Abstract
Background:
Obesity is a suspected risk factor for respiratory depression following neuraxial morphine for postcesarean analgesia, however monitoring guidelines for obese obstetric patients are based on small, limited studies. We tested the hypothesis that clinically significant respiratory depression following neuraxial morphine occurs more commonly in women with body mass index (BMI) ≥40 kg/m2 compared with BMI <40 kg/m2.
Methods:
We conducted a single-center, retrospective chart review (2006–2017) of obstetric patients with clinically significant respiratory depression following neuraxial morphine, defined as: (1) opioid antagonist administration; (2) rapid response team activation (initiated in April 2010); or (3) tracheal intubation due to a respiratory event. The incidence of respiratory depression was compared between women with BMI ≥40 kg/m2 and BMI <40 kg/m2.
Results:
In total, 11 327 women received neuraxial morphine (n=1945 BMI ≥40 kg/m2; n=9382 BMI <40 kg/m2). Women with BMI ≥40 kg/m2 had higher rates of sleep apnea, hypertensive disorders, and magnesium administration. Sixteen cases of clinically significant respiratory depression occurred within seven days postpartum. The incidence did not significantly differ between groups (odds ratio 2.2, 95% CI 0.6 to 6.9, P=0.174). Neuraxial morphine was not deemed causative in any case, however women with BMI ≥40 kg/m2 had higher rates of tracheal intubation unrelated to neuraxial morphine (2/1945 vs. 0/9382, P=0.029).
Conclusions:
Respiratory depression in this population is rare. A larger sample (~75 000) is required to determine whether the incidence is higher with BMI ≥40 kg/m2. Tracheal intubation was higher among the BMI ≥40 kg/m2 cohort, likely due to more comorbidities.
Keywords: Cesarean delivery, Neuraxial morphine, Respiratory depression, Respiratory monitoring, Obesity
Introduction
Currently reported incidences of neuraxial morphine-related respiratory depression following cesarean delivery (CD) vary greatly, due partly to the lack of a standard definition.1 In obstetric studies using modern dosing of neuraxial morphine for post-cesarean analgesia, the reported incidence of respiratory depression ranges from 0% to 1.3% using intermittent clinical measures to a high of 18–32% using continuous carbon dioxide or oxygen saturation monitoring for detection of respiratory depression.2–8 A recent systematic review of the literature explored the incidence of ‘clinically significant’ respiratory depression (i.e. airway intervention, oxygen therapy, pharmacologic opioid antagonism, excessive sedation) in pregnant women due to neuraxial morphine or diamorphine for post-cesarean analgesia and estimated the rate at 1.08 per 10 000 (95% confidence interval [CI] 0.24 to 7.22) for low-dose neuraxial morphine and 8.67 per 10 000 (95% CI 4.20 to 15.16) for all neuraxial morphine doses.9
The obesity epidemic in the United States is of increasing concern in the pregnant population, with nearly 30% of women meeting criteria for obesity based on pre-pregnancy body mass index (BMI) ≥30 kg/m2.10 There has been little research into the possible association between obesity and the risk of respiratory depression in women receiving neuraxial morphine. One retrospective study (n=5036) found no cases of respiratory depression (defined by naloxone administration or rapid response team activation) after administration of neuraxial morphine for CD among a cohort in which 63% were obese (mean BMI 34 kg/m2) and 18% had a BMI ≥40 kg/m2.11 Two studies demonstrated that desaturation events were more common in obese women and those with a positive Berlin questionnaire, a validated screening questionnaire for obstructive sleep apnea in non-pregnant populations.4,12 Despite little data on this topic, the Society for Obstetric Anesthesia and Perinatology (SOAP) consensus statement for prevention and detection of respiratory depression with neuraxial morphine for post-cesarean analgesia lists obesity (BMI ≥40 kg/m2) as a risk factor for considering more frequent or continuous respiratory monitoring.13
In an effort to better inform respiratory monitoring guidelines for obese parturients, we undertook this retrospective data analysis to compare rates of clinically significant respiratory depression in women with BMI ≥40 kg/m2 with those of BMI <40 kg/m2 who had received neuraxial morphine for post-cesarean analgesia. We hypothesized that clinically significant respiratory depression occurs more frequently in women with obesity class III (BMI ≥40 kg/m2).
Methods
This single-center study was approved by the Institutional Review Board at Vanderbilt University Medical Center and adheres to the applicable STROBE guidelines.14 All pregnant patients from 2006 to 2017 who received neuraxial morphine for post-cesarean analgesia were included, and data were retrospectively collected from the Vanderbilt Perioperative Information Management System. Included patients were identified via a diagnosis-related group code for CD with a surgery date within the study period, in addition to intrathecal or epidural morphine administration documented in the anesthetic care record. Subjects were excluded if data on BMI were missing or if neuraxial morphine dosing was presumed inaccurate because it was outside the range of reasonable clinical doses (intrathecal 0.1–0.5 mg or epidural 1–10 mg). The primary outcome was the incidence of ‘clinically significant’ postoperative respiratory depression following neuraxial morphine administration in women with BMI ≥40 kg/m2 compared with those with BMI <40 kg/m2. As a secondary outcome, we also compared rates of respiratory events in women with all obesity classes (BMI ≥30 kg/m2) compared with non-obese women (BMI <30 kg/m2). Data were collected for all events occurring within seven days of CD. Clinically significant respiratory depression was defined as: 1. naloxone administration for opioid reversal (excluding administration to treat pruritus); 2. rapid response team activation for a respiratory event (response team established in April 2010); or 3. tracheal intubation due to a respiratory event. Naloxone administration was identified from the medication administration record, and rapid response team activation was identified via the paging log from LifeF-light, which broadcasts all code and rapid responses throughout the hospital. Postoperative tracheal intubation was identified by the presence of an anesthesia airway note, which is completed for all nonoperating room intubations.
Information was collected on baseline patient demographics, comorbidities, anesthesia management, and frequency of concomitant administration of sedating medications (which are commonly administered postpartum at our institution for nausea (e.g. haloperidol, prochlorperazine), pruritus (e.g. diphenhydramine), shivering (e.g. meperidine), anxiety (e.g. lorazepam) or breakthrough pain (e.g. gabapentin, hydromorphone, morphine). It is routine clinical practice at our institution for parturients to receive neuraxial morphine for CD unless a contraindication is present. The typical neuraxial morphine dose during the study period was 0.2 mg intrathecally or 3 mg epidurally. In addition to morphine, the typical intrathecal injection included hyperbaric bupivacaine with either fentanyl or clonidine. Epidural labor analgesia was maintained with a continuous infusion of bupivacaine and fentanyl through most of the study period until the introduction of programmed intermittent bolus in 2015. Epidural anesthesia for CD was initiated with lidocaine 2% with epinephrine 1:200 000. The typical postoperative analgesic regimen during this time period included scheduled ketorolac for 24 h followed by ibuprofen as needed, acetaminophen every 6 h as needed, and occasional low-dose oral opioids (e.g. oxycodone 5 mg as needed) for breakthrough pain. Respiratory monitoring during the study period included respiratory rate and pulse oximetry measurements every four hours for 24 h. Standing nursing orders exist to administer naloxone and call the anesthesia team for respiratory distress, opioid reversal or a respiratory rate <8 breaths per minute. The rapid response team is called for staff concern, abnormal respiratory rate (<8 or >30 breaths per minute) or change in oxygenation (<90% oxygen saturation or oxygen requirement >8 L/min), in addition to other non-respiratory conditions. Tracheal intubation is performed by the anesthesia team when an emergency call is placed for respiratory distress or failure.
For each primary outcome event, the precipitating circumstances were evaluated via medical record review. Two independent authors (RD with LS, BR or SD) determined whether neuraxial morphine was causative, contributory, or unrelated to the respiratory event, while a third author (JB) resolved discrepancies through consensus. The following definitions were used to assess causality: 1. causative – neuraxial morphine was the primary reason for the respiratory event; 2. contributory – neuraxial morphine either may have been or cannot be ruled out as a reason for the respiratory event; 3. unrelated - neuraxial morphine was definitively not a reason for the respiratory event. The time period was selected to include the years following conver
The time period was selected to include the years following conversion to electronic health record charting but prior to the transition to a new electronic health record in 2017. Demographic data were compared between groups using the Pearson's chi-square test or Fisher’s exact test as appropriate for categorical variables and using the Wilcoxon rank sum test for quantitative variables. Despite the possibility of confounding due to comorbidities that are more frequently present in obese versus non-obese women, the rarity of respiratory complications prevents statistical adjustment for these potential confounders. Thus, we report unadjusted comparisons across groups. Given the very low incidence of respiratory depression following neuraxial morphine, we report the incidence (per 10 000 cases) and 95% CI using the Wilson binomial method.15 In addition, for the primary and secondary outcome we compute the odds ratio, 95% CI, and P-value for comparison between groups using the Fisher exact method. P-values <0.05 were considered statistically significant. All statistical analyses were performed using R (v4.0.3) and the ‘epitools’ (v0.5–10.1) add-on package.16,17
Results
From 2006 to 2017, 11 761 women underwent CD and received neuraxial morphine. After excluding 305 with unavailable BMI value and 79 with incorrectly charted neuraxial morphine dose, 11 327 women were included in the final analysis, 1945 with BMI ≥40 kg/m2 (17%) and 9382 with BMI <40 kg/m2 (83%) (Fig. 1). Women in the BMI ≥40 kg/m2 group had higher American Society of Anesthesiologists physical status and Centers for Medicare and Medicaid Services-Hierarchical Condition Categories comorbidity index as well as higher rates of obstructive sleep apnea, hypertensive disorders of pregnancy, and intrapartum magnesium administration (Table 1). The use of high-dose intrathecal morphine (>0.15 mg) was not significantly different between groups, however high-dose epidural morphine (>3 mg) was administered slightly more frequently in the BMI ≥40 kg/m2 group. There was no significant difference between the groups in the use of general anesthesia and the administration rate of other sedating medications. Of note, administration of concomitant sedating medications in addition to neuraxial morphine was common in our cohort (43% in BMI ≥40 kg/m2 group and 41% in BMI <40 kg/m2 group).
Fig. 1.
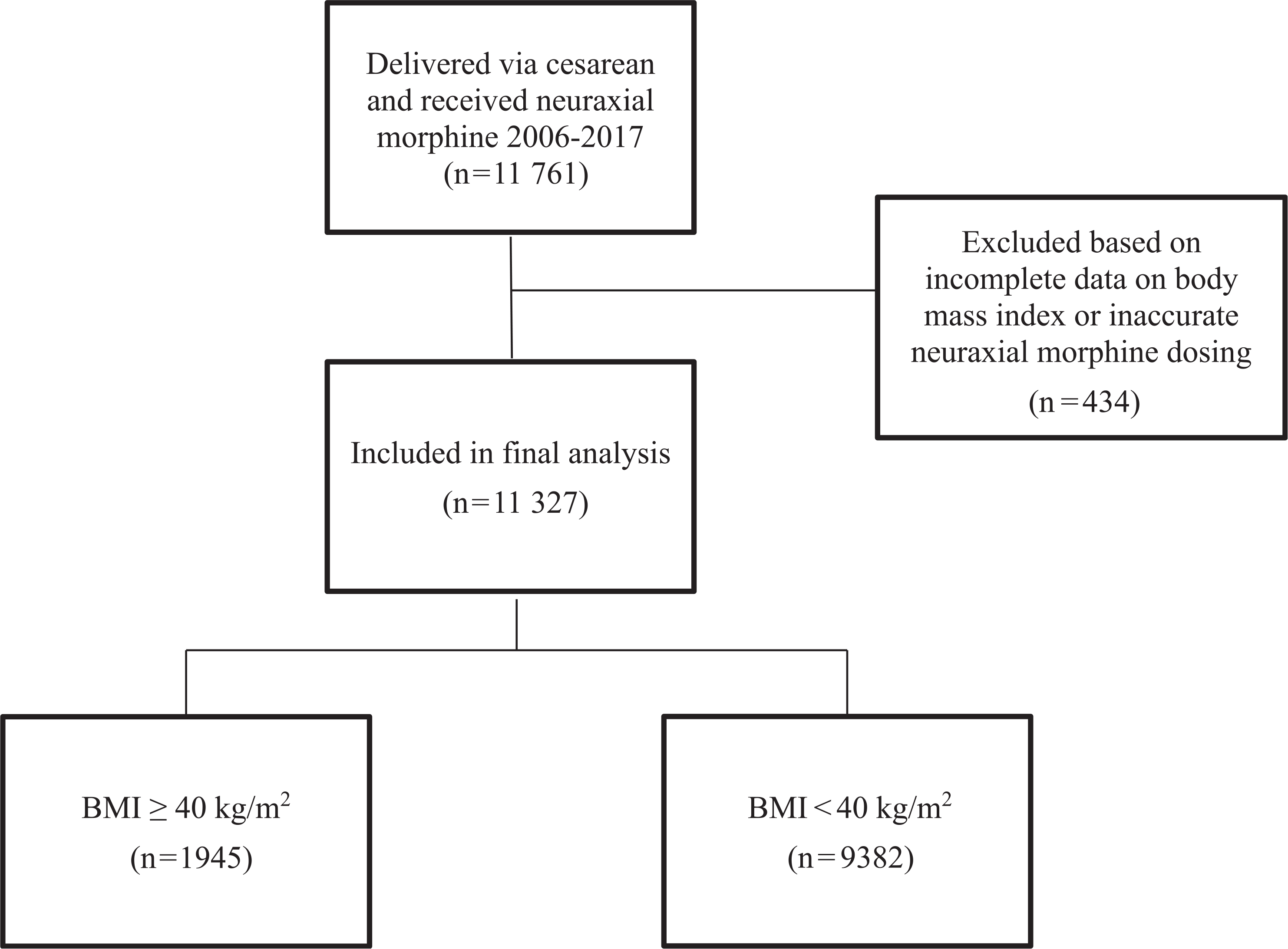
Flow chart of included patients
Table 1.
Baseline patient and medication data
| BMI ≥40 kg/m2 n = 1945 | BMI <40 kg/m2 n=9382 | P-valuea | |
|---|---|---|---|
| Age (y) | 28.6±5.6 | 28.8±6.0 | 0.369 |
| Body mass index (kg/m2) | 46.8±7.3 | 30.6±4.8 | <0.001 |
| ASA Physical Status classification | |||
| 1 | 5 (0%) | 42 (0%) | |
| 2 | 1050 (54%) | 7917 (84%) | |
| 3 | 744 (38%) | 1339 (14%) | <0.001 |
| 4 | 146 (8%) | 83 (1%) | |
| 5 | 0 (0%) | 1 (0%) | |
| CMS-HCC Surgical Comorbidity Index | 1.03±0.18 | 0.93±0.19 | <0.001 |
| Obstructive sleep apnea | 29 (1%) | 13 (0%) | <0.001 |
| Chronic hypertension | 133 (7%) | 206 (2%) | <0.001 |
| Gestational hypertension | 26 (1%) | 65 (1%) | 0.004 |
| Preeclampsia | 412 (21%) | 948 (10%) | <0.001 |
| Magnesium administration | 443 (23%) | 1204 (13%) | <0.001 |
| General anesthesia | 58 (3%) | 314 (3%) | 0.411 |
| High-dose intrathecal morphineb | 1037 (53%) | 5120 (55%) | 0.200 |
| Low-dose intrathecal morphineb | 66 (3%) | 392 (4%) | |
| High-dose epidural morphineb | 62 (3%) | 180 (2%) | 0.002 |
| Low-dose epidural morphineb | 780 (40%) | 3690 (39%) | |
| Any sedating medication | 836 (43%) | 3867 (41%) | 0.151 |
| Meperidine | 184 (9%) | 1250 (13%) | <0.001 |
| Hydromorphone | 59 (3%) | 305 (3%) | 0.621 |
| Diphenhydramine | 627 (32%) | 2530 (27%) | <0.001 |
| Haloperidol | 32 (2%) | 157 (2%) | 0.930 |
| Prochlorperazine | 3 (0%) | 13 (0%) | 0.867 |
| Lorazepam | 2 (0%) | 20 (0%) | 0.314 |
| Gabapentin | 14 (1%) | 56 (1%) | 0.529 |
All values are reported as mean±SD or n (%). BMI: body mass index. ASA: American Society of Anesthesiologists. CMS-HCC: Centers for Medicare and Medicaid Services-Hierarchical Condition Categories.
P-values computed using the Pearson chi-square test or Fisher’s exact test as appropriate for categorical variables and the Wilcoxon rank sum test for quantitative variables.
High and low neuraxial doses as defined by the Society for Obstetric Anesthesia and Perinatology Respiratory Monitoring Guidelines (high-dose intrathecal >0.15 mg, low-dose intrathecal ≤0.15 mg, high-dose epidural >3 mg, low-dose epidural ≤3 mg).13
Sixteen cases met the definition of clinically significant respiratory depression within seven days of CD. Of the 16, four had normal BMI, six had class I obesity (BMI 30.0–34.9 kg/m2), one had class II obesity (BMI 35.0–39.9 kg/m2), and five had class III obesity (BMI ≥40 kg/m2). The majority of cases (13/16) occurred within 24 h of delivery. The only two intubations were in women with BMI >45 kg/m2 and occurred on postpartum days 2 and 6, respectively. Both were deemed unrelated to neuraxial morphine administration. Additional details pertaining to these cases can be found in the Supplementary Material. The overall rate of clinically significant respiratory depression in women receiving neuraxial morphine was 25.7 per 10 000 for BMI ≥40 kg/m2 (95% CI 11.0 to 60.0) and 11.7 per 10 000 for BMI <40 kg/m2 (95% CI 6.5 to 21.0). When combining women of all obesity classes (BMI ≥30 kg/m2) the incidence was 16.9 per 10 000 (95% CI 9.7 to 29.6), with non-obese women demonstrating an incidence of 9.4 per 10 000 (95% CI 3.7 to 24.3). The estimated odds ratio among women with BMI ≥40 kg/m2 compared with women with BMI <40 kg/m2 was 2.2 (95% CI 0.6 to 6.9). While no statistically significant differences were noted between groups (Table 2), the CI included clinically important differences. Neuraxial morphine was not deemed causative for any respiratory event; however, neuraxial morphine was found to be contributory in five respiratory events (two BMI ≥40 kg/m2 and three BMI <40 kg/m2). Additionally, women with BMI ≥40 kg/m2 had higher intubation rates unrelated to neuraxial morphine compared with those with BMI <40 kg/m2 (2/1945 vs. 0/9382, P=0.029).
Table 2.
Respiratory event outcomes by body mass index group and neuraxial morphine dose
| Type of event | BMI ≥40 kg/m2 n=1945 | BMI <40 kg/m2 n = 9382 | Odds ratioa (95% CI) | P-valuea |
|---|---|---|---|---|
| Intubation performed | 2, 10.3 (2.8 to 37.4) | 0, 0.0 (0.0 to 4.1) | Inf (0.9, Inf) | 0.029 |
| Naloxone administered | 2, 10.3 (2.8 to 37.4) | 5, 5.3 (2.3 to 12.5) | 1.9 (0.2 to 11.8) | 0.344 |
| Rapid response called | 1, 5.1 (0.9 to 29.1) | 6, 6.4 (2.9 to 13.9) | 0.8 (0.0 to 6.6) | 1.000 |
| Total events | 5, 25.7 (11.0 to 60.0) | 11, 11.7 (6.5 to 21.0) | 2.2 (0.6 to 6.9) | 0.174 |
| Type of event | BMI ≥30 kg/m2 n = 7092 | BMI < 30 kg/m2 n = 4235 | Odds ratioa (95% CI) | P-valuea |
| Intubation performed | 2, 2.8 (0.8 to 10.3) | 0, 0.0 (0.0 to 9.1) | Inf (0.1 to Inf) | 0.532 |
| Naloxone administered | 5, 7.1 (3.0 to 16.5) | 2, 4.7 (1.3 to 17.2) | 1.5 (0.2 to 15.7) | 1.000 |
| Rapid response called | 5, 7.1 (3.0 to 16.5) | 2, 4.7 (1.3 to 17.2) | 1.5 (0.2 to 15.7) | 1.000 |
| Total events | 12, 16.9 (9.7 to 29.6) | 4, 9.4 (3.7 to 24.3) | 1.8 (0.5 to 7.6) | 0.439 |
| Type of event | High-dose neuraxial morphineb (n = 6390) | Low-dose neuraxial morphineb (n = 4937) | Odds ratioa (95% CI) | P-valuea |
| Intubation performed | 1, 1.6 (0.3 to 8.9) | 1, 2.0 (0.4 to 11.5) | 0.8 (0.0 to 60.6) | 1.000 |
| Naloxone administered | 4, 6.3 (2.4 to 16.1) | 3, 6.1 (2.1 to 17.9) | 1.0 (0.2 to 7.0) | 1.000 |
| Rapid response called | 3, 4.7 (1.6 to 13.8) | 4, 8.1 (3.2 to 20.8) | 0.6 (0.1 to 3.4) | 0.478 |
| Total events | 8, 12.5 (6.3 to 24.7) | 8, 16.2 (8.2 to 31.9) | 2.2 (0.6 to 6.9) | 0.623 |
BMI: body mass index. All incidences are reported as n (total number of patients), estimated incidence per 10 000 with 95% confidence interval [CI], calculated using the Wilson binomial method.
Odds ratios and P-values were computed using the Fisher’s exact method.
High and low neuraxial doses as defined by the Society for Obstetric Anesthesia and Perinatology Respiratory Monitoring Guidelines (high-dose intrathecal >0.15 mg, low-dose intrathecal ≤0.15 mg, high-dose epidural >3 mg, low-dose epidural ≤3 mg).13
Discussion
In this single-center retrospective study of women who received neuraxial morphine for post-cesarean analgesia, we found that the incidence of clinically significant respiratory depression in women with BMI ≥40 kg/m2 was 25.7 per 10 000 (95% CI 11.0 to 60.0). We found a higher intubation rate unrelated to neuraxial morphine in women with BMI ≥40 kg/m2 compared with those with a lower BMI, likely due to the higher overall rates of comorbidities. No clinically significant respiratory depression cases were attributed to neuraxial morphine alone, and most incidents of respiratory depression were determined to be unrelated to neuraxial morphine administration.
In comparing the incidence of respiratory depression in women with BMI ≥40 kg/m2 with women with BMI <40 kg/m2, the odds ratio was not statistically significantly different; however, the CI did include clinically significant differences. Although this study evaluated outcomes in 11 761 women, due to the rarity of significant respiratory depression in this population, the evidence was inconclusive due to inadequate power of our study. In a post-hoc power analysis, we determined that a sample of nearly 75 000 women would be necessary to detect an odds ratio of 2.2 with 90% power.
Despite the inability to definitively compare rates of respiratory depression between obese and non-obese women, this study still adds important information to the body of evidence attempting to elucidate the incidence of clinically significant respiratory depression following neuraxial morphine administration for CD. Unlike Crowgey et al., who examined rapid response or naloxone administration in a similar obstetric population (n=5036) and found no instances of respiratory depression, we did find cases of respiratory depression in which the administration of neuraxial morphine for CD may have been contributory.11 In a recently published systematic review, Shawari et al. attempted to estimate the incidence of respiratory depression following neuraxial morphine or diamorphine administration for CD by evaluating all published reports in the English language with no date restriction.9 They defined clinically significant respiratory depression as non-invasive airway interventions, pharmacologic reversal, oxygen therapy, or stimulation for sedation. By combining data from randomized controlled trials, prospective observational studies, and retrospective studies, they identified 16 cases of clinically significant respiratory depression in 18 452 patients for an incidence of 8.67/10 000 (95% CI 4.20 to 15.16). This estimate included patients receiving both contemporary low-dose neuraxial morphine (≤0.15 mg intrathecal, ≤3 mg epidural), as well as historically higher neuraxial morphine doses. While their combined sample size was large, data were pooled from studies reporting varying definitions of respiratory depression, and no stratification by BMI was possible.
Our study, therefore, provides important additional knowledge to understanding the incidence of respiratory depression in the context of neuraxial morphine administration for CD. Firstly, our reported rate estimates in non-obese women receiving either high- or low-dose neuraxial morphine (9.4/10 000, 95% CI 3.7 to 24.3) are comparable to those reported by Shawari et al. and serve to corroborate those findings in a single cohort of patients with a more homogeneous definition of respiratory depression. More importantly, however, our data provide additional estimates in more specific populations of interest, namely women with all-class obesity (16.9/10 000, 95% CI 9.7 to 29.6) and obesity class III (25.7/10 000, 95% CI 11.0 to 60.0).
We further report that tracheal intubation seemingly unrelated to neuraxial morphine occurred more frequently in women with BMI ≥40 kg/m2 compared with those with lower BMI, a finding that, to our knowledge, has not been previously reported. The two cases of intubation were for pulmonary edema in the setting of preeclampsia. For this reason, more frequent or intensive respiratory monitoring in morbidly obese women may be warranted in the setting of certain high-risk comorbidities. Whether this increased respiratory monitoring allows providers to identify respiratory events sooner or reduce adverse outcomes will require further study.
The incidence of clinically significant respiratory depression reported in this study should be considered in the setting of high administration rates (>40%) of concomitant sedating medications such as meperidine, gabapentin, benzodiazepines, diphenhydramine, dopamine antagonists, and other opioids. In addition, during the study period, anesthesiologists at our institution did not direct the postoperative pain management of women who received neuraxial morphine. In a recent analysis of the American Society of Anesthesiologists Closed Claims Database, concomitant administration of additional sedating medications and multiple prescribing physicians were both found to be frequent contributors to postoperative opioid-induced respiratory depression.18
There are several limitations to consider in the interpretation of our results. Firstly, we report only respiratory emergencies and not less severe forms of respiratory depression, such as oxygen administration, hypercapnia, or physical stimulation of a sedated patient. Additionally, the type and frequency of postpartum respiratory monitoring were not reliably reported in the medical record and could not be compared between groups. Due to national recommendations for increased monitoring in obese patients, it is possible that those subjects received more intensive or frequent monitoring which could have affected the event rates captured in different populations. In addition, there are many comorbidities which occur more commonly in obese patients, and these may confound these results. We were unable to adjust for these potential confounders or to perform robust regression analysis due to the rarity of respiratory events. Finally, due to the study being performed at a single institution, our results may not be generalizable to other obstetric populations.
In conclusion, our study reports the estimated incidence of clinically significant respiratory depression following neuraxial morphine administration for post-cesarean analgesia in women of varying obesity classes. We were unable to compare these risk estimates between obese and non-obese women because the study was underpowered. Larger multi-center studies are needed to provide definitive evidence comparing these risks.
Supplementary Material
Funding
R. Freundlich receives funding from NIH-NHLBI 1K23HL148640.
Disclosure of interests
R. Freundlich receives grant funding and consulting fees from Medtronic and owns stock in 3M, Gilead Sciences, and Pfizer.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijoa.2021.103187.
References
- 1.Ko S, Goldstein DH, VanDenKerkhof EG. Definitions of “respiratory depression” with intrathecal morphine postoperative analgesia: A review of the literature Définitions de la “dépression respiratoire” de l’analgésie postopératoire réalisée avec de la morphine intrathécale : une revue documentaire. Can J Anaesth. 2003;50:679–688. 10.1007/BF03018710. [DOI] [PubMed] [Google Scholar]
- 2.Dualé C, Frey C, Bolandard F, Barrière A, Schoeffler P. Epidural versus intrathecal morphine for postoperative analgesia after caesarean section. Br J Anaesth. 2003;91:690–694. 10.1093/bja/aeg249. [DOI] [PubMed] [Google Scholar]
- 3.Kato R, Shimamoto H, Terui K, Yokota K, Miyao H. Delayed respiratory depression associated with 0.15 mg intrathecal morphine for cesarean section: A review of 1915 cases. J Anesth. 2008;22:112–116. 10.1007/s00540-007-0593-z. [DOI] [PubMed] [Google Scholar]
- 4.Abouleish E, Rawal N, Rashad MN. The addition of 0.2 mg subarachnoid morphine to hyperbaric bupivacaine for cesarean delivery: A prospective study of 856 cases. Reg Anesth.. 1991;16:137–140. [PubMed] [Google Scholar]
- 5.Fuller JG, McMorland GH, Douglas MJ, Palmer L. Epidural morphine for analgesia after caesarean section: A report of 4880 patients. Can J Anaesth.. 1990;37:636–640. 10.1007/BF03006481. [DOI] [PubMed] [Google Scholar]
- 6.Kotelko DM, Dailey PA, Shnider SM, Rosen MA, Hughes SC, Brizgys RV. Epidural morphine analgesia after cesarean delivery. Obstet Gynecol. 1984;63:409–413. [PubMed] [Google Scholar]
- 7.Bauchat JR, McCarthy R, Fitzgerald P, Kolb S, Wong CA. Transcutaneous carbon dioxide measurements in women receiving intrathecal morphine for cesarean delivery: A prospective observational study. Anesth Analg. 2017;124:872–878. 10.1213/ANE.0000000000001751. [DOI] [PubMed] [Google Scholar]
- 8.Dalchow S, Lubeigt O, Peters G, Harvey A, Duggan T, Binning A. Transcutaneous carbon dioxide levels and oxygen saturation following caesarean section performed under spinal anaesthesia with intrathecal opioids. Int J Obstet Anesth. 2013;22:217–222. 10.1016/j.ijoa.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Sharawi N, Carvalho B, Habib AS, Blake L, Mhyre JM, Sultan P. A systematic review evaluating neuraxial morphine and diamorphine-associated respiratory depression after cesarean delivery. Anesth Analg. 2018;127:1385–1395. 10.1213/ANE.0000000000003636. [DOI] [PubMed] [Google Scholar]
- 10.Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016–2019. NCHS Data Brief. 2020;1–8. [PubMed] [Google Scholar]
- 11.Crowgey TR, Dominguez JE, Peterson-Layne C, Allen TK, Muir HA, Habib AS. A retrospective assessment of the incidence of respiratory depression after neuraxial morphine administration for postcesarean delivery analgesia. Anesth Analg. 2013;117:1368–1370. 10.1213/ANE.0b013e3182a9b042. [DOI] [PubMed] [Google Scholar]
- 12.Ladha KS, Kato R, Tsen LC, Bateman BT, Okutomi T. A prospective study of postcesarean delivery hypoxia after spinal anesthesia with intrathecal morphine 150 ug. Int J Obstet Anesth. 2017;32:48–53. 10.1016/j.ijoa.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauchat JR, Weiniger CF, Sultan P, et al. Society of Obstetric Anesthesia and Perinatology consensus statement: Monitoring recommendations for prevention and detection of respiratory depression associated with administration of neuraxial morphine for cesarean delivery analgesia. Anesth Analg. 2019;129:458–474. 10.1213/ANE.0000000000004195. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. BMJ (Clinical research ed). 2007;335:806–808. 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newcombe RG. Interval estimation for the difference between independent proportions: Comparison of eleven methods. Stat Med. 1998;17:873–890. . [DOI] [PubMed] [Google Scholar]
- 16.R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/. Accessed November 5, 2020.
- 17.Epitools: Epidemiology tools r package version 0.5–10.0. 2020. https://CRAN.Rproject.org/package=epitools. Accessed November 5, 2020.
- 18.Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: A closed claims analysis. Anesthesiology. 2015;122:659–665. 10.1097/ALN.0000000000000564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


