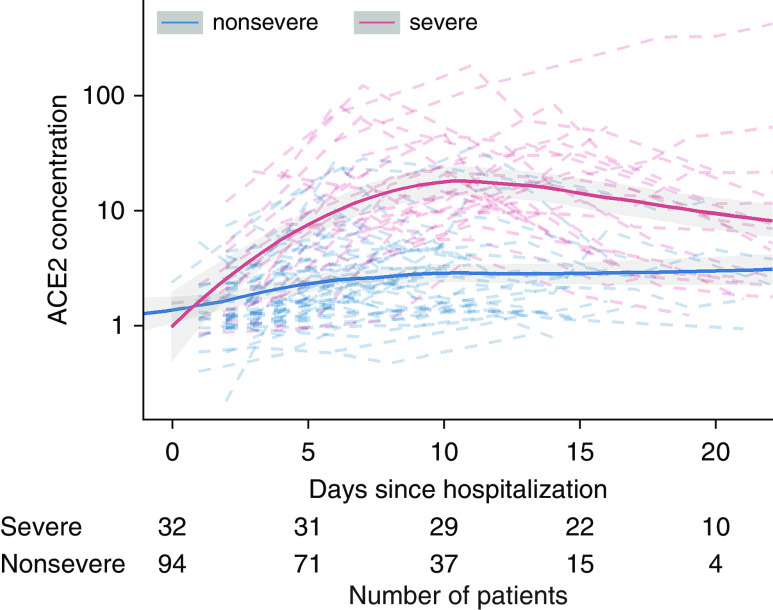
Baseline characteristics, covariables, and clinical outcomes of the study patients (patients with COVID-19 [stratified by disease severity] and patients with influenza) are provided in Table 1. Antivirals and immunomodulatory medication differed by COVID-19 severity. As shown in Figure 1, ACE2 in patients with COVID-19 increased over time, especially in severe patients, in whom ACE2 reached a peak of 15.1 ng/ml during the late time interval (Days 9–11), compared with 3.2 ng/ml in nonsevere patients (P < 0.001). ACE2 during the early time interval (Days 0–3) was 2.1 ng/ml in patients with severe COVID-19 and 1.3 ng/ml in patients with nonsevere COVID-19 (P = 0.078).
Table 1.
Demographics, Clinical Characteristics, and RAS Profiles of Patients with COVID-19 and Influenza
| Baseline Characteristics, Medication, and Outcome | |||||
|---|---|---|---|---|---|
| COVID-19 (n = 126) |
Comparator: Influenza (n = 27) |
P Value |
|||
| Nonsevere COVID-19 (n = 94) | Severe COVID-19 (n = 32) | Nonsevere vs. Severe COVID-19 | Severe COVID-19 vs. Influenza | ||
| Demographics | |||||
| Age, yr | 59 (47–77) | 68 (53–74) | 58 (49–64) | 0.207 | <0.05 |
| Sex, F | 34 (36.2) | 7 (21.9) | 11 (40.7) | 0.136 | 0.067 |
| BMI | 27.2 (24.5–30.6) | 25.7 (20.5–42.5) | n.a. | 0.740 | n.a. |
| Comorbidities | |||||
| Diabetes | 28 (30.1) | 11 (35.5) | 3 (13.6) | 0.577 | 0.075 |
| Hypertension | 47 (50.5) | 20 (64.5) | 2 (9.1) | 0.176 | <0.001 |
| COPD | 7 (7.5) | 6 (19.4) | 3 (13.6) | 0.063 | 0.585 |
| RAS inhibitor comedication | |||||
| ACEi | 16 (17.2) | 2 (6.5) | 2 (9.1) | 0.141 | 0.720 |
| ARB | 14 (15.1) | 8 (25.8) | 2 (9.1) | 0.175 | 0.125 |
| Antiviral medication | |||||
| Hydroxychloroquine | 8 (8.5) | 6 (18.8) | n.a. | 0.111 | n.a. |
| Lopinavir/ritonavir | 19 (20.2) | 11 (34.4) | n.a. | 0.104 | n.a. |
| Remdesivir | 4 (4.3) | 10 (31.2) | n.a. | <0.001 | n.a. |
| Convalescent plasma | 2 (2.1) | 4 (12.5) | n.a. | <0.05 | n.a. |
| Camostat | 25 (26.6) | 0 (0.0) | n.a. | <0.001 | n.a. |
| Immunomodulatory medication | |||||
| Tocilizumab | 1 (1.1) | 6 (18.8) | n.a. | <0.001 | n.a. |
| Steroids | 8 (8.6) | 11 (34.4) | n.a. | <0.001 | n.a. |
| Laboratory values | |||||
| Baseline CRP,* mg/L | 46.7 (19.5–82.7) | 120.90 (65.0–283.7) | 69.8 (32.1–226.1) | <0.001 | 0.188 |
| Maximum CRP,† mg/L | 58.9 (19.9–112.5) | 188.70 (102 –324.3) | 314.0 (211.9–338.2) | <0.001 | <0.05 |
| Baseline D-dimer,* mg/L | 0.96 (0.62–1.97) | 1.81 (0.82–4.27) | n.a. | <0.05 | n.a. |
| Maximum D-dimer,† mg/L | 1.63 (0.68–3.00) | 6.45 (2.67–15.55) | n.a. | <0.001 | n.a. |
| Baseline creatinine,* mg/dl | 0.86 (0.71–1.12) | 1.20 (0.78–1.50) | 1.29 (0.91–1.67) | <0.05 | 0.316 |
| Maximum creatinine,† mg/dl | 0.94 (0.76–1.17) | 1.42 (0.93–2.24) | 2.13 (1.47–3.46) | <0.001 | <0.05 |
| Baseline IL-6,* pg/ml | 25.1 (9.8–50.2) | 152.00 (68.8–369.3) | n.a. | <0.001 | n.a. |
| Maximum IL-6,† pg/ml | 25.8 (9.8–56.6) | 368.50 (138.5–1,448.5) | n.a. | <0.001 | n.a. |
| Severity of disease | |||||
| SOFA score | n.a. | 9 (8–11) | 9 (8–11) | n.a. | 0.551 |
| Outcome | |||||
| Length of hospital stay, d | 16 (10–26.5) | 31 (28–45) | 41 (25–66) | <0.001 | <0.05 |
| Death | 4 (4.3) | 11 (34.4) | 8 (29.6) | <0.001 | 0.581 |
| RAS Profiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 |
P Value |
|||||||||
| Nonsevere COVID-19 |
Severe COVID-19 |
Nonsevere: Early vs. Late COVID-19 | Severe: Early vs. Late COVID-19 | Early: Severe vs. Nonsevere COVID-19 | Late: Severe vs. Nonsevere COVID-19 | Severe COVID-19 vs. Influenza | ||||
| Days 0–3 (Early) | Days 9–11 (Late) | Days 0–3 (Early) | Days 9–11 (Late) | Comparator: Influenza | ||||||
| ACE2, ng/ml | 1.3 (1.0–2.4) | 3.2 (1.8–4.7) | 2.1 (1.3–3.0) | 15.1 (9.8–31.8) | 3.5 (2.3–6.6) | <0.001 | <0.001 | 0.078 | <0.001 | <0.001 |
| Angiotensin II, pmol/L | 47.7 (16.3–131.7) | 32.9 (9.3–78.2) | 165.7 (61.2–680.6) | 95.7 (37.5–250.0) | 114.4 (27.0–435.2) | 0.105 | 0.259 | <0.01 | <0.01 | 0.378 |
| Angiotensin 1–7, pmol/L | 1.5 (1.5–5.0) | 1.5 (1.5–3.7) | 10.8 (5.1–50.7) | 49.8 (14.6–132.0) | 21.0 (59–92.1) | 0.681 | 0.103 | <0.001 | <0.001 | 0.551 |
| Angiotensin-1–7–to–angiotensin-II ratio | 0.05 (0.03–0.15) | 0.10 (0.04–0.22) | 0.07 (0.04–0.13) | 0.31 (0.21–0.72) | 0.17 (0.10–0.47) | 0.129 | <0.001 | 0.545 | <0.001 | 0.671 |
Definition of abbreviations: ACE2 = angiotensin-converting enzyme 2; ACEi = ACE inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease; CRP=C-reactive protein; n.a. = not applicable; RAS = renin–angiotensin system; SOFA = Sequential Organ Failure Assessment.
Continuous variables are presented as medians (quartile 1–quartile 3); binary variables are presented as n (%). Baseline characteristics and medication were compared using Student’s t, χ2, and Fisher’s exact tests, when appropriate, whereas laboratory data and ACE2 and RAS metabolite concentrations were compared by Mann-Whitney U tests. RAS metabolites and ACE2 in influenza samples were compared with the average value across all available time points in patients with severe COVID-19. The influenza cohort was assembled retrospectively using serum samples obtained for clinical routine and stored at the Department of Virology of the Medical University of Vienna. Longitudinal sampling in individuals with influenza was therefore not available for RAS profiling, as samples were obtained at different time points during the course of their disease (ranging from Day 0 to Day 21 after hospitalization). Significant P values are given in bold.
First value.
Highest value. Regarding the comparisons between patients with influenza and those with severe COVID-19, note that these comparisons were done between single measurements per patient (in patients with influenza) and averages across all time points per patient (in patients with severe COVID-19).
Figure 1.
Systemic ACE2 (angiotensin-converting enzyme 2) concentration in coronavirus disease (COVID-19) over time stratified by severity of disease. ACE2 increased in patients with severe COVID-19, peaking during the Day 9–Day 11 time interval after hospitalization. On Day 10 of follow-up, 29 of all 32 patients with severe COVID-19 were still alive, and the respective renin–angiotensin system (RAS) data were included in RAS profile analysis, reducing a potential bias introduced by the overall high mortality rate in severe COVID-19 that could impact ACE2 and RAS metabolite trajectories. Continuous lines represent local regression curves with 95% local confidence intervals, whereas dashed lines represent individual patient-level data, with blue and red lines for nonsevere and severe COVID-19, respectively. Numbers at the bottom indicate number of patients available for analysis at each time point. ACE2 concentration is reported in ng/ml.
On the RAS metabolite level, we analyzed substrate (angiotensin II) and product (angiotensin 1–7) of ACE2. Early angiotensin II concentrations were significantly higher in patients with severe versus nonsevere COVID-19 (165.7 vs. 47.7 pmol/L; P < 0.01) but subsequently decreased in both groups. Simultaneously, angiotensin 1–7 concentrations increased from 10.8 pmol/L (early) to 49.8 pmol/L (late) in patients with severe COVID-19 (Table 1). The angiotensin-1–7–to–angiotensin-II ratio increased from 7% (early) to 31% (late) in patients with severe COVID-19, suggesting an increase in the formation of angiotensin 1–7 from angiotensin II. In patients with nonsevere COVID-19, we observed no statistically significant increase in alternative RAS metabolites.
ACE2 in patients with influenza was significantly lower compared with patients with severe COVID-19 but showed a similar time-dependent pattern, with higher values measured in samples obtained at later time points after intubation (2.4 ng/ml and 5.3 ng/ml for the Day 0–3 time interval and after Day 5, respectively; P < 0.05).
We used a mixed-effects model to identify factors associated with ACE2 (on the log scale) in COVID-19 (overall goodness of fit: r2 = 0.789). To account for the nonlinear time trends as well as the distinct behaviors observed in Figure 1, we introduced a term for severity of disease and linear and quadratic terms for time (i.e., days since hospitalization) as well as interaction terms for the time-dependent variables and severity of disease. In a parsimonious model, we further included patient sex and history of diabetes and hypertension on the basis of significant model improvements tested via likelihood ratio tests. Additional covariables that were tested but not selected for the final model were RAS-inhibitor comedication at time of hospitalization, treatment with corticosteroids, history of chronic obstructive pulmonary disease, and age (they did not improve the overall performance of the model).
Factors significantly associated with ACE2 trajectories were COVID-19 severity, days after hospitalization (linear and quadratic), and the interaction terms. The significant interactions supported the observation of increasing ACE2 in patients with severe COVID-19, with a subsequent decline, realized in the model via a linear positive effect for time (on antilog scale: factor, 1.42; 95% confidence interval [CI], 1.32–1.53 per day) and a nonlinear negative effect for square of time (factor, 0.99; 95% CI, 0.98–0.99). Additional factors that were associated with higher ACE2 were male sex (factor, 1.57; 95% CI, 1.18–2.09) and history of diabetes (factor, 1.42; 95% CI, 1.05–1.91). Among key laboratory parameters (IL-6, C-reactive protein, serum creatinine, and D-dimer), only IL-6 concentrations were significantly associated with ACE2 concentration in the mixed-effects model.
Regarding the influence of immunomodulatory therapy and antivirals, median ACE2 concentrations in patients treated with corticosteroids (compared with no corticosteroids) were 9.9 (interquartile range [IQR], 6.9–17.4) ng/ml and 11.3 (IQR, 6.4–18.1) ng/ml, respectively (P = 0.969). Median ACE2 concentrations in patients receiving antiinflammatory treatment with tocilizumab (compared with no tocilizumab) were 12.6 (IQR, 6.0–31.8) ng/ml and 10.6 (IQR, 7.1–17.2) ng/ml, respectively (P = 0.760). Antiviral treatment did not have a statistically significant impact on ACE2 concentrations or RAS metabolites (data not shown).
Discussion
In the present study, the main finding was a sevenfold increase in ACE2 in patients with severe COVID-19 from early to late time periods during their disease course. ACE2 was associated with IL-6, supporting a link with inflammation. Immunomodulatory treatment with corticosteroids or tocilizumab, however, did not have an impact on ACE2 concentrations.
The observed increase in ACE2 in severe COVID-19 was accompanied by an increase in the alternative RAS metabolite angiotensin 1–7 and an increase in the angiotensin-1–7–to–angiotensin-II ratio, indicating a shift toward the potentially protective alternative RAS. Angiotensin 1–7 signaling via the MAS receptor promotes antifibrosis and antagonizes angiotensin II–mediated effects such as vasoconstriction, fibrosis, and thrombogenesis (2).
Higher ACE2 concentrations later during the course of disease in the influenza cohort as well as previously published data on elevated ACE2 in patients with acute respiratory distress syndrome (8) point to a potentially uniform response in those with (virus-induced) severe lung injury. However, ACE2 in patients with severe COVID-19 was significantly higher compared with patients with influenza with similar disease severity based on the Sequential Organ Failure Assessment score (Table 1) and requirement for mechanical ventilation in both groups. Different intubation strategies may hamper direct comparability. Longitudinal analyses of changes in RAS enzyme activity and metabolites are therefore required to draw more definite conclusions on RAS (dys)regulation in other critically ill patients. Currently, systemic application of human recombinant soluble ACE2 is tested as a treatment strategy in severe COVID-19 (9).
Immunomodulatory treatment, antivirals, or RAS medication at the time of hospitalization did not have a statistically significant impact on ACE2, but numbers of patients in respective groups were small. Additional data are necessary to determine how different treatment strategies may impact ACE2 concentrations in COVID-19. A pronounced increase of ACE2 in severe COVID-19 was nevertheless observed across all groups. Systemic ACE2 in severe COVID-19 increased to concentrations that could directly affect systemic angiotensin concentrations, balancing the RAS toward the alternative axis. The observed increase in plasma ACE2 activity in severe COVID-19 may therefore reflect an inflammation-driven, pathophysiological mechanism aimed at counterbalancing an excess of angiotensin II.
Footnotes
Supported by the Austrian Science Fund (FWF grant KLI 861-B) and by the Medical-scientific fund of the Mayor of the federal capital Vienna (grant MA 40-GMWF-COVID027).
Originally Published in Press as DOI: 10.1164/rccm.202101-0142LE on February 18, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garvin MR, Alvarez C, Miller JI, Prates ET, Walker AM, Amos BK, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;9:e59177. doi: 10.7554/eLife.59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder C, Poglitsch M, Agibetov A, Duca F, Zotter-Tufaro C, Nitsche C, et al. Angs (angiotensins) of the alternative renin-angiotensin system predict outcome in patients with heart failure and preserved ejection fraction. Hypertension. 2019;74:285–294. doi: 10.1161/HYPERTENSIONAHA.119.12786. [DOI] [PubMed] [Google Scholar]
- 7.Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol. 2017;69:805–819. doi: 10.1016/j.jacc.2016.11.064. [DOI] [PubMed] [Google Scholar]
- 8.Krenn K, Höbart P, Poglitsch M, Croizé A, Ullrich R. Equilibrium angiotensin metabolite profiling in patients with acute respiratory distress syndrome indicates angiotensin-converting enzyme inhibition. Am J Respir Crit Care Med. 2020;202:1468–1471. doi: 10.1164/rccm.201912-2504LE. [DOI] [PubMed] [Google Scholar]
- 9.Zoufaly A, Poglitsch M, Aberle JH, Hoepler W, Seitz T, Traugott M, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]