Abstract
Rationale: Patients with severe coronavirus disease (COVID-19) require supplemental oxygen and ventilatory support. It is unclear whether some respiratory support devices may increase the dispersion of infectious bioaerosols and thereby place healthcare workers at increased risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Objectives: To quantitatively compare viral dispersion from invasive and noninvasive respiratory support modalities.
Methods: This study used a simulated ICU room with a breathing-patient simulator exhaling nebulized bacteriophages from the lower respiratory tract with various respiratory support modalities: invasive ventilation (through an endotracheal tube with an inflated cuff connected to a mechanical ventilator), helmet ventilation with a positive end-expiratory pressure (PEEP) valve, noninvasive bilevel positive-pressure ventilation, nonrebreather face masks, high-flow nasal oxygen (HFNO), and nasal prongs.
Measurements and Main Results: Invasive ventilation and helmet ventilation with a PEEP valve were associated with the lowest bacteriophage concentrations in the air, and HFNO and nasal prongs were associated with the highest concentrations. At the intubating position, bacteriophage concentrations associated with HFNO (2.66 × 104 plaque-forming units [PFU]/L of air sampled), nasal prongs (1.60 × 104 PFU/L of air sampled), nonrebreather face masks (7.87 × 102 PFU/L of air sampled), and bilevel positive airway pressure (1.91 × 102 PFU/L of air sampled) were significantly higher than those associated with invasive ventilation (P < 0.05 for each). The difference between bacteriophage concentrations associated with helmet ventilation with a PEEP valve (4.29 × 10–1 PFU/L of air sampled) and bacteriophage concentrations associated with invasive ventilation was not statistically significant.
Conclusions: These findings highlight the potential differential risk of dispersing virus among respiratory support devices and the importance of appropriate infection prevention and control practices and personal protective equipment for healthcare workers when caring for patients with transmissible respiratory viral infections such as SARS-CoV-2.
Keywords: COVID-19, critical care, simulation, bioaerosol, bacteriophage
At a Glance Commentary
Scientific Knowledge on the Subject
Patients with severe coronavirus disease (COVID-19) require supplemental oxygen and ventilatory support. It is unclear whether some respiratory support devices may increase the dispersion of infectious bioaerosols and thereby place healthcare workers at increased risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
What This Study Adds to the Field
Comparing viral dispersion from invasive and noninvasive respiratory support modalities, our findings highlight the potential differential risk of dispersing virus among respiratory support devices and the importance of appropriate infection prevention and control practices and personal protective equipment for healthcare workers when caring for patients with transmissible respiratory viral infections such as SARS-CoV-2.
The ongoing coronavirus disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in an unprecedented number of patients requiring hospitalization globally. Over one-fifth of people hospitalized with COVID-19 require supplemental oxygen, and up to 10–25% may require ICU admission and ventilatory assistance (1–4).
One of the major healthcare challenges of the COVID-19 pandemic is the safe delivery of respiratory support. The volume of patients needing advanced respiratory support has led to shortages of mechanical ventilators in some jurisdictions (5, 6). High-flow nasal oxygen (HFNO) therapy and noninvasive ventilation (NIV) have been widely used for patients with COVID-19 and may reduce the need for invasive ventilation (2, 7). However, bioaerosol dispersion in the environment, which may increase the risk of SARS-CoV-2 transmission to others, has been a concern when using HFNO and NIV (8, 9). During the severe acute respiratory syndrome outbreak in 2003, nurses caring for patients receiving NIV may have been at higher risk of infection (10). During the COVID-19 pandemic, the World Health Organization has recommended that HFNO and NIV should be used in selected patients suffering from hypoxemic respiratory failure with airborne precautions in place because of the uncertain potential to generate infectious aerosols (11). HFNO has emerged as a common therapy in one-third to two-thirds of critically ill patients with COVID-19 in Wuhan (China) and Lombardi (Italy) (2, 12–14).
There is an important need to investigate the infectious potential of viral bioaerosols by different modes of oxygen delivery and ventilatory support (15). A limited number of studies are available; these include models investigating nonviral particles, such as smoke, respiratory droplets, water, yeast, and bacteria, or bedside bacterial dispersion data from critically ill patients to investigate aerosol dispersion using noninvasive respiratory support systems (16–20). This lack of evidence about aerosol generation (21, 22) underscores the importance of quantitatively investigating viral dispersion/infectivity as a function of various respiratory treatment modalities in a critical care context.
Methods
Simulation Facility
A simulated negative-pressure ICU room with a patient bed (Hillrom) was established in a 4.5-m (length) × 3-m (width) × 2.75-m (height) space at Sunnybrook Health Sciences Centre. The room-ventilation parameters were adjusted to achieve a −2.5-Pa pressure difference through supply and return airflows of 3.7 and 7.3 m3/min, respectively, with 12 exhaust air exchanges/h. All exfiltrated air was high-efficiency particulate air (HEPA) filtered before entering the heating, ventilation, and air-conditioning system.
The patient simulator was developed to mimic a spontaneously breathing patient with mild-to-moderate respiratory distress and to exhale a constant breath-to-breath viral load. The design consisted of a SimMan Classic (Laerdal Medical) recommissioned mannequin with a head attached to a 2-L neoprene reservoir bag via a 15-mm connector to serve as the tracheobronchial tree. The esophagus was clamped to prevent inadvertent system leaks. The mannequin head closely replicated the anatomy of a male human head, inclusive of anatomical structures of the trachea, larynx, oropharynx, mouth, and nose that may contribute to flow patterns. The patient simulator was positioned in a standard hospital bed (Hillrom) with the head of the bed in the flat position.
An LTV 900 transport ventilator (Pulmonetic Systems, Inc.) with a HEPA filter was used to simulate patient breathing patterns. The ventilator allowed for continuous volume delivery. To mimic a spontaneously breathing patient with mild-to-moderate respiratory distress, the ventilator supplying air to the lung-reservoir bag was set to a respiratory rate of 29 breaths/min and an inspiratory-to-expiratory ratio of 1:2 (23). To ensure that set parameters matched what was delivered at the mannequin’s oropharynx, the model was calibrated with a ventilator tester (Performance Test System 2000 and BreathLab Performance Test System software [version 2.0, revision B]; Mallinckrodt Puritan Bennett) to confirm the absence of a flow leak in the system. The average and maximum airflow at the patient simulator’s mouth was measured to be 34 and 60 L/min, respectively.
The aerosolized DNA bacteriophage ϕX174 (HER 36) was used to model bioaerosol dispersion from the lower respiratory tract into the environment. To introduce the bacteriophage in the model’s lower respiratory tract, an in-line Aerogen Solo vibrating mesh nebulizer (Aerogen Inc.) was attached to the distal end of the ventilator’s outflow tubing immediately before the 2-L neoprene reservoir bag. Comparison of Aerogen nebulizers with an atomizer (model 9302; TSI Inc.) and a six-jet Collison nebulizer (BGI) showed a similar recovery yield for the bacteriophage ϕX174 (24). The bacteriophage solutions consisted of amplified bacteriophage lysate at a concentration of 1:27 in phosphate-buffered saline (PBS). Before each experiment, 6 ml of the bacteriophage solution at a viral concentration of approximately 108 plaque-forming units (PFU)/ml was added to the nebulizer.
A schematic of the experimental setup is presented in Figure E1 in the online supplement.
Experimental Design
Six different respiratory scenarios were examined. The first scenario examined invasive ventilation through an endotracheal tube with an inflated cuff connected to a mechanical ventilator (Puritan Bennett 980 ventilator; Medtronic) that used a Vt of 250 ml, a respiratory rate of 16 breaths/min, and an N99 HEPA filter attached to the expiratory port of the ventilator (control). This scenario examined invasive mechanical ventilation once the closed-loop system was established and did not examine the act of intubation itself. The second scenario examined helmet ventilation with a positive end-expiratory pressure (PEEP) valve (Subsalve), an oxygen–air mixer (MaxVenturi), gas-outlet pressure of 8 cm H2O, and an N99 HEPA filter placed upstream of the PEEP valve. The third scenario examined bilevel positive airway pressure (V60; Philips) ventilation with a leak calibration of <10 L/min between the mask and the mannequin’s face, an inspiratory/expiratory pressure of 10/6 cm H2O, an respiratory rate of 10, and an inspiratory time of 0.9 seconds. The fourth scenario examined the use of a nonrebreather face mask (Hi-Ox; Novus Medical) with an O2 flow rate of 15 L/min and an N99 HEPA filter. The fifth scenario examined the use of HFNO (AIRVO 2; Fisher and Paykel Healthcare) with a nasal cannula and a target flow rate of 40 L/min (37°C and 21% O2). The sixth scenario examined the use of nasal prongs (HCSU4514 soft-touch nasal oxygen cannula; Medline Industries, Inc.) with an O2 flow rate of 4 L/min. During the examination of HFNO, invasive ventilation, and helmet ventilation with a PEEP valve, an in-line heated humidifier (MR850; Fisher and Paykel Healthcare) was used to heat (to 37°C) and humidify the airflow. The environmental temperature and relative humidity were measured to be within 21.2–23.4°C and 41.4–64.2%, respectively, throughout all experiments.
In addition to these six scenarios, the use of a helmet operated with a mechanical ventilator (Puritan Bennett 980 ventilator; Medtronic) with a Vt of 250 ml and a respiratory rate of 16 breaths/min was also examined, and the results are presented separately in Tables E1–E3.
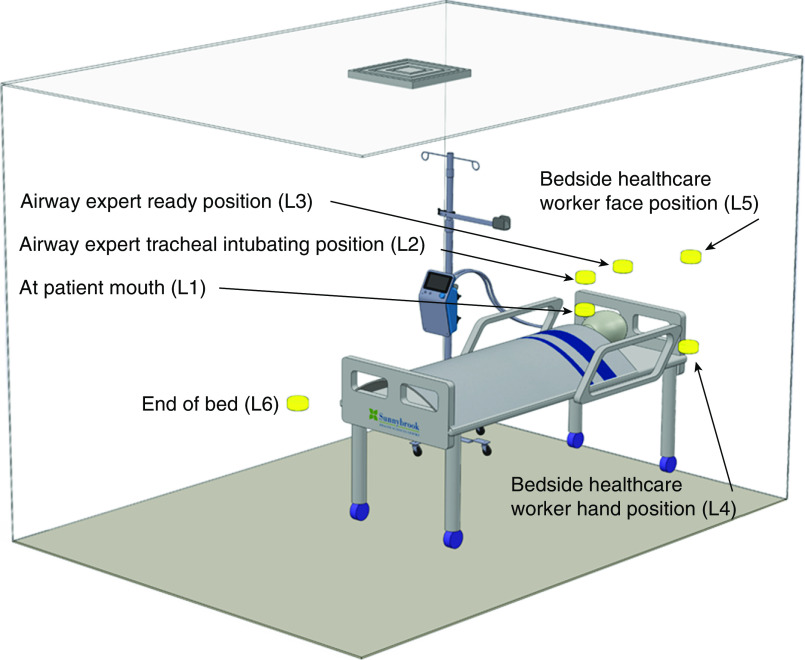
Six different locations were chosen to collect air samples. Locations 1–3 (10 cm above the level of the mouth, 40 cm vertically above the mouth, and 40 cm above and 30 cm behind the mouth, respectively) were chosen in close proximity to the patient mouth, where a healthcare worker may be located during aerosol-generating medical procedures such as endotracheal intubation. Locations 4 and 5 (100 cm above the ground and beside the bed and 150 cm above the ground and beside the bed) were representative of an assisting healthcare worker’s hands and face. Finally, location 6 (100 cm above the ground and 275 cm from the mouth) was toward the foot of the bed. See Figures 1 and E2 for the schematic of the simulation facility and clinical relevance of air-sampling locations.
Figure 1.
Schematic of the simulation facility and sensor positions. L1 is 10 cm above the level of the mouth, L2 is 40 cm vertically above the mouth (intubating position), L3 is 40 cm above and 30 cm behind the mouth (airway expert position), L4 is 100 cm above the ground and beside the bed (assistant hand position), L5 is 275 cm above the ground and beside the bed (assistant face position), and L6 is 100 cm above the ground and 180 cm from the mouth toward the foot of the bed. For the clinical relevance of L1–L6, also see Figure E1 in the online supplement. L1–L6 = locations 1–6.
Aerosol Sampling and Viral Particle Recovery
Air samples were collected using 37-mm cassettes (SKC Inc.) with 1.0-μm polytetrafluoroethylene filters for a duration of 10 minutes per location. The cassettes were connected to a constant-flow air-sampling pump (Gilian Gilair-5; Sensidyne Industrial Health and Safety Instrumentation). Pumps were calibrated (Gilian Gilibrator-3 calibrator; Sensidyne Industrial Health and Safety Instrumentation) before each experiment at a flow rate of 3.5 L/min. Two positive controls were taken from the nebulizer before and after each experiment to quantify the bacteriophage concentration in the nebulizer for all experiments. Two negative controls were taken at location 1 and location 6 before each experiment to ensure that no bacteriophage residue was present within the patient simulator or ambient environment. Collection of negative controls was conducted by sampling the air at these two locations for 10 minutes while nebulizing PBS. Immediately after the 10-minute air-sampling period concluded, 3 ml of trypticase soy broth (TSB) was added to each filter by removing the top cap of the polytetrafluoroethylene cassette and pipetting through the inlet. Then the top cap was placed back on and the cassette was placed on a vortex at maximum velocity for 2 minutes. The cassettes were rotated and flipped upside down during vortexing to ensure that all the viral particles that may have adhered to the sidewalls of the cassette during sampling were recovered.
Plaque Assay for Bacteriophage Quantification
Plaque assays were conducted to quantify the amount of viable bacteriophage recovered from each experiment (25). The bacteriophage ϕX174 (HER 36) and Escherichia coli (HER 1036) were used to perform plaque assays. ϕX174 and its E. coli host were obtained from the Félix d’Hérelle Reference Center for Bacterial Viruses (Laval University). Trypticase soy agar was used for the culture media. ϕX174 was propagated in TSB with its E. coli host at 37°C under agitation. Once an optical density at 600 nm reached 0.1, the bacteriophage was added to the culture broth and the incubation was left overnight. The bacteriophage lysate was then centrifuged at 3,500 revolutions/min for 15 minutes to remove any bacterial cells or debris. The bacteriophage-containing supernatant was then filtered using a 0.45-μm pore-size syringe filter and stored at −80°C. Bacteriophage amplifications produced approximately 3.0 × 109 PFU/ml, as determined by plaque assay. Plaque assays were conducted in duplicate using TSB, trypticase soy agar (1.5%), and TSB soft agar (0.7%).
Particle Count Measurements
To measure the concentration of 0.3-, 1.0-, 2.5-, and 5.0-μm particles (measured as the particle count/L of sampled air), an optical particle counter (Aerotek 9303; TSI Inc.) was used. Particle concentrations were measured before starting each experiment (as a baseline measurement and to ensure no residual particles remained in the room from a previous experiment) as well as at the end of each 10-minute nebulization.
Data Calculation
The bacteriophage concentration obtained from each air sampler was normalized by the average of the two positive control bacteriophage concentrations. For ease of comparison, the normalized value was multiplied by 108 before base-10 logarithmic transformations were performed. Raw data expressed in PFU/L of sampled air are presented in Table E1.
Statistical Analyses
For graphical presentation, bacteriophage concentrations were described using means and SDs after using logarithmic transformation. The Kruskal-Wallis test was used at each location to test whether any statistically significant difference exists among the six modes and whether there is an overall difference, and each mode was compared with invasive ventilation. No adjustment for multiple comparisons was made. SAS version 9.4 (SAS Institute Inc.) was used for all analyses. Comparisons were considered significant at P ≤ 0.05.
Results
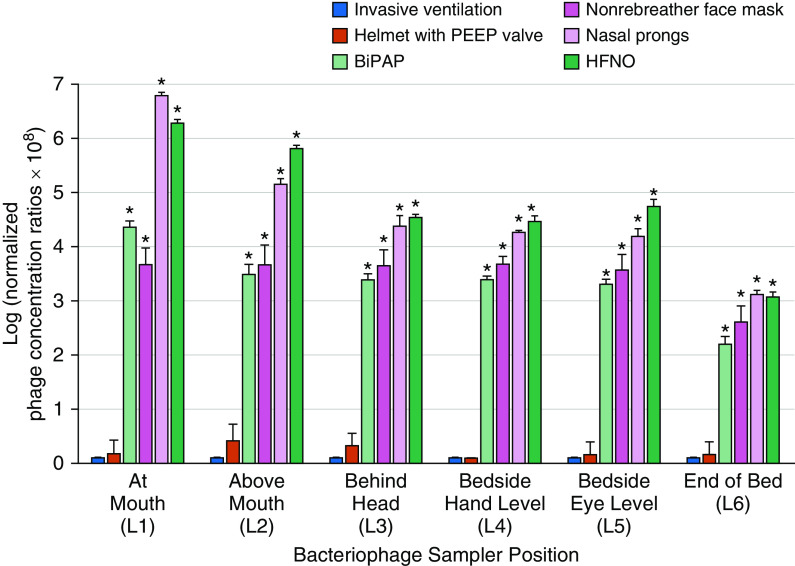
A summary of the results is presented in Figure 2. Bacteriophage concentrations and a summary of statistical analysis are presented in Tables E1–E3. The bacteriophage concentrations in the air varied substantially with respiratory support intervention and position (relative to the mouth) in the room. Across all locations, the lowest concentrations of the bacteriophage were achieved with closed-circuit invasive ventilation and noninvasive helmet ventilation with a PEEP valve. HFNO was associated with the highest bacteriophage concentration (with the exception of location 1). Generally, across the six locations, bacteriophage concentrations were highest at the mouth and above the mouth (location 1 and location 2) and lowest at the end of the bed (location 6).
Figure 2.
Logarithmic normalized bacteriophage concentration of six respiratory modalities at L1–L6. The asterisks represent the statistically significant modalities when compared with invasive ventilation at each location using the Kruskal-Wallis method (P < 0.05; a summary of pairwise comparisons is presented in Table E3 of the online supplement). Each experiment was run in triplicate (n = 3), and the error bars represent the SDs. BiPAP = bilevel positive airway pressure; HFNO = high-flow nasal oxygen; L1–L6 = locations 1–6; PEEP = positive end-expiratory pressure.
Discussion
One of the major considerations for the administration of suitable respiratory modalities for patients in ICUs is the risk associated with the generation and dispersion of infectious pathogens from patients’ airways to healthcare workers and other patients. In this simulated critical care setting, a quantitative approach was taken to compare the viral dispersion associated with six respiratory support interventions.
The results revealed that closed-circuit invasive ventilation and helmet ventilation with a PEEP valve were associated with the lowest amount of dispersion of the infectious bacteriophage and that use of HFNO and nasal prongs was associated with the highest amount of dispersion. In general, bacteriophage concentrations were highest at the mouth and nose of the patient simulator and decreased with increasing distance. These results highlight the importance of appropriate infection prevention and control practices and personal protective equipment for healthcare workers performing aerosol-generating medical procedures for patients with COVID-19 (26).
To date, only a few studies have addressed the potential exposure risk for healthcare workers performing aerosol-generating medical procedures using detection of the virus in the air surrounding a patient. Intubation is commonly cited as an aerosol-generating procedure (27). We demonstrated that after an endotracheal tube is placed in the airway, the cuff is inflated, the tube is connected to a closed-circuit system, and a filter is connected to the exhalation port of the ventilator, very little aerosolized bacteriophage material was dispersed throughout the room.
NIV using a face mask can reduce the need for invasive ventilation in selected patients with acute respiratory failure (acute respiratory distress syndrome). However, about half of patients with acute respiratory distress syndrome will require a transition from NIV to invasive ventilation because of intolerance to the device, worsening oxygenation or ventilation. An alternative strategy to face-mask NIV is to provide noninvasive support via a helmet (28, 29). The findings from the present work suggest that viral concentrations in the air around a patient treated with a helmet and a PEEP valve were very low, in agreement with other published literature (30, 31).
Only a few studies have previously considered the dispersion of viruses associated with HFNO in comparison with other oxygenation and ventilation support devices. This is of particular importance during the COVID-19 pandemic because of the frequency of HFNO use and the potential benefit in reducing the need for mechanical ventilation, and also in reducing the need for mechanical ventilators, which have been in short supply for healthcare facilities in areas of the world suffering the greatest number of infections (5, 6, 32).
In this study, the use of HFNO led to higher concentrations of the bacteriophage at key locations where healthcare workers are in contact with patients but was not associated with substantially more dispersion of the bacteriophage than oxygen delivery by nasal prongs. Previous studies of oxygen-support devices using an exhaled-smoke model have demonstrated variable distances of dispersion with HFNO, nonrebreather, and Venturi masks (17, 33), as have studies using water and yeast dispersion during HFNO use (18, 19). However, smoke, yeast, and water models may not reflect the behavior of viral pathogens (34).
The concentrations of the bacteriophage associated with the use of nasal prongs (at an O2 flow rate of 4 L/min) at all six locations were found to be higher than those associated with use of the nonrebreather face mask (at an O2 flow rate of 15 L/min). This interesting, yet significant, finding of the present study suggests that the flow rate may not be the only responsible parameter in the dispersion of infectious particles. This behavior is in agreement with the findings of other studies (30, 35) and highlights the importance of other factors (e.g., interface seal quality between the device and patient) in addition to airflow when considering infectious-particle dispersion in the patient environment.
Previous studies of exhaled breath and cough aerosols from patients with various respiratory infections have shown prominent similarities in aerosol size distributions, with a predominance of pathogens in small particles (i.e., <5 μm [36]). In a recent study, the median particle diameter generated from the respiratory tracts of 10 healthy individuals exposed to various modes of oxygen delivery, including nasal prongs, face masks, HFNO, and bilevel positive airway pressure, was reported to be within 1.01–1.53 μm (20). Hence, a vibrating mesh nebulizer that generates aerosols with a small particle size was deemed to be clinically relevant for the generation of the viral aerosol in our model.
Limitations and Future Directions
As with any simulation study, the present work has limitations. First, even though ϕX174 and SARS-CoV-2 are unrelated viruses with dissimilar structural biology that may lead them to behave differently during aerosolization, ϕX174 provides a model that mimics viruses like SARS-CoV-2 more closely than nonbiological tracers can. E. coli bacteriophages generally pose low risk to humans and require less biocontainment. Bacteriophages as models for human viruses have been extensively used in various workplace settings to understand the behavior of viruses under different experimental conditions (37–40), making them a feasible surrogate for SARS-CoV-2 in this study. Using a variety of nonpathogenic (to humans) bacteriophages with different features (e.g., enveloped and nonenveloped, RNA and DNA, single- and double-stranded) represents an additional line of investigation for future studies (36). Until then, these findings provide information to clinicians in critical care areas on the relative potential risks of aerosol dispersion with common respiratory support procedures. It is also important to underscore that experimentally generated, nebulized bioaerosols do not directly reflect the number and size distribution or infectivity of bioaerosols generated by patients with COVID-19. Second, the model lacks fluids or mucus in the patient simulator’s airway, which may change viral dispersion properties. Third, in this study, a negative-pressure room was used to mimic the realistic isolation room that would be used to house patients suffering from a highly transmissible infectious disease under airborne precautions. Hence, the results may not be generalized to a standard hospital room. Fourth, the experiments were performed in a simulated environment and therefore cannot encompass all dynamic aspects encountered during clinical care of patients (e.g., ventilator-circuit disconnects during noninvasive or invasive ventilation), which might increase the risk of viral dispersion. Fifth, the reservoir in which the bacteriophage was instilled was below the level of the endotracheal cuff, simulating more lower-respiratory-tract viral replication and not viral replication in the upper airways. Sixth, a single-limb circuit with NIV was used, whereas some guidelines recommend a dual-limb circuit when treating patients with COVID-19. Seventh, the present results do not imply that there is limited viral dispersion in all patients with COVID-19 who are intubated. These experiments do, however, provide a quantitative assessment of the relative risk of a surrogate pathogen dispersion by directly comparing various common respiratory support devices under controlled conditions to help clinicians and infection prevention and control practitioners with risk assessments. Eighth, throughout the experiments, the same medical mannequin head was used in the patient simulator; thus, the potential impacts of varying facial morphologies were not studied.
Conclusions
Invasive ventilation and helmet ventilation with a PEEP valve were associated with the lowest amount of dispersion of the infectious bacteriophage, and HFNO and nasal prongs were associated with the highest amount of dispersion, in a simulated critical care setting. These findings highlight the potential differential risk of the aerosolizing virus among respiratory support devices and the importance of appropriate infection prevention and control practices and personal protective equipment for healthcare workers when caring for patients with COVID-19.
Acknowledgments
Acknowledgment
The authors thank Marie-Ève Dubuis and Nathalie Turgeon for their help with bacteriophage nebulization and plaque-assay quantification. They also thank Roman Tymchal, Susan DeSousa, and Jose Cornejo for technical assistance during this study.
Footnotes
Supported by a postdoctoral fellowship from the Fond de Recherche du Québec–Nature et Technologie and the Laboratory Exchange Visitor Program Award from the Canadian Society for Virology (H.M.), an H. Barrie Fairley Professorship of Critical Care at the University Health Network and Interdepartmental Division of Critical Care Medicine (R.A.F.), the Sunnybrook Foundation and QuestCap (S.M.), and the Canadian Institute of Health Research Canadian Masters Graduate Scholarship (R.J.H.).
Author Contributions: R.A.F., H.M., and S.M. developed the concept. H.A. and A.A.R. developed the patient-simulation model. H.A., R.J.H., and A.A.R. designed the experiments. H.A. and R.J.H. performed aerosolization and air sampling. R.J.H. performed the plaque assays. H.A., R.A.F., and H.M. assisted in the interpretation of data. S.M. provided funding and supervised the entire project with the help of H.A., R.A.F., and H.M. H.M. wrote the original draft with help from H.A., R.J.H., and A.A.R. R.P. conducted the statistical analysis, and H.A. assisted in the development of analysis methodology. H.A., A.A.R., and H.M. produced the figures. H.A. and R.J.H. contributed equally as co–first authors. A.L., J.N., H.K.-J., P.K., and S.W.P. helped in the design of the experiment and data collection. All authors approved the final version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202008-3070OC on February 3, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [Published erratum appears in JAMA 323:2098.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [Published erratum appears in Lancet Respir Med 8:e26.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al. Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [Published erratum appears in Lancet Respir Med 8:e42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranney ML, Griffeth V, Jha AK. Critical supply shortages - the need for ventilators and personal protective equipment during the COVID-19 pandemic. N Engl J Med. 2020;382:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 7.Demoule A, Vieillard Baron A, Darmon M, Beurton A, Géri G, Voiriot G, et al. High-flow nasal cannula in critically III patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202:1039–1042. doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odor PM, Neun M, Bampoe S, Clark S, Heaton D, Hoogenboom EM, et al. Anaesthesia and COVID-19: infection control. Br J Anaesth. 2020;125:16–24. doi: 10.1016/j.bja.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler RA, Guest CB, Lapinsky SE, Sibbald WJ, Louie M, Tang P, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169:1198–1202. doi: 10.1164/rccm.200305-715OC. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva, Switzerland: World Health Organization; 2020. Clinical management of COVID-19. [accessed 2020 Jun 1]. Available from: https://www.who.int/publications-detail/clinical-management-of-covid-19. [Google Scholar]
- 12.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [Published erratum appears in Lancet 395:496.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Ehrmann S. High-flow aerosol-dispersing versus aerosol-generating procedures. Am J Respir Crit Care Med. 2020;202:1069–1071. doi: 10.1164/rccm.202008-3317ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui DS, Chow BK, Lo T, Tsang OTY, Ko FW, Ng SS, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53:1802339. doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 17.Kotoda M, Hishiyama S, Mitsui K, Tanikawa T, Morikawa S, Takamino A, et al. Assessment of the potential for pathogen dispersal during high-flow nasal therapy. J Hosp Infect. 2020;104:534–537. doi: 10.1016/j.jhin.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung CCH, Joynt GM, Gomersall CD, Wong WT, Lee A, Ling L, et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101:84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Loh NW, Tan Y, Taculod J, Gorospe B, Teope AS, Somani J, et al. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anaesth. 67:893–894. doi: 10.1007/s12630-020-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaeckle NT, Lee J, Park Y, Kreykes G, Evans MD, Hogan CJ., Jr Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am J Respir Crit Care Med. 2020;202:1115–1124. doi: 10.1164/rccm.202006-2309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kluytmans-van den Bergh MFQ, Buiting AGM, Pas SD, Bentvelsen RG, van den Bijllaardt W, van Oudheusden AJG, et al. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3:e209673. doi: 10.1001/jamanetworkopen.2020.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai X, Wang M, Qin C, Tan L, Ran L, Chen D, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3:e209666. doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.L’Her E, Deye N, Lellouche F, Taille S, Demoule A, Fraticelli A, et al. Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med. 2005;172:1112–1118. doi: 10.1164/rccm.200402-226OC. [DOI] [PubMed] [Google Scholar]
- 24.Turgeon N, Toulouse MJ, Martel B, Moineau S, Duchaine C. Comparison of five bacteriophages as models for viral aerosol studies. Appl Environ Microbiol. 2014;80:4242–4250. doi: 10.1128/AEM.00767-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panec M, Katz DS. Washington, DC: American Society for Microbiology; 2010. Plaque assay protocols. [accessed 2020 Mar 12]. Available from: https://www.asmscience.org/content/education/protocol/protocol.3073. [Google Scholar]
- 26.Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from asymptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11:940. doi: 10.3390/v11100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315:2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duca A, Memaj I, Zanardi F, Preti C, Alesi A, Della Bella L, et al. Severity of respiratory failure and outcome of patients needing a ventilatory support in the emergency department during Italian novel coronavirus SARS-CoV2 outbreak: preliminary data on the role of Helmet CPAP and non-invasive positive pressure ventilation. EClinicalMedicine. 2020;24:100419. doi: 10.1016/j.eclinm.2020.100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferioli M, Cisternino C, Leo V, Pisani L, Palange P, Nava S. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev. 2020;29:200068. doi: 10.1183/16000617.0068-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui DS, Chow BK, Lo T, Ng SS, Ko FW, Gin T, et al. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest. 2015;147:1336–1343. doi: 10.1378/chest.14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells CR, Fitzpatrick MC, Sah P, Shoukat A, Pandey A, El-Sayed AM, et al. Projecting the demand for ventilators at the peak of the COVID-19 outbreak in the USA. Lancet Infect Dis. 2020;20:1123–1125. doi: 10.1016/S1473-3099(20)30315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ip M, Tang JW, Hui DS, Wong AL, Chan MT, Joynt GM, et al. Airflow and droplet spreading around oxygen masks: a simulation model for infection control research. Am J Infect Control. 2007;35:684–689. doi: 10.1016/j.ajic.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One. 2010;5:e15100. doi: 10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hui DSC, Chan MTV, Chow B. Aerosol dispersion during various respiratory therapies: a risk assessment model of nosocomial infection to health care workers. Hong Kong Med J. 2014;20:9–13. [PubMed] [Google Scholar]
- 36.Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8:914–924. doi: 10.1016/S2213-2600(20)30323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubuis M-E, Dumont-Leblond N, Laliberté C, Veillette M, Turgeon N, Jean J, et al. Ozone efficacy for the control of airborne viruses: bacteriophage and norovirus models. PLoS One. 2020;15:e0231164. doi: 10.1371/journal.pone.0231164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turgeon N, Michel K, Ha T-L, Robine E, Moineau S, Duchaine C. Resistance of aerosolized bacterial viruses to Four germicidal products. PLoS One. 2016;11:e0168815. doi: 10.1371/journal.pone.0168815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verreault D, Marcoux-Voiselle M, Turgeon N, Moineau S, Duchaine C. Resistance of aerosolized bacterial viruses to relative humidity and temperature. Appl Environ Microbiol. 2015;81:7305–7311. doi: 10.1128/AEM.02484-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vyskocil JM, Turgeon N, Turgeon J-G, Duchaine C. Ozone treatment in a wind tunnel for the reduction of airborne viruses in swine buildings. Aerosol Sci Technol. 2020;54:1471–1478. [Google Scholar]