Despite advances in the treatment of idiopathic pulmonary fibrosis (IPF), it remains a progressive and ultimately fatal disease. Barriers to expedited development of new treatments include costs, prolonged trial duration, and difficulty determining treatment response. Given the natural history of ongoing disease progression in IPF, deciphering treatment response for an individual patient is challenging if not impossible. Change in FVC over 12 months is the currently accepted efficacy endpoint for IPF clinical trials. Biomarkers of early treatment response are lacking. A validated measure of treatment response in IPF would 1) improve clinical trial feasibility by enabling early determination of treatment efficacy, thereby decreasing overall trial duration; and 2) improve patient care by enabling treatment plans to be tailored to an individual patient. The proposed use of collagen-targeted positron emission tomography (PET) imaging as a novel biomarker of IPF treatment response was the winner of the 2020 American Thoracic Society Building Education to Advance Research (BEAR) Cage Innovation Award.
What Can Collagen-targeted PET Offer to IPF?
Excessive deposition of collagen is the hallmark of fibrosis. Current measures of monitoring IPF, such as pulmonary function tests and computed tomography (CT), detect the physiologic and structural changes resulting from collagen deposition; however, visualization of collagen has, in the past, required histology. This proposal hinges on the application of 68Ga-CBP8 to IPF. 68Ga-CBP8 is the first collagen-targeted PET probe to be translated into humans. 68Ga-CBP8 binds with high specificity to type I collagen, thereby enabling noninvasive direct collagen visualization and quantification (1, 2). In mouse models of fibrosis, this probe detected and staged the degree of collagen and measured response to therapy with an anti-αvβ6 antibody (1). In explanted lung tissue, the degree of probe uptake highly correlated with the amount of lung collagen. We have performed the first-in-human studies of this probe in healthy volunteers and participants with IPF (3). 68Ga-CBP8 displays rapid renal clearance and minimal background uptake in the lungs of healthy volunteers. When compared with healthy volunteers, probe uptake was significantly increased in the lungs of participants with IPF, consistent with increased collagen deposition. When compared with CT images, areas of high PET signal were visualized both in fibrotic regions as well as in areas of lung parenchyma that appeared normal. This suggests that 68Ga-CBP8 can detect areas of disease activity prior to the development of CT-apparent structural damage. Further research is ongoing to determine the sensitivity of this probe to detect early changes reflective of fibrosis compared with CT and if the degree of PET signal associates with disease progression as determined by pulmonary function testing.
Collagen-targeted PET as a Surrogate Marker of IPF Treatment Response
I proposed to use 68Ga-CBP8 to assess for early treatment response to antifibrotic therapies (Figure 1). There are several reasons why 68Ga-CBP8 is primed for this application. First, reducing collagen deposition is the current goal of antifibrotic therapies. Therefore, changes in the degree of 68Ga-CBP8 uptake could be a true surrogate marker of drug effect. Second, 68Ga-CBP8 appears to be more sensitive to binding recent collagen than to older collagen (Figure 2). For example, in mouse models, minimal uptake is detected in skin and bone, both of which contain high amounts of type I collagen. Although specific for type I collagen, the probe’s affinity for collagen is not high, and thus it is more apt to bind during states in which the rate of collagen deposition is high. Collagen that is less organized, thus less mature, has more binding sites available to the probe. I proposed to perform 68Ga-CBP8 PET imaging before and 3 months after initiation of U.S. Food and Drug Administration–approved antifibrotic therapies to determine if treatment reduced 68Ga-CBP8 uptake in the lungs. The degree of change in probe uptake over 3 months would be compared with change in FVC over 12 months, as the goal is to develop a marker of disease response that outperforms the current clinical measure. Although the first step would be a single center study, there are several aspects that make large-scale translation across multiple centers feasible. First, PET measurements can be quantified in a standardized manner through standardized uptake values. Although we have performed 68Ga-CBP8 PET with magnetic resonance imaging, it could easily be performed using PET-CT scanners, which are widely available. Second, although 68Ga-CBP8 is not commercially available at the current time, the radiochemistry protocol is a simple one-step process that involves adding 68GaCl3 to the CBP8 precursor. 68Ga is easily acquired, and many large academic centers have a 68Ga generator. Third, based on the probe’s half-life, PET imaging can occur 1 hour after injection, thereby minimizing inconvenience to patients. Lastly, no requirements exist for fasting prior to probe injection, as is the case with the clinically utilized 18F-fluorodeoxyglucose probe. The main limitation of PET imaging is cost; however, if 68Ga-CBP8 could inform as to treatment efficacy, or lack thereof, it could potentially be cost saving by sparing a patient from a costly medication that is determined not to provide any disease-modifying benefit.
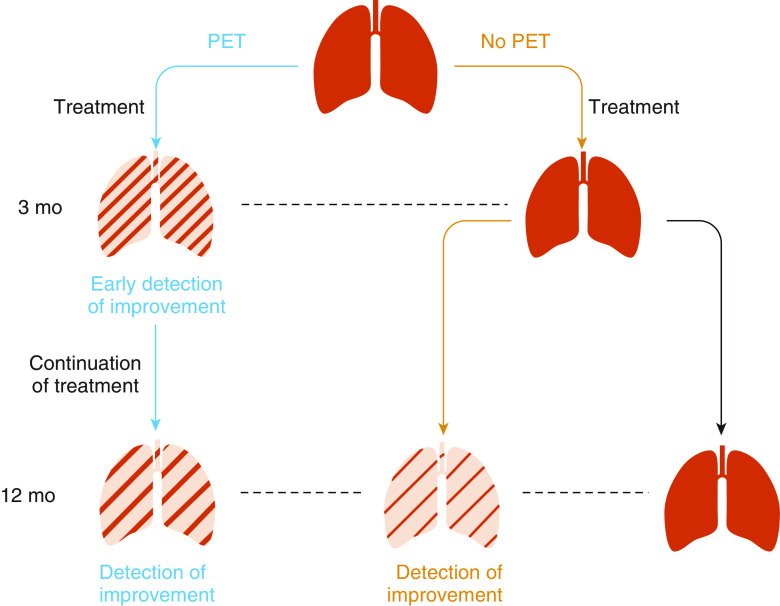
Figure 1.
Proposed use of 68Ga-CBP8 positron emission tomography (PET) to detect treatment response in idiopathic pulmonary fibrosis. PET detects changes with treatment earlier than conventional methods of monitoring. PET information can be used to guide treatment decisions at an early time point. With conventional methods of monitoring, treatment effect is not detected until later, thereby precluding an opportunity for early modification of the treatment plan if needed. Note this is a hypothetical scenario, and these studies have not been performed. Adapted by permission from Reference 4.
Figure 2.
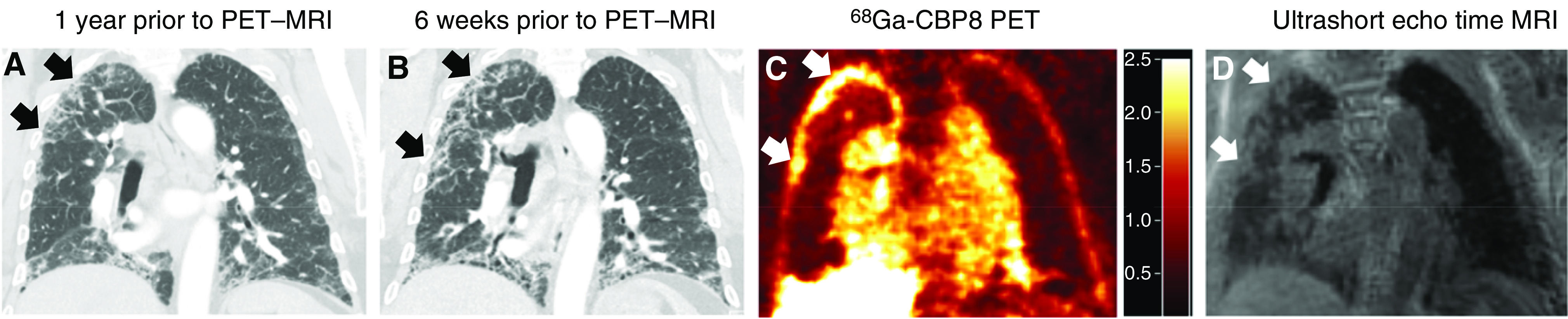
68Ga-CBP8 positron emission tomography (PET) in pulmonary fibrosis. (A) Computed tomography (CT) performed 1 year prior to PET–magnetic resonance imaging (MRI). (B) CT performed 6 weeks prior to PET–MRI. Note the progression of interstitial fibrosis on the CT over time (arrows). (C) 68Ga-CBP8 PET demonstrates areas of high probe uptake in peripheral portions of the right upper lobe (arrows), corresponding to reticulation and ground-glass opacity (interstitial fibrosis) seen on ultrashort echo time MRI (D). The high probe uptake (C) is seen predominately in regions of progressive fibrosis in the peripheral right upper lobe. The uptake in the heart and vasculature as well as in the liver is to be expected based on the probe’s half-life and biodistribution. Scale bar denotes standardized uptake values for C.
Ongoing Clinical Translation and Additional Areas of Application
We have already performed the first-in-human studies of this probe under an investigational new drug (IND) application with no safety concerns identified (clinicaltrials.gov identifier NCT: 03535545). Early data from short-interval test–retest imaging in a limited number of subjects with IPF appear promising, and should these results be confirmed, we should be sensitive to detect small changes in probe uptake with treatment. The next steps would be a single-center study to determine if 68Ga-CBP8 uptake in the lungs is reduced with IPF treatment, followed by a multicenter study to determine if 68Ga-CBP8 PET can detect IPF treatment response before current clinical measurements (i.e., FVC). The ultimate goal is to develop a validated surrogate endpoint to be used in clinical trials and clinical care. Whether 68Ga-CBP8 may add value as part of the diagnostic evaluation for other ILD states, such as early disease or acute exacerbations, remains to be determined. This work builds on an expanding foundation of molecular imaging as applied to fibrosis (4, 5). As collagen deposition is the hallmark of pulmonary fibrosis and fibrosing diseases in general, 68Ga-CBP8 may have wide-ranging applicability to other fibrotic lung diseases and other types of organ fibrosis.
The BEAR Cage Innovation Award
Currently in its sixth year, the BEAR Cage Competition, sponsored by the American Thoracic Society Drug Device Discovery and Development Committee, provides early career investigators the opportunity to pitch a new technology as an innovative solution to a pressing human health need. Finalists receive feedback from members of academia and industry, opening doors for further project development and collaborations. The winner of the BEAR Cage Innovation Award benefits from dedicated mentorship with the goal of accelerating clinical translation and, ultimately, impacting patient care.
Acknowledgments
Acknowledgment
The author thanks Joel Moss, Craig Conoscenti, and Mark Forshag of the American Thoracic Society Drug Device Discovery and Development Committee and Peter Caravan, Iris Zhou, Subba Digumarthy, and Rachel Knipe of Massachusetts General Hospital for their input into this piece.
Footnotes
S.B.M. is supported by the NHLBI (K23 HL150331) and the Scleroderma Foundation. Funding has been received for this work from the American Thoracic Society.
Originally Published in Press as DOI: 10.1164/rccm.202010-3994ED on February 5, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Désogère P, Tapias LF, Hariri LP, Rotile NJ, Rietz TA, Probst CK, et al. Type I collagen-targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci Transl Med. 2017;9:eaaf4696. doi: 10.1126/scitranslmed.aaf4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Désogère P, Tapias LF, Rietz TA, Rotile N, Blasi F, Day H, et al. Optimization of a collagen-targeted PET probe for molecular imaging of pulmonary fibrosis. J Nucl Med. 2017;58:1991–1996. doi: 10.2967/jnumed.117.193532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montesi SB, Izquierdo-Garcia D, Désogère P, Abston E, Liang LL, Digumarthy S, et al. Type I collagen-targeted positron emission tomography imaging in idiopathic pulmonary fibrosis: first-in-human studies. Am J Respir Crit Care Med. 2019;200:258–261. doi: 10.1164/rccm.201903-0503LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montesi SB, Désogère P, Fuchs BC, Caravan P. Molecular imaging of fibrosis: recent advances and future directions. J Clin Invest. 2019;129:24–33. doi: 10.1172/JCI122132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Désogère P, Montesi SB, Caravan P. Molecular probes for imaging fibrosis and fibrogenesis. Chemistry. 2019;25:1128–1141. doi: 10.1002/chem.201801578. [DOI] [PMC free article] [PubMed] [Google Scholar]