Abstract
Rationale: Symptoms and morbidities associated with obstructive sleep apnea (OSA) vary across individuals and are not predicted by the apnea–hypopnea index (AHI). Respiratory event duration is a heritable trait associated with mortality that may further characterize OSA.
Objectives: We evaluated how hypopnea and apnea durations in non-REM (NREM) sleep vary across demographic groups and quantified their associations with physiological traits (loop gain, arousal threshold, circulatory delay, and pharyngeal collapsibility).
Methods: Data were analyzed from 1,546 participants from the Multi-Ethnic Study of Atherosclerosis with an AHI ≥5. Physiological traits were derived using a validated model fit to the polysomnographic airflow signal. Multiple linear regression models were used to evaluate associations of event duration with demographic and physiological factors.
Measurements and Main Results: Participants had a mean age ± SD of 68.9 ± 9.2 years, mean NREM hypopnea duration of 21.73 ± 5.60, and mean NREM apnea duration of 23.87 ± 7.44 seconds. In adjusted analyses, shorter events were associated with younger age, female sex, higher body mass index (P < 0.01, all), and Black race (P < 0.05). Longer events were associated with Asian race (P < 0.01). Shorter event durations were associated with lower circulatory delay (2.53 ± 0.13 s, P < 0.01), lower arousal threshold (1.39 ± 0.15 s, P < 0.01), reduced collapsibility (−0.71 ± 0.16 s, P < 0.01), and higher loop gain (−0.27 ± 0.11 s, P < 0.05) per SD change. Adjustment for physiological traits attenuated age, sex, and obesity associations and eliminated racial differences in event duration.
Conclusions: Average event duration varies across population groups and provides information on ventilatory features and airway collapsibility not captured by the AHI.
Keywords: obstructive sleep apnea, sleep, epidemiology, phenotype, MESA
At a Glance Commentary
Scientific Knowledge on the Subject
Obstructive sleep apnea (OSA) is characterized by repetitive airway collapse leading to sleep fragmentation and hypoxia. The most common disease-defining metric, the apnea–hypopnea index, incompletely describes OSA pathology and severity. Measurements providing information on physiological mechanisms are needed to phenotype individuals with sleep apnea. The duration of apnea and hypopnea events, measured in standard polysomnographic records, has been shown to be a heritable OSA trait and to predict mortality. Nevertheless, little is known about its variation in the population or its specific association with mechanistic traits.
What This Study Adds to the Field
Although respiratory event duration is measured routinely with polysomnography, no prior study has examined its distribution across demographic groups, or its association with major health conditions or physiological traits. We characterized the distribution of the durations of non-REM hypopnea and apnea events in a large, diverse community-based cohort who underwent standardized polysomnography. Adjusted analyses showed that shorter events are found in women, and in individuals who are younger, more obese, and of Black race, whereas longer events are found in those of Asian heritage. Respiratory event duration also associates with physiological traits that reflect underlying physiological subphenotypes, including markers of chemoreflexes that characterize OSA. These findings suggest that event duration, reflecting mechanisms associated with chemoreflex/arousal responses, may help improve phenotyping of OSA and identify subgroups that could benefit from targeted treatment of nonanatomical traits.
Obstructive sleep apnea (OSA) is a heterogeneous disorder characterized by intermittent pharyngeal obstruction causing recurrent desaturations and arousals. Untreated OSA is associated with increased mortality (1), cardiovascular disease (CVD) (2), and vehicular motor crashes (3), but these outcomes can substantially vary between individuals. Although the apnea–hypopnea index (AHI) is commonly used to quantify the severity of OSA, this frequency measurement provides an incomplete descriptor of physiological stressors and has variable association with symptom burden, outcomes, or treatment adherence (4). Therefore, there is a need to identify markers that better characterize physiological variability and can inform risk stratification.
An initial step toward this goal is to better characterize the underlying pathophysiology of each patient and quantify the contributions of anatomic (i.e., upper airway collapsibility) and nonanatomic factors (i.e., arousal threshold and ventilatory control system sensitivity such as loop gain) to disease severity (5–8). The contribution of these physiologic traits to OSA varies among individuals by age, sex, and body mass index (BMI) (9–11). These traits are beginning to be used to test targeted interventions (12). Advanced signal analysis has been validated to estimate these traits from polysomnogram (PSG) data (13, 14), but extraction and analysis require additional processing not routinely available in clinical laboratories.
Respiratory event duration may be a useful parameter for improving OSA phenotyping because it can provide an integrated output of the major pathophysiology physiologic traits that characterize OSA (15, 16). It varies widely in patients with similar AHI levels, suggesting different information content than AHI (17). Event duration also has higher heritability than the AHI (18). Genome-wide association studies identified several genetic variants that associate with event duration, including ARRB1 that regulates hypoxic-inducible factor-A, and PLCB1, which is highly expressed in the brain and associated with insomnia (19). We also recently showed that, paradoxically, shorter respiratory events are associated with mortality, for reasons that remain unclear (20). These data suggest that event duration, a measurement currently extracted from polysomnograms, is an inherited trait that may help characterize OSA subtypes.
To better understand the clinical utility of event duration, it is necessary to understand its variation across the population and characterize the physiological basis for this variation. The aims of our study, therefore, were to investigate how event durations vary with demographic factors, obesity, physiologic traits, and routine polysomnography metrics in a large, diverse community cohort. We focused on non-REM (NREM) events as these are most reflective of the metabolic respiratory control system and algorithms measuring the physiological traits of interest have been validated in NREM sleep. Some of the results of these studies have been previously reported in the form of an abstract (21).
Methods
Sample Characteristics
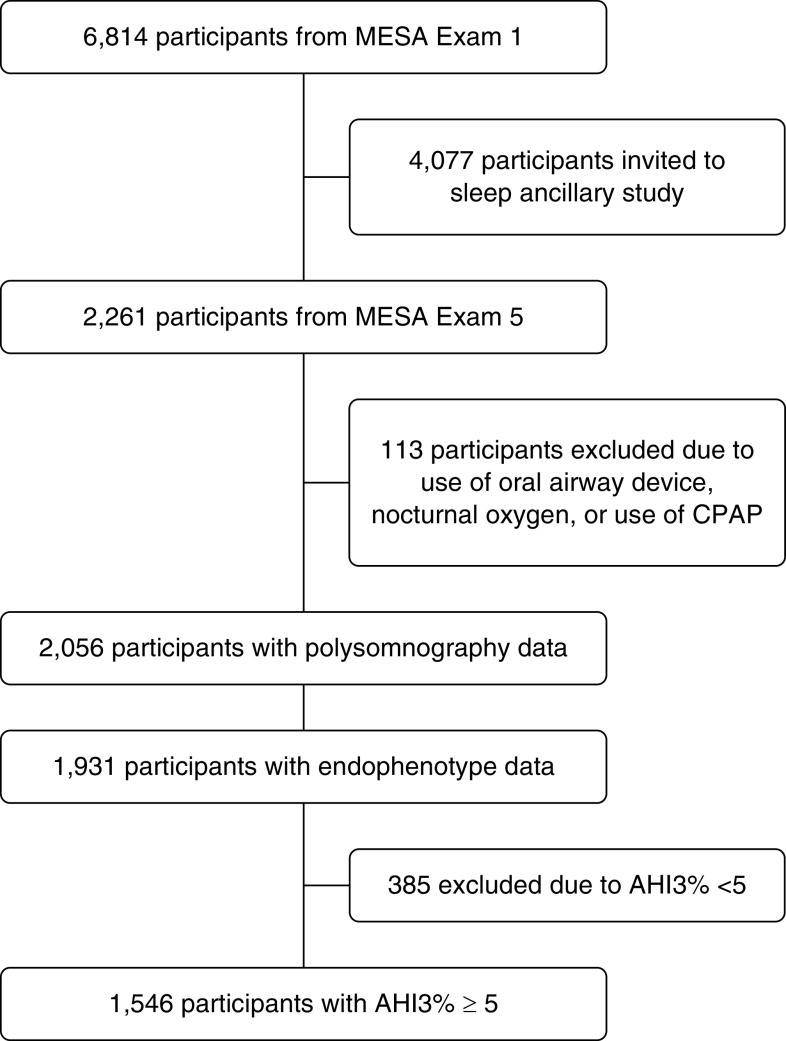
Data were derived from MESA (Multi-Ethnic Study of Atherosclerosis), a prospective cohort study designed to investigate risk factors for CVD. At baseline (exam 1; 2000–2002), 6,814 men and women, ages 45–84 years without clinically recognized CVD, were recruited and followed longitudinally with serial assessments. During exam 5 (2010–2013), a total of 2,261 participated in the sleep exam, which included polysomnography and questionnaires as detailed (22). Of these, 1,931 had PSG with adequate data to derive physiological traits. Analyses were limited to participants with AHI ≥5 to ensure sufficient events to calculate event duration (n = 1,546; Figure 1). The institutional review board at each site approved the study. All participants provided written informed consent.
Figure 1.
Subject selection. AHI3% = the sum of all apneas and hypopneas, each associated with ≥3% desaturation divided by total sleep duration; CPAP = continuous positive airway pressure; MESA = Multi-Ethnic Study of Atherosclerosis.
Sleep Protocol
Participants underwent in-home PSG using a 15-channel monitor, which were scored by research polysomnologists at a centralized reading center (22). Apneas were defined as >90% airflow reduction of thermocouple signal from preevent baseline for ≥10 seconds. Hypopneas were defined as ≥30% airflow reduction of the nasal pressure or inductance plethysmography signals of the preevent baseline for ≥10 seconds. The AHI was the sum of apneas and hypopneas, each associated with ≥3% desaturation, divided by sleep time.
Event duration was measured based on a “trough to trough” marking on the thermistor or flow signal. Time was measured between when the first reduced breath was identified to the breath preceding the end of each respiratory event. Inter- and intrascorer reliability for event duration were 0.91 and 0.89, respectively.
Symptoms of insomnia and excessive daytime sleepiness were assessed using the Women’s Health Initiative Insomnia Rating Scale (23) and Epworth Sleepiness Scale (24), respectively.
Physiologic Traits
A validated model fit to the polysomnographic airflow signal (14) was used to estimate loop gain (magnitude of reflex ventilatory drive response to a change in ventilation; dimensionless) (25), circulatory delay (latency between ventilation drop and increased ventilatory drive) (25), arousal threshold (level of ventilatory drive that causes arousal from sleep) (26), and collapsibility (Vpassive; ventilation occurring at eupneic ventilatory drive; lower values reflect greater collapsibility) (27).
We measured cardiac and arousal responses to respiratory events based on the heart rate response to respiratory events (ΔHR) and arousal intensity (ArI), respectively (28, 29), expressed in relationship to each individual’s mean area under the desaturation curve (ΔSpO2, defined as the total area under the respiratory event–related desaturation curve divided by the number of respiratory events) (30). Normalized mean heart rate (ΔHR/ΔSpO2) and arousal intensity (ArI/ΔSpO2) indicate cardiac/arousal sensitivity or responsiveness to respiratory-related hypoxemia.
Covariates
Sociodemographic characteristics and health habits, including age, sex, smoking status (current, former, or never), and race and ethnicity (Black, Asian [predominantly Chinese], or White and/or Hispanic), were self-reported. BMI was derived from measured height and weight. The definitions of comorbidities are provided in the online supplement.
Statistical Analysis
Analyses were performed using SAS (v. 9.3; SAS Institute). Associations were evaluated using ANOVA, Spearman correlations, and multivariable linear regression models with event duration as the outcome. To account for heteroscedasticity of average event duration, we used weighted least squares, where each observation was weighted by the inverse of its variance, estimated by the squared outcome. A series of models were fit to understand associations with demographic factors, smoking status, and BMI (model 1) and these factors plus physiological traits (models 2–6). We examined hypopnea and obstructive apnea duration separately.
Results
The cohort consisted of older men and women, representing each of the race and ethnicity groups sampled (Table 1). The majority of participants were hypertensive. A large proportion had impaired fasting glucose or diabetes (43%). The prevalence of heart failure (3.3%) and chronic obstructive pulmonary disease (COPD) (1.5%) was low. Average lung function was within normal limits. The average AHI was in the moderate range (AHI 24.4 ± 18.4). Average Epworth Sleepiness Scale was within the nonsleepy range (6.2 ± 4.2; normal <11). Mean apnea duration was approximately 2 seconds longer than hypopnea duration.
Table 1.
Sample Characteristics
| Demographics | Frequency (%) or Mean ± SD | Sleep Characteristics | Mean ± SD |
|---|---|---|---|
| Age, yr | 68.9 ± 9.2 | AHI3% (events/h) | 24.4 ± 18.4 |
| Sex, M | 780 (50.5) | NREM AHI3% (events/h) | 21.7 ± 19.6 |
| Race/ethnicity | REM AHI3% (events/h) | 36.3 ± 21.8 | |
| Non-Hispanic white | 557 (36.0) | OAI (events/h) | 5.0 ± 8.9 |
| Asian | 189 (12.2) | % Sleep time <90% oxygen saturation | 4.7 ± 9.6 |
| Black | 422 (27.3) | Oxygen desaturation index at 3% | 26.4 ± 18.4 |
| Hispanic | 378 (24.5) | Oxygen desaturation index at 4% | 17.6 ± 16.4 |
| Body mass index, kg/m2 | 29.3 ± 5.5 | Hypoxic burden | 68.39 ± 67.67 |
| Smoking use | Total sleep time (min) | 358.8 ± 80.1 | |
| Current smoker | 109 (7.1) | Sleep efficiency (%) | 75.4 ± 13.2 |
| Former smoker | 720 (46.8) | Stage N1 (%) | 15.4 ± 9.6 |
| Never smoked | 708 (46.1) | Stage N2 (%) | 57.2 ± 10.3 |
| Hypertension | 901 (58.4) | Stage N3 (%) | 9.7 ± 8.8 |
| Diabetes | 656 (42.9) | % Time in REM | 17.6 ± 6.7 |
| Heart failure | 51 (3.3) | ||
| FVC, % predicted | 96.2 ± 17.5 | Epworth sleepiness scale (0–24) | 6.2 ± 4.2 |
| FEV1, % predicted | 95.0 ± 19.2 | Women's Health Initiative Insomnia Rating scale | 7.4 ± 4.4 |
| Antidepressant use | 110 (9.8) | NREM hypopnea, seconds | 21.73 ± 5.60 |
| Sedative use | 218 (14.1) | NREM obstructive apnea, seconds | 23.87 ± 7.44 |
Definition of abbreviations: AHI3% = the sum of all apneas and hypopneas, each associated with ≥3% desaturation divided by total sleep duration; NREM = non-REM; OAI = obstructive apnea index.
Oxygen desaturation index: the average number of desaturation episodes per hour. Desaturation episodes were defined as a decrease in the mean oxygen saturation by either ≥3 or ≥4% (over the last 120 s) that lasts for at least 10 seconds. Hypoxic burden: respiratory event–associated area under the desaturation curve from preevent baseline.
Relationship of Respiratory Event Duration to Demographics, Prevalent Health Conditions, and Polysomnogram Characteristics
The variation of NREM hypopnea and apnea durations by demographics and comorbidities is summarized in Table 2. In unadjusted analyses, hypopnea duration was shorter in association with younger age, female sex, higher BMI, current smoking, and COPD. Black participants had the shortest hypopnea durations (20.4 ± 5.4 s), whereas Asians had the longest durations (24.3 ± 6.2 s, P < 0.001). Event duration did not vary with the presence of hypertension, diabetes, heart failure, or use of sedatives or hypnotics. Similar associations were observed for NREM apnea variation, except that apnea duration was not associated with smoking status.
Table 2.
Variation of NREM Event Duration by Demographics and Comorbidities (N = 1,998)
| NREM Hypopnea Duration |
NREM Obstructive Apnea Duration |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Mean ± SD | Spearman's ρ | P Value | Mean ± SD | Spearman's ρ | P Value |
| Age, yr | 0.16 | <0.001 | 0.20 | <0.001 | ||
| <65 | 20.8 ± 4.9 | 22.2 ± 6.4 | ||||
| ≥65 | 22.3 ± 5.9 | 24.8 ± 7.8 | ||||
| BMI, kg/m2 | −0.33 | <0.001 | −0.23 | <0.001 | ||
| <30 | 22.8 ± 5.8 | 24.8 ± 7.8 | ||||
| ≥30 | 20.0 ± 4.7 | 22.3 ± 6.5 | ||||
| Sex | — | <0.001 | — | <0.001 | ||
| M | 23.2 ± 5.9 | 25.4 ± 7.4 | ||||
| F | 20.3 ± 4.9 | 22.1 ± 7.1 | ||||
| Race/ethnicity | — | <0.001 | — | <0.001 | ||
| Non-Hispanic white | 21.9 ± 5.5 | 23.8 ± 7.7 | ||||
| Asian | 24.3 ± 6.2 | 27.1 ± 8.5 | ||||
| Black | 20.4 ± 5.4 | 22.3 ± 6.4 | ||||
| Hispanic | 21.7 ± 5.2 | 23.9 ± 7.0 | ||||
| Smoking status | — | <0.001 | — | 0.084 | ||
| Current | 19.6 ± 4.7 | 24.0 ± 0.6 | ||||
| Former | 21.6 ± 5.7 | 23.9 ± 7.3 | ||||
| Never | 22.1 ± 5.5 | 22.0 ± 6.1 | ||||
| Comorbidities | ||||||
| Hypertension | — | 0.232 | — | 0.887 | ||
| Presence | 21.6 ± 5.7 | 23.9 ± 7.7 | ||||
| Absence | 21.9 ± 5.4 | 23.8 ± 7.0 | ||||
| Impaired fasting glucose or diabetes | — | 0.329 | — | 0.461 | ||
| Presence | 21.6 ± 5.3 | 24.0 ± 7.3 | ||||
| Absence | 21.8 ± 5.8 | 23.7 ± 7.6 | ||||
| Heart failure | — | 0.879 | — | 0.580 | ||
| Presence | 21.6 ± 5.4 | 24.5 ± 7.5 | ||||
| Absence | 21.7 ± 5.6 | 23.8 ± 7.4 | ||||
| COPD | — | 0.026 | — | 0.003 | ||
| Presence | 19.3 ± 4.2 | 20.3 ± 4.2 | ||||
| Absence | 21.8 ± 5.6 | 23.9 ± 7.5 | ||||
| Sedative use | — | 0.211 | — | 0.143 | ||
| Presence | 21.3 ± 5.3 | 23.1 ± 6.9 | ||||
| Absence | 21.8 ± 5.6 | 24.0 ± 7.5 | ||||
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; NREM = non-REM.
Relationship of Event Duration to Physiological Traits and Sleep Characteristics
The variations of NREM hypopnea and apnea durations with physiologic traits are shown in Table 3. Shorter hypopnea duration was associated with higher loop gain, lower arousal threshold, shorter circulatory delay, and less-severe collapsibility (higher Vpassive). Similar associations were observed after adjusting for demographic factors, BMI and smoking, and apnea duration, although a slightly stronger association was observed between shorter apnea duration and less-severe collapsibility.
Table 3.
Estimated Differences in Hypopnea and Apnea Duration (in Seconds) per SD Change in Physiologic Traits
| Characteristic | NREM Hypopnea Duration |
NREM Obstructive Apnea Duration |
||||
|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 1 |
Model 2 |
Model 3 |
|
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Loop gain (sensitivity) | −1.04 (0.13)* | −0.80 (0.12)* | −0.27 (0.11)† | −0.61 (0.21)* | −0.38 (0.19)† | −0.36 (0.20) |
| Arousal threshold (%eupnea) | 1.97 (0.14)* | 1.48 (0.13)* | 1.39 (0.15)* | 2.37 (0.22)* | 1.91 (0.21)* | 1.92 (0.25)* |
| Circulatory delay, s | 2.81 (0.13)* | 2.17 (0.13)* | 2.53 (0.13)* | 3.04 (0.21)* | 2.28 (0.22)* | 3.10 (0.21)* |
| Collapsibility per Vpassive‡ (%eupnea) | −1.89 (0.16) | −1.43 (0.14)* | −0.71 (0.16)* | −2.16 (0.23)* | −1.72 (0.22)* | −0.95 (0.25)* |
Definition of abbreviation: NREM = non-REM.
Model 1: unadjusted. Model 2: adjusted for age, sex, BMI, race, and smoking status. Model 3: adjusted for other three physiologic traits.
P < 0.01.
P < 0.05.
Higher Vpassive indicates less-severe collapsibility; thus, longer events are associated with more severe collapsibility.
Table E1 in the online supplement illustrates how event duration varies with polysomnographic measures and sleep symptoms with and without adjustment for age, sex, race, BMI, and smoking. NREM hypopnea and apnea duration increased in association with indices of more severe frequent respiratory events, with the strongest association observed between event duration and hypoxic burden (r = 0.26, P < 0.02). Durations increased with greater time in stages N1 and N3 sleep and decreased with higher sleep efficiency and more time in N2 and REM sleep. No significant associations were observed with the sleepiness or insomnia scale scores in adjusted analyses, although unadjusted analyses showed a weak negative association with the Insomnia Rating Scale with hypopnea duration (r = −0.075; P < 0.01).
Results of Multivariable Models Predicting NREM Hypopnea Duration
To understand the extent to which demographic factors and physiological traits are associated with hypopnea duration, a sequence of models was run with various adjustments (Table 4). Model 1 (hypopnea duration as outcome) was adjusted for age, sex, BMI, race and ethnicity, and smoking. Models 2–5 were adjusted for physiological traits in addition to the covariates included in model 1. Model 6 was a fully adjusted model, adjusting all covariates and physiological traits.
Table 4.
Multivariate Linear Regression of the Associations between NREM Hypopnea Duration and Sample Characteristics
| Model 1 [β (SE)] | + Loop Gain [β (SE)] | + Arousal Threshold [β (SE)] | + Circulatory Delay [β (SE)] | + Collapsibility [β (SE)] | + All Physiologic Traits [β (SE)] | |
|---|---|---|---|---|---|---|
| Intercept | 20.62 (0.24) | 20.45 (0.23) | 21.08 (0.23) | 20.80 (0.22) | 21.08 (0.23) | 21.19 (0.20) |
| Age, yr | 0.65 (0.13)* | 0.71 (0.13)* | 0.61 (0.12)* | 0.40 (0.12)* | 0.53 (0.13)* | 0.41 (0.11)* |
| Sex | ||||||
| M | 2.38 (0.25)* | 2.56 (0.25)* | 1.57 (0.25)* | 1.91 (0.23)* | 1.60 (0.25)* | 0.99 (0.21)* |
| F | REF | REF | REF | REF | REF | REF |
| Race | ||||||
| Non-Hispanic white | REF | REF | REF | REF | REF | REF |
| Asian | 1.34 (0.46)* | 1.43 (0.45)* | 0.80 (0.44)† | 1.20 (0.42)* | 0.73 (0.44)† | 0.49 (0.38) |
| Black | −0.79 (0.31)‡ | −0.52 (0.31)† | −0.81 (0.29)* | −0.43 (0.28) | −0.71 (0.30)‡ | −0.04 (0.26) |
| Hispanic | 0.14 (0.33) | 0.13 (0.32) | 0.07 (0.31) | 0.01 (0.29) | 0.02 (0.31) | −0.02 (0.26) |
| BMI, kg/m2 | −1.18 (0.13)* | −1.00 (0.13)* | −1.19 (0.12)* | −0.46 (0.12)* | −1.30 (0.12)* | −0.50 (0.11)* |
Definition of abbreviations: BMI = body mass index; NREM = non-REM; REF = reference.
Weighted least-squares regression analysis, with weights taken to be the inverse of the squared outcome, estimating observation-specific variance. Model 1: age, sex, BMI, race, and smoking status. Model 2: model 1 + loop gain. Model 3: model 1 + arousal threshold. Model 4: model 1 + circulatory delay. Model 5: model 1 + collapsibility (per Vpassive; lower values represent greater collapsibility). Model 6: model 1 + loop gain, arousal threshold, circulatory delay, passive collapsibility threshold. n = 1,532 in all models.
P < 0.01.
P < 0.10.
P < 0.05.
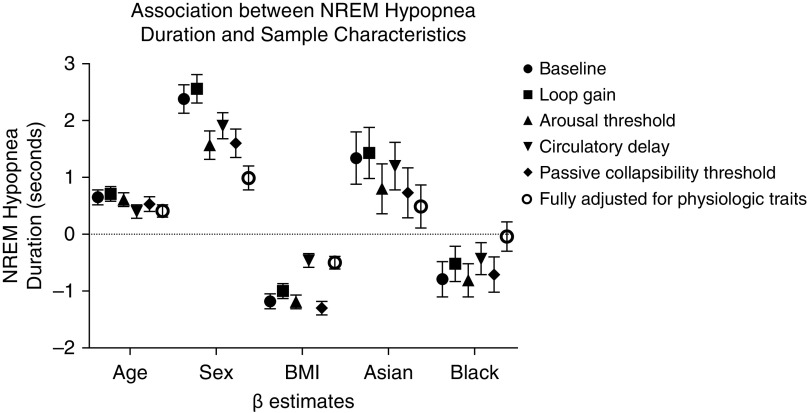
In adjusted models that did not include physiological traits, shorter hypopnea duration was associated with younger age, female sex, higher BMI, and Black race; Asian race was associated with longer events (Table 4). When adjusting for loop gain, the magnitude of association of duration modestly decreased with BMI (15% reduction) and Black race (21% reduction), but the associations with Asian race, age, and sex increased. When adjusting for arousal threshold, the magnitude of the associations with sex and Asian race decreased by 34% and 40%, respectively. When adjusting for circulatory delay, the association with age decreased by 39%, with sex by 20%, with Black race by 46%, and BMI by 61%. Adjusting for passive collapsibility decreased the sex association by 33% and the association with Asian race by 46%; other associations changed by less than 15%. When all physiologic traits were considered together, association with age, sex, and BMI persisted but were attenuated. Race associations were no longer significantly associated with hypopnea duration when all physiological traits were included in the model. Figure 2 illustrates the β estimates for each of the demographic variables after adjustments for physiologic traits. Similar findings were found in unweighted analyses (see Table E2) for comparison. Similar patterns were observed when obstructive apneas were modeled (see Table E2).
Figure 2.
Association between NREM hypopnea duration and sample characteristics. Physiologic traits were derived from polysomnographic data. β estimates (±SE) of age (yr), male sex, body mass index (kg/m2), Asian ethnicity compared with white, and Black race compared with white are plotted after normalization of each physiologic trait. BMI = body mass index; NREM = non-REM.
We explored associations between event duration with two novel metrics of cardiac autonomic and arousal responsiveness to event-related desaturation (ΔHR/ΔSpO2, ArI/ΔSpO2).
Shorter apneas were associated with greater cardiac autonomic responsiveness (apnea duration vs. ΔHR/ΔSpO2; Spearman correlation coefficient: −0.23, N = 1,017, P < 0.0001) and greater arousal responsiveness (apnea duration vs. ArI/ΔSpO2; Spearman −0.45, respectively (N = 1,013, P < 0.0001). Similar associations were found between these metrics and hypopnea duration (vs. ΔHR/ΔSpO2, Spearman: −0.21, N = 1,328, P < 0.0001; vs. ArI/ΔSpO2, Spearman: −0.38, N = 1,307, P < 0.0001).
Discussion
A step toward improving the personalization of therapy is to better characterize each patient’s underlying disease pathophysiology (6, 8). Recent data indicate that event duration has a strong heritable component (18) and predicts mortality (20), suggesting that this parameter may help characterize OSA subtypes and risk stratification. Although event duration is readily measurable using routine polysomnography and home sleep apnea tests, it is rarely considered in routine clinical practice or in epidemiological studies, possibly because of the limited understanding of the distribution of event duration with clinical and physiological correlates in the population. In this study, we report novel data describing the variation of respiratory event durations during NREM sleep with age, sex, race, BMI, smoking, health conditions, physiological traits, and standard polysomnography measures in a large, diverse community sample. In unadjusted and adjusted analyses, we show that shorter event duration is associated with younger age, female sex, higher BMI, and Black race, whereas longer event duration is associated with Asian race. Validated physiological endotypes derived from polysomnography are also associated with event duration; shorter event duration is associated with decreased arousal threshold, decreased circulatory delay, increased loop gain, and less-severe pharyngeal collapsibility. Once we considered these physiological traits, the associations with demographic factors and BMI were attenuated, suggesting that much of the population variation in event duration is explained by measurements capturing chemoreflexes, respiratory arousal threshold, and collapsibility. In addition, the contributions of these physiological mechanisms varied across race/ethnic groups. Taken together, these data suggest significant population variability in event duration, which is partly explained by several physiological mechanisms.
Associations with Physiological Traits
Events were shorter in association with shorter circulatory delay, lower arousal threshold, less-severe collapsibility, and increased loop gain. Thus, shorter events, in general, are seen in conjunction with a greater responsiveness to (i.e., reduced tolerance of) pharyngeal airway obstruction; a shorter chemoreflex time and higher loop gain may reflect a more brisk response to chemoreflex stimuli, and the lower arousal threshold may reflect an earlier, more sensitive cortical arousal response to rising chemical (hypoxic and/or hypercapnic) stimuli. Greater physiological responsiveness is also supported by the finding that shorter events were associated with greater cardiac autonomic and arousal responses to event-related desaturations. Our findings are consistent with prior literature that show that higher arousal threshold is associated with longer event durations (26) and increased circulation time in heart failure is associated with longer events (31). The observed association between shorter events and milder collapsibility in the current study is intriguing because physiologically a more severe obstruction should lead to earlier event termination. It is possible that earlier event termination (lower arousal threshold) may prevent the progression of more complete collapse; alternatively, more severe collapse over time may promote adaptative mechanisms that reduce arousability and lengthen respiratory events (32). Notably, the lack of a single predominant mechanism that correlates with event duration suggests that event duration is a compound trait that encapsulates more than a single physiological mechanism, providing aggregate information on several ventilatory features and collapsibility not routinely captured using traditional PSG data.
Sex Differences
There is increasing interest in understanding why men and women with OSA differ in symptoms, polysomnographic findings, and susceptibility to cardiovascular disease. Women have lower AHI measured in NREM but not REM sleep (33), and they are less likely to experience deep desaturation with respiratory events but more likely to experience cortical arousals (34) and insomnia symptoms (35). Our data show that respiratory events are significantly shorter in women than in men. These findings are consistent with a prior study that reported that women with OSA had an apnea duration approximately 3 seconds shorter than that in men with OSA (36). Our findings in a large, community-based sample not selected for OSA extend these findings, showing that such associations are independent of age, BMI, and race and are partly explained by differences in arousal threshold, circulatory delay, and collapsibility (10). The latter physiological differences are consistent with laboratory studies that have demonstrated that women have less-severe pharyngeal collapsibility compared with men, particularly in NREM sleep (37, 38). In analyses unadjusted for sex, we also found that shorter event duration was associated with more insomnia symptoms. Our findings provide support for the existence of a sleep apnea phenotype more common in women than men that is characterized by short respiratory event duration, likely due to increased arousability to respiratory stimuli, and possibly, autonomic hyperresponsiveness. Given the recent finding of increased mortality observed in individuals with shorter respiratory events (20), and findings of an inverse association between event duration and cardiac and arousal responses to desaturation, short event duration may be a marker of augmented autonomic activity and may help identify individuals who may benefit from strategies to dampen hyperreflexive autonomic, chemoreflex, and/or arousal responses to obstruction (e.g., increasing the arousal threshold).
Obesity Associations
Obesity increases risk of OSA severity through multiple pathways, including direct effects of upper airway fat deposition on nasopharyngeal patency and collapsibility, and in reducing lung volumes. We observed a negative association between event duration and BMI that was attenuated after adjusting for circulatory delay and loop gain, suggesting that abnormal chemoreflexes may partly mediate obesity-associated ventilatory responses. Alternatively, shortened delays could be due to elevated (nocturnal) Q̇, a consequence of the heightened metabolic demand of obesity, or an activated sympathetic nervous system (39, 40). The shorter event duration in more obese individuals may reflect an underlying propensity for adverse outcomes, as discussed for females. However, adverse events in obese individuals with OSA are likely due to multiple factors, some of which may relate to the underlying physiological characteristics associated with airway obstruction, and others related to independent pathways that increase morbidity and mortality.
Age Associations
In this community cohort, event duration increased with older age. The association between apnea duration and advanced age has been reported in a clinical cohort (41). The predominant mechanism that explained the association between age and event duration in our analysis was circulatory delay, which was positively associated with event duration. An increase in circulatory delay with age may be due to age-associated blunting of chemoreflexes (42). Because long events result in more severe event-associated hypoxemia, which contributes to cardiovascular morbidity (30), there is a potential for a bidirectional and amplifying mechanism whereby individuals with delayed circulatory times and reduced Q̇ have longer events that predispose to hypoxemia and further cardiac dysfunction. However, compared with older individuals, OSA has been reported to be more strongly associated with CVD in younger individuals (43), whom we find to have the shortest respiratory events. Our findings raise the possibility that a mechanism apart from event-related hypoxemia, reflected by shorter events and increased cardiac autonomic and arousal sensitivity to event-related desaturations, may contribute to the cardiovascular morbidity observed in younger and middle-aged individuals.
Race/Ethnicity Associations
Racial and ethnic differences in OSA have been reported in multiple prior studies (44, 45) and appear to relate to genetic factors (46, 47) and environmental factors (45). A prior study from MESA showed that individuals of Asian background have the highest age- and BMI-adjusted AHI levels compared with other racial or ethnic groups (44). Additionally, Asians had the most marked increase in AHI with incremental increases in adiposity. This may suggest more propensity for OSA, possibly due to craniofacial structural factors (44). The current analysis further shows that Asians have longer event durations compared with the other race/ethnic groups. However, this difference was eliminated after considering the physiological endotypes, particularly arousal threshold and collapsibility. These findings identify an OSA phenotype in Asians that relates to reduced arousability and increased collapsibility, suggesting that Asians may be predisposed to hypoxemic burden owing to long events. Treatments that raise arousal threshold further may be ineffective or detrimental.
In contrast, in age-, sex-, BMI-, and smoking-adjusted analyses, Black participants had shorter respiratory events compared with the other groups. This association was attenuated after accounting for loop gain and circulatory delay. This suggests that chemosensitivity may underlie a propensity for early event termination in Black individuals. The marked differences in event duration between racial and ethnic groups suggest that the pathophysiology of OSA likely varies across population groups. This may correspond to differences in responsiveness to alternative treatments.
Other Factors
We also explored other factors that may influence the associations between event duration and demographic and anthropometric factors. Surprisingly, we did not observe differences in event duration by cardiometabolic conditions; the underlying population differences may outweigh the effects of these comorbidities in this community-based sample studied when in stable health. Shorter event duration was associated with COPD and current smoking, suggesting that nicotine or sleep fragmentation may reduce the arousal threshold, raise chemosensitivity, and/or reduce collapsibility. We previously observed this same relationship between current smoking and reduced event duration in an independent cohort (20), and our recent work suggests that that active smoking lowers the arousal threshold (S. A. Sands and colleagues, unpublished results). Self-reported sedative use was not associated with event duration, although it was associated with an increased arousal threshold. Age-, sex-, and BMI-adjusted associations with traditional polysomnography variables showed weak associations with event duration, with some evidence that event durations were longer in association with a higher AHI, greater hypoxemia, a higher arousal index, more N1 and N3, and less N2 and REM sleep.
Strengths and Limitations of the Study
This study is the first to comprehensively assess the clinical and pathophysiological determinants of event duration, a readily accessible polysomnographic measurement, in a large community sample. We studied a diverse cohort, allowing assessments across sex, race and ethnicity, and a wide range of AHI and BMI. Rigorous collection of sleep data and covariates allowed us to evaluate both PSG variables and other health and exposure variables.
Several limitations should be noted. This is a cross-sectional study, and temporal and selection biases may occur. The sample was predominantly older, and we could not assess factors associated with menopause. A strength and limitation of the current study is that traits and event duration are taken from the same inherent signals data. Although these measurements are therefore not completely independent, arousal threshold and collapsibility are both determined by measurements relating to the amplitude of ventilation and ventilatory drive (respectively) rather than the timing and expected to be largely independent of event duration. Loop gain and circulatory delay are derived from the chemical drive response to airflow obstruction; thus, longer events (and attendant increase in time for drive to build) could feasibly influence measured loop gain and delay. However, we emphasize that any dependencies between loop gain or delay and event duration are considered authentic rather than spurious; indeed, supplemental oxygen raises obstructive event duration while lowering loop gain and increasing delay (25). Also, event durations track the shorter circulatory delays at high altitude (48) and longer delays in patients with heart failure (31).
The physiologic traits were based on a noninvasive method and, although validated, may not as accurately quantify physiological mechanisms as laboratory-based assessments. Because of this, muscle responsiveness was not investigated. However, use of noninvasive calculations (vs. invasive measures) is expected to yield random error or misclassification, reducing the observed effects (type II error) rather than enhancing them (type I error) or introducing bias. Additionally, this study is limited to NREM events as the algorithm used to assess physiologic traits has not been validated in REM. Future research is needed to validate physiologic traits in REM and explore if REM-associated event durations have similar associations. Assessment of the effects of medications was limited by use of self-report data and absence of information specifically on opioids. Finally, the extent to which shorter event duration is a marker versus a mediator of adverse outcomes could not be assessed. In particular, additional research is needed that comprehensively assesses nocturnal cardiac and autonomic nervous system activity in association with event duration and other physiological traits.
In summary, this study shows that NREM respiratory event durations demonstrate characteristic variations with age, obesity, sex, and race and ethnicity and that these associations are partially influenced by physiologic traits. Women and Black individuals have the shortest events, whereas Asian and older individuals have the longest events. Although measuring event duration may not provide information on a single specific mechanism, its measurement may help identify individuals likely to have low or high autonomic, arousal, and/or chemoreflex responses. This may help identify patients that are more or less likely to respond to interventions that target different ventilatory control mechanisms (49, 50). Event duration, as described before (20), may also have prognostic implications, although the competing influences of phenotypes characterized by hyperarousal (short event) compared with increased hypoxic burden (long event) need to be carefully studied in prospective studies. Further research should evaluate if event duration and associated physiological endotypes help define subgroups of patients with the most deleterious clinical outcomes or differential responses to therapy.
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the NHLBI, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. This research was also supported by NHLBI grants HL098433, HL110350, K23 KL094760, HL113338, R21HL140377, R35HL135818, R24HL11447, and AHA15SDG25890059, the American Thoracic Society ASPIRE (Academic Sleep Pulmonary Integrated Research/Clinical) Fellowship, and the Sleep Research Society Career Development Award 018-JP-18.
Author Contributions: P.V.B., T.S., M.P.B., H.W., A.W., S.A.S., and S.R. designed the research and analyzed data. P.V.B., A.W., S.A.S., and S.R. interpreted the data and wrote the manuscript. M.R., T.S., A.A., H.W., and S.A.S. analyzed data.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202005-1808OC on December 7, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 2.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 3.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–581. [PMC free article] [PubMed] [Google Scholar]
- 4.Bosi M, De Vito A, Kotecha B, Viglietta L, Braghiroli A, Steier J, et al. Phenotyping the pathophysiology of obstructive sleep apnea using polygraphy/polysomnography: a review of the literature. Sleep Breath. 2018;22:579–592. doi: 10.1007/s11325-017-1613-3. [DOI] [PubMed] [Google Scholar]
- 5.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens RL, Edwards BA, Eckert DJ, Jordan AS, Sands SA, Malhotra A, et al. An integrative model of physiological traits can be used to predict obstructive sleep apnea and response to non positive airway pressure therapy. Sleep (Basel) 2015;38:961–970. doi: 10.5665/sleep.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanchina M, Robinson K, Corrao W, Donat W, Sands S, Malhotra A. Clinical use of loop gain measures to determine continuous positive airway pressure efficacy in patients with complex sleep apnea: a pilot study. Ann Am Thorac Soc. 2015;12:1351–1357. doi: 10.1513/AnnalsATS.201410-469BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73:472–480. doi: 10.1136/thoraxjnl-2017-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards BA, Wellman A, Sands SA, Owens RL, Eckert DJ, White DP, et al. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep (Basel) 2014;37:1227–1236. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Won CH, Reid M, Sofer T, Azarbarzin A, Purcell S, White D, et al. Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis. Sleep. 2020;43:zsz274. doi: 10.1093/sleep/zsz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leppänen T, Kulkas A, Mervaala E, Töyräs J. Increase in body mass index decreases duration of apneas and hypopneas in obstructive sleep apnea. Respir Care. 2019;64:77–84. doi: 10.4187/respcare.06297. [DOI] [PubMed] [Google Scholar]
- 12.Edwards BA, Redline S, Sands SA, Owens RL. More than the sum of the respiratory events: personalized medicine approaches for obstructive sleep apnea. Am J Respir Crit Care Med. 2019;200:691–703. doi: 10.1164/rccm.201901-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) 2013;114:911–922. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakayama H, Kobayashi M, Tsuiki S, Yanagihara M, Inoue Y. Obstructive sleep apnea phenotypes in men based on characteristics of respiratory events during polysomnography. Sleep Breath. 2019;23:1087–1094. doi: 10.1007/s11325-019-01785-8. [DOI] [PubMed] [Google Scholar]
- 16.Leppänen T, Kulkas A, Oksenberg A, Duce B, Mervaala E, Töyräs J. Differences in arousal probability and duration after apnea and hypopnea events in adult obstructive sleep apnea patients. Physiol Meas. 2018;39:114004. doi: 10.1088/1361-6579/aae42c. [DOI] [PubMed] [Google Scholar]
- 17.Muraja-Murro A, Nurkkala J, Tiihonen P, Hukkanen T, Tuomilehto H, Kokkarinen J, et al. Total duration of apnea and hypopnea events and average desaturation show significant variation in patients with a similar apnea-hypopnea index. J Med Eng Technol. 2012;36:393–398. doi: 10.3109/03091902.2012.712201. [DOI] [PubMed] [Google Scholar]
- 18.Liang J, Cade BE, Wang H, Chen H, Gleason KJ, Larkin EK, et al. Comparison of heritability estimation and linkage analysis for multiple traits using principal component analyses. Genet Epidemiol. 2016;40:222–232. doi: 10.1002/gepi.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cade BE, Chen H, Stilp AM, Gleason KJ, Sofer T, Ancoli-Israel S, et al. Genetic associations with obstructive sleep apnea traits in Hispanic/Latino Americans. Am J Respir Crit Care Med. 2016;194:886–897. doi: 10.1164/rccm.201512-2431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler MP, Emch JT, Rueschman M, Sands SA, Shea SA, Wellman A, et al. Apnea-hypopnea event duration predicts mortality in men and women in the sleep heart health study. Am J Respir Crit Care Med. 2019;199:903–912. doi: 10.1164/rccm.201804-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borker PV, Mariani S, Weng J, Sands SA, Sofer T, Butler MP, et al. Apnea hypopnea duration: epidemiological and physiological correlates [abstract] Am J Respir Crit Care Med. 2018;197:A2944. [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Levine DW, Kripke DF, Kaplan RM, Lewis MA, Naughton MJ, Bowen DJ, et al. Reliability and validity of the Women’s Health Initiative insomnia rating scale. Psychol Assess. 2003;15:137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 24.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 25.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45:408–418. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sands SA, Terrill PI, Edwards BA, Taranto Montemurro L, Azarbarzin A, Marques M, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep. 2018;41:zsx183. doi: 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sands SA, Edwards BA, Terrill PI, Taranto-Montemurro L, Azarbarzin A, Marques M, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197:1187–1197. doi: 10.1164/rccm.201707-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azarbarzin A, Ostrowski M, Moussavi Z, Hanly P, Younes M. Contribution of arousal from sleep to postevent tachycardia in patients with obstructive sleep apnea. Sleep. 2013;36:881–889. doi: 10.5665/sleep.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep. 2014;37:645–653. doi: 10.5665/sleep.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efken C, Bitter T, Prib N, Horstkotte D, Oldenburg O. Obstructive sleep apnoea: longer respiratory event lengths in patients with heart failure. Eur Respir J. 2013;41:1340–1346. doi: 10.1183/09031936.00082212. [DOI] [PubMed] [Google Scholar]
- 32.Loewen A, Ostrowski M, Laprairie J, Atkar R, Gnitecki J, Hanly P, et al. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep. 2009;32:1355–1365. doi: 10.1093/sleep/32.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian S, Hesselbacher S, Mattewal A, Surani S. Gender and age influence the effects of slow-wave sleep on respiration in patients with obstructive sleep apnea. Sleep Breath. 2013;17:51–56. doi: 10.1007/s11325-011-0644-4. [DOI] [PubMed] [Google Scholar]
- 34.Wimms A, Woehrle H, Ketheeswaran S, Ramanan D, Armitstead J. Obstructive sleep apnea in women: specific issues and interventions. BioMed Res Int. 2016;2016:1764837. doi: 10.1155/2016/1764837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B, Wing Y-K. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 36.Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep. 2000;23:165–170. [PubMed] [Google Scholar]
- 37.Jordan AS, Wellman A, Edwards JK, Schory K, Dover L, MacDonald M, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol (1985) 2005;99:2020–2027. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trinder J, Kay A, Kleiman J, Dunai J. Gender differences in airway resistance during sleep. J Appl Physiol (1985) 1997;83:1986–1997. doi: 10.1152/jappl.1997.83.6.1986. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 40.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koo BB, Mansour A. Correlates of obstructive apnea duration. Lung. 2014;192:185–190. doi: 10.1007/s00408-013-9510-4. [DOI] [PubMed] [Google Scholar]
- 42.Paleczny B, Niewiński P, Rydlewska A, Piepoli MF, Borodulin-Nadzieja L, Jankowska EA, et al. Age-related reflex responses from peripheral and central chemoreceptors in healthy men. Clin Auton Res. 2014;24:285–296. doi: 10.1007/s10286-014-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep (Basel) 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R, Dong Y, Weng J, Kontos EZ, Chervin RD, Rosen CL, et al. Associations among neighborhood, race, and sleep apnea severity in children: a six-city analysis. Ann Am Thorac Soc. 2017;14:76–84. doi: 10.1513/AnnalsATS.201609-662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H, Cade BE, Gleason KJ, Bjonnes AC, Stilp AM, Sofer T, et al. Multiethnic meta-analysis identifies RAI1 as a possible obstructive sleep apnea-related quantitative trait locus in men. Am J Respir Cell Mol Biol. 2018;58:391–401. doi: 10.1165/rcmb.2017-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Cade BE, Sofer T, Sands SA, Chen H, Browning SR, et al. Admixture mapping identifies novel loci for obstructive sleep apnea in Hispanic/Latino Americans. Hum Mol Genet. 2019;28:675–687. doi: 10.1093/hmg/ddy387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lombardi C, Meriggi P, Agostoni P, Faini A, Bilo G, Revera M, et al. HIGHCARE Investigators. High-altitude hypoxia and periodic breathing during sleep: gender-related differences. J Sleep Res. 2013;22:322–330. doi: 10.1111/jsr.12012. [DOI] [PubMed] [Google Scholar]
- 49.Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D, Marshall NS, Duffin J, Yee BJ, Wong KK, Noori N, et al. Phenotyping interindividual variability in obstructive sleep apnoea response to temazepam using ventilatory chemoreflexes during wakefulness. J Sleep Res. 2011;20:526–532. doi: 10.1111/j.1365-2869.2011.00931.x. [DOI] [PubMed] [Google Scholar]