Abstract
Background
Capnocytopha ga is a gram-negative, facultative anaerobe. Human infection is rare but can lead to devastating outcomes. Capnocytophaga canimorsus can cause sepsis following an animal bite, whereas human-oral–associated Capnocytophaga infections were reported in immunocompromised patients. Current data on these infections are not robust. Our goal is to provide a contemporary description of a unique characteristic of Capnocytophaga infections.
Methods
We performed a retrospective review of all patients with Capnocytophaga infection from January 2010 to August 2020 at 3 main hospitals of Mayo Clinic in Rochester, Minnesota; Scottsdale, Arizona; and Jacksonville, Florida. We collected baseline demographic data, clinical characteristics, microbiological data, and outcomes of C. canimorsus and human-oral–associated Capnocytophaga infection.
Results
Among 82 patients with Capnocytophaga infection, 46 patients (56.0%) had bacteremia. The most common species identified in this group was C. sputigena (57.9%), followed by C. canimorsus (34.8%). Patients with human-oral–associated Capnocytophaga bacteremia were often immunocompromised, presented with neutropenic fever, and had worse 6-month all-cause mortality compared to C. canimorsus bacteremia (36.4% vs 6.2%, P = .03). They also had a higher β-lactamase production rate (36.4% vs 0.0%, P = .02). Among patients without bacteremia, the main clinical syndrome was polymicrobial head and neck infections (47.2%).
Conclusions
Human-oral–associated Capnocytophaga bacteremia occurs primarily in immunocompromised patients, particularly those with hematologic malignancy. In contrast, C. canimorsus bacteremia is more likely to present with community-onset infection related to zoonotic exposure. Human-oral–associated Capnocytophaga infection without bacteremia is frequently isolated in polymicrobial infection; this phenomenon’s significance is yet to be fully understood.
Keywords: Capnocytophaga, bacteremia, mortality, zoonosis
The clinical and microbiological characteristics of Capnocytophaga bacteremia are unique. Our study found that human-oral–associated Capnocytophaga bacteremia primarily occurs in immunocompromised patients, especially those with hematologic malignancies. They have higher 6-month all-cause mortality compared to C. canimorsus bacteremia.
Capnocytophaga species are a slow-growing, facultative anaerobic, gram-negative bacilli that can cause various clinical syndromes in humans [1]. There are 9 species of Capnocytophaga, which can be classified into 2 main categories: human-oral associated and zoonosis associated. The species C. gingivalis, C. granulosa, C. haemolytica, C. leadbetteri, C. ochracea, and C. sputigena are a part of human oral microbiota, whereas C. canimorsus, C. canis, and C. cynodegmi are commensal organisms in the mouths of dogs or cats [1–3].
Capnocytophaga infection in humans is rare. Previous studies were focused on C. canimorsus, as it is the most common among the genus to cause human disease [4]. According to 1 population-based study, incidence of infection was approximately 0.67 infections per million population [5]. Bacteremia can occur with both zoonotic and human-oral–associated Capnocytophaga [5, 6]. Other previously reported clinical manifestations of C. canimorsus infection include fever of unknown origin, meningitis, brain abscess, endocarditis, mycotic aneurysm, respiratory tract infection, and orthopedic infections such as septic arthritis and soft tissue infections [7–11].
In contrast to C. canimorsus, clinical syndromes from human-oral–associated Capnocytophaga infection other than bacteremia are sporadic and have not been well studied. Sepsis is common in immunocompromised populations, especially in patients with hematologic malignancies [12]. Some studies reported human-oral–associated Capnocytophaga as a rare cause of pleural empyema, chorioamnionitis, and neonatal sepsis [13–15].
Our study aims to highlight the key differences in clinical characteristics of C. canimorsus and human-oral–associated Capnocytophaga bloodstream infections, as well as to outline the clinical manifestations and outcomes of such infections.
METHODS
Study Design and Participants
We conducted a retrospective review of all patients diagnosed with Capnocytophaga infection from 1 January 2010 to 31 August 2020, at 3 main hospitals of Mayo Clinic in Rochester, Minnesota; Scottsdale, Arizona; and Jacksonville, Florida. We used Mayo Clinic Advance Text Explorer software and the Division of Clinical Microbiology laboratory database to identify all adult patients (age 18 years or older) who had Capnocytophaga isolated from their clinical specimens.
We manually reviewed all the medical records to determine the clinical significance of each positive culture. We deemed that the culture was clinically significant if the patient developed a clinical syndrome consistent with Capnocytophaga infection and was documented as such by the treating providers. All clinical data were collected and managed using REDCap electronic data capture tools hosted at Mayo Clinic [16, 17].
We gathered patient characteristics such as age, sex, reported animal bite/scratch, and comorbidities. Comorbidities of interest included host immune status in addition to known risk factors for Capnocytophaga infections [13, 18, 19]. Hospitalization data including an initial clinical syndrome, length of hospital stay, intensive care unit (ICU) admission, type and duration of antimicrobial therapy, and surgical management were also included. Additionally, we collected all-cause mortality at the time of hospital discharge and 6 months for bacteremia patients.
Patient Consent Statement
The study was reviewed and approved by the Mayo Clinic Institutional Review Board. The study was granted an exemption from patient consent, as it does not include factors necessitating patient consent.
Microbiological Data
Matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS) was used for species identification after subculturing. β-lactamase testing was done using Cefinase (Becton Dickinson, catalog number 231650). Cefinase disks are impregnated with a cephalosporin nitrocefin solution, which is normally yellow but changes color to red when a β-lactamase hydrolyses the β-lactam ring.
Statistical Analysis
We used Fisher exact or χ 2 test for categorical data and Wilcoxon-Mann-Whitney test for nonparametric quantitative data. All analyses were performed using BlueSky Statistics version 7.10 software (BlueSky Statistics LLC, Chicago, Illinois).
RESULTS
Clinical Characteristics
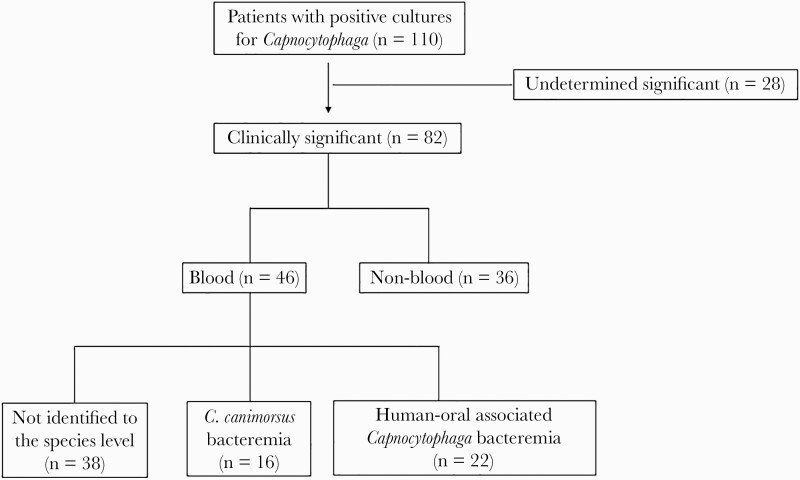
We identified 110 patients who had positive cultures for Capnocytophaga species. Of those, 82 patients had clinically significant culture results and were included in the study (Figure 1). Forty-six patients (56.1%) had positive cultures from the blood (bacteremia group), and 36 patients (43.9%) had positive cultures from non-blood clinical specimens (nonbacteremia group). The median age was 59 years (interquartile range [IQR], 49.3–68.8 years), and the majority of patients were male. Thirteen patients (15.9%) reported a history of an animal bite or scratch, while it was not documented in the rest of the cases. The median Charlson comorbidity index was 3 (IQR, 2–5). Forty-seven patients (57.3%) had at least 1 comorbidity. Twenty-eight patients (34.1%) actively received immunosuppressive medications.
Figure 1.
Study design and participants.
Overall, 79 patients required hospitalization (96.3%), with 23 patients (29.1%) admitted to the ICU. The median length of stay was 7 days (IQR, 3.5–13.0 days). Surgical management was needed in 32 patients (39%). The most common antimicrobial regimens involved combination β-lactam/β-lactamase inhibitors (n = 25 [30.5%]), third/fourth-generation cephalosporins (n = 25 [30.5%]), and carbapenems (n = 25 [30.5%]). The median duration of antimicrobial therapy was 14 days (IQR, 10–28 days). Other detailed clinical characteristics were summarized in Table 1.
Table 1.
Characteristics of Patients With Capnocytophaga Infection
| Characteristic | No. (%) |
|---|---|
| Age, y, median (IQR) | 59 (49.3–68.8) |
| Male sex | 45 (54.9) |
| Charlson comorbidity index, median (IQR) | 3 (2.0–5.0) |
| Comorbidities | |
| Active immunosuppressive medicationsa | 28 (34.1) |
| Diabetes | 15 (18.3) |
| Hematopoietic stem cell transplantation | 13 (15.9) |
| Active hematologic malignancy | 12 (14.6) |
| Active solid organ malignancy | 7 (8.5) |
| End-stage renal disease | 4 (4.9) |
| Alcohol use disorder | 3 (3.7) |
| Splenectomy | 3 (3.7) |
| Solid organ transplantation | 3 (3.7) |
| Reported history of animal bite or scratch | |
| Dog | 9 (11) |
| Cat | 4 (4.9) |
| Hospitalization | |
| Requiring hospitalization | 79 (96.3) |
| Length of stay, d, median (IQR) | 7 (3.5–13.0) |
| Requiring ICU admission | 23 (29.1) |
| Specific antibiotic therapy | |
| β-lactam/β-lactamase inhibitorb | 25 (30.5) |
| Cephalosporinc | 25 (30.5) |
| Carbapenemd | 25 (30.5) |
| Other antibioticse | 6 (7.3) |
| No antibiotic due to hospice care | 1 (1.2) |
| Capnocytophaga species (n = 62) | |
| C. sputigena | 28 (45.2) |
| C. canimorsus | 16 (25.8) |
| C. ochracea | 10 (16.1) |
| C. gingivalis | 5 (8.1) |
| C. cynodegmi | 1 (1.6) |
| C. granulosa | 1 (1.6) |
| C. leadbetteri | 1 (1.6) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
aActive immunosuppressive medications included daily steroid >20 mg of prednisone equivalence for >1 month; active chemotherapy within 3 months; calcineurin inhibitor; other biologic agent; cytostatic agent other than chemotherapy.
bβ-lactam/β-lactamase inhibitor included amoxicillin/clavulanate, ampicillin/sulbactam, and piperacillin/tazobactam.
cCephalosporin included ceftriaxone and cefepime.
dCarbapenem included ertapenem and meropenem.
eOther antibiotics included levofloxacin, moxifloxacin, and clindamycin.
Bacteremia Group
Forty-three patients (93.5%) had a monomicrobial bloodstream infection. The clinical syndromes associated with bacteremia were neutropenic fever (n = 19 [41.3%]), sepsis/septic shock in nonneutropenia (n = 16 [34.8%]), skin and soft tissue infection (n = 8 [17.4%]), septic joint (n = 1 [2.2%]), postoperative respiratory failure (n = 1 [2.2%]), and meningitis (n = 1 [2.2%]). Identification to the species level was done in 38 isolates (82.6%). Capnocytophaga canimorsus bacteremia comprised 42.1% of cases (n = 16), while 57.9% (n = 22) had human-oral–associated Capnocytophaga bacteremia (C. sputigena, C. ochracea, and C. gingivalis). The median time to blood culture positivity was 70 hours (IQR, 57–96 hours). Nine isolates (24.3%) produced β-lactamase, which was found only in the human-oral–associated Capnocytophaga group. The detail of antibiotic therapy based on β-lactamase production can be found in Supplementary Table 1.
There were no differences in age or sex between C. canimorsus and human-oral–associated Capnocytophaga groups. Hematopoietic stem cell transplantation was more common in the human-oral–associated Capnocytophaga group (P < .001). Sixteen patients (72.7%) in the human-oral–associated Capnocytophaga group were taking immunosuppressive medications compared with 2 patients (12.5%) in the C. canimorsus group (P < .001). Unsurprisingly, all patients with a reported history of animal bite/scratch were in the C. canimorsus group. Detailed clinical syndromes of each group and sources of bacteremia were described in Table 2.
Table 2.
Comparison of Capnocytophaga canimorsus and Human-Oral–Associated Capnocytophaga Bacteremia
| Characteristic | Capnocytophaga canimorsus Bacteremia (n = 16) | Human-Oral–Associated Capnocytophaga Bacteremia (n = 22) | P Value |
|---|---|---|---|
| Baseline characteristic | |||
| Age, y, median (IQR) | 57 (48.0–74.3) | 59.5 (47.3–68.5) | .69 |
| Male sex | 10 (62.5) | 13 (59.1) | .832 |
| Risk factors | |||
| Alcohol use disorder | 2 (12.5) | 0 (0.0) | .171 |
| Splenectomy | 2 (12.5) | 0 (0.0) | .171 |
| Diabetes | 3 (18.8) | 4 (18.2) | 1 |
| End-stage renal disease | 1 (6.2) | 1 (4.5) | 1 |
| Solid organ transplantation | 1 (6.2) | 1 (4.5) | 1 |
| Hematopoietic stem cell transplantation | 0 (0.0) | 12 (54.5) | <.001 |
| Active solid organ malignancy | 0 (0.0) | 2 (9.1) | .499 |
| Active hematologic malignancy | 1 (6.2) | 9 (40.9) | .025 |
| Active immunosuppressive | 2 (12.5) | 16 (72.7) | <.001 |
| At least 1 risk factor presence | 8 (50) | 22 (100) | <.001 |
| Reported animal bite/scratch | 11 (68.8) | 0 (0.0) | <.001 |
| Clinical syndromes | <.001 | ||
| Neutropenic fever | 0 (0.0) | 17 (77.3) | |
| Sepsis/septic shock without neutropenia | 8 (50.0) | 3 (13.6) | |
| Skin and soft tissue infection of extremities | 6 (37.5) | 1 (4.5) | |
| Septic joint | 1 (6.2) | 0 (0.0) | |
| Postoperative respiratory failure | 0 (0.0) | 1 (4.5) | |
| Meningitis | 1 (6.2) | 0 (0.0) | |
| Source of bacteremia | .001 | ||
| Unable to identify the source of bacteremia | 6 (37.5) | 6 (27.3) | |
| Central catheter associated | 0 | 10 (45.5) | |
| Skin and soft tissue infection of extremities | 8 (50.0) | 0 (0.0) | |
| Neutropenic colitis/mucositis | 0 (0.0) | 4 (18.2) | |
| Jaw osteomyelitis | 0 (0.0) | 1 (4.5) | |
| Septic arthritis | 1 (6.2) | 0 (0.0) | |
| Upper/lower respiratory tract infection | 0 (0.0) | 1 (4.5) | |
| Meningitis | 1 (6.2) | 0 (0.0) | |
| Outcome | |||
| Mortality on hospital discharge | 0 (0.0) | 2 (9.1) | .306 |
| All-cause mortality at 6 mo | 1 (6.2) | 8 (36.4) | .03 |
Data are presented as No. (%) unless otherwise indicated. Abbreviation: IQR, interquartile range.
The median length of stay was 8 days (IQR, 5.0–16.0 days) in the human-oral–associated Capnocytophaga group and 4.5 days (IQR, 2.8–8.0 days) in the C. canimorsus group (P = .025). There was no difference in the rates of ICU admission between the groups. All-cause mortality at discharge was not different between the 2 groups. However, 6-month all-cause mortality was higher in the human-oral–associated Capnocytophaga group (36.4% vs 6.2%, P = .03).
Nonbacteremia Group
Capnocytophaga was isolated from 33 of 36 patients (91.7%) as part of a polymicrobial infection. Twenty-four isolates (66.7%) were identified to the species level; all were human-oral–associated Capnocytophaga species. Seven isolates (33.3%) produced β-lactamase (Supplementary Table 1). The most common clinical syndrome was head and neck infections (n = 17 [47.2%]), such as neck abscess, facial cellulitis, facial abscess, osteomyelitis of mandible/maxilla, retropharyngeal abscess, and dental root infection. This was followed by osteomyelitis of extremities (n = 4 [11.1%]), thoracic empyema (n = 4 [11.1%]), respiratory tract infection (n = 4 [11.1%]), and intra-abdominal abscess (n = 2 [5.6%]). Other rare clinical syndromes included surgical site infection, skin abscess, breast abscess, brain abscess, and vascular graft infection (1 patient each).
DISCUSSION
Our study illustrated the clinical characteristics of Capnocytophaga infection at 3 main hospitals of Mayo Clinic in Rochester, Minnesota; Scottsdale, Arizona; and Jacksonville, Florida during the past 10 years. More than half of all Capnocytophaga infections in our study were bloodstream infections. We found that C. sputigena was the most prevalent species among all Capnocytophaga infections, followed by C. canimorsus. One previous study revealed that >70% of C. canimorsus infection presented with bacteremia [20]. However, the actual incidence of bacteremia in other Capnocytophaga infections is unknown.
Traditional risk factors associated with severe C. canimorsus infections are a history of dog bite, asplenia, cirrhosis, and alcohol use disorder [19–21]. Risk factors for human-oral–associated Capnocytophaga infections are less well studied. Our study found that not all patients with C. canimorsus bloodstream infection had classical risk factors; overall, the numbers of patients with asplenia, cirrhosis, and alcohol use disorder were approximately only 4%. Additionally, only 68% of our C. canimorsus bacteremia patients were linked to animal bites or scratches. These findings are consistent with a nationwide survey from the Netherlands. They found that only half of the patients reported a history of animal contact and a low prevalence of splenectomy and alcohol use disorder [5]. In the clinical setting, the decision to obtain blood cultures in patients presenting with animal bite skin and soft tissue infection is often determined based on the severity of presentation, the presence of systemic features of sepsis, and specific host risk factors.
In contrast, human-oral–associated Capnocytophaga bacteremia was implicated in immunocompromised patients, especially with neutropenia secondary to chemotherapy for hematologic malignancy and hematopoietic stem cell transplantation. This finding was observed in previous case reports and small case series [12, 18, 22]. Blood cultures were obtained as part of routine investigations for febrile episodes in neutropenic patients; approximately 27% of these episodes were without a clear source, whereas the rest were attributed to translocation through the oral or gastrointestinal mucosa or labeled as central venous catheter–associated bloodstream infections. Consequently, the growth of small gram-negative bacilli in anaerobic blood culture bottles after 48 hours in a neutropenic patient should raise the suspicion for human-oral–associated Capnocytophaga, and empiric antibiotic coverage should be reviewed to ensure appropriate coverage. Once identified, a close evaluation of the oral mucosa, assessment for differential time to positivity to detect line-related infection, and abdominal imaging may be indicated.
According to the literature, mortality from C. canimorsus ranged from 10% to 30% [5, 6, 11, 20]. Our study found that 6-month all-cause mortality was higher in the human-oral–associated Capnocytophaga group. This could be from poorer baseline clinical status in the human-oral–associated Capnocytophaga group. We found that patients with C. canimorsus bacteremia had fewer comorbidities compared to human-oral–associated Capnocytophaga bacteremia.
Polymicrobial infections involving Capnocytophaga species are less defined in the literature. Recent advancements in the microbiology field, especially in the era of MALDI-TOF MS [23], may explain the increased detection of Capnocytophaga in various other clinical specimens, often as part of polymicrobial infection. Their exact role in the disease process is difficult to interpret as many other virulent bacteria were isolated. As normal commensal microbiota of the human-oral cavity, it is not surprising that they are implicated in head and neck infections or respiratory tract infections. As we have found, almost all of the nonbacteremia group had polymicrobial infection. The treatment and choice of antibiotic regimens are also unlikely to be affected by Capnocytophaga species’ presence.
Currently, there is no standard antimicrobial susceptibility guide for Capnocytophaga species. Previous in vitro susceptibility is based on the breakpoint for anaerobes, which is not widely available [24]. β-lactamase detection is an alternative way to help guide antimicrobial therapy. However, this result may take days to become available due to the slow-growing nature of Capnocytophaga. Our rate of β-lactamase production is almost 30% and it was solely found in the human-oral–associated Capnocytophaga group. These findings were previously described in past studies [25–27]. Previous exposure to antibiotics could contribute to this finding, especially among patients with hematologic malignancies and other immunocompromising conditions.
Owing to the higher prevalence of β-lactamase production among the human-oral Capnocytophaga group, stable agents against β-lactamases should be utilized for definitive therapy. This includes a combination of β-lactam/β-lactamase inhibitors, advanced-generation cephalosporins (third or fourth generation), or carbapenems. We have not noted treatment failure with advanced-generation cephalosporins in our cohort. On the contrary, β-lactamase production was rare among C. canimorsus, making penicillin a viable choice for C. canimorsus monomicrobial infections. The duration of therapy in both bacteremia and nonbacteremia is often dictated by the primary syndrome.
To our knowledge, this is the largest cohort evaluating clinical characteristics of Capnocytophaga infection. However, there are some limitations. First, not all patients in the nonbacteremia group had blood culture collection at the time of diagnosis; therefore, it is possible that some patients in this group had bacteremia. Second, the nature of cross-sectional study limited the ability to define the association and causation of the variables. Third, not all isolates were identified to the species level, limiting the accuracy of the conclusions regarding the significance of species identification and association between particular species and specific syndromes or populations at risk. Finally, there may be reporting bias. Most of our patients had multiple comorbidities; therefore, these findings may not be generalizable to Capnocytophaga infection in other populations.
In conclusion, our study provides contemporary data on the host factors, clinical presentations, and management trends of Capnocytophaga infection. We also described the difference between human-oral–associated vs zoonosis- associated Capnocytophaga infection.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This research was made possible through support from the Center for Clinical and Translational Science (grant number UL1TR002377).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. Zangenah S, Abbasi N, Andersson AF, Bergman P. Whole genome sequencing identifies a novel species of the genus Capnocytophaga isolated from dog and cat bite wounds in humans. Sci Rep 2016; 6:22919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suzuki M, Kimura M, Imaoka K, Yamada A. Prevalence of Capnocytophaga canimorsus and Capnocytophaga cynodegmi in dogs and cats determined by using a newly established species-specific PCR. Vet Microbiol 2010; 144:172–6. [DOI] [PubMed] [Google Scholar]
- 3. Idate U, Bhat K, Kotrashetti V, et al. Molecular identification of Capnocytophaga species from the oral cavity of patients with chronic periodontitis and healthy individuals. J Oral Maxillofac Pathol 2020; 24:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaastra W, Lipman LJ. Capnocytophaga canimorsus. Vet Microbiol 2010; 140:339–46. [DOI] [PubMed] [Google Scholar]
- 5. van Dam AP, Jansz A. Capnocytophaga canimorsus infections in the Netherlands: a nationwide survey. Clin Microbiol Infect 2011; 17:312–5. [DOI] [PubMed] [Google Scholar]
- 6. Hästbacka J, Hynninen M, Kolho E. Capnocytophaga canimorsus bacteremia: clinical features and outcomes from a Helsinki ICU cohort. Acta Anaesthesiol Scand 2016; 60:1437–43. [DOI] [PubMed] [Google Scholar]
- 7. Piau C, Arvieux C, Bonnaure-Mallet M, Jolivet-Gougeon A. Capnocytophaga spp. involvement in bone infections: a review. Int J Antimicrob Agents 2013; 41:509–15. [DOI] [PubMed] [Google Scholar]
- 8. Hansen M, Crum-Cianflone NF. Capnocytophaga canimorsus meningitis: diagnosis using polymerase chain reaction testing and systematic review of the literature. Infect Dis Ther 2019; 8:119–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janda JM, Graves MH, Lindquist D, Probert WS. Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis 2006; 12:340–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prasil P, Ryskova L, Plisek S, Bostik P. A rare case of purulent meningitis caused by Capnocytophaga canimorsus in the Czech Republic–case report and review of the literature. BMC Infect Dis 2020; 20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mader N, Lührs F, Langenbeck M, Herget-Rosenthal S. Capnocytophaga canimorsus—a potent pathogen in immunocompetent humans–systematic review and retrospective observational study of case reports. Infect Dis (Lond) 2020; 52:65–74. [DOI] [PubMed] [Google Scholar]
- 12. Martino R, Rámila E, Capdevila JA, et al. Bacteremia caused by Capnocytophaga species in patients with neutropenia and cancer: results of a multicenter study. Clin Infect Dis 2001; 33:E20–2. [DOI] [PubMed] [Google Scholar]
- 13. Bonatti H, Rossboth DW, Nachbaur D, et al. A series of infections due to Capnocytophaga spp in immunosuppressed and immunocompetent patients. Clin Microbiol Infect 2003; 9:380–7. [DOI] [PubMed] [Google Scholar]
- 14. Lopez E, Raymond J, Patkai J, et al. Capnocytophaga species and preterm birth: case series and review of the literature. Clin Microbiol Infect 2010; 16:1539–43. [DOI] [PubMed] [Google Scholar]
- 15. Howlett AA, Mailman TL, Ganapathy V. Early cystic lung disease in a premature neonate with perinatally acquired Capnocytophaga. J Perinatol 2007; 27:68–70. [DOI] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Minor BL, et al. REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mendes FR, Bruniera FR, Schmidt J, et al. Capnocytophaga sputigena bloodstream infection in hematopoietic stem cell transplantations: two cases report and review of the literature. Rev Inst Med Trop Sao Paulo 2020; 62:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lion C, Escande F, Burdin JC. Capnocytophaga canimorsus infections in human: review of the literature and cases report. Eur J Epidemiol 1996; 12:521–33. [DOI] [PubMed] [Google Scholar]
- 20. Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis 2015; 34:1271–80. [DOI] [PubMed] [Google Scholar]
- 21. Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982–1995: review of 39 cases. Clin Infect Dis 1996; 23:71–5. [DOI] [PubMed] [Google Scholar]
- 22. Isabel R. Capnocytophaga sputigena bacteremia in a neutropenic host. IDCases 2019; 17:e00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singhal N, Kumar M, Kanaujia PK, Virdi JS. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol 2015; 6:791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jolivet-Gougeon A, Buffet A, Dupuy C, et al. In vitro susceptibilities of Capnocytophaga isolates to beta-lactam antibiotics and beta-lactamase inhibitors. Antimicrob Agents Chemother 2000; 44:3186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ehrmann E, Handal T, Tamanai-Shacoori Z, et al. High prevalence of β-lactam and macrolide resistance genes in human oral Capnocytophaga species. J Antimicrob Chemother 2014; 69:381–4. [DOI] [PubMed] [Google Scholar]
- 26. Jolivet-Gougeon A, Guérin J, Tamanai-Shacoori Z, et al. Influence of previous antimicrobial therapy on oral carriage of beta-lactamase producing Capnocytophaga isolates. Acta Paediatr 2008; 97:964–7. [DOI] [PubMed] [Google Scholar]
- 27. Maury S, Leblanc T, Rousselot P, et al. Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of β-lactamase-producing strains. Clin Infect Dis 1999; 28:1172–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.