Abstract
Despite evidence that ADHD is associated with disruptions in emotion regulation, few studies have examined the biological correlates of emotion dysregulation among children with this disorder. Prior work has pointed to roles of the parasympathetic and sympathetic nervous system, as indexed via respiratory sinus arrhythmia (RSA) and cardiac pre-ejection period (PEP), respectively. Work in typically developing populations suggests that parenting behavior and parental emotion expression may shape the development of these systems. To date, a single study has examined the independent and interactive roles of autonomic nervous system functioning and parent emotion expression in youth with ADHD. This study seeks to extend that work. 86 children (42 with ADHD), aged 8–12 years, and a parent completed a parent-child interaction task, while electrocardiography and impedance cardiography data were recorded to derive RSA and PEP. Parent and child emotion word use (positive and negative valence) were coded from recordings of the task. Parents of youth with ADHD used fewer positive emotion words throughout the task. Additionally, throughout the task, children with ADHD engaged in excessive RSA withdrawal from baseline. Further, the association between RSA reactivity and ADHD diagnosis was moderated by parent positive emotion word use. Specifically, those with RSA augmentation and parents displaying high positive affect across the task conditions were least likely to have an ADHD diagnosis. If replicated and extended, these results support the use of interventions specifically designed to increase parental modeling of positive emotions, while simultaneously focusing on building emotion regulation skills in youth with ADHD.
Keywords: Attention-deficit/hyperactivity disorder, Emotion regulation, Parenting
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent disorder affecting 7.8–11% of children, aged 4–17 years, in the United States (Polanczyk et al. 2014; Vitola et al. 2017). ADHD is characterized by inattention, hyperactivity, and/or impulsivity, as well as associated impairment across contexts (American Psychiatric Association 2013). Attempts to characterize ADHD have focused almost exclusively on behavioral, cognitive, and executive functioning deficits, as well as related neural underpinnings (American Psychiatric Association 2013; Barkley 1997; Castellanos and Tannock 2002; Willcutt et al. 2005). Yet, it is becoming increasingly clear that emotional functioning plays an important role in ADHD (Graziano and Garcia 2016; Petrovic and Castellanos 2016; Shaw et al. 2014).
Within the last decade or so, there has been increased recognition of the need to integrate emotional functioning into the conceptualization of, and clinical care for, youth with ADHD (Graziano and Garcia 2016; Karalunas et al. 2015; Martel 2009; Musser et al. 2011, Musser and Nigg 2017; Petrovic and Castellanos 2016; Shaw et al. 2014). For example, a recent meta-analysis of 77 studies suggests that ADHD is associated with disruptions in both emotion reactivity and emotion dysregulation (d = .75–.94), with effect sizes equal to those of executive function (d = .46–.69 range; Graziano and Garcia 2016), and a recent systematic review corroborates these findings (Shaw et al. 2014). Importantly, the aforementioned results take into account comorbid diagnoses, making it clear there is a unique association between ADHD and disruptions in emotional functioning (Graziano and Garcia 2016; Shaw et al. 2014). However, what is less clear is how emotion dysregulation develops in youth with ADHD. Prior work suggests roles for both parent emotional functioning and autonomic-linked pathophysiology in the development of emotion regulation difficulties in youth with ADHD (McQuade and Breaux 2017; Musser et al. 2011). This study seeks to examine the individual and interactive roles of parent emotional expression and autonomic-linked indexes of emotion regulation in childhood ADHD via a multimethod approach in the context of a challenging parent-child interaction task.
Parental Emotion and Children’s Emotional Development
Prior theory and research suggests that children learn to regulate their emotions in part via observational learning, modeling and social referencing; that is, parents’ emotional reactions and regulatory strategies may serve to socialize and teach children effective emotion regulation skills (Cole et al. 2009; Hersh and Hussong 2009). One way in which parents influence the development of emotion regulation is by directly engaging in emotional displays and interactions, thereby affecting the overall emotional tone of the home and family context via their own emotional expression, while also serving as models to their children (Denham et al. 1997; McCarty and Weisz 2002). Prior work has examined the emotional tone of the family via assessments of parental expressed emotion, which is classically understood as a two-dimensional coded construct composed of criticism and emotional overinvolvement (Miklowitz et al. 1984; Leff and Vaughn 1984). The criticism domain is designed to index negativity or resentment directed toward the child, while the emotional overinvolvement category indexes behaviors which are overprotective or overly self-sacrificing (Leeb et al. 1991; Magaña et al. 1986). One frequently used metric is parental expressed emotion assessed and coded during a five-minute speech sample (Baker et al. 2000; Leeb et al. 1991; Magaña et al. 1986; Miklowitz et al. 1984). Coding the five-minute speech sample for parental expressed emotion typically involves a consideration of the respondent’s speech content, including the use of emotion-laden words related to both positive and negative valence and tone (Magaña et al. 1986).
Importantly, disruptions in the emotional tone of the family and/or modeling of maladaptive parent emotional behavior has been linked to negative outcomes in youth (Hale et al. 2016). For example, high parental expressed emotion (and high parental criticism, in particular) has been associated with both oppositional/aggressive behavior and ADHD symptom severity (Asarnow et al. 2001; Cartwright et al. 2011; Caspi et al. 2004; Keown 2012; McCarty and Weisz 2002; Musser et al. 2016; Peris and Baker 2000; Peris and Hinshaw 2003; Peris and Miklowitz 2015; Pfiffner et al. 2005; Psychogiou et al. 2007, 2008; Sonuga-Barke et al. 2008, 2009, 2013). Similarly, expressions of parental criticism and negative emotion have been shown to be predictive of the continuation of externalizing behavior problems (including ADHD symptoms) across the middle childhood to adolescent age-range (Musser et al. 2016).
In sum, there is evidence that parental emotional expression shapes the development of child emotion regulation and that parental emotion expressions characterized by more negative emotional content/tone (including, criticism and anger) are associated with externalizing problems in youth, including ADHD. However, what is less clear is the mechanism by which this occurs. Prior work has primarily relied on parent-report, child-report, or behavioral codes of child emotion as indexes of emotion regulation (Bunford et al. 2015), while few studies have examined the independent and interactive associations among parental emotional expression and child physiological indexes of emotion regulation in the context of childhood ADHD.
Autonomic-Linked Indexes of Emotion Regulation in Childhood
The autonomic nervous system plays a key role in emotion reactivity and regulation (for a review see Beauchaine 2012). Cardiac-based indexes of both the sympathetic and parasympathetic branches have been developed based on substantive theoretical and empirical work (Beauchaine 2012). Specifically, parasympathetic-linked cardiac activity has been indexed via respiratory sinus arrhythmia (RSA), a marker of vagal influence over the heart, as verified by pharmacological blockade studies (Porges 2007). RSA has been repeatedly associated with parasympathetic-based regulation across context, including: behavioral, cognitive, emotional, and social (Porges 2007). Although it is somewhat context dependent, task-appropriate levels of RSA-withdrawal (i.e., reductions from rest) in response to a stressor are typically viewed as an adaptive emotion regulatory response (Beauchaine 2012; Porges 2007), while excessive RSA-withdrawal or RSA-augmentation (i.e., increases from rest) are typically viewed as maladaptive. Relatedly, sympathetic-linked cardiac activity has been indexed via cardiac pre-ejection period (PEP), a commonly used index of beta-adrenergic influence over the heart, which represents the time between depolarization of the left ventricle and the onset of ejection of blood into the aorta (Kelsey 2012; Newlin and Levenson 1979). PEP has been associated with emotional reactivity/arousal, reward sensitivity, and approach behaviors (Brenner et al. 2005; Brenner and Beauchaine 2011; Richter and Gendolla 2009). Generally, a shortening of PEP in response to a stressor is associated with an increase in sympathetic activation (Kelsey 2012; Newlin and Levenson 1979).
Over the past decade, a substantial literature has demonstrated that ADHD is associated with disruptions in emotion regulation, as indexed via RSA, (and to a lesser degree PEP reactivity) to a variety of tasks, including cognitive challenge, emotion induction and suppression, and reward-based tasks (Musser et al. 2011; Rash and Aguirre-Camacho 2012; Tenenbaum et al. 2017; Ward et al. 2015). This work has shown that ADHD is associated with: 1) reductions in positive emotional reactivity indexed by parasympathetic-linked (i.e., RSA) indexes of reactivity to positive emotion induction (Musser et al. 2011), 2) emotion dysregulation, indexed by RSA, during negative emotion suppression (Musser et al. 2011), 3) reduced coherence (i.e., correspondence, correlation) among emotional systems, including RSA and facial affective behavioral indexes (Musser et al. 2016), and 4) hyper-reactivity to reward at the behavioral level, but hyporeactivity to reward and dysregulation at the autonomic level of analysis, indexed by PEP and RSA, respectively (Tenenbaum et al. 2017). Thus, there is ample evidence to support an association between autonomic indexes of emotion reactivity and regulation in ADHD. However, it remains unclear whether, or in what way, environmental factors, such as parenting behavior or parental emotional expression, may interact with relevant autonomic indexes of emotional functioning in childhood ADHD.
Children’s Autonomic-Linked Emotion Regulation Interacts with Parental Emotion Expression in Shaping Child Outcomes
With respect to the associations among parental emotional expression, children’s autonomic-linked emotion regulation, and child outcomes, a large body of evidence suggests that a child’s autonomic-linked emotion (dys)regulation may serve as a protective (or risk) factor which modulates the impact that the family environment has on child outcomes, measured both cross-sectionally and longitudinally (for a review see El-Sheikh and Erath 2011).
However, to date, only a single study has examined these associations using autonomic indexes in youth with ADHD. Specifically, Breaux et al. (2017) examined whether parent-reported emotion socialization practices predicted parent-reported child emotion regulation, as well as autonomic indexes of child emotion regulation (including RSA and skin conductance obtained during an impossible puzzle and social rejection task), one year later. Breaux et al. (2017) further examined whether the association between parent emotion socialization and child emotion regulation was moderated by child ADHD symptoms in a sample of 61 children with and without clinically significant symptoms of ADHD. Here, it was determined that positive parent emotion socialization may play a protective role in the development of child emotion regulation (Breaux et al. 2017). While this study has a number of notable strengths, including its multimethod design (i.e., utilizing both parent-report and autonomic-indexes) and well-characterized sample, there are also several notable limitations, including: 1) the use of parental-report to assess parent emotion socialization which may introduce positive bias or issues of shared method variance, 2) child emotion regulation via autonomic indexes was measured outside the context of parent-child interaction resulting in questions related to specificity of the emotion regulatory effect, and 3) the relatively small sample size. Our study seeks to add to this small literature by using a multi-method design to assess the associations among childhood ADHD, and autonomic-based emotion regulation and parent emotional expression, as indexed during a parent-child interaction task.
Present Study
The current study seeks to examine parental emotional expression and autonomic-linked emotion regulation in youth with and without ADHD using a multi-method approach. We utilize specialized emotion word counting software to examine parental use of emotional words (in the domains of positive and negative valence), as well as electrocardiography and impedance cardiography (to derive RSA and PEP, respectively) to assess child autonomic-linked emotion regulation, in the context of a challenging parent-child interaction task. Dyads completing the task, included a parent and a child either with or without an ADHD diagnosis (provided via well-characterized, gold-standard diagnostic procedures). It was expected that youth with ADHD would engage in excessive RSA withdrawal (i.e., reduction from baseline, indexing parasympathetic-based dysregulation) and reduced PEP shortening (indexing sympathetic-based hypo-arousal) in the context of the parent-child interaction task, in line with prior literature on autonomic regulation in youth with ADHD. Additionally, parents of youth with ADHD were expected to engage in more negative and less positive emotional expression across the task, in line with prior work demonstrating increased criticism expression among parents of youth with ADHD. Finally, moderation models were utilized to examine the interactive roles of child autonomic regulation and parent emotion expression. Here, it was expected that childhood ADHD would be associated with excessive RSA withdrawal, and reduced PEP shortening, as well as high levels of negative expression and low levels of positive expression. These expectations were derived based on prior work in typically developing populations demonstrating that appropriate parental emotional modeling is associated with adaptive autonomic-linked emotion regulation, and that both harsh parenting/inappropriate parental emotion modeling and maladaptive child emotion regulation are associated with more negative outcomes (for a review see El-Sheikh and Erath 2011).
Participants
Participants were 86 children aged 8 to 12 years (mean age = 9.38, SD = 0.72). 42 met DSM 5 criteria for ADHD (39 combined, 2 inattentive, and 1 hyperactive/ impulsive presentation; American Psychiatric Association 2013). All parents provided written informed consent, and all children provided written assent. The local Institutional Review Board approved the study, which conformed to the Ethical Principles of Psychology and Code of Conduct (American Psychological Association 2017).
Procedures
Recruitment and Identification Procedures
Sample recruitment, assessment, and diagnostic assignment procedures followed those outlined in detail elsewhere <<masked for review>>. In brief, all families (both those of children with and without ADHD) were recruited via the community through public advertisements and mailings. Those families volunteering for the study passed through a multi-gate screening process to establish eligibility and diagnosis.
A parent and a teacher completed the ADHD Rating Scale (DuPaul et al. 1998) and Conner’s Rating Scale-3rd Edition (Conners 2008). A parent also completed the Kiddie Schedule for Affective Disorders and Schizophrenia-Epidemiological version (KSADS-E; Puig-Antich and Ryan 1996) with a master-degree level clinician. Children completed an IQ screening, including Block Design, Vocabulary, and Information sub-tests from the Wechsler Intelligence Scales for Children, Fourth Edition (WISC-IV; Wechsler 2003).
Final ADHD and other Diagnoses
Results from the KSADS-E, parent and teacher rating scales, and child IQ tests were presented to a diagnostic team (a board-certified child psychiatrist and licensed neuropsychologist) each of whom arrived independently at a “best estimate” diagnosis for ADHD, ADHD DSM 5 presentation, and all other disorders assessed by the KSADS-E, using DSM 5 criteria. Agreement rates were satisfactory (k > .75 for all disorders). Disagreements were resolved by conference, with exclusion if consensus was not easily achieved. To be included in the ADHD group, ADHD had to be the primary diagnosis.
Exclusion Criteria
Exclusion criteria included Full Scale IQ < 75, neurological impairment, seizures, or traumatic brain injury, current major depressive episode, lifetime mania or psychosis, and pervasive developmental disorder. Other disorders were free to vary. Children were excluded if taking psychoactive medications, which could not be washed out for the study.
Medication Washout
Children taking stimulant medication completed a medication washout of 24–48 h, depending on the type of stimulant preparation they were prescribed, as stimulant medications have been shown to impact autonomic functioning. As an extra precaution, stimulant prescription status (present or absent) was covaried.
Parent-Child Interaction Task Procedure
Children were video-taped with a parent as they completed two five-minute tasks designed by Kochanska and Aksan (1995, 2004) to require cooperation while inducing mild to moderate levels of frustration. In the first task, the parent and child were instructed to copy a line-drawing of a house using an Etch-A-Sketch (a drawing toy with two dials, with each dial controlling either horizontal or vertical lines). The parent was instructed to use only the vertical dial, and the child, to use only the horizontal dial. To complete the drawing within the five-minute time limit, both parties must collaborate. In the second task, the parent and child were asked to move a marble through a tilting-maze with dead ends and holes through which the marble could drop, requiring the dyad to begin again. The tilting and maneuvering action was again controlled by two dials, one of which was controlled by the parent and the other of which was controlled by the child. The dyad was told that most families complete the maze task in the five-minute limit; however, in reality the task is meant to be quite challenging and impossible to finish within five minutes. The task conditions are thought to be minimally and moderately frustrating, respectively.
Language Inquiry and Word Count (LIWC) Software
Both interaction tasks were transcribed separately for parents and children. Transcriptions were coded for total word count, as well as for positive and negative affective words (separately for parents and children) using Language Inquiry and Word Count (LIWC) software (Pennebaker et al. 2015). LIWC is a text analysis program that counts words in preset categories (Tausczik and Pennebaker 2010). For the present study, word usage in the domains of positive (e.g., best, fun, share) and negative emotional valence (e.g., fight, hate, inadequate), as well as total word counts were obtained.
Physiological Recording
Overview
Psychophysiological data acquisition procedures followed those outlined elsewhere <<masked for review>>. In brief, disposable silver/silver-chloride electrodes were placed in a standard lead II electrocardiogram (ECG) and impedance cardiography (ICG) configuration. ECG and ICG were recorded continuously throughout the baseline and each parent-child interaction task. The R-R series was sampled at 1000 Hz. ECG and IMP motion artifacts were examined and removed using the MindWare software and visual inspection; with 25% completed by two raters and inter-rater agreement readily achieved (k > 0.9 for each task epoch).
Respiratory Sinus Arrhythmia (RSA)
RSA was indexed by extracting the high frequency component (>0.15 Hz) of the R-R peak time series using MindWare® Heart Rate Variability V. 2.6. RSA was derived using spectral analysis of the R-R time series from the ECG (Berntson et al. 1997). The time series was detrended and submitted to a Fourier transformation. The high frequency band (ln(ms2)) was set over the respiratory frequency band of 0.24 to 1.040 Hz, which was derived from the impedance cardiograph signal (Zo) ensuring that these signals remained within the analytical bandwidth.
Cardiac Pre-Ejection Period (PEP)
PEP was derived from ECG and ICG in 60 s epochs, using MindWare Impedance Cardiography V. 2.6, allowing for simultaneous editing of motion artifacts in the data obtained from ECG and ICG. PEP was indexed as the time interval in milliseconds from the onset of the Q-wave to the B point of the dZ/dt wave, using the method outlined by Berntson and colleagues (Berntson et al. 2004).
Analytic Plan
Primary Hypothesis Test of Group Comparisons
In order to test primary hypotheses of interest, separate group comparisons across primary variables of interest (i.e., parent LIWC -rated positive valence words, parent LIWC-rated negative valence words, RSA reactivity scores [i.e., task-rest], and PEP reactivity scores [i.e., task-rest]) were conducted using separate, mixed-models via repeated-measure analysis of covariance (RM-ANCOVA) with ADHD diagnostic status as the independent variable. Thus, a total of four RM-ANCOVA were performed to test primary study hypotheses. Simple effects by group are presented in Table 2, but only examined in text when justified by the results of higher order effects. SPSS version 24 was utilized for all analyses.
Table 2.
Simple effect-based differences in parent and child Linguistic Inquiry and Word Count (LIWC) word counts, affect words, and child respiratory sinus arrhythmia (RSA; ms2) and pre-ejection (PEP; ms) by task epoch according to group
| Variable | ADHD (mean, s.e.) | Control (n = 42) | F-value (n = 44) | Partial eta2 |
|---|---|---|---|---|
| LIWC—Child Scores | ||||
| Etch-A-Sketch | ||||
| Word Count | 156.95, 16.26 | 159.82, 15.48 | 0.01 | .000 |
| Positive affect | 6.07, 0.80 | 5.87, 0.77 | 0.03 | .000 |
| Negative affect | 1.51, 0.31 | 0.40, 0.30 | 4.81 | .060* |
| Maze | ||||
| Word Count | 146.55, 15.00 | 173.56, 14.28 | 1.25 | .016 |
| Positive affect | 3.89, 0.56 | 4.95, 0.53 | 1.41 | .018 |
| Negative affect | 1.47, 0.30 | 1.56, 0.29 | 0.03 | .000 |
| LIWC--Parent Scores | ||||
| Etch-A-Sketch | ||||
| Word Count | 381.01, 24.93 | 366.13, 23.73 | 0.14 | .002 |
| Positive affect | 7.66, 0.64 | 10.22, 0.61 | 6.17 | .074* |
| Negative affect | .066, 0.12 | 0.75, 0.11 | 0.22 | .003 |
| Maze | ||||
| Word Count | 259.54, 108.01 | 413.15, 102.82 | 0.78 | .010 |
| Positive affect | 7.82, 0.73 | 9.41, 0.70 | 1.82 | .023 |
| Negative affect | 1.11, 0.21 | 0.99, 0.20 | 0.13 | .002 |
| Baseline physiology data | ||||
| RSA | 7.74, 0.22 | 7.25, 0.21 | 1.87 | .023 |
| PEP | 100.28, 2.16 | 102.39, 2.06 | 0.37 | .005 |
| Task physiology raw scores | ||||
| Etch-a-sketch | ||||
| RSA | 6.73, 0.24 | 6.93, 0.23 | 0.28 | .004 |
| PEP | 99.67, 1.89 | 100.14, 1.82 | 0.02 | .000 |
| Marble Maze | ||||
| RSA | 6.73, 0.18 | 6.84, 0.17 | 0.12 | .002 |
| PEP | 101.07, 1.98 | 101.00, 1.95 | 0.00 | .000 |
| Task physiology change scores | ||||
| Etch-A-Sketch - Rest Baseline | ||||
| RSA | −1.01, 0.21 | −0.32, 0.18 | 4.00 | .046* |
| PEP | −0.78, 1.38 | −2.72, 1.34 | 0.75 | .010 |
| Marble Maze - Rest Baseline | ||||
| RSA | −0.95, 0.19 | −0.43, 0.18 | 2.97 | .036 |
| PEP | 0.57, 1.24 | −0.86, 1.20 | 0.50 | .006 |
Differing superscripts (*) indicate comparisons of simple group effects that were significant p < .05 for continuous Language Inquiry Word Count (LIWC) variables, as well as respiratory sinus arrhythmia (RSA) and cardiac preejection period (PEP) via ANCOVA, including the following covariates: child sex, estimated full scale IQ, child medication status, and whether the child has any comorbid diagnosis
Follow-Up Moderation
Moderation models were tested using the procedures proposed by Hayes and Matthes (2009) and Hayes (2012), to examine whether parent affect language use (i.e. positive and negative valence in separate analyses) during the parent-child interaction task moderated the association between child psychophysiological indexes (i.e., RSA and PEP reactivity scores in separate analyses) and ADHD diagnosis. Thus, a total of four moderation models were examined. The PROCESS macro (Hayes 2012) was used to conduct logistic regression-based moderation analyses in SPSS version 24 and examine the interaction of relevant word count variables and RSA (and separately PEP) reactivity in predicting ADHD as a category and dimensionally. Predictor variables were mean centered prior to analysis. The PROCESS macro provides conditional effects (www.processmacro.org). Johnson-Neyman conditional effects are presented for moderation results and indicate the significance of the conditional effect across a range of values of the moderator (Bauer and Curran 2005).
Power Analysis
G*Power was used (Faul et al. 2007). Using our sample size of 86 participants, power was 0.99 to detect an ADHD vs. non-ADHD group difference across two measurements correlated at 0.5 or greater with a significance level set at p < 0.05 and an effect size estimated to be partial eta2 of 0.05 or greater, which is a small effect size (Cohen 1992). Adding covariates (e.g., child sex, IQ, the use of stimulant medications, comorbid disorders) reduces this power estimate only slightly.
Results
Preliminary Analyses
Descriptive and Diagnostic Overview of Sample
Descriptive statistics for demographic and clinical characteristics are provided in Table 1. Groups did not differ with respect to child age,ethnicity/race, family income, or parent biological sex (all F < 1.28, all p > .26). However, youth with ADHD were more likely to be male, prescribed a stimulant medication, and have a somewhat lower IQ than typically developing youth (all F > 8.28, all p < .01; see Table 1). These characteristics (i.e., child sex, stimulant use, and IQ) were included as covariates in all analyses. Comorbid diagnostic statistics are also presented in Table 1. Groups did not differ with respect to frequency of diagnoses of mood or conduct disorders (all X2 < 3.26, p > .07), but youth with ADHD were more likely to be diagnosed with either an anxiety disorder or ODD (all X2 > 5.56, p < .02; see Table 1), as is typical among youth with ADHD. Presence of comorbid diagnosis was included as a covariate in all analyses.
Table 1.
Descriptive and diagnostic statistics for ADHD and control groups
| Variable | ADHD (n = 42) | Control (n = 44) | F or Xb | Partial-etab |
|---|---|---|---|---|
| Demographics | ||||
| Age (months; mean, SD) | 9.41, 0.78 | 9.35, 0.66 | 0.13 | .002 |
| Child biological sex (% male) | 81.0% | 40.9% | 14.41 | .409* |
| Ethnicity and Race (% White) | 88.1% | 81.8% | 0.66 | .088 |
| Fam. Income (% < $50 K) | 35.7% | 27.3% | 0.71 | .091 |
| Stimulant Med. (% on med.) | 61.9% | 0.0% | 35.48 | .642* |
| WISC-IVa FSIQb (mean, SD) | 107.93, 13.97 | 116.11, 12.39 | 8.28 | .090* |
| Parent biological sex (% male) | 14.3% | 6.8% | 1.28 | .122 |
| ADHD-RSc-Parent Report (mean, SD) | ||||
| Hyperactive/Impulsive T-score | 71.38, 15.15 | 44.09, 7.63 | 112.83 | .573* |
| Inattentive T-score | 71.05, 11.90 | 44.32, 8.62 | 143.36 | .631* |
| Total T-score | 72.74, 13.47 | 44.02, 8.38 | 142.36 | .629* |
| Hyperactive Symptoms | 5.26, 2.68 | 0.27, 0.90 | 136.63 | .619* |
| Inattentive Symptoms | 6.10, 2.77 | 0.32, 1.43 | 150.17 | .641* |
| ADHD-RS-Teacher Report (mean, SD) | ||||
| Hyperactive/Impulsive T-score | 56.23, 9.16 | 44.62, 4.72 | 47.98 | .397* |
| Inattentive T-score | 56.71, 7.07 | 43.12, 4.18 | 102.99 | .585* |
| Total T-score | 56.90, 7.80 | 43.43, 4.28 | 86.51 | .542* |
| Hyperactive Symptoms | 3.76, 3.06 | 0.16, 0.44 | 51.55 | .414* |
| Inattentive Symptoms | 4.84, 2.98 | 0.25, 0.73 | 84.62 | .537* |
| CD Symptoms | 0.27, 0.65 | 0.00, 0.00 | 6.54 | .082* |
| ODD Symptoms | 1.38, 2.27 | 0.05, 0.32 | 12.75 | .149* |
| Comorbid Disorders (%; K-SADSd,e) | ||||
| Mood Disorder (past year) | 7.1% | 0.0% | 3.26 | .195 |
| Anxiety Disorder | 26.2% | 4.5% | 7.85 | .302* |
| Conduct Disorder (CD) | 2.4% | 0.0% | 1.06 | .111 |
| Oppositional Defiant (ODD) | 11.9% | 0.0% | 5.56 | .254* |
| Any Comorbid Diagnosis | 38.1% | 4.5% | 14.62 | .412* |
Differing superscripts (*) indicate comparisons that were significant p < .05 for continuous variables via ANOVA, including: age, estimated full-scale IQ, ADHD-RS and Conner's parent and teacher rating scales; and for categorical variables va Chi-square, including: child sex, race, family income, parent gender, child medication status, and comorbid disorders
WISC-IV: Wechsler Intelligence Scales for Children
Full-Scale Intelligence Quotient (estimated)
Attention-Deficit/Hyperactivity Disorder Rating Scale
Kiddie Schedule of Affective Disorders and Schizophrenia
0% of the sample had autism (or other pervasive developmental disorder), eating disorders, current mood disorder, post-traumatic stress disorder, psychosis, or substance use disorders
Task Manipulation Checks
To verify that parents and children found the Marble Maze task to be more evocative of negative emotion than the Etch-A-Sketch task, paired-sample t-tests were conducted to examine mean differences in parent and child positive and negative affective word use. As expected, it was observed that children expressed more positive affect words during the Etch-A-Sketch (M = 5.88, SD = 3.91) than Marble Maze tasks (M = 4.46, SD = 2.69; t(85) = 4.47, p < .001), and children expressed more negative affect words during the Marble Maze (M = 1.56, SD = 1.49) than the Etch-A-Sketch task (M = 0.95, SD = 1.57; t(85) = 3.01, p = .003). Additionally, parents expressed more negative affect words during the Marble Maze (M = 1.02, SD =1.03) than the Etch-A-Sketch task (M = 0.72, SD = 0.61; t(85) = 2.83, p = .006). However, no significant difference in positive word use was observed (t(85) = 1.63, p = .107). Thus, the task conditions were successful in eliciting the expected pattern of emotion for parents and children.
Primary Analyses
Child and Parent Affective Word use during Parent-Child interaction tasks
Table 2 presents descriptive statistics for LIWC coded child and parent word counts (i.e., total words, total positive and negative affect words), as well as simple effects comparisons, which are only interpreted when justified (below). Here, 2*2 repeated measures ANCOVA were used to examine differences in LIWC coded child and parent word counts.
Child Affective Word Use
Beginning with total words used by children, the main effects of task condition (F(1,83) = 0.02, p =.90, np2 < .01) and group (F(1,83) = 0.45, p = .51, np2 < .01) were each non-significant, as was the interaction of group*task condition (F(1,83) = 1.07, p = .30, np2 < .01; see Table 2 for simple effects). A similar pattern was observed for child use of positive affective words; such that, the main effects of task condition (F(1,83) = 0.17, p = .69, np2 < .01) and group (F(1,83) = 0.19, p = .66, np2 < .01) were each non-significant, as was the interaction of group*task condition (F(1,83) = 1.53, p = .22, np2 < .01; see Table 2 for simple effects). Thus, there were no meaningful differences in either total word use or positive word use by children according to task condition and/or group.
With respect to child use of negative affective words, again the main effects of task condition (F(1,83) = 1.76, p = .19, np2 = .02) and group (F(1,83) = 1.88, p = .17, np2 = .02) were each non-significant; however, the interaction of group*task condition was significant (F(1,83) = 3.78, p = .04, np2 = .05). Youth in the ADHD group (M = 1.51, S.E. = 0.31) used significantly more negative affective words during the Etch-A-Sketch task than typically developing youth (M = 0.40, S.E. = 0.30; F(1,83) = 4.81, p = .03, np2 = .06). In contrast, groups did not differ in negative affect word use during the Marble Maze task (F(1,83) = 0.03, p = .86, np2 < .01; see Table 2 for simple effects). Thus, youth with ADHD tended to use more negative affect words in the Etch-A-Sketch task.
Parent Affective Word Use
Turning to parent total word use, the main effects of task condition (F(1,83) = 0.03, p = .85, np2 < .01) and group (F(1,83) = 0.58, p = .45, np2 = .01) were each non-significant, as was the interaction of group*task condition (F(1,83) = 0.93, p = .34, np2 = .01; see Table 2 for simple effects). A similar pattern was observed for parent use of negative affective words; such that, the main effects of task condition (F(1,83) = 0.01, p = .96, np2 < .01) and group (F(1,83) = 0.01, p = .94, np2 < .01) were each non-significant, as was the interaction of group*task condition (F(1,83) = 0.40, p = .53, np2 = .01; see Table 2 for simple effects). Thus, there were no differences in either total word use or negative word use by parents according to task condition and/or group.
With respect to parent use of positive affective words, again the main effects of task condition (F(1,83) = 0.01, p = .92, np2 < .01) was non-significant. However, there was a significant main effect of group (F(1,83) = 4.53, p = .04, np2 = .06). Specifically, parents of youth in the ADHD group (M = 7.74, S.E. = 0.61) used significantly fewer positive affect words when averaging across the two tasks than typically developing youth (M = 9.81, S.E. = 0.58). With respect to parent positive affect word use, the interaction of group*task condition was non-significant (F(1,83) = 0.86, p = .36, np2 = .01; see Table 2 for simple effects). Thus, parents of youth with ADHD tended to use fewer positive affect words across the task conditions.
Child Psychophysiology Data during Parent-Child Interaction Tasks
Table 2 presents descriptive statistics for child psychophysiology variables (i.e., RSA and PEP) according to group and task condition. 2*2 repeated measures ANCOVA were used to examine differences for child psychophysiological variables.
Baseline Effects
During the resting baseline period, there were no significant group differences in RSA or PEP (all F < 1.87, all p > .15; see Table 2 for simple effects).
Parent-Child Interaction Task Effects
With respect to child RSA reactivity during the parent-child interaction task, the main effects of task condition (F(1,83) = 0.13, p = .72, np2 < .01) was non-significant, as was the group*task condition interaction (F(1,83) = 0.41, p = .53, np2 = .01). However, there was a significant main effect of group (F(1,83) = 4.37, p = .04, np2 = .05; see Table 2 for simple effects). Specifically, youth in the ADHD group (M = −1.00, S.E. = 0.18) engaged in significantly more RSA withdrawal from baseline across the two tasks than typically developing youth (M = −0.37, S.E. = 0.17). Thus, children with ADHD tended to respond to the tasks with excessive RSA withdrawal from baseline when compared to typically developing youth. With respect to PEP reactivity, none of the effects were significant (all F < 0.94, p > .3).
Test of Moderation Effects
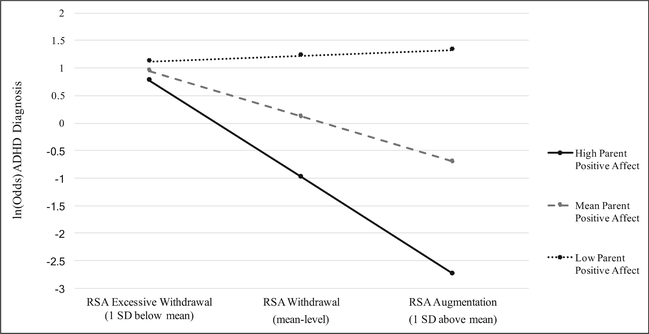
In line with our primary hypothesis, the interaction of RSA reactivity by parental positive affect was significant (z = −2.27, b = −0.26, p = 0.03; see Fig. 1). Conditional effects indicated that when high levels of parental positive affect were present, augmented RSA (i.e., increase from baseline) was associated with a decreased likelihood of an ADHD diagnosis. However, when low parental positive affect was present, augmented RSA was associated with an increased likelihood of an ADHD diagnosis. Importantly, this was not the case for children demonstrating excessive RSA withdrawal, as these youths appeared to be an elevated likelihood of an ADHD diagnosis irrespective of parent affect (see Fig. 1 and Table 3). This pattern held even after covarying child negative affect during the task(z = −2.19, b = −0.32, p = 0.03), suggesting that the moderating effect of parent positive affect was independent of child affect during the task.
Fig. 1.
Interaction between parent positive affect and child RSA reactivity in predicting the likelihood of child ADHD diagnosis, when averaging across the parent-child interaction tasks. Note: Odds of an ADHD diagnosis are greatest for those experiencing excessive RSA withdrawal across the task conditions; however, for children with parents displaying low positive affect, augmented RSA was associated with greater odds of being in the ADHD group. For children whose parents offer high positive affect, RSA augmentation was associated with the lowest odds of being in the ADHD group (z = −2.27, b = −0.46, p = 0.03). Of note, results are presented based on the mean and ± 1 SD for illustrative purposes only. Continuous measures of parent affect and respiratory sinus arrhythmia were utilized in all analyses
Table 3.
Conditional effects of child respiratory sinus arrhythmia reactivity (RSA; ms2) on ADHD outcome by level of the moderator (parent positive/negative affect expression) when averaging across the parent-child interaction tasks
| Outcome | Parent affect* RSA | b | SE | z | 95% CI | p |
|---|---|---|---|---|---|---|
| Positive | ||||||
| ADHD diagnosis | − 1 SDa | 0.10 | .67 | 0.15 | −1.21, 1.41 | .88 |
| Mean | −0.81 | .45 | −1.72 | −1.73, 0.11 | .09 | |
| 1 SDb | −1.72 | .79 | −2.17 | −3.26, −0.17 | .03* | |
| Negative | ||||||
| ADHD diagnosis | − 1 SDa | 0.32 | .44 | 0.72 | −0.55, 1.19 | .47 |
| Mean | −0.81 | .28 | −0.29 | −0.64, 0.47 | .77 | |
| 1 SDb | −0.48 | .736 | −1.33 | −1.18, 0.24 | .19 | |
Differing superscripts (*) indicate significance p < .05; simple effects are presented based on the mean and ± 1 SD for illustrative purposes only. Continuous measures of parent affect and respiratory sinus arrhythmia were utilized in all analyses.
−1 SD below the mean indicates excess withdrawal in response to the task.
1 SD above the mean indicates average withdrawal or augmentation of RSA in response to the task
With respect to parental negative affect’s moderating role in the association between RSA reactivity and risk of ADHD diagnosis, the interaction fell short of statistical significance (z = −1.39, b = −0.57, p = 0.16; see Table 3 for conditional effects). Finally, none of the moderation models examining the interaction of PEP reactivity with parental affect words were significant (all Z < 0.81, all p > .42); thus, these models were not examined further.
Discussion
This study examined the independent and interactive associations among parental emotional expression (i.e., counts of positive and negative valence word use), autonomic-linked emotion regulation (i.e., RSA and PEP), and childhood ADHD, during an emotionally evocative parent-child interaction task. Results were generally in line with hypotheses and prior literature with some caveats. Further, results should be interpreted in the context of several preliminary analyses of the task conditions. Specifically, both parents and children utilized significantly more negative affect words during the Marble Maze than the Etch-A-Sketch task, while children utilized more positive affect words during the Etch-A-Sketch than Marble Maze task. This pattern of results was expected and suggests that, as designed, the Marble Maze task was more challenging/emotionally evocative (Kochanska and Aksan 1995, 2004).
With respect to the primary results, youth with ADHD were found to use significantly more negative affect words during the Etch-A-Sketch task than typically developing youth, while the groups did not differ in negative affect word use during the Marble Maze task or positive affect word use across task conditions. Specifically, youth with ADHD utilized an equivalent number of negative affect words across the task conditions, while typically developing youth utilized significantly fewer negative affect words during the Etch-A-Sketch than the Marble Maze task. This suggests that youth with ADHD found both task conditions to be equally unpleasant, while typically developing youth found the Marble Maze to be more unpleasant. This is in line with the only prior study to examine parent emotion socialization and children’s emotion regulation in ADHD youth (Breaux et al. 2017), which found that parents (and teachers) of youth with elevations in ADHD symptoms were more likely to rate their children as high in negative emotional lability. However, it is notable that the effect size for the group difference in negative affect word use during the Etch-A-Sketch task was in the small range, and the clinical utility of such a difference is unknown. With this in mind, it may be that youth with ADHD are slightly more likely to display negative emotion than their typically developing peers, particularly during low level emotionally evocative situations, when it may be less adaptive.
Moving on to parent affective word use, parents of youth with ADHD were found to use significantly fewer positive affect words during the Etch-A-Sketch task. Thus, parents of youth with ADHD were found to be less positive when interacting with their children than parents of typically developing youth, during a mildly emotionally evocative task, but not during a more strongly emotionally evocative task. This finding is somewhat consistent with prior work that shows reduced positive parenting practices among dyads including children with ADHD compared to those without an ADHD diagnosis (Edwards et al. 2001; Johnston and Mash 2001; Lifford et al. 2008).
In examining child parasympathetic regulation (indexed via RSA reactivity), youth with ADHD engaged in significantly greater amounts of RSA withdrawal from baseline across the Etch-A-Sketch and Marble Maze tasks than typically developing youth. This difference was primarily driven by excessive RSA withdrawal by ADHD youth in the Etch-A-Sketch task. This is in line with prior work showing excessive RSA withdrawal during challenging tasks among children with externalizing problems (for a meta-analysis see Graziano and Derefinko 2013) and those with ADHD (Ward et al. 2015). Thus, youth with ADHD demonstrated parasympathetic-based dysregulation compared to typically developing youth, and of particular interest, youth with ADHD displayed excessive RSA withdrawal during a lower level emotionally evocative task. Such withdrawal may be particularly maladaptive.
Importantly, upon examining the role of parent affect word use in moderating the association between child RSA reactivity and ADHD diagnosis, it was determined that excessive RSA withdrawal during the parent-child interaction task was associated with ADHD diagnosis, and this was true regardless of parent’s positive affect word use. Similarly, it appears that low levels of parent positive affect word use were associated with child ADHD diagnosis, regardless of child RSA reactivity to the task conditions. However, average levels of RSA withdrawal and/or augmentation of the RSA response were associated with ADHD only when parent positive affect word use was below average. Thus, it appears that both parasympathetic-based regulation and low levels of parent positive affect are associated with child ADHD, while the combination of adaptive child parasympathetic-based regulation and parent positive affect word use is negatively associated with child ADHD. These results are similar to, but distinct from, prior work by Breaux et al. (2017), which showed that supportive (i.e., positive) parenting practices are associated with improved child emotion regulation (as indexed via parent report and an index of sympathetic nervous system reactivity), particularly among youth with elevated symptoms of ADHD (Breaux et al. 2017).
Limitations and Future Directions
Key limitations should be noted. First, the sample size was relatively small, and too small to evaluate other clinical characteristics in explaining heterogeneity in these results. Although the effects reported here cannot be explained by the presence of comorbid disorders, given that the presence of comorbid diagnosis was treated as a covariate, it should be noted that youth with ADHD in this sample were more likely to have both ODD and anxiety disorders. Future studies should carefully consider this, and may identify more severe dysregulation in subgroups of children with ADHD, for example, those with comorbid anxiety or ODD, given the associations between those diagnoses and emotion dysregulation. Next, while the use of emotional words may approximate the emotional tone of the family (as in the expressed emotion literature), emotion words are only one indicator of emotional expression. Future studies should incorporate a multimethod analysis of parent emotional expression including behavioral indexes. Additionally, this study was cross-sectional, so direction of effects cannot be determined, and it is likely that there are bi-directional effects at play. Thus, in order to further examine the directionality of these effects longitudinal designs are needed. Finally, it will be of interest in larger samples to identify which children with ADHD have this pattern of dysregulation and whether it is predictive of course, impairment, response to treatment, or other outcomes.
Clinical Implications
Despite noted limitations, this study has several strengths and important clinical implications. Specifically, if replicated, these results may support the use of interventions designed to increase parental expressions of positive emotions and modeling appropriate emotion regulation skills. Such interventions could simultaneously focus on building emotion regulation skills in youth with ADHD, while helping youth become more aware of physiological responses to emotion.
Acknowledgements
We are grateful to the families and teachers who gave their time to participate in this study. Additionally, we would like to thank Joel T. Nigg, Ph.D. whose generous support made this project possible.
Funding Funding for this project was provided by NIMH (R03MH110812-02, first author [Musser]; R01 MH59105, Joel Nigg, Ph.D.)
Footnotes
Conflicts of Interest Erica D. Musser, Yulie Lugo, Anthony R. Ward, Rachel B. Tenenbaum, Stephanie Morris, Nisha Brijmohan and Jessica Martinez declare that they have no conflicts of interest.
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were completed in accordance with the ethical standards of the institutional and/or national research committee and with the 1064 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent and assent (for children under age 18 years) were obtained from all participants included in this study.
Experiment Participants All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- American Psychological Association. (2017). Ethical principles of psychologists and code of conduct (2002, Amended June 1, 2010 and January 1, 2017). 10.1037/0003-066X.57.12.1060. [DOI]
- Asarnow JR, Tompson M, Woo S, & Cantwell DP (2001). Is expressed emotion a specific risk factor for depression or a nonspecific correlate of psychopathology? Journal of Abnormal Child Psychology, 29(6), 573–583. 10.1023/A:1012237411007. [DOI] [PubMed] [Google Scholar]
- Baker BL, Heller TL, & Henker B (2000). Expressed emotion, parenting stress, and adjustment in mothers of young children with behavior problems. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 41(7), 907–915. 10.1111/1469-7610.00678. [DOI] [PubMed] [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, & Curran PJ (2005). Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behavioral Research, 40(3), 373–400. 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2012). Physiological measures of emotion from a developmental perspective: State of the science: Physiological markers of emotion and behavior dysregulation in externalizing psychopathology. Monographs of the Society for Research in Child Development, 77(2), 79–86. 10.1111/j.1540-5834.2011.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Bigger TJ, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … Van Der Molen MW (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ, & Cacioppo JT (2004). Where to Q in PEP. Psychophysiology, 41(2), 333–337. 10.1111/j.1469-8986.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Breaux RP, McQuade JD, Harvey EA, & Zakarian RJ (2017). Longitudinal associations of parental emotion socialization and children’s emotion regulation: The moderating role of ADHD symptomatology. Journal of Abnormal Child Psychology, 1–13. 10.1007/s10802-017-0327-0. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP, & Sylvers PD (2005). A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology, 42(1), 108–115. 10.1111/j.1469-8986.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Brenner SL, & Beauchaine TP (2011). Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: A pilot study. Psychophysiology, 48(11), 1588–1596. 10.1111/j.1469-8986.2011.01230.x. [DOI] [PubMed] [Google Scholar]
- Bunford N, Evans SW, & Wymbs F (2015). ADHD and emotion dysregulation among children and adolescents. Clinical Child and Family Psychology Review, 18(3), 185–217. 10.1007/s10567-015-0187-5. [DOI] [PubMed] [Google Scholar]
- Cartwright KL, Bitsakou P, Daley D, Gramzow RH, Psychogiou L, Simonoff E, … Sonuga-Barke EJ (2011). Disentangling child and family influences on maternal expressed emotion toward children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 50(10), 1042–1053. 10.1016/j.jaac.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Morgan J, Rutter M, Taylor A, Arseneault L, et al. (2004). Maternal expressed emotion predicts children’s antisocial behavior problems: Using monozygotic-twin differences to identify environmental effects on behavioral development. Developmental Psychology, 40(2), 149–161. 10.1037/0012-1649.40.2.149. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, & Tannock R (2002). Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews Neuroscience, 3(8), 617–628. 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cohen J (1992). A power primer. Psychological Bulletin, 112(1), 155–159. 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cole PM, Dennis TA, Smith-Simon KE, & Cohen LH (2009). Preschoolers’ emotion regulation strategy understanding: Relations with emotion socialization and child self-regulation. Social Development, 18(2), 324–352. 10.1111/j.1467-9507.2008.00503.x. [DOI] [Google Scholar]
- Conners KC (2008). Conners 3rd Edition Manual. New York: Multi-Health Systems, Inc.. [Google Scholar]
- Denham SA, Mitchell-Copeland J, Strandberg K, Auerbach S, & Blair K (1997). Parental contributions to preschoolers’ emotional competence: Direct and indirect effects. Motivation and Emotion, 21(1), 65–86. 10.1023/A:1024426431247. [DOI] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, & Reid R (1998). ADHD Rating Scale-IV: Checklists, Norms, & Clinical Interpretation. New York: Guilford. 10.1177/0734282905285792. [DOI] [Google Scholar]
- Edwards G, Barkley RA, Laneri M, Fletcher K, & Metevia L (2001). Parent-adolescent conflict in teenagers with ADHD and ODD. Journal of Abnormal Child Psychology, 29(6), 557–572. 10.1023/A:1012285326937. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, & Erath SA (2011). Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Development and Psychopathology, 23(2), 703–721. 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Graziano P, & Derefinko K (2013). Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology, 94(1), 22–37. 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P, & Garcia A (2016). Attention-deficit hyperactivity disorder and children’s emotion dysregulation: A meta-analysis. Clinical Psychology Review, 46, 106–123. 10.1016/j.cpr.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Hale WW, Crocetti E, Nelemans SA, Branje SJ, Lier PA, Koot HM, & Meeus WH (2016). Mother and adolescent expressed emotion and adolescent internalizing and externalizing symptom development: A six-year longitudinal study. European Child & Adolescent Psychiatry, 25(6), 615–624. 10.1007/s00787-015-0772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, & Matthes J (2009). Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods, 41(3), 924–936. 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2012). PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. http://www.afhayes.com/public/process2012.pdf
- Hersh MA, & Hussong AM (2009). The association between observed parental emotion socialization and adolescent self-medication. Journal of Abnormal Child Psychology, 37(4), 493–506. 10.1007/s10802-008-9291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, & Mash EJ (2001). Families of children with attention-deficit/hyperactivity disorder: Review and recommendations for future research. Clinical Child and Family Psychology Review, 4(3), 183–207. 10.1023/A:1017592030434. [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Fair D, Musser ED, Aykes K, Iyer S, & Nigg JT (2015). Toward biologically based nosology: ADHD subtyping using temperament dimensions. Journal of the American Medical Association: Psychiatry, 71(9), 1015–1024. 10.1001/jamapsychiatry.2014.763. [DOI] [Google Scholar]
- Kelsey RM (2012). Beta-adrenergic cardiovascular reactivity and adaptation to stress: The cardiac pre-ejection period as an index of effort. In Wright RA & Gendolla GHE (Eds.), How motivation affects cardiovascular response: Mechanisms and applications (pp. 43–60). Washington, DC: American Psychological Association. [Google Scholar]
- Keown LJ (2012). Predictors of boys’ ADHD symptoms from early to middle childhood: The role of father-child and mother-child interactions. Journal of Abnormal Child Psychology, 40(4), 569–581. 10.1007/s10802-011-9586-3. [DOI] [PubMed] [Google Scholar]
- Kochanska G, & Aksan N (1995). Mother-child mutually positive affect, the quality of child compliance to requests and prohibitions, and maternal control as correlates of early internalization. Child Development, 66(1), 236–254. 10.2307/1131203. [DOI] [Google Scholar]
- Kochanska G, & Aksan N (2004). Development of mutual responsiveness between parents and their young children. Child Development, 75(6), 1657–1676. 10.1111/j.1467-8624.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- Leeb B, Hahlweg K, Goldstein MJ, Feinstein E, Mueller U, Dose M, & Magana-Amato A (1991). Cross-national reliability, concurrent validity, and stability of a brief method for assessing expressed emotion. Psychiatry Research, 39(1), 25–31. 10.1016/0165-1781(91)90005-A. [DOI] [PubMed] [Google Scholar]
- Leff J, & Vaughn C (1984). Expressed emotion in families: Its significance for mental illness. Guilford Press. [Google Scholar]
- Lifford KJ, Harold GT, & Thapar A (2008). Parent–child relationships and ADHD symptoms: A longitudinal analysis. Journal of Abnormal Child Psychology, 36(2), 285–296. 10.1007/s10802-007-9177-5. [DOI] [PubMed] [Google Scholar]
- Magaña AB, Goldstein MJ, Karno M, Miklowitz DJ, Jenkins J, & Falloon IR (1986). A brief method for assessing expressed emotion in relatives of psychiatric patients. Psychiatry Research, 17(3), 203–212. 10.1016/0165-1781(86)90049-1. [DOI] [PubMed] [Google Scholar]
- Martel MM (2009). Research review: A new perspective on attention-deficit/hyperactivity disorder: Emotion dysregulation and trait models. Journal of Child Psychology and Psychiatry, 50(9), 1042–1051. 10.1111/j.1469-7610.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- McCarty CA, & Weisz JR (2002). Correlates of expressed emotion in mothers of clinically-referred youth: An examination of the five-minute speech sample. Journal of Child Psychology and Psychiatry, 43(6), 759–768. 10.1111/1469-7610.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade JD, & Breaux RP (2017). Are elevations in ADHD symptoms associated with physiological reactivity and emotion dysregulation in children? Journal of Abnormal Child Psychology, 45(6), 1091–1103. 10.1007/s10802-016-0227-8. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Goldstein MJ, Falloon IR, & Doane JA (1984). Interactional correlates of expressed emotion in the families of schizophrenics. The British Journal of Psychiatry, 144(5), 482–487. 10.1192/bjp.144.5.482. [DOI] [PubMed] [Google Scholar]
- Musser ED, Backs RW, Schmitt CF, Ablow JC, Measelle JR, & Nigg JT (2011). Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD). Journal of Abnormal Child Psychology, 39(6), 841–852. 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Karalunas SL, Dieckmann N, Peris TS, & Nigg JT (2016). Attention-deficit/hyperactivity disorder developmental trajectories related to parental expressed emotion. Journalof Abnormal Psychology, 125(2), 182–195. 10.1037/abn0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, & Nigg JT (2017). Emotion dysregulation across emotion systems in attention-deficit/hyperactivity disorder (ADHD). Journal of Clinical Child and Adolescent Psychology. 10.1080/15374416.2016.1270828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, & Levenson RW (1979). Pre-ejection period: Measuring beta-adrenergic influences upon the heart. Psychophysiology, 16(6), 546–552. 10.1111/j.1469-8986.1979.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Booth RJ, Boyd RL, & Francis ME (2015). Linguistic inquiry and word count: LIWC2015. Austin, TX: Pennebaker Conglomerates; Retrieved from www.LIWC.net. [Google Scholar]
- Peris TS, & Baker BL (2000). Applications of the expressed emotion construct to young children with externalizing behavior: Stability and prediction over time. Journal of Child Psychology and Psychiatry, 41(4), 457–462. [PubMed] [Google Scholar]
- Peris TS, & Hinshaw SP (2003). Family dynamics and preadolescent girls with ADHD: The relationship between expressed emotion, ADHD symptomatology, and comorbid disruptive behavior. Journal of Child Psychology and Psychiatry, 44(8), 1177–1190. 10.1111/1469-7610.00199. [DOI] [PubMed] [Google Scholar]
- Peris TS, & Miklowitz DJ (2015). Parental expressed emotion and youth psychopathology: New directions for an old construct. Child Psychiatry and Human Development, 46(6), 863–873. 10.1007/s10578-014-0526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, & Castellanos FX (2016). Top-down dysregulation—from ADHD to emotional instability. Frontiers in Behavioral Neuroscience, 10(70). 10.3389/fnbeh.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfiffner LJ, McBurnett K, Rathouz PJ, & Judice S (2005). Family correlates of oppositional and conduct disorders in children with attention deficit/hyperactivity disorder. Journal of Abnormal Child Psychology, 33(5), 551–563. 10.1007/s10802-005-6737-4. [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, & Rohde LA (2014). ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43(2), 434–442. 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychogiou L, Daley DM, Thompson MJ, & Sonuga-Barke EJ (2007). Mothers’ expressed emotion toward their school-aged sons. European Child & Adolescent Psychiatry, 16(7), 458–464. 10.1007/s00787-007-0619-y. [DOI] [PubMed] [Google Scholar]
- Psychogiou L, Daley D, Thompson MJ, & Sonuga-Barke E (2008). Testing the interactive effect of parent and child ADHD on parenting in mothers and fathers: A further test of the similarity-fit hypothesis. British Journal of Developmental Psychology, 25(3), 419–433. 10.1348/026151006X170281. [DOI] [Google Scholar]
- Puig-Antich J, & Ryan N (1996). Kiddie Schedule for Affective Disorders and Schizophrenia. Pittsburgh, PA: Western Psychiatric Institute. [Google Scholar]
- Rash JA, & Aguirre-Camacho A (2012). Attention-deficit hyperactivity disorder and cardiac vagal control: A systematic review. Attention Deficit and Hyperactivity Disorders, 4(4), 167–177. 10.1007/s12402-012-0087-1. [DOI] [PubMed] [Google Scholar]
- Richter M, & Gendolla GH (2009). The heart contracts to reward: Monetary incentives and preejection period. Psychophysiology, 46(3), 451–457. 10.1111/j.1469-8986.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, & Leibenluft E (2014). Emotion dysregulation in attention deficit hyperactivity disorder. American Journal of Psychiatry, 171(3), 276–293. 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Lasky-Su J, Neale BM, Oades R, Chen W, Franke B, et al. (2008). Does parental expressed emotion moderate genetic effects in ADHD? An exploration using a genome wide association scan. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 147(8), 1359–1368. 10.1002/ajmg.b.30860. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Oades RD, Psychogiou L, Chen W, Franke B, Buitelaar J, et al. (2009). Dopamine and serotonin transporter genotypes moderate sensitivity to maternal expressed emotion: The case of conduct and emotional problems in attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry, 50(9), 1052–1063. 10.1111/j.1469-7610.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Cartwright KL, Thompson MJ, Brown J, Bitsakou P, Daley D, et al. (2013). Family characteristics, expressed emotion, and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 52(5), 547–548. 10.1016/j.jaac.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Tausczik YR, & Pennebaker JW (2010). The psychological meaning of words: LIWC and computerized text analysis methods. Journal of Language and Social Psychology, 29(1), 24–54. 10.1177/0261927X09351676. [DOI] [Google Scholar]
- Tenenbaum R, Musser ED, Raiker J, Gnagy E, Coles E, & Pelham W (2017). Heterogeneity in autonomic reactivity and regulation underlying reward processing among children with disruptive behavior disorders. Journal of Abnormal Child Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitola ES, Bau CHD, Salum GA, Horta BL, Quevedo L, Barros FC, … Grevet EH (2017). Exploring DSM-5 ADHD criteria beyond young adulthood: Phenomenology, psychometric properties and prevalence in a large three-decade birth cohort. Psychological Medicine, 47(4), 744–754. 10.1017/S0033291716002853. [DOI] [PubMed] [Google Scholar]
- Ward AR, Alarcon G, Nigg JT, & Musser ED (2015). Variation in parasympathetic dysregulation moderates short-term memory problems in childhood attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology, 43(8), 1573–1583. 10.1007/s10802-015-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children, Fourth Edition: Administration and scoring manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, & Pennington BF (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57(11), 1336–1346. 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]