Abstract
Background
Neutralizing monoclonal antibodies (MAbs) are a promising therapy for early coronavirus disease 2019 (COVID-19), but their effectiveness has not been confirmed in a real-world setting.
Methods
In this quasi-experimental pre-/postimplementation study, we estimated the effectiveness of MAb treatment within 7 days of symptom onset in high-risk ambulatory adults with COVID-19. The primary outcome was a composite of emergency department visits or hospitalizations within 14 days of positive test. Secondary outcomes included adverse events and 14-day mortality. The average treatment effect in the treated for MAb therapy was estimated using inverse probability of treatment weighting and the impact of MAb implementation using propensity-weighted interrupted time series analysis.
Results
Pre-implementation (July–November 2020), 7404 qualifying patients were identified. Postimplementation (December 2020–January 2021), 594 patients received MAb treatment and 5536 did not. The primary outcome occurred in 75 (12.6%) MAb recipients, 1018 (18.4%) contemporaneous controls, and 1525 (20.6%) historical controls. MAb treatment was associated with decreased likelihood of emergency care or hospitalization (odds ratio, 0.69; 95% CI, 0.60–0.79). After implementation, the weighted probability that a given patient would require an emergency department visit or hospitalization decreased significantly (0.7% per day; 95% CI, 0.03%–0.10%). Mortality was 0.2% (n = 1) in the MAb group compared with 1.0% (n = 71) and 1.0% (n = 57) in pre- and postimplementation controls, respectively. Adverse events occurred in 7 (1.2%); 2 (0.3%) were considered serious.
Conclusions
MAb treatment of high-risk ambulatory patients with early COVID-19 was well tolerated and likely effective at preventing the need for subsequent emergency department or hospital care.
Keywords: novel coronavirus, COVID-19, SARS-CoV-2, monoclonal antibody, casirivimab, imdevimab, bamlanivimab
Monoclonal antibodies (MAbs) designed to avidly bind to the receptor binding domain of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein are an emerging neutralizing passive immune therapy for coronavirus disease 2019 (COVID-19). In fall 2020, bamlanivimab (Eli Lilly, Indianapolis, IN, USA) and casirivimab/imdevimab (Regeneron, Tarrytown, NY, USA) received US Food and Drug Administration Emergency Use Authorization (EUA) for treatment of mild/moderate symptomatic COVID-19 in ambulatory patients at higher risk for hospitalization [1, 2] and were distributed for administration under a Department of Health and Human Services program. Secondary end points from phase II/III clinical trials suggest that early administration of these MAbs may prevent emergency department (ED) visits or hospitalization, particularly in the subgroups at highest risk for progression to severe COVID-19 [3–5]. Despite promising trial data, widespread implementation of MAb therapy is limited by challenging logistics and competing demands on resources. A clearer understanding of the magnitude of treatment effects and adverse event rates in a real-world setting is needed to inform decisions about MAb deployment. Here, we describe adverse event rates and use a causal inference methodology to evaluate the effectiveness of MAbs for COVID-19 after implementation in a large integrated health care system.
METHODS
Setting and Data Repository
Intermountain Healthcare is a regional integrated health care system that provides care to more than 1.5 million patients each year. During the COVID-19 pandemic, Intermountain has offered SARS-CoV-2 testing at urgent care facilities, emergency departments, and community drive-up testing sites in Utah and Southeastern Idaho. As part of patient notification and clinical trial enrollment processes, a report of all positive test results is generated daily and includes clinical and demographic data. Clinical data for this study were extracted from the Intermountain enterprise data warehouse and Intermountain Prospective Observational COVID-19 (IPOC) database. Comorbidities were defined using the Charlson and Elixhauser definitions [6, 7]. Comorbidity data were complete for patients with prior encounters within the integrated health system; patients with missing prior encounter data were excluded.
Monoclonal Antibody Eligibility and Delivery
At the time of EUA approval, which was a period of high community transmission, patients eligible for MAb using the EUA criteria far exceeded infusion capacity. To address resource scarcity, the Scarce Medications Allocation Subcommittee of the Utah Crisis Standards of Care (CSC) Workgroup was convened with the dual aims of targeting available MAb infusions to the patients most likely to benefit and ensuring equity in delivery [8]. A simple clinical prediction score for predicting severe COVID-19 resulting in hospitalization or mortality among ambulatory patients was validated in a large cohort of Utah patients and adopted for use in MAb allocation [9]. The score weights age, gender, shortness of breath, comorbidities, and non-White race or Hispanic/Latinx ethnicity to address recognized disparities in poor COVID-19 outcomes in these populations.
Clinical eligibility for MAb was defined by the CSC committee as the following: (1) at or above the risk score threshold (set at ≥7.5 points, which identified approximately the top decile of estimated risk among COVID-19-positive patients), (2) laboratory-confirmed COVID-19 by nucleic acid amplification test or antigen detection, (3) symptomatic disease with symptom onset within no more than 7 days. Patients were ineligible to receive MAb therapy for the following conditions: (1) hospitalized due to COVID-19, (2) new COVID-related hypoxemia (defined as peripheral oxygen saturation <90% at rest or new supplemental oxygen requirement, or for those with chronic hypoxia, a new change in baseline saturation or oxygen demand, (3) pregnant, or (4) known hypersensitivity to other monoclonal drugs. Eligibility criteria for pediatric patients differed from those for adults, and patients <18 years of age are not included in this study.
To provide equitable access to treatment, 16 regional infusion sites (7 hospital-based infusion centers and 9 urgent care facilities) were selected based on population density, prevalence of underserved patients, and travel time for patients in rural communities. Two pathways for patient identification were developed: first, a hotline and email address were set up to receive referrals from patients and providers in the state. Second, recognizing that patients with poorer health care access might have less awareness about treatment availability and be less likely to have a referring provider, a process was implemented to proactively identify patients at the time of test positivity. To do this, the risk calculator result was electronically integrated into the daily report of all new positive cases. Each day, a clinician using the report reviewed available medical records for candidate patients with new positive tests and attempted to contact patients by telephone to verify eligibility for MAb infusion. A telephone interpreter service was used for all patients with a primary language other than English. Eligible patients were then scheduled at the nearest infusion site with appointment availability.
At a daily huddle, all infusion sites reported infusion-associated adverse events, defined as symptoms requiring clinical evaluation or management during the infusion or 1-hour observation period. The first bamlanivimab infusions were provided in infusion centers and urgent care clinics on December 1, 2020 (to patients with positive tests as of November 28, 2020), and the first casirivimab/imdevimab infusions were administered on December 30, 2020. Due to drug preparation requirements, when casirivimab/imdevimab became available, it replaced bamlanivimab as the sole product used at infusion centers, whereas urgent care sites continued to administer only bamlanivimab.
Study Design and Statistical Analysis
Because observational studies are particularly prone to bias due to unrelated secular trends in outcomes over time and/or by residual confounding by indication when comparing treated and untreated patients, we designed this study with 2 co-primary analyses, each using causal inference methods to address limitations inherent to the other. First, we estimated the average treatment effect in those treated with MAbs using propensity weighting in a cohort of patients after MAb program implementation. Second, we evaluated the impact of the MAb program on patient-level outcomes pre- and postimplementation using interrupted time series (ITS) analysis to mitigate temporal bias and propensity weighting to promote a balanced comparison.
For these analyses, we identified 2 cohorts: (1) a pre-implementation cohort comprised of ambulatory patients with positive COVID-19 tests performed between July 1 and November 27, 2020 (historical controls), and (2) a postimplementation cohort of patients with positive tests during the period when MAb therapy was available (November 28, 2020, through January 28, 2021), comprising patients treated with MAbs and untreated contemporaneous controls. All patients had at least 14 days of follow-up from the time of testing. We limited the entire cohort (pre- and postimplementation) to patients who would have been screened for MAb eligibility based on a risk prediction score of at least 7.5 points, calculated using the same electronic method applied in the actual patient identification process (Supplementary Table 1). We then excluded patients who were either admitted at the time of COVID-19 testing or within 72 hours following testing. By doing this, we excluded patients who would not have been eligible for MAbs because of hospitalization or hypoxia at the time of screening and those who would not have had sufficient time to receive MAb treatment and derive benefit had treatment been available.
Because accurate time-from-symptom-onset data were not available, we also did a sensitivity analysis to explore whether there may have been differences in the time from testing to clinical deterioration and subsequent presentation at the ED or hospital between the pre-implementation controls, contemporaneous controls, and MAb-treated groups. The rationale for this was to evaluate whether patients in different groups may have been presenting for testing at a different stage in their course relative to symptom onset, potentially affecting the time to deterioration and need for medical evaluation.
The primary outcome used in both co-primary analyses was a composite of subsequent ED visits or hospitalizations in the 14 days after testing. This composite outcome was chosen to account for a change made to the standardized clinical criteria for hospital admission during the pre-implementation period that represented a risk for secular bias. In November 2020, an ED-based program was instituted triaging patients with exertional but not resting hypoxemia and normal inflammatory markers to be discharged home with supplemental oxygen and remote vital sign monitoring. This program did not change the likelihood of a patient initially presenting for ED care, however. Because no other changes to hospital admission criteria were made after November, we were also able to perform a planned sensitivity analysis looking at the treatment effect of MAbs on the outcome of hospitalization alone in the postimplementation cohort.
To estimate the effect of MAb therapy on subsequent ED visits or hospitalization, we first developed a propensity model using multivariable logistic regression to estimate the propensity for MAb treatment among all patients in the postimplementation group [10]. Propensity model variables included demographics, comorbidities, symptoms at time of testing, and geographic testing location and were selected on the basis of expert opinion, previous evidence, plausibility, and results of a focus group of clinicians involved in the MAb screening process (see Supplementary Table 3 for variables in the model). We then used the propensity model to conduct inverse probability of treatment weighting (IPTW) to estimate the average treatment effect in the treated (ATT) for MAb treatment [11]. Adequate covariate balance after IPTW was assessed using standardized mean differences. We used the same methodology for the analysis using the 14-day hospitalization outcome only and did not adjust for multiple comparisons because this was a sensitivity analysis. To aid in comparing these results with those from randomized trials, we used the Chatellier method of estimating the number needed to treat (NNT) from the odds ratio [12].
We then conducted ITS analysis using segmented regression with propensity weighting to estimate the change in the probability that a given patient would require ED or hospital care before and after MAb implementation [13]. The segmented regression for ITS analysis was conducted at the individual patient level with days as the time series unit, applying IPTW using the propensity model developed in the postimplementation cohort. The output of the model includes the baseline trend in the pre-implementation group and the level change and trend change postimplementation in the per-day probability of subsequent emergency department visit or hospitalization. This analysis was designed to isolate the effect of MAbs as much as possible from other measurable factors (changes in demographics, symptoms, comorbidities, age) and unmeasured factors (secular changes) by estimating the difference in probability that any given patient, weighted equally likely to be treated, would later require ED/hospitalization after MAb implementation.
Finally, we conducted a planned exploratory analysis to compare the efficacy of casirivimab/imdevimab with bamlanivimab. Methods for this analysis are described in the Supplementary Data. Statistical analyses were performed using R, version 4.3 (Vienna, Austria). Study elements fulfilled STROBE reporting guidelines for cohort studies.
Patient Consent
This observational study was reviewed by the Intermountain Institutional Review Board, which determined that it qualified for exemption from full board review and that it met qualifications for waiver of informed consent.
RESULTS
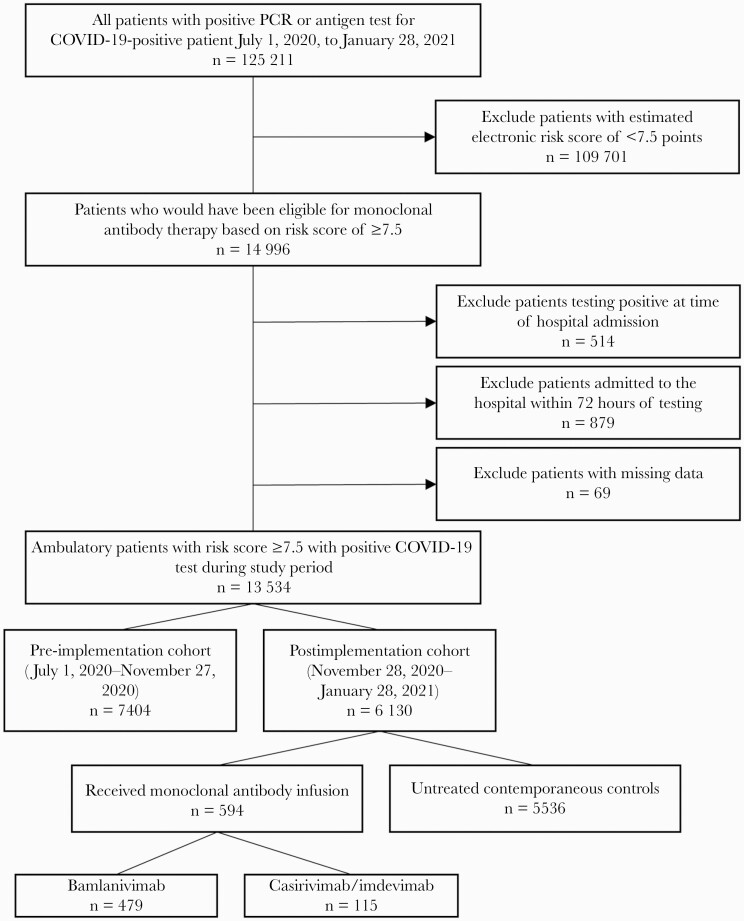
A total of 13 534 ambulatory patients within 7 days of onset of symptomatic, laboratory-confirmed COVID-19 with a risk score ≥7.5 points were included in the study, including 7404 patients in the pre-implementation period and 6130 in the postimplementation period (Figure 1). Of the latter, 594 (9.7%) patients received a monoclonal antibody infusion; 479 (80.6%) received bamlanivimab and 115 (19.4%) received casirivimab/imdevimab. Demographic and clinical characteristics by group are displayed in Table 1. The distribution of infusions by identification source was 462 (77.8%) program-initiated contact, 112 (18.8%) provider referral, and 20 (3.4%) self-referral. Urgent care was the most common infusion site (n = 317 [53%]), followed by infusion centers (272 [46%]) and 1 rural emergency department (5 [1%]). The median time from SARS-CoV-2 test sample collection to infusion (interquartile range [IQR]) was 53 (49–74) hours; this was consistent across sites, with a range of median time by site of 48.9–67.7 hours (Supplementary Table 2). Among patients who received MAb treatment, the mean age (SD) was 65 (13) years, and the median number of comorbidities (IQR) was 5 (3–6), of which obesity (397 [67%]), diabetes mellitus (390 [66%]), and chronic pulmonary disease (347 [58%]) were common. Factors included in the propensity model are listed in Supplementary Table 3. IPTW effectively reduced imbalance in the treated vs contemporaneous controls, as demonstrated by the standardized mean differences plot (Supplementary Figure 1).
Figure 1.
CONSORT-style flow diagram. Abbreviations: COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction.
Table 1.
Clinical Features by Treatment and Nontreatment Groups
| Variable | All | Monoclonal Treatment Group | Contemporaneous Control Group | Pre-implementation Cohort |
|---|---|---|---|---|
| No. | 13 534 | 594 | 5536 | 7404 |
| Age, mean (SD), y | 61 (15) | 65 (13) | 62 (15) | 60 (15) |
| Female, No. (%) | 6064 (44.8) | 240 (40.4) | 2531 (45.7) | 3293 (44.5) |
| Race, No. (%) | ||||
| American Indian or Alaska Native | 223 (1.7) | 3 (0.5) | 77 (1.4) | 143 (1.9) |
| Asian | 232 (1.7) | 5 (0.8) | 89 (1.6) | 138 (1.9) |
| Black or African American | 156 (1.2) | 4 (0.7) | 62 (1.1) | 90 (1.2) |
| Native Hawaiian or Pacific Islander | 585 (4.3) | 15 (2.5) | 170 (3.1) | 400 (5.4) |
| White | 11 437 (84.5) | 548 (92.3) | 4787 (86.5) | 6102 (82.4) |
| Ethnicity, No. (%) | ||||
| Hispanic or Latinx | 2737 (20.2) | 70 (11.8) | 933 (16.9) | 1734 (23.4) |
| Communities of colora | 4295 (31.7) | 99 (16.7) | 1494 (27.0) | 2702 (36.5) |
| Testing location, No. (%) | ||||
| Drive-up testing | 11 713 (86.5) | 562 (94.6) | 4868 (87.9) | 6283 (84.9) |
| Emergency department | 1379 (10.2) | 29 (4.9) | 523 (9.4) | 827 (11.2) |
| Other | 445 (3.3) | 2 (0) | 142 (2.6) | 284 (3.8) |
| Symptoms at testing, No. (%) | ||||
| Fever or chills | 4791 (35.4) | 181 (30.5) | 1826 (33.0) | 2784 (37.6) |
| Cough | 8411 (62.1) | 370 (62.2) | 3390 (61.2) | 4651 (62.8) |
| Shortness of breath or difficulty breathing | 4928 (36.4) | 173 (29.1) | 1928 (34.8) | 2827 (38.2) |
| Muscle or body aches | 7274 (53.7) | 328 (55.2) | 2984 (53.9) | 3962 (53.5) |
| Congestion or runny nose | 5925 (43.8) | 256 (43.1) | 2402 (43.4) | 3267 (44.1) |
| New loss of taste or smell | 2464 (18.2) | 78 (13.1) | 965 (17.4) | 1421 (19.2) |
| Sore throat | 4549 (33.6) | 198 (33.3) | 1815 (32.8) | 2536 (34.3) |
| Diarrhea | 2407 (17.8) | 91 (15.3) | 942 (17.0) | 1374 (18.6) |
| Total comorbidities, median (IQR) | 4 (3–5) | 5 (3–6) | 4 (3–5) | 4 (3–5) |
| Individual comorbidities, No. (%) | ||||
| Immunosuppressionb | 579 (4.3) | 34 (5.7) | 236 (4.3) | 309 (4.2) |
| Diabetes mellitus | 6719 (49.6) | 390 (65.7) | 2656 (48.0) | 3673 (49.6) |
| Coronary artery disease | 1346 (9.9) | 82 (13.8) | 529 (9.6) | 735 (9.9) |
| Active malignancy | 440 (3.3) | 23 (3.9) | 191 (3.5) | 226 (3.1) |
| Chronic pulmonary disease | 7183 (53.1) | 347 (58.4) | 2928 (52.9) | 3908 (52.8) |
| Chronic kidney disease | 2612 (19.3) | 188 (31.6) | 1077 (19.5) | 1347 (18.2) |
| Chronic liver disease | 3546 (26.2) | 170 (28.6) | 1476 (26.7) | 1900 (25.7) |
| Cerebrovascular disease | 2176 (16.1) | 117 (19.7) | 904 (16.3) | 1155 (15.6) |
| Hypertension | 10 699 (79.1) | 537 (90.4) | 4392 (79.3) | 5770 (77.9) |
| Chronic neurological disease | 2141 (15.8) | 93 (15.7) | 903 (16.3) | 1145 (15.5) |
| Congestive heart failure | 2033 (15.0) | 145 (24.4) | 827 (14.9) | 1061 (14.3) |
| Cardiac arrhythmia | 5677 (41.9) | 294 (49.5) | 2323 (42.0) | 3060 (41.3) |
| Obesityc | 8323 (61.5) | 397 (66.8) | 3416 (61.7) | 4510 (60.9) |
| Outcomes, No. (%) | ||||
| Emergency department visit (14 d) | 2442 (18.0) | 71 (12.0) | 944 (17.1) | 1427 (19.3) |
| Hospital admission (14 d) | 1412 (10.4) | 23 (3.9) | 538 (9.7) | 851 (11.5) |
| Mortality (14 d) | 129 (1.0) | 1 (0.2) | 57 (1.0) | 71 (1.0) |
| Composite outcome (14 d) | 2618 (19.3) | 75 (12.6) | 1018 (18.4) | 1525 (20.6) |
| Time from testing to first ED or admission, mean (SD), d | 6.9 (5.9) | 6.7 (5.8) | 7.1 (6.2) |
Abbreviations: ED, emergency department; IQR, interquartile range.
aSelf-identification as non-White race or Hispanic/Latinx ethnicity.
bSolid organ or hematopoietic stem cell transplant recipient, HIV, or currently receiving chemotherapy.
cBody mass index ≥30 kg/m2.
The primary outcome occurred in 75 (12.6%) patients in the MAb group, 1018 (18.4%) in the contemporaneous nontreated group, and 1525 (20.6%) in the pre-implementation cohort (Table 1). In the MAb treatment group, 23 (3.9%) patients were admitted to the hospital within 14 days of testing compared with 538 (9.7%) in the post- and 851 (11.5%) in the pre-implementation cohort. All-cause mortality at 14 days was 0.2% (n = 1) in the MAb treatment group compared with 1.0% (n = 71; P = .08) and 1.0% (n = 57; P = .05) in untreated patients in the pre- and postimplementation periods, respectively.
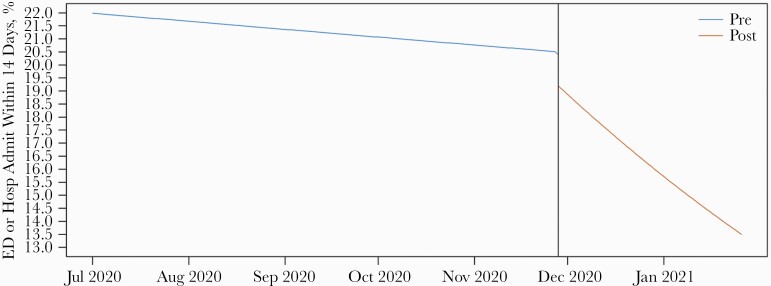
After IPTW, MAb-treated patients were significantly less likely to have an ED visit or hospitalization within 14 days of testing compared with contemporaneous controls (odds ratio estimating the ATT, 0.69; 95% CI, 0.60–0.79). This corresponds to an estimated NNT to prevent 1 medically attended visit of 7.6. In the sensitivity analysis, the odds of 14-day hospital admission were also significantly decreased in MAb-treated patients (odds ratio, 0.43; 95% CI, 0.35–0.53). In the interrupted time series analysis, the propensity-weighted probability of ED visit or hospitalization decreased by an additional 0.7% per day (95% CI, 0.03%–0.10%) after implementation of MAb treatment (Figure 2; Supplementary Table 4). The results of the comparison between bamlanivimab and casirivimab/imdevimab are listed in Table 2, and in the Supplementary Data and Supplementary Tables 5 & 6.
Figure 2.
Interrupted time series analysis estimating differences in the daily probability of emergency department visit or hospital admission pre- and postimplementation of monoclonal therapy. Abbreviation: ED, emergency department.
Table 2.
Clinical Features by Monoclonal Product
| Variable | All | Bamlanivimab | Casirivimab/Imdevimab |
|---|---|---|---|
| No. (%) | 594 | 479 (80.6) | 115 (19.4) |
| Age, mean (SD), y | 65 (13) | 65 (13) | 66 (15) |
| Female, No. (%) | 240 (40.4) | 186 (38.8) | 54 (47.0) |
| Race, No. (%) | |||
| American Indian or Alaska Native | 3 (0.5) | 3 (0.6) | 0 (0) |
| Asian | 5 (0.8) | 4 (0.8) | 1 (0.9) |
| Black or African American | 4 (0.7) | 3 (0.6) | 1 (0.9) |
| Native Hawaiian or Pacific Islander | 15 (2.5) | 14 (2.9) | 1 (0.9) |
| White | 548 (92.3) | 440 (91.9) | 108 (93.9) |
| Ethnicity | |||
| Hispanic or Latinx | 70 (11.8) | 55 (11.5) | 15 (13.0) |
| Communities of colora | 99 (16.7) | 80 (16.7) | 19 (16.5) |
| Total comorbidities, median (IQR) | 5 (3–6) | 5 (3–6) | 4 (3–5) |
| Individual comorbidities, No. (%) | |||
| Immunosuppressionb | 34 (5.7) | 28 (5.8) | 6 (5.2) |
| Diabetes mellitus | 390 (65.7) | 317 (66.2) | 73 (63.5) |
| Coronary artery disease | 82 (13.8) | 66 (13.8) | 16 (13.9) |
| Active malignancy | 23 (3.9) | 17 (3.5) | 6 (5.2) |
| Chronic pulmonary disease | 347 (58.4) | 282 (58.9) | 65 (56.5) |
| Chronic kidney disease | 188 (31.6) | 153 (31.9) | 35 (30.4) |
| Chronic liver disease | 170 (28.6) | 136 (28.4) | 34 (29.6) |
| Cerebrovascular disease | 117 (19.7) | 97 (20.3) | 20 (17.4) |
| Hypertension | 537 (90.4) | 435 (90.8) | 102 (88.7) |
| Chronic neurological disease | 0 (0) | 0 (0) | 0 (0) |
| Congestive heart failure | 145 (24.4) | 126 (26.3) | 19 (16.5) |
| Cardiac arrhythmia | 294 (49.5) | 237 (49.5) | 57 (49.6) |
| Obesityc | 397 (66.8) | 335 (69.9) | 62 (53.9) |
| Hours from test to infusion, median (IQR) | 53 (49–74) | 54 (49–74) | 52 (44–74) |
| Infusion location | |||
| Emergency department | 5 (0.8) | 5 (10.4) | 0 (0) |
| Infusion center | 272 (45.8) | 157 (32.8) | 115 (100) |
| Urgent care | 317 (53.4) | 317 (66.2) | 0 (0) |
| Infusion-associated adverse events | |||
| Any | 7 (1.2) | 6 (1.3) | 1 (0.9) |
| Mild | 5 (0.8) | 4 (0.8) | 1 (0.9) |
| Severed | 2 (0.3) | 2 (0.4) | 0 (0) |
| Outcomes | |||
| Emergency department visit (14 d) | 71 (12.0) | 62 (12.9) | 9 (7.8) |
| Hospital admission (14 d) | 23 (3.9) | 22 (4.6) | 1 (0.9) |
| Mortality (14 d) | 1 (0.2) | 1 (0.2) | 0 (0) |
| Composite outcome (14 d) | 75 (12.6) | 65 (13.6) | 10 (8.7) |
Abbreviation: IQR, interquartile range.
aSelf-identification as non-White race or Hispanic/Latinx ethnicity.
bSolid organ or hematopoietic stem cell transplant recipient, HIV, or currently receiving chemotherapy.
cBody mass index ≥30 kg/m2.
dSevere adverse events defined as requiring referral to emergency department for management.
Both monoclonal antibody products were well tolerated. A total of 7 (1.2%) patients experienced infusion-associated adverse events (Table 3). Two events (0.3%) were considered severe—1 patient with known coronary disease developed chest pain during infusion, and another had a syncopal episode; both patients were managed in the emergency department, with good outcomes. Five (0.8%) mild reactions were observed: pruritis, hives, rigors, nausea/vomiting, and oral tingling. None of the mild reactions required premature discontinuation of the infusion, and all were self-limited.
Table 3.
Infusion-Associated Adverse Events
| Reported Symptom | Drug | Outcome | Serious Adverse Event? |
|---|---|---|---|
| Chest pain | Bamlanivimab | Known coronary disease, resolved with anti-angina treatment in the emergency department | Yes |
| Oral tingling | Bamlanivimab | Self-limited | No |
| Pruritis without rash | Bamlanivimab | Infusion paused and safely resumed, treated with diphenhydramine | No |
| Hives | Casirivimab/imdevimab | Completed infusion, treated with diphenhydramine and short course of oral methylprednisolone | No |
| Rigors | Bamlanivimab | Infusion paused, resumed a slower rate, no hypotension | No |
| Nausea and emesis | Bamlanivimab | Completed infusion, palliated with ondansetron, persistent nausea lasted 4 d | No |
| Syncope | Bamlanivimab | Infusion terminated, patient was triaged to the emergency department and diagnosed with likely vasovagal etiology, no anaphylactoid features | Yes |
DISCUSSION
In this real-world evaluation of SARS-CoV-2 monoclonal antibody infusion therapy, administration of MAb treatment to a high-risk population of ambulatory patients with COVID-19 within 7 days of symptom onset was associated with significant reductions in subsequent emergency department visits and hospital admissions. At an estimated NNT of <8 to prevent 1 medically attended visit, the effect size is comparable to subgroup analyses of higher-risk patients reported in randomized controlled trials (bamlanivimab absolute difference, –10.1%; 95% CI, –21.4% to 1.2%; NNT = 10; casirivimab/imdevimab absolute difference [vs placebo], −9; 95% CI, −29 to 11; NNT = 11) [3–5]. It appears likely that focusing MAb treatment on patients both at greater risk for poor outcomes and earlier in the symptom course than specified by the EUA criteria [1, 2] may enhance the expected effectiveness of the therapy.
These results lend additional support to the concept that passive immune therapies [14] are effective when administered early after symptom onset when viral replication is highest [15] and in patients who fail to mount a robust early humoral response [5]. Of note, while the EUA for both agents authorizes administration for up to 10 days after symptom onset [1, 2], symptom onset beyond day 7 was an exclusion criterion in the casirivimab/imdevimab trial [5], and the median time from symptom onset to infusion in the bamlanivimab trial [3] was 4 days. Our patients’ experience also corroborates safety data from clinical trials, suggesting that MAb treatment is well tolerated. Serious events were rare, and no anaphylactic-type events were observed. However, data from larger populations will be necessary to accurately characterize the incidence of rare events.
Our study has several limitations. First, despite a target trial emulation design and complementary causal inference analyses, it is impossible to fully mitigate biases inherent to observational data. We recognize that in the postimplementation group, unmeasurable or unmeasured confounding factors may influence the estimates for counterfactual treatment effect. While impossible to fully mitigate, we are reassured that the complementary interrupted time series analysis does corroborate the effect estimate among those treated with MAbs. However, it is possible that unmeasured factors may have also influenced time series estimates. Although no other COVID-19 therapies for ambulatory patients have proven effective and use is uncommon for ambulatory patients in our health system, we were unable to measure concomitant prescription of other repurposed therapies such as corticosteroids.
An important limitation was that the date of symptom onset was not accurately available for all patients. While we attempted to limit the cohort by excluding patients who required medical care within 72 hours of test positivity, we acknowledge that it would have strengthened inference if we could have included the stage of illness via time from symptom onset in propensity models. There may also have been differences in patients who were identified through proactive screening vs provider or self-referral, but we could not assign a likely referral source for historical controls. Similarly, in the contemporaneous group, a common factor impacting the likelihood of being treated was whether a patient answered the phone on the day they were identified by screening, which may indeed correlate with unmeasured but potentially important confounding clinical characteristics. Although our integrated health system provides care for more than two-thirds of all COVID-19 hospitalizations in our region, it is possible that some patients may have been admitted to an ED or hospital outside of our system after testing, resulting in outcome misclassification.
Despite intentional and programmatic efforts at both the state and integrated health care network levels to address health care disparities in MAb delivery, we did observe a significantly lower rate of MAb infusion to patients from communities of color. This can be partially understood by the dramatic intercurrent decline in the proportion of non-White patients among positive cases in Utah during the study period, from 56.3% in July 2020 to 27.5% by February 2021. However, this does not fully account for differences in the postimplementation group and highlights the ongoing need to address equity in health care access to COVID-19 care. Finally, our sample size was not sufficiently powered to detect rare adverse events or to make conclusions regarding comparative effectiveness between agents.
CONCLUSIONS
In a real-world implementation targeting high-risk ambulatory patients with COVID-19 within 7 days of symptom onset, anti-SARS-CoV-2 monoclonal neutralizing antibody infusion appears to be well tolerated and likely effective at preventing subsequent ED visits or hospitalizations. These data may help guide patient selection to maximize the effectiveness of MAb therapy, an important consideration given logistical constraints of MAb delivery, and suggest that MAbs could play a greater role in preventing clinical deterioration and the need for hospitalizations or oxygen therapy if widely deployed in areas with high transmission and low vaccination rates. Additional investigation is needed to elucidate the impact on mortality or symptomatic improvement, the incidence of rare adverse events, and the comparative effectiveness of different Mab products against emerging variant strains.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. Study concept: B.J.W., J.B., W.B., T.V., I.D.P., S.M.B. Study design: B.J.W., W.B., S.M.B., A.M.B. Data collection: N.G., B.J.W. Statistical analysis: A.M.B., B.J.W., J.B., I.D.P., S.M.B. Interpretation of results: all authors. Manuscript preparation: all authors. Critical review of the manuscript: all authors.
Potential conflicts of interest. I.D.P. reports salary support through a grant from the National Institutes of Health (NIH) and, outside of the present study, grant support from the Centers for Disease Control and Prevention (CDC), Janssen, and support to his institution from Asahi Kasei Pharma. S.M.B. reports salary support from the US NIH, the CDC, and the Department of Defense; he also reports receiving support for chairing a data and safety monitoring board for a respiratory failure trial sponsored by Hamilton, effort paid to Intermountain for steering committee work for Faron Pharmaceuticals and Sedana Pharmaceuticals for ARDS work, support from Janssen for influenza research, and royalties for books on religion and ethics from Oxford University Press/Brigham Young University. B.J.W. reports partial salary support from a grant from the US Agency for Healthcare Research and Quality (AHRQ). E.S. receives partial salary support through grants from the CDC and AHRQ. E.S.S. serves on Gilead Sciences’ Advisory Board for remdesivir. At the time of submission, Intermountain Healthcare and the University of Utah have participated in COVID-19 trials sponsored by: AbbVie, Genentech, Gilead, Regeneron, Roche, the US NIH ACTIV, ACTT and PETAL clinical trials networks, and the Department of Defense; several authors (B.W., I.D.P., J.B., S.M.B., E.S., E.S.S.) were site investigators on these trials but received no direct or indirect remuneration for their effort. E.S.S., B.J.W., S.M.B., and M.S. are members of the Utah Crisis Standards of Care Scarce Medications Allocation Subcommittee. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. An EUA for bamlanivimab—a monoclonal antibody for COVID-19. JAMA. 2021; 325: 880– 1. [DOI] [PubMed] [Google Scholar]
- 2. An EUA for casirivimab and imdevimab for COVID-19. Med Lett Drugs Ther 2020; 62:201–2. [PubMed] [Google Scholar]
- 3. Chen P, Nirula A, Heller B, et al. ; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021; 384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021; 325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinreich DM, Sivapalasingam S, Norton T, et al. ; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 7. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 8. Utah Department of Health and Utah Hospital Association. Available at: https://www.utahhospitals.org/images/pdfs-doc/Utah_CSC_Monoclonal_Ab_Guidelines_v11_04202021.pdf. Accessed 12 July 2021. [Google Scholar]
- 9. Webb BJ, Levin NM, Grisel N, et al. Simple scoring tool to estimate risk of hospitalization and mortality in ambulatory and emergency department patients with COVID-19. MedRxiv 2021.02.22.21252171 [Preprint]. 23 February 2021. Available at: 10.1101/2021.02.22.21252171. Accessed 12 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 127:757–63. [DOI] [PubMed] [Google Scholar]
- 11. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chatellier G, Zapletal E, Lemaitre D, et al. The number needed to treat: a clinically useful nomogram in its proper context. BMJ 1996; 312:426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309. [DOI] [PubMed] [Google Scholar]
- 14. Libster R, Pérez Marc G, Wappner D, et al. ; Fundación INFANT–COVID-19 Group. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 2021; 384:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2021; 2:e13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.