Abstract
Recent advances in cancer neuroscience necessitate the systematic analysis of neural influences in cancer as potential therapeutic targets in oncology. Here, we outline recommendations for future preclinical and translational research in this field.
Research over the past 10 years has revealed a key role for peripheral neurons in cancer progression1. Neural influences in cancer should be regarded as a bi-directional relationship between cancer cells and nerves (Figure 1), as both have been shown to enhance their mutual growth or outgrowth. These interactions can be summarized as follows:
Figure 1. Neural Influences in Cancer.
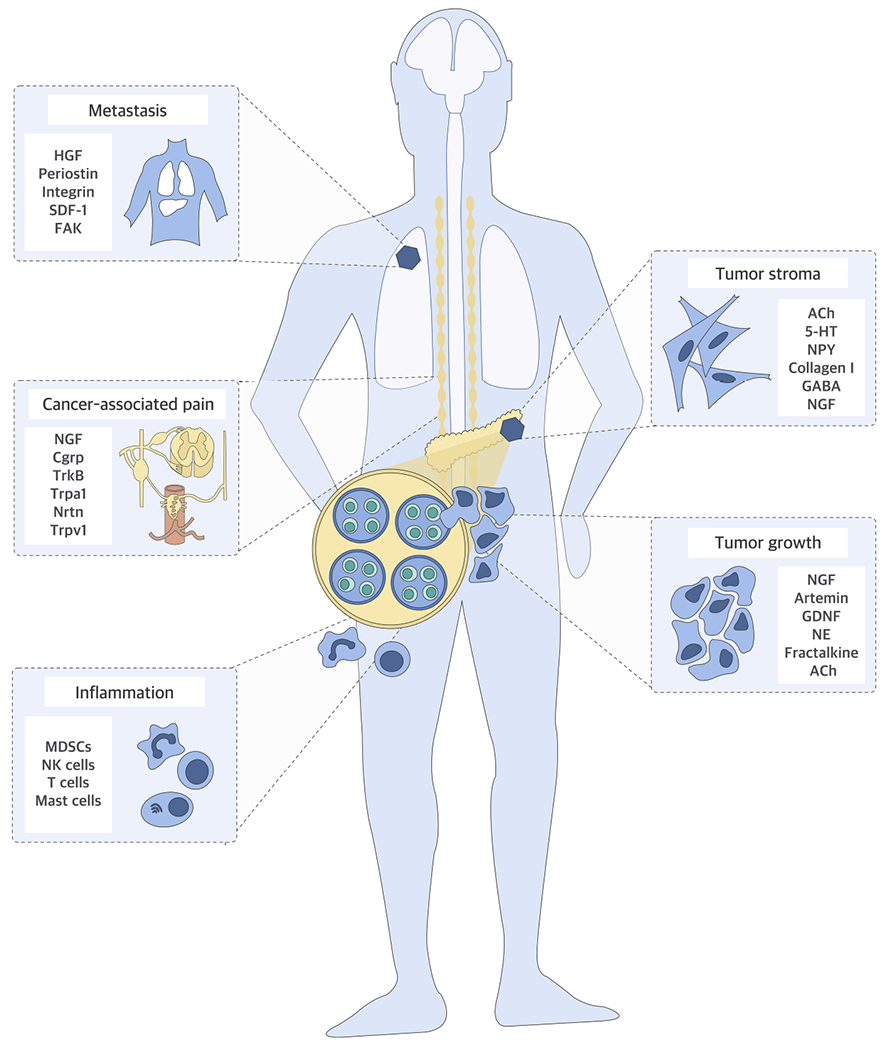
Nerves have been shown to impact multiple aspects of tumorigenesis. They can enhance the growth of primary or metastatic cancer cells directly through secereted factors and their receptors e.g. nerve growth factor (NGF), Artemin, glial-cell-line-derived neurotrophic factor (GDNF), fractalkine, neurturin (Nrtn), tropomyosin kinase receptors (Trk), stromalcell derived factor-1 (SDF-1), hepatocyte growth factor (HGF), periostin, integrin subclasses, focal adhesion kinase (FAK) or indirectly by promoting tumor-associated, pro-tumorigenic inflammation involving myeloid-derived suppressor cells (MDSC), natural killer (NK) cells. Nerves are also major modulators of the stroma, in which vascular or lymphatic endothelial cells, or cancer-associated fibroblasts are responsive to neural signals e.g. norepinephrine (NE), acetylcholine (Ach) and 5-HT, owing to their expression of neurotransmitter receptors such as GABA receptors. Pain is the most unpleasant symptom associated with cancer, and cancer-secreted neuropeptides such as calcitonin-generelated peptide (CGRP) and neuropeptide Y (NPY) overstimulate the damaged peripheral nerve endings to cause neuropathic pain.
Nerves as promoters of tumor growth
Nerves and glia are recruited into growing tumors, where they provide the cancer tissue with growth factors and neurotransmitters, reduce the rate of cell death, and/or contribute to cancer stem cell expansion1. However, specific autonomous neural influences may differ between cancer types. For example, cholinergic tumor-promotion has been observed in gastric and prostate cancers, but cholinergic growth-inhibition has been reported in pancreatic and breast cancers1. In hematologic malignancies, adrenergic signals can mitigate tumor progression2.
Cancer-induced neural invasion
Cancer-induced neuritogenesis is accompanied by the frequent presence of cancer cells in the multi-layered sheaths of nerves, a process termed neural invasion3, 4. The severity of neural invasion can be scored histologically and is an independent prognostic factor for worsened overall survival in several cancers including pancreatic cancer, carcinoma of the esophagogastric junction or head and neck cancers4, 5.
Nerves as modulators of immune responses
Neurons are pleiotropic regulators of immune cells and participate in immunomodulatory neural circuits6. Emerging evidence suggests that both the parasympathetic and sympathetic circuits modulate inflammatory responses under tumor conditions6. Parasympathetic and sympathetic signals can further reduce or augment the expression of inhibitory immune checkpoint proteins (e.g. PD1, PDL1), for example in breast cancer7.
Nerves as promoters of cancer pain
Increased pain in cancer is associated with poor prognosis3, 8, which can be exacerbated by emotional or physical stress. Optimized pain management has been shown to be associated with improvement of the overall survival of the cancer patients8. Nociceptive nerve fiber activation by cancer-secreted mediators [e.g., TNFα, NGF, GM-CSF] results in pro-inflammatory neuropeptides released from peripheral nerve endings, activation of the body’s immune system, and thus has a protective effect against cancer progression. Conversely, many types of cancer cells express receptors for neuropeptides with proliferative effects on cancer cells [e.g., RAMP1, NK1R], suggesting a pro-tumorigenic effect of neuropeptides1.
Nerves as mediators of effects on tumor stromal cells
Mesenchymal cells, such as cancer-associated fibroblasts, and endothelial cells are responsive to neural signals as they express adrenergic receptors such as Adrb21. For instance, blockade of beta-adrenergic receptor signaling was shown to favorably attenuate desmoplasia and metastasis in orthotopic mouse models of breast cancer7. Adrenergic signals were also shown to be necessary for the maintenance of a glycolytic state in endothelial cells during angiogenesis in the prostate cancer tissue1, but also for lymphangiogenesis in different cancers1. Thus, nerves can act on stromal cells in the tumor microenvironment and influence tumors also indirectly.
Recommendations and best practice
Successful clinical translation of cancer neuroscience findings depends on our ability to identify the druggable components of the interaction of cancer cells with neurons and to rapidly test the impact of targeting these components in preclinical studies using human-like models. However, despite the advances in the cancer neuroscience field over the last few years, our knowledge of the specific cellular and molecular interactions of neuronal and cancer cells and the extent of neuron- and glia-specific molecular alterations in different cancer types remains scarce. Therefore, focusing on understanding the underlying biology and improving discovery of potential therapeutic targets relating to cancer neuroscience should be a priority (Figure 2). To that end, we recommend the following strategies:
Figure 2. Areas of future research focus for the cancer neuroscience field.
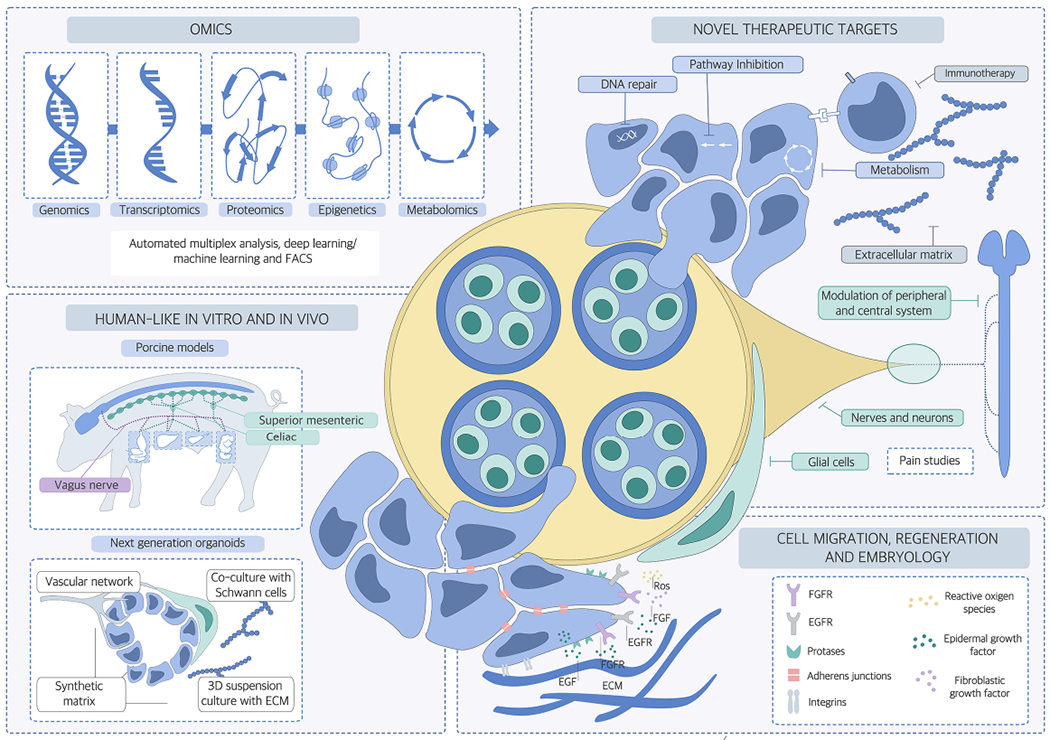
The following areas of research should be increasingly addressed in future cancer neuroscience studies: 1) Improved target discovery through advanced in vivo and in vitro models, for example by increased application of porcine models which show major similarities to human anatomy and pathophysiology; or of organoids owing to their 3D growing ability; 2) OMICs studies that apply genomic, epigenomic, proteomic, metabolomics, FACS, deep and machine learning approaches to cancer cells, neuronal and glial and cell subclasses and also other tumor microenvironment cell types such as immune cells, to uncover the molecular signatures pertinent to neuroglial activation in the cancer microenvironment; and 3) transfer of knowledge of molecular actors such growth factor receptors, proteases, adherence molecules from embryology and early development to cancer neuroscience research. These research tracks will likely permit the identification of therapeutic targets that can be tested and hopefully integrated into current and evolving oncological treatment regimens in the future.
1). Better in vitro and in vivo models:
In in vitro cancer neuroscience studies neurons are frequently co-cultured with cancer cells, often without other cells of the tumor microenvironment4. Emulating the in vivo conditions would require including additional cell types such as glia/Schwann cells, immune cells and cancer-associated fibroblasts4, 9, as well as cancer cells grown in a 3D tumor microenvironment setting, such as tumor organoids9. Such conditions can be generated by adding the natural components of the tumor extracellular matrix (ECM) into the 3D culture environment, e.g. the specifically enriched collagen subtypes and growth factors, and the specific stromal cell subclasses9.
Similarly, mouse modelling of cancer does not always fully recapitulate the human disease, which holds particularly true in cancer neuroscience studies. For example, although Kras-based mouse pancreatic cancer models exhibit invasion of nerves, they do not reflect the typical configuration of the adhesive neural invasion observed in human pancreatic cancer5. Development of mouse models that emulate human cancer types more faithfully should be a priority for this field in the coming years. The molecular signature behind the severely neuropathic-neuroplastic phenotypes of human cancers can be exploited for genetic manipulation in mice and for the generation of such human-like, novel mouse models. Additionally, humanized patient-derived xenografts (PDXs) should be tested for their ability to induce a similar extent of neuritogenesis and neural invasion in a mouse host. Moreover, other species such as porcine models may offer advantages for recapitulating human pathophysiology over mouse models, since pigs exhibit several similarities to humans with regard to organ anatomy, size and physiology.
When modelling cancer neurobiology there is a need for organ- and site-specific, inducible targeting of neurons and glia. This can be achieved by 1) precise stereotactic injection of shRNA-expressing viral vectors into selected regions of the central and peripheral nervous system10, and 2) optogenetic or chemogenetic activation or inactivation of central and peripheral neurons10, including enteric neural circuits. Similarly, ex vivo preparations can be isolated for subsequent electrophysiological recording of neuronal activity in the presence of a peripheral solid tumor (e.g., colon-pelvic nerve-L6 DRG ex vivo preparation11). Furthermore, current single-cell transcriptomic and proteomic analyses increasingly reveal markers of such cell subpopulations, which could be used to generate targeted, inducible genetic knockin, knockout or dual recombinase mouse lines for mechanistic studies.
2). Cancer neuroscience omics studies:
Although bulk tissue analyses may mask the contribution of specific neuronal or glial subclasses and the contributing molecules, identification of novel therapeutic targets based on neuron-cancer interactions could be achieved by exploring the single-cell landscapes in different tumor types12. Even though single-cell datasets analyzing the neuronal or glial component of human or mouse tumor samples are scarce, the emergence of such studies for other tumor-associated cell types raise the expectation that such efforts will also materialize in the cancer neuroscience field. For solid cancers, the bioinformatic dissection of the transcriptomic, proteomic, and epigenetic landscape and signature of neuronal cell subclasses, glia cells and cancer cell subgroups should be a priority. Moreover, the molecular alterations that are induced specifically by adherent or physically interacting cells (PIC), can be deciphered via PIC-seq, a method that allows the bioinformatic deconvolution of interacting cells and that could yield important insights for the molecular interactions between neurons and cancer cells12.
3). Imaging at microscopic resolution and at the molecular level:
Imaging techniques such as time-lapse microscopy and histology have greatly contributed to our understanding of the interaction of cancer cells, neurons and glia cells13. More advanced approaches such as fluorescence molecular tomography systems together with molecule-specific fluorescent tracers or intravital microscopy could help to elucidate the contribution of specific neuro-glial mediators to cancer progression13. Furthermore, high-resolution imaging technologies such as electron microscopy or atomic force microscopy will be required to resolve the interaction of neurites and glial projections with cancer cell protrusions. Tissue clearing methods with light sheet microscopy can help obtain 3D maps of the neural anatomy of tumors or ogans in animal models as well as patient specimens. This is particularly important for visualizing neuron interactions with the tumor front, which may guide future clinical efforts for improved local tumor control, for example through locally ablative drugs, radiotherapy, or precision surgery. Advanced imaging of the bone marrow remains challenging, but would be especially useful in hematologic cancers. Using reporter mice that highlight specific nerve subtypes (e.g. sensory or sympathetic nerves) could further enhance the application and readouts of cancer neuroscience imaging approaches .
4). Understanding the embryology and organ regeneration mechanisms:
Given the remarkable parallels between the molecular and morphological mechanisms of regeneration and reactive neuro-glial alterations during carcinogenesis, the analysis of cancer neuroscience in each organ should start with the study of the corresponding embryology1. In particular, the differences in the embryological development of anatomic segments of organs may provide clues for the differential cancer presentation and extent of neural influences in tumors at different locations. Studying the contribution to tumor development and progression of molecular cues such as axon guidance molecules, which are usually activated only during organogenesis or regeneration1, is a promising research route.
5). Identification of functional neuronal types involved in different aspects of nerve cancer interactions:
The nerves that innervate and regulate solid cancers and, in the case of hematologic malignancies, the bone marrow are complex and can contain autonomic motor axons arising from either pre- or post- ganglionic neurons, as well as sensory axons arising from vagal or spinal sensory ganglia6. Identification of these different populations is critical as each type of neuron has the potential to communicate with developing tumors through the release of different neurotransmitters and neuromodulators, such as neuropeptides, and as different cancers may exhibit a different spatial distribution of neurites. In addition, different types of neurons express immunomodulatory genes, such as TLR receptors, interleukins and their receptors, that contribute to the immune status of peripheral tissue in which tumors reside6.
6). Pain studies:
Neurobiology and pain mechanisms discovered in animal models often do not translate to patients. For example, neurokinin-1 or NMDA receptor antagonism showed great promise as analgesics in animal models, yet failed in human clinical trials and produced many side effects14. Therefore, when studying pain in the context of cancer neuroscience it would also be important to perform reverse translational studies, to confirm the effectiveness of drugs approved in humans also in terms of pain in animals14. This approach would help determine how well the animal models match the patient’s pain experience with a view to retaining only those models that recapitulate the human situation. It is also important to evaluate pain and nociceptive sensory neuron activity for the duration of the disease. Lack of patient-reported pain or nociceptive behaviour early in cancer does not necessarily mean that nociceptive sensory neurons are not active. Rather, endogenous opioid signalling mediated by the immune system8 may mask pain signalling in early stages. Subsequent exhaustion of this immune-mediated anti-nociception effect may result in spontaneous breakthrough cancer pain, often with a neuropathic pain component, in later stages of cancer progression.
7). Neuro-immune interactions in cancer:
Neuro-immune communication regulates homeostasis as well as responses to non-cancerous pathologies6. Neurons express receptors for immune cell-derived mediators, such as cytokines and histamines, and also release neurotransmitters and cytokines that can act directly on immune cells. These shared signalling pathways indicate that effects on tumor growth and response to therapy that were previously attributed specifically to either nerves or immune cells may be the result of tri-directional cell communication6. It would be important to identify and study the immune cell types that interact with cancer and neuronal cells in this manner at high spatial and temporal resolution. Some techniques that can be applied in the context of cancer neuroscience research are 1) automated multiplex immunofluorescence staining of tumor samples, 2) fluorescence-activated cell sorting (FACS) for subsequent single-cell omics analyses, and 3) machine learning approaches for the correlation of immune cell subclasses based on their proximity to neuronal and glial subclasses. The enhanced implementation in the cancer-neuron context of such state-of-the-art techniques, which are current common tools in the field of immunology, should be a major priority for advancing the cancer neuroscience field.
Translating preclinical findings to the clinic
As outlined in the previous sections, to identify therapeutic targets relating to cancer neuroscience it is essential to first understand the underlying biology of cancer-neuron cellular and molecular interactions. Druggable targets are likely to be identified by deciphering the molecular signatures of interacting neurons and cancer cells derived from human specimens. Moreover, neural invasion is present in most solid cancers, meaning that translationally relevant mediators of cancer-neuronal interactions could be extracted from high-resolution imaging and omics analyses. Further insight into therapeutically relevant processes and molecules will be gained by studying the neuronally-influenced tumor microenvironment. Complementing research on human specimens with preclinical in vitro and animal models that recapitulate the human disease fatithfully is also essential. The proposed multidisciplinary fundamental research approaches using human-like preclinical models will hopefully reveal molecules sufficiently influential in nerve-cancer interactions to test in early-phase human clinical trials. However, when designing interventional clinical trials using inhibitory or modulatory agents relating to cancer neuroscience, it is essential to consider their potential adverse effects on the nervous system. For example, the tropomyosin receptor kinase (Trk) receptors TrkA, TrkB, and TrkC are critical for growth and differentiation of neuronal subclasses, but also found to be highly active in human solid cancers such as pancreatic or lung cancer15. As such, Trk inhibtiors were reported to cause several central nervous system side effects such as ataxia and tremor15. Although both the peripheral and central nervous systems are protected from non-selective exposure to components of the blood stream through the blood-nerve and the blood-brain barriers, the ability of compounds to cross these barriers is very difficult to predict. For targets that are identified to be profoundly altered in their expression or activity within specific areas of the central nervous system, one potential non-pharmacological way to achieve translation may be neuromodulation, for example by transcranial magnetic stimulation of central cerebral or spinal areas. The ability of such techniques to influence cancer progression awaits testing in preclinical and clinical trials.
Cancer neuroscience stands at the intersection of several disciplines. As such, beyond guiding researchers towards adopting robust preclinical and translational research practices, research teams working at this interface need to be well-versed in the principles of neuroscience, oncology and – given its increasingly important role in giving rise to neuritogenesis, cancer progression and pain – also in immunology. In addition to building multidisciplinary teams and fostering collaborations between experts in these distinct areas, the curricula of graduate and clinician-scientist training programs should consider cancer neuroscience educational tracks with a view to integrating items from these three fields. This educational approach together with the multidisciplinary preclinical research strategies listed above, should ensure that the field continues to develop and to yield actionable findings for successful cancer therapies.
Acknowledgments
Financial disclosures
The consensus recommendations in this article have been gathered during the 1st Neural Influences in Cancer (NIC) International Think Tank Meeting, which took place November 7-9, 2019 in Heidelberg, Germany. IED was awarded with the ENIGMA Young Investigator Prize of the Society for Scientific Meetings in Biomedicine to organize the NIC meeting. We thank Society for Scientific Meetings in Biomedicine (Verein für wissenschaftliche Fachtagungen in der Biomedizin e.V. / VWFB e.v; www.vwfb.de) and its Chair Hellmut Augustin for support. The meeting was also supported by grants from the German Research Foundation (Deutsche Forschungsgemeinschaft/DFG, DE2428/7-1) and the Fritz Thyssen Foundation (30.19.0.128MN). IED was also funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 329628492 – SFB 1321. MM is a New York Stem Cell Foundation (NYSCF) Druckenmiller fellow and was supported by the EMBO European Commission FP7 (Marie Curie Actions; EMBOCOFUND2012, GA-2012-600394, ALTF 447-2014). TCW is a Consultant for Novo Nordisc and Glaxo SmithKline and on the Scientific Advisory Board for Cygnal Therapeutics. The remaining authors report no financial disclosures or no conflicts of interest.
The Neural Influences in Cancer (NIC) International Research Consortium
Ihsan Ekin Demir1,2,3,4,*, Carmen Mota Reyes1,3,4, Wasfi Alrawashdeh5, Güralp O. Ceyhan2, Sylvie Deborde6, Helmut Friess1,3,4, Kıvanç Görgülü, Rouzanna Istvanffy1,3,4, David Jungwirth1,3,4, Rohini Kuner8, Maria Maryanovich9,10, Shorook Na’ara11,12, Simon Renders13, Jami L. Saloman14, Nicole N. Scheff15, Hendrik Steenfadt1,3,4, Pavel Stupakov1,3,4, Vera Thiel13, Divij Verma9,10, Bengi Su Yilmaz1,3,4, Ruth A. White16, Timothy C. Wang17, Richard J. Wong6, Paul S. Frenette9,10,18, Ziv Gil11,12, and Brian M. Davis19
1Department of Surgery, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany
2Department of General Surgery, HPB-Unit, School of Medicine, Acibadem Mehmet Ali Aydinlar University, Istanbul, Turkey
3German Cancer Consortium (DKTK), Partner Site Munich, Germany
4CRC 1321 Modelling and Targeting Pancreatic Cancer, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany
5Department of HPB and Transplant Surgery, The Freeman Hospital, Newcastle upon Tyne, Tyne and Wear, United Kingdom
6Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York, USA
7 Comprehensive Cancer Center Munich, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany
8Department of Molecular Pharmacology, Institute of Pharmacology, Heidelberg University, Heidelberg, Germany
9Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Medicine Research, Albert Einstein College of Medicine, Bronx, NY 10461, USA
10Department of Cell Biology, Albert Einstein College of Medicine, Bronx, NY 10461, USA
11Department of Otolaryngology, Head and Neck Surgery, and the Laboratory for Applied Cancer Research, Rappaport Institute of Medicine and Research, The Technion, Israel Institute of Technology, Haifa, Israel.
12 Head and Neck Center, Rambam Healthcare Campus, Haifa, Israel
13Division of Stem Cells and Cancer, German Cancer Research Center (DKFZ) and DKFZ-ZMBH Alliance, 69120 Heidelberg, Germany; Heidelberg Institute for Stem Cell Technology and Experimental Medicine (HI-STEM gGmbH), 69120 Heidelberg, Germany
14Department of Medicine, Division of Gastroenterology, Hepatology, & Nutrition, Center for Neuroscience at the University of Pittsburgh, Pittsburgh Center for Pain Research and Department of Neurobiology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
15Hillman Cancer Center and Department of Neurobiology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
16Division of Hematology ad Oncology, Department of Medicine, Columbia University, College of Physicians and Surgeons, New York, NY, USA
17Division of Digestive and Liver Diseases, Department of Medicine, Columbia University, College of Physicians and Surgeons, New York, NY, USA
18Department of Medicine, Albert Einstein College of Medicine, Bronx, NY 10461, USA
19Center for Neuroscience at the University of Pittsburgh, Pittsburgh Center for Pain Research and Department of Neurobiology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
References
- 1.Zahalka AH & Frenette PS Nerves in cancer. Nat Rev Cancer 20, 143–157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arranz L et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512, 78–81 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Ceyhan GO et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology 136, 177–186 e1 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Amit M, Na'ara S & Gil Z Mechanisms of cancer dissemination along nerves. Nat Rev Cancer 16, 399–408 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Demir IE, Friess H & Ceyhan GO Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol 12, 649–59 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Saloman JL, Cohen JA & Kaplan DH Intimate neuro-immune interactions: breaking barriers between systems to make meaningful progress. Curr Opin Neurobiol 62, 60–67 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Kamiya A et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. NatNeurosci 22, 1289–1305 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Mantyh PW Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 7, 797–809 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Pastula A et al. Three-Dimensional Gastrointestinal Organoid Culture in Combination with Nerves or Fibroblasts: A Method to Characterize the Gastrointestinal Stem Cell Niche. Stem Cells Int 2016, 3710836 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faber CL, Matsen ME, Meek TH, Krull JE & Morton GJ Adaptable Angled Stereotactic Approach for Versatile Neuroscience Techniques. J Vis Exp (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith-Edwards KM et al. Extrinsic Primary Afferent Neurons Link Visceral Pain to Colon Motility Through a Spinal Reflex in Mice. Gastroenterology 157, 522–536 e2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giladi A et al. Dissecting cellular crosstalk by sequencing physically interacting cells. Nat Biotechnol 38, 629–637 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Deborde S et al. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest 126, 1538–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burma NE, Leduc-Pessah H, Fan CY & Trang T Animal models of chronic pain: Advances and challenges for clinical translation. J Neurosci Res 95, 1242–1256 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Bailey JJ, Schirrmacher R, Farrell K & Bernard-Gauthier V Tropomyosin receptor kinase inhibitors: an updated patent review for 2010-2016 - Part II. Expert Opin Ther Pat 27, 831–849 (2017). [DOI] [PubMed] [Google Scholar]


