Abstract
Background
To understand the clinical, bacterial, and host characteristics associated with recurrent Staphylococcus aureus bacteremia (R-SAB), patients with R-SAB were compared to contemporaneous patients with a single episode of SAB (S-SAB).
Methods
All SAB isolates underwent spa genotyping. All isolates from R-SAB patients underwent pulsed-field gel electrophoresis (PFGE). PFGE-indistinguishable pairs from 40 patients underwent whole genome sequencing (WGS). Acute phase plasma from R-SAB and S-SAB patients was matched 1:1 for age, race, sex, and bacterial genotype, and underwent cytokine quantification using 25-analyte multiplex bead array.
Results
R-SAB occurred in 69 (9.1%) of the 756 study patients. Of the 69 patients, 30 experienced relapse (43.5%) and 39 reinfection (56.5%). Age, race, hemodialysis dependence, presence of foreign body, methicillin-resistant Staphyloccus aureus, and persistent bacteremia were individually associated with likelihood of recurrence. Multivariate risk modeling revealed that black hemodialysis patients were nearly 2 times more likely (odds ratio [OR] = 9.652 [95% confidence interval [CI], 5.402–17.418]) than white hemodialysis patients (OR = 4.53 [95% CI, 1.696–10.879]) to experience R-SAB. WGS confirmed PFGE interpretations in all cases. Median RANTES (regulated on activation, normal T cell expressed and secreted) levels in acute phase plasma from the initial episode of SAB were higher in R-SAB than in matched S-SAB controls (P = .0053, false discovery rate < 0.10).
Conclusion
This study identified several risk factors for R-SAB. The largest risk for R-SAB is among black hemodialysis patients. Higher RANTES levels in R-SAB compared to matched controls warrants further study.
Keywords: Staphylococcus aureus, bacteremia, recurrence, whole genome sequencing, health disparity
This study identified several risk factors for recurrent Staphylococcus aureus bacteremia (R-SAB). The largest risk for R-SAB is among black hemodialysis patients. Higher RANTES levels in R-SAB compared to matched controls warrants further study.
Staphylococcus aureus bacteremia (SAB) is a common and potentially lethal infection [1]. Approximately 2–20% of patients with an initial episode of SAB will develop a recurrent S. aureus bacteremia (R-SAB) after the resolution of the initial infection [2–4]. Although several previous studies have sought to identify risk factors for R-SAB [5–11], none have simultaneously considered clinical, bacterial, and host inflammatory characteristics.
In the current investigation, we used a large prospective cohort of patients with SAB to identify clinical characteristics associated with R-SAB as compared with patients who had only a single episode of SAB (S-SAB). Next, we genotyped the paired bacterial isolates from the repeat SAB episodes to differentiate patients with recurrent SAB due to a persistent source (relapse) from patients with a new episode of SAB (reinfection) using 3 molecular techniques. Finally, we tested the possibility that patients with R-SAB exhibited fundamental differences in their response to S. aureus that predisposed them to recurrence by comparing the cytokines from acute phase plasma of matched patients with R-SAB and S-SAB.
METHODS
Study Population
Since September 1994, the SAB Group Prospective Cohort Study (SABG-PCS) has prospectively enrolled all eligible adult (age ≥ 18 years) hospitalized nonneutropenic (absolute neutrophil count > 1×109/L) patients with monomicrobial SAB at Duke University Medical Center. Demographics, past medical history, history of surgery within the previous 30 days, site of acquisition of SAB, APACHE (Acute Physiology and Chronic Health Evaluation) II score calculated on the day of the index positive blood culture, metastatic complications, and patient outcome were collected on a standardized case report form and entered into an electronic database. Using the SABG-PCS data between January 2008 and May 2015, patients with SAB were selected. Patients included in this study are part of a larger cohort discussed elsewhere [12] and were previously presented in part at IDWeek 2018 [13]. This study was approved by the Duke Institutional Review Board. Patients (or legal representative) provided written informed consent.
Definitions
R-SAB was defined as a second episode of SAB after the resolution of the first episode occurring at least 14 days from the date of the last positive blood culture of the first episode [14]. SAB was categorized as community-acquired, healthcare-associated, or hospital-acquired, as described elsewhere [15]. A foreign body was defined as any device that was inserted for an extended period of time (eg, tunneled intravascular catheter, synthetic intravascular graft, arthroplasty, orthopedic hardware, prosthetic valves, and cardiac devices). Persistent bacteremia was defined as ≥ 5 days of positive blood cultures after appropriate treatment was initiated. Patients were considered to have hematogenous metastatic infections from their bacteremia if they developed any of the following conditions during their hospitalization for SAB after the first positive culture: infective endocarditis, vertebral osteomyelitis, septic arthritis, septic emboli, septic thrombophlebitis, metastatic abscess, or other deep tissue abscess (ie, epidural or psoas abscess).
Laboratory Studies
Bacterial isolates were speciated by the Duke Clinical Microbiology Laboratory using standard techniques. Minimum inhibitory concentration values were determined using an automated broth microdilution method (MicroScan WalkAway plus System, Beckman Coulter, Brea, CA, USA). Methicillin susceptibility was defined according to Clinical and Laboratory Standards Institute (CLSI) guidelines.
Pulsed-field Gel Electrophoresis (PFGE)
PFGE was performed for all S. aureus blood isolates from patients with R-SAB, as previously described [16]. PFGE patterns were compared between isolates for each patient with R-SAB by visual inspection and interpreted as either “indistinguishable” or “discordant” using established guidelines [17] by 2 experienced investigators (B. K. S.; B. W.) blinded to clinical category of the source patient.
Defining and Differentiating Relapse Versus Reinfection in Patients With R-SAB
R-SAB patients with PFGE-discordant isolate pairs were defined as having reinfection. In R-SAB patients with PFGE-indistinguishable isolate pairs, the time between the first episode of SAB and the subsequent recurrent episode (ΔT) was used to differentiate relapse from reinfection [18, 19]. Patients in whom the PFGE-indistinguishable pair of isolates occurred at ΔT ≥ 150 days apart were defined as having reinfection with an identical strain. By contrast, patients in whom the PFGE-indistinguishable pair of isolates occurred < 150 days apart were defined as having relapse.
Spa Genotyping
The initial S. aureus bloodstream isolate from all study patients underwent spa typing as described elsewhere [20]. Assignment of spa types was performed using Ridom StaphTypeTM (Ridom GmbH, Wurzburg, Germany). Spa types were clustered into spa clonal complexes (spa-CCs) using the Based Upon Repeat Pattern (BURP) algorithm at a cost setting of ≤ 4 and excluding spa types with < 5 repeats.
Whole Genome Sequencing
Whole genome Illumina sequencing (paired end, read length 150 bp) was carried out on 40 isolate pairs (80 isolates total). One sample failed sequencing; it and its pair were not considered further (seeSupplementary methods).
Cytokine Analysis
Acute phase plasma was obtained from consenting study participants within ~3 days after initial positive blood cultures for SAB. To conduct cytokine analysis, 21 patients with R-SAB were matched 1:1 to patients with S-SAB based upon age, sex, race, and spa-CC of the bloodstream S. aureus isolate. For 18 of the 21 samples, all 4 variables were matched. The remaining 3 patients were matched on as many of these variables as possible. Plasma cytokine and chemokine concentrations were assayed using a 25-analyte multiplex bead array (GM-CSF, Eotaxin, interferon [IFN]-α, induced protein [IP]-10, IFN-γ, monocyte chemoattractant protein [MCP]-1, interleukin [IL]-1β, monokine induced by interferon-γ [MIG], IL-1RA, macrophage inflammatory protein [MIP]-1α, IL-2, MIP-1β, IL-2R, RANTES [regulated on activation, normal T cell expressed and secreted], IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 [p40/p70], IL-13, IL-15, IL-17, tumor necrosis factor [TNF]-α; Invitrogen; Carlsbad) prepared according to the manufacturer’s recommended protocol and read using a Bio-Plex 200 suspension array reader (Bio-Rad). Data were analyzed using Bio-Plex manager software v6.1 (Bio-Rad; Hercules, CA, USA).
Statistical Analysis
Continuous variables were compared using the Wilcoxon 2-sample rank-sum test and categorical variables using Fisher exact test. The cytokine levels of baseline plasma were compared using Wilcoxon signed rank test between matched pairs. Logistic regression analysis was performed to identify independent risk factors for R-SAB. Variables with P < .10 on univariate analysis were included in a multivariate logistic regression analysis. Where variables were strongly correlated, the correlation structure was accounted for. Multiple comparisons adjustment was made as appropriate with false discovery rate (FDR) 10%.
RESULTS
Inclusion of the Study Patients and Differentiation Between Relapse and Reinfection
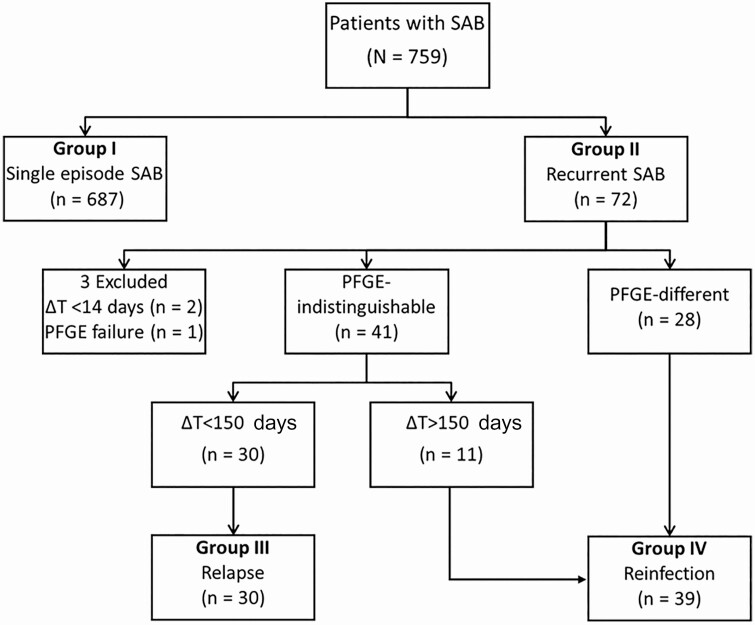
During the 7-year study period, a total of 759 patients with SAB were enrolled. Of these, 687 patients experienced S-SAB and 72 patients (9.5%) had R-SAB. After the exclusion of 2 patients whose ΔT was < 14 days and 1 patient whose isolates failed to show any PFGE band in repeated tests, 69 patients with R-SAB were included in the study analyses. Of these 69 patients, 55 experienced a single recurrence (2 total episodes of SAB) and 14 experienced multiple recurrences (≥3 episodes of SAB). Patients with 1 recurrence did not differ significantly from those with multiple recurrences (Table S1). For patients with multiple recurrences, only the first and second episodes were analyzed. Overall, the median duration of ΔT until the first recurrence was 143 days (interquartile range [IQR] 77d – 354d). PFGE performed on sequential isolates corresponding to the first recurrence resulted in 41 indistinguishable pairs and 28 different pairs (Figure S4). All sequential pairs with ΔT < 60 days were caused by indistinguishable strains (eg, relapse). The first occurrence of R-SAB due to a PFGE-different strain appeared during the third month, and 75% of the sequential pairs with different PFGE profiles (eg, reinfection) occurred at ΔT ≥ 150 days. For this reason, if a sequential pair with indistinguishable PFGE profiles occurred at ΔT ≥ 150 days, it was interpreted as reinfection with an identical strain for the purposes of the present analysis. By contrast, PFGE-indistinguishable pairs obtained < 150 days apart were classified as relapse. PFGE-discordant pairs were all considered to be reinfections. Using this definition, the study participants were classified into 2 groups: relapse (30 patients; 43.5%) and reinfection (39 patients; 56.5%) (Figure 1).
Figure 1.
Flow of classification of study patients based on PFGE and cutoff value of the time between the first episode of SAB) and the subsequent episode of recurrent SAB (ΔT). Abbreviations: PFGE, pulsed-field gel electrophoresis; SAB, S. aureus bacteremia.
Patients With R-SAB Versus Patients With S-SAB
As compared to patients with S-SAB, R-SAB patients were significantly younger (56y vs 61y, P = .0003); more frequently black (63.8% vs 29.8%, P < .0001) and hemodialysis dependent (55.1% vs 15.6%, P < .0001); more likely to have a foreign body (82.6% vs 59.7%, P = .0001), more likely to exhibit persistent bacteremia (39.1% vs 24.7%, P = .0138), and be infected with methicillin-resistant S. aureus (MRSA) (56.5% vs 43.7%, P = .0429) (Table 1). Conversely, patients with S-SAB were more likely to have a diagnosed neoplasm (25.5% vs 11.6%, P = .008) and to have had surgery within the 30 days preceding infection (23.7% vs 11.6%, P = .0229). Surgery within 30 days, presence of a foreign body, age, and APACHE II scores showed significant pairwise correlation. In addition, there was a strong relationship between race and hemodialysis dependence. To explore this further, we constructed a multilevel variable to capture potential interactions between race and hemodialysis dependence by defining 3 levels: “black and hemodialysis dependent,” “white and hemodialysis dependent,” and “not hemodialysis dependent.”
Table 1.
Clinical Characteristics of Patients With Single Episode of Staphylococcus aureus Bacteremia (SAB) (Group I), Patients With Recurrent SAB (Group II), Patients With Relapse of SAB (Group III), and Patients With Reinfection of SAB (Group IV)
| Characteristics | Group I Single episode (N = 687) | Group II Recurrence (N = 69) | P value, I vs II | Group III Relapse (N = 30) | P value I vs III | Group IV Reinfection (N = 39) | P value I vs IV | P value III vs IV |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Median age (IQR) | 61.0 (50.0–71.0) | 56.0 (40.0–63.0) | .0003 | 58.0 (51.0–63.0) | .0615 | 46.0 (35.0–64.0) | .001 | .3061 |
| Male sex | 419 (61.0) | 40 (58.0) | .70 | 23 (76.7) | .0882 | 17 (43.6) | .0423 | .0073 |
| Racea | <.0001 | .0080 | <.0001 | .2021 | ||||
| White | 460(67.2) | 24 (34.8) | 13 (43.3) | 11 (28.2) | ||||
| Black | 205 (29.8) | 44 (63.8) | 16 (53.3) | 28 (71.8) | ||||
| Other | 20 (2.9) | 1 (1.4) | 1 (3.3) | 0 | ||||
| Acquisitionb | .43 | .1001 | .6466 | .1387 | ||||
| Hospital-acquired | 120 (17.5) | 8 (11.8) | 1 (3.3) | 7 (17.9) | ||||
| Healthcare-associated | 473 (68.9) | 52 (76.5) | 23 (76.7) | 29 (74.4) | ||||
| Community-acquired | 94 (13.7) | 8 (11.8) | 5 (16.7) | 3 (7.7) | ||||
| Underlying disease/condition | ||||||||
| Diabetes mellitus | 279 (40.6) | 34 (49.3) | .1995 | 16 (53.3) | .19 | 18 (46.2) | .5070 | .6307 |
| Hemodialysis dependence | 107 (15.6) | 38 (55.1) | <.0001 | 16 (53.3) | <.0001 | 22 (56.4) | <.0001 | .8123 |
| Injection drug use | 28 (4.1) | 1 (1.4) | .51 | 0 | .62 | 1 (2.56) | 1.00 | 1.00 |
| Neoplasm | 175 (25.5) | 8 (11.6) | .008 | 6 (20.0) | .6681 | 2 (5.13) | .0019 | .0702 |
| Transplantation | 59 (8.6) | 6 (8.7) | 1.00 | 4 (13.3) | .3253 | 2 (5.13) | .7641 | .3920 |
| Steroid use | 163 (23.7) | 10 (14.5) | .098 | 6 (20.0) | .8264 | 4 (10.3) | .0517 | .3121 |
| HIV infectionc | 16 (2.3) | 3 (4.4) | .24 | 2 (6.7) | .1636 | 1 (2.56) | .6146 | .5712 |
| Foreign bodyd | 410 (59.7) | 57 (82.6) | .0001 | 26 (86.7) | .0034 | 31 (79.5) | .0172 | .5316 |
| Surgery within 30 dayse | 163 (23.7) | 8 (11.6) | .0229 | 3 (10.0) | .0814 | 5 (12.8) | .1226 | 1.00 |
| Clinical features of SAB | ||||||||
| Median APACHE II score (IQR) | 16.0 (12.0 – 22.0) | 15.0 (12.0 – 18.0) | .07 | 15.0 (12.0 – 19.0) | .18 | 16.05 (12.0 – 18.0) | .30 | .7520 |
| Persistent bacteremia | 170 (24.7) | 27 (39.1) | .0138 | 14 (46.7) | .0103 | 13 (33.3) | .2551 | .3229 |
| Infective endocarditise | 122 (17.8) | 15 (21.7) | .3590 | 5 (16.7) | 1.00 | 10 (25.6) | .2011 | .3829 |
| Metastatic abscesse | 78 (11.4) | 12 (17.4) | .1013 | 7 (23.3) | .0384 | 5 (12.82) | .5853 | .3426 |
| Metastatic arthritise | 54 (7.9) | 4 (5.8) | .81 | 3 (10.0) | .48 | 1 (2.56) | .51 | .3148 |
| Septic thrombophlebitise | 40 (5.8) | 5 (7.2) | .4658 | 3 (10.0) | .2644 | 2 (5.13) | 1.00 | .6480 |
| Microbiologic characteristics | ||||||||
| Methicillin resistance | 300 (43.7) | 39 (56.5) | .0429 | 22 (73.3) | .0022 | 17 (43.6) | 1.00 | .0160 |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
aData missing for 2 patients from Group I; P-values reported are for black vs white.
bData missing for 1 patient from Group II, originating from Group III.
cData missing for 1 patient from Group III.
d Data missing for 1 patient from Group I.
eData missing for 6 patients from Group II, 2 originating from Group III, and 4 from group IV.
A multivariate logistic regression model was generated based on predictors that had P < .10 in initial models (Table S2) and using the race and hemodialysis combination variable. Variables were eliminated based on a lack of independent predictive value with clinical relevance used to judge selection among correlated variables. In the final model, the combination variable of race/hemodialysis dependence was an independent risk factor for R-SAB (P < .001). Blacks on hemodialysis were at the highest risk with an adjusted odds ratio of 9.652 (95% confidence interval [CI], 5.402–17.418), followed by hemodialysis dependent whites (4.53 [95% CI, 1.696–10.879]) compared to nonhemodialysis dependent controls. An APACHE II score above median value was also a significant predictor of recurrence (1.869 [95% CI, 1.081–3.271], P = .0263) (Table 2).
Table 2.
Multiple Logistic Regression Model Predicting Recurrent Staphylococcus aureus Bacteremia
| Final Regression ModelC = 0.736 | ||
|---|---|---|
| Risk Factor | Adjusted Odds Ratio (95% CI) | P-value |
| Dialysis and race | <.0001 | |
| Dialysis dependent blacks | 9.652 (5.402–17.418) | |
| Dialysis dependent whites | 4.53 (1.696–10.879) | |
| APACHE score above median | 1.869 (1.081–3.271) | .0263 |
Abbreviation: CI, confidence interval.
Survival, mortality, and recurrence are part of the criteria that define S-SAB versus R-SAB study design and could therefore only be evaluated in the S-SAB group. Within S-SAB, blacks and whites had virtually identical survival (68.8% and 67.6%, respectively) and attributable mortality (18.1% and 19.4%) rates.
Risk Factors for Relapse and Reinfection R-SAB
As compared to patients with S-SAB, blacks (compared to whites), hemodialysis dependence, and the presence of an indwelling foreign body were significantly associated with both relapse and reinfection (Table 1). Persistent bacteremia (46.7% vs 24.7%, P = .0103), methicillin resistance (73.3% vs 43.7%, P = .0022), and metastatic abscess (23.3% vs 11.4%, P = .0384) were more frequent in patients with relapsed SAB than patients with S-SAB. Patients with reinfection were younger (46y vs 61y; P = .0010), more likely to be female (56.4% vs 39.0%, P < .0423) and less likely to have a neoplasm (5.13% vs 25.5%, P = .0019) compared to patients with S-SAB.
Isolate Genotypes
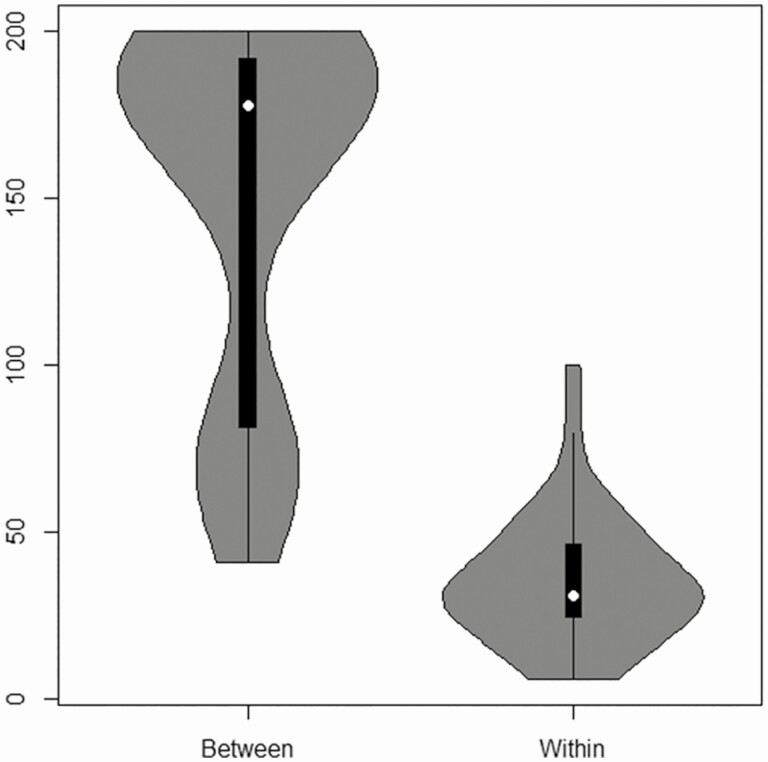
We sought to understand whether whole genome sequencing (WGS) provided additional insights not apparent in the PFGE analysis. The paired isolates selected for sequencing all had highly related or identical PFGE profiles. WGS data revealed that these paired isolates shared MLST ST types and differed by ≤ 100 single-nucleotide polymorphisms (SNPs). This finding suggests that these highly related isolates either arise from a single persistent patient source of infection or from multiple unrelated infections with a single successful clone [21]. To see if we could separate these possibilities, we compared all possible pairs of isolates between different individuals. A small subset had fewer than 200 SNPs (n = 72 out of 1040 pairs of isolates). These 72 pairs are combinations of isolates originating from a few patients. We compared the PFGE among these patients and found that the patterns are a visual match, indicating that the SNP counts agree with PFGE (Figure S5). This implies that we have identified a few successful clones in our sample. These successful clones are a small subset of the most frequently occurring Spa-types in this population: MLST ST105/5 (Spa-CC002) and ST8 (Spa-CC008). We compared the distribution of SNPs in the PFGE matched isolates from different patients to the PFGE matched isolate pairs from the same individual (Figure 2). The distributions are different with fewer SNP differences in the pairs from the same patient (median = 31 vs 178, P < .0001). However, the range observed is similar in both cases. This similarity indicates that the number of SNPs alone is insufficient to distinguish between independent reinfection from a successful clone, reinfection from a clone in an external reservoir, or a relapse of an incompletely resolved infection within a single patient. We conclude that the amount of SNP variation by WGS is consistent with the PFGE and that no novel insights were provided by the WGS in these data.
Figure 2.
Distribution of the number of SNPs for isolate pairs between individuals with fewer than 200 SNPs (between) compared to pairs of isolates from the same individual with a PFGE match (within). Supplementary Figure 5 indicates that the isolate pairs between individuals are a visual PFGE match. Abbreviations: PFGE, pulsed-field gel electrophoresis; SNP, single-nucleotide polymorphism.
Genotype of S. aureus Isolates in Patients With R-SAB and S-SAB
The most common spa-CCs in our sample were CC2 and CC8 (Table 3). Although CC8 was more frequently observed in the relapse group (40%), this was not significantly different from the S-SAB group (29.7%, P = .1341) or the reinfection group (25.6%, P = .8411). The distribution of CC2 was similar in all groups.
Table 3.
Distribution of Spa-clonal Complexes (CC) of Patients for Each Categorization of SAB
| Spa-clonal Complex | Group I Single Episode (n = 687) | Group II Recurrence (n = 69)a | P-value, I vs II | Group III Relapse (n = 30)b | P-value, I vs III | Group IV Reinfection (n = 39)c | P-value, I vs IV |
|---|---|---|---|---|---|---|---|
| CC2 | 208 (30.3) | 21 (30.4) | .2922 | 8 (26.7) | .8276 | 13 (33.3) | .1695 |
| CC8 | 204 (29.7) | 22 (31.9) | .1341 | 12 (40.0) | .0736 | 10 (25.6) | .8411 |
| Otherd | 275 (40.0) | 13 (18.8) | 5 (16.7) | 8 (20.5) |
Abbreviation: SAB, Staphylococcus aureus bacteremia.
aData missing from 13 patients.
bData missing for 5 patients.
cData missing for 8 patients.
dThis category includes spa-types CC12, CC148, CC164, CC189, CC216, CC4, CC78/81, CC84, nontypeable, and not available; statistical comparison of these subgroups was not performed due to low cell counts.
Acute Phase Plasma Cytokines in Patients With R-SAB and S-SAB
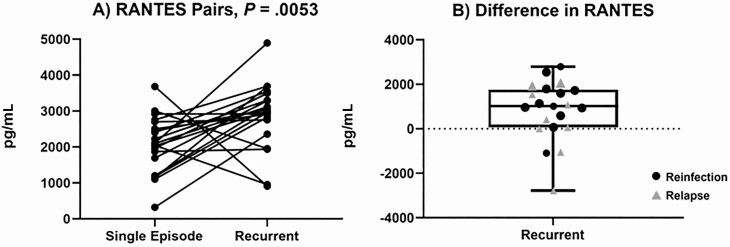
Next, we evaluated acute phase plasma cytokine levels from the initial episode of bacteremia in patients with R-SAB and S-SAB. Twenty-one patients with R-SAB were selected based on the availability of their baseline (first episode) plasma. Among the 25 cytokines tested (Figure S6), only RANTES exhibited significant differences between patients with R-SAB and S-SAB (Figure 3). We define ΔRANTES to be the difference in RANTES values between a case of R-SAB and its matched S-SAB control (ΔRANTES = RANTES R-SAB–RANTES control). ΔRANTES was positive for 18 of the 21 cases of R-SAB (Figure 3A; WRS P = .0053, false discovery rate < 0.10) [22]. Three cases of R-SAB with a negative ΔRANTES all came from perfectly matched pairs. RANTES values were not significantly correlated (range (−0.13 to 0.26; median −0.02) with any other cytokines, and although ΔRANTES was positive for most matched pairs, equivalent metrics for the majority of other cytokines were negative.
Figure 3.
A, Comparison of baseline plasma RANTES level between patients with recurrent SAB) and age/sex/race/genotype-matched patients with a resolving single episode of SAB. B, Difference in RANTES values between recurrent SAB and their matched single SAB. We also examined a more conservative set of fewer than 100 SNPs. Here the median for the “across individual” set was 74 and still significantly different from the “same individual” pairs (P < .0001). Abbreviations: RANTES, regulated on activation, normal T cell expressed and secreted; SAB, Staphylococcus aureus bacteremia; SNP, single-nucleotide polymorphism.
Discussion
The prevalence of R-SAB in our study was ~9%, similar to earlier reports [6, 7]. However, the documented prevalence of R-SAB varies widely (Table S3). This variation is influenced by study design, definitions of recurrence, and the proportion of MRSA. For example, recurrence rates varied from 7.1% in a study where MRSA accounted for < 1% of the sample [11] to >20% in a study limited to MRSA infections [9]. Kreisel et al reported a high rate of R-SAB (~17%), but excluded patients who died before the completion of antibiotics [8]. Conversely, Albertson et al reported a lower R-SAB rate of 6.3%, but excluded recurrent cases more than 180 days after the initial episode of SAB [5]. Thus, our rate of R-SAB is consistent with what has been previously reported in the literature.
Blacks comprised ~ one-third of the entire study cohort. In the multivariate model, black hemodialysis patients were ~ 2 times more likely than white hemodialysis counterparts to develop R-SAB. This is consistent with previous research showing that there is a substantial health disparity in the incidence of invasive MRSA infections compared to white patients [23, 24].
Socioeconomic status (SES), factors such as poverty, crowding, and the availability and affordability of medical care have been shown to contribute [25–27] to heath disparities in SAB. Indeed, See et al reported that after controlling for socioeconomic factors in a mediation anlysis, there was no disparity between black and white patients in MRSA infection [25]. This is supported by data from outside the United States, suggesting that lower SES is associated with higher rates of MRSA infection [26, 27]. In addition, there is some evidence to suggest a relationship between SES and host colonization with S. aureus [28, 29]. For example, Freitas et al found self-reported black race as a risk factor for colonization with a highly virulent strain of MRSA (OR, 1.81 [95% CI, 1.38–2.38]) [30]. Differences in allele frequencies within HLA Class II and other genetic factors controlling innate immunity also influence host susceptibility to S. aureus infection in both black and white subjects [31, 32].
In our study, black race in combination with hemodialysis dependence conferred significant risk of R-SAB, with an OR of over 9. These patients were twice as likely to have R-SAB as white hemodialysis dependent patients (OR, 4.5). This is consistent with previous studies. In a study that evaluated the impact of race on risk for invasive MRSA, blacks were at an increased risk for both healthcare-associated MRSA infections in general (adjusted rate ratio [aRR], 3.84 [95% CI, 2.94–5.01]) and hemodialysis-associated infection in particular (aRR, 1.83 [95% CI, 1.72–1.96]) [23]. In the United States, non-Hispanic blacks have the highest lifetime risk of developing end-stage renal disease (ESRD) [33] and are subsequently at an increased risk of becoming hemodialysis-dependent [34]. In addition, Saunders et al demonstrated that hemodialysis-dependent blacks are less likely to use hemodialysis facilties that are rated highly by federal quality reporting programs [35].
Although hemodialysis dependent blacks were more likely to experience R-SAB than white hemodialysis dependent patients, the 2 groups had similar APACHE II scores, rates of metastatic infections, and persistent bacteremia. In addition, survival and SAB-attributable mortality for black and white patients in the S-SAB group were virtually identical. These findings are consistent with a previous study suggesting that race did not influence risk-adjusted mortality in patients with sepsis despite a health disparity in the incidence [36].
Higher APACHE II scores were also a significant predictor of R-SAB in our multivariate model. This association between measures of illness severity and risk of recurrence has been previously demonstrated [5]. APACHE II scores are widely used, easily interpreted, and include estimates for both acute and chronic illness severity estimates, which can reduce the number of variables included in a multivariable model [8, 11]. Thus, we used APACHE II in our final multivariable model as a parsimonious strategy to address patient comorbidity. Using this strategy, we found that patients with higher levels of acute illness severity and more comorbid conditions, represented by APACHE II > 15, were 1.869 (95% CI, 1.081–3.271) times more likely to develop R-SAB.
R-SAB includes bacteremia due to both relapse and reinfection. Differentiating these syndromes is clinically important, but often challenging. Relapsing SAB typically suggests an inability to eradicate an established S. aureus infection or potentially an additional episode of bacteremia with the same bacterial strain. Nasal carriage of S. aureus is a significant risk factor for developing SAB [37, 38] and is often the source of the isolate causing bacteremia [39]. In our study and previous research, persistent bacteremia [5], metastatic abscesses, and MRSA [8] were associated with relapse. This is due in part to their association with infections involving high bacterial load, unremoved foci, and/or insufficient antimicrobial treatment [3, 6]. On the other hand, new episodes of reinfection in the same host suggest an increased susceptibility to recurrent bouts of S. aureus infection. This increased susceptibility may be due to a medical (eg, long-term catheter), environmental (injection drug use), or genetic condition. In our study, reinfection was associated with younger age, a finding that aligns with previous reports [5].
Despite their increased risk to develop R-SAB, hemodialysis-dependent black patients were no more likely than nonhemodialysis patients to relapse compared to reinfection (P = .20). This finding suggests that the observed health disparity reflects a general susceptibility to recurrence as opposed to an inability to successfully clear infection or exposure to an untreated reservoir. This interpretation is supported by the similar time to recurrence observed in black hemodialysis-dependent patients, white hemodialysis-dependent patients, and nonhemodialysis patients (P = .60).
Host inflammatory response was associated with R-SAB. The median level of chemokine ligand 5 (CCL5), also known as RANTES, was significantly higher in the baseline plasma of the 21 patients with R-SAB than in the baseline plasma of 21 matched subjects with S-SAB. There was no association between race and RANTES levels. RANTES is a chemotactic cytokine that recruits leukocytes to infection sites [40]. In a recent study investigating cytokine responses to SAB, levels of RANTES rose significantly in SAB patients by day 7 and remained persistently elevated up to 14 days in patients with complicated SAB [41]. Although the biological mechanism is yet to be determined, our findings suggest that RANTES may play a role in the host immune response to SAB. Further study is needed to validate this discovery and understand the underlying mechanism.
This study has limitations. First, we categorized PFGE-indistinguishable strains into relapse and reinfection based on the time point at which the second episode of SAB occurred. Thus, we may have inaccurately classified patients who relapsed after 149 days as having experienced a reinfection. Second, patients may have sought medical attention at other hospitals for their recurrent episode of SAB. Third, the impact of antimicrobial and surgical therapy on the occurrence of R-SAB was not evaluated in this study. Fourth, it is not possible to compare outcomes among patients with R-SAB and S-SAB due to the definition of these 2 groups. Finally, this is a single-center study with a small sample size for certain events and measurements.
In conclusion, the effects of SAB can be compounded by recurrent episodes in some patients. Our study demonstrated a racial health disparity in risk for R-SAB that is consistent with previous literature on racial disparities in SAB. Further research is needed to fully elucidate the mechanisms driving this disparity. In addition, illness severity increases the likelihood of recurrence. Finally, in a small matched pair cohort, RANTES at baseline was higher in patients who went on to develop R-SAB than in patients with S-SAB, and this trend was not associated with race. Further study on the mechanisms of R-SAB are warranted. In particular, adequately powered studies of black patients are critical.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Funding. This research was supported in part by the following grants from the National Institutes of Health: U01-AI124319, R01-AI068804, R01-GM128193, U2-CES030167. Dr. Fowler was supported by National Institutes of Health Grant K24-AI093969. Biomarker profiling was performed under the management of Dr Andrew N. Macintyre and direction of Dr Gregory D. Sempowski in the Immunology Unit of the Duke Regional Biocontainment Laboratory (RBL), which received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6-AI058607).
Potential conflicts of interest. V. G. F. reports grant/ research support from the National Institutes of Health, MedImmune, Cerexa/Forest/Actavis/Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Cubist/Merck; Medical Biosurfaces; Locus; Affinergy; Contrafect; Karius; Gtenentech, Regeneron, BasileaPaid Consultant: Pfizer, Novartis, Galderma, Novadigm, Durata, Debiopharm, Genentech, Amphliphi Biosciences, Integrated Biotherapeutics, C3J, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Cubist, Basilea, Affinergy, Janssen, xBiotech, Contrafect, Regeneron, Basilea, Destiny. Membership: Merck Co-Chair V710 Vaccine. Educational fees: Green Cross, Cubist, Cerexa, Durata, Theravance; Debiopharm. Royalties from UpToDate. Patent pending for host gene expression signature diagnostic for sepsis. All other authors have no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fowler VGJ, Holland TL. Clinical approach to Staphylococcus aureus bacteremia in adults. In: Lowy FD, ed. UpToDate. Waltham, MA: UpToDate Inc. [Google Scholar]
- 2. Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 3. Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med 2007; 167:1861–7. [DOI] [PubMed] [Google Scholar]
- 4. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albertson J, McDanel JS, Carnahan R, et al. Determination of risk factors for recurrent methicillin-resistant Staphylococcus aureus bacteremia in a Veterans Affairs healthcare system population. Infect Control Hosp Epidemiol 2015; 36:543–9. [DOI] [PubMed] [Google Scholar]
- 6. Chang FY, Peacock JE Jr, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–9. [DOI] [PubMed] [Google Scholar]
- 7. Fowler VG Jr, Kong LK, Corey GR, et al. Recurrent Staphylococcus aureus bacteremia: pulsed-field gel electrophoresis findings in 29 patients. J Infect Dis 1999; 179:1157–61. [DOI] [PubMed] [Google Scholar]
- 8. Kreisel K, Boyd K, Langenberg P, Roghmann MC. Risk factors for recurrence in patients with Staphylococcus aureus infections complicated by bacteremia. Diagn Microbiol Infect Dis 2006; 55:179–84. [DOI] [PubMed] [Google Scholar]
- 9. Liao CH, Lai CC, Chen SY, Huang YT, Hsueh PR. Strain relatedness of meticillin-resistant Staphylococcus aureus isolates recovered from patients with repeated bacteraemia. Clin Microbiol Infect 2010; 16:463–9. [DOI] [PubMed] [Google Scholar]
- 10. Walker TM, Bowler IC, Bejon P. Risk factors for recurrence after Staphylococcus aureus bacteraemia: a retrospective matched case-control study. J Infect 2009; 58:411–6. [DOI] [PubMed] [Google Scholar]
- 11. Wiese L, Mejer N, Schønheyder HC, et al. ; Danish Staphylococcal Bacteraemia Study Group . A nationwide study of comorbidity and risk of reinfection after Staphylococcus aureus bacteraemia. J Infect 2013; 67:199–205. [DOI] [PubMed] [Google Scholar]
- 12. Souli M, Ruffin F, Choi SH, et al. Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis 2019; 69:1868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi SH, Ruffin F, Park L, et al. 1060. Risk factors for recurrent Staphylococcus aureus bacteremia. Open Forum Infect Dis 2018; 5(suppl_1): S317. [Google Scholar]
- 14. Chong YP, Moon SM, Bang KM, et al. Treatment duration for uncomplicated Staphylococcus aureus bacteremia to prevent relapse: analysis of a prospective observational cohort study. Antimicrob Agents Chemother 2013; 57:1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 16. Sharma-Kuinkel BK, Rude TH, Fowler VG Jr. Pulse field gel electrophoresis. Methods Mol Biol 2016; 1373:117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995; 33:2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hooton TM, Gupta K. Recurrent simple cystitis in women. In: Calderwood SB, ed. UpToDate. Waltham, MA: UpToDate Inc. [Google Scholar]
- 19. Johnson JR. Reinfection versus relapse in urinary tract infection. Clin Infect Dis 2005; 40: 495; author reply -6. [DOI] [PubMed] [Google Scholar]
- 20. Mathema B, Mediavilla J, Kreiswirth BN. Sequence analysis of the variable number tandem repeat in Staphylococcus aureus protein A gene: spa typing. Methods Mol Biol 2008; 431:285–305. [DOI] [PubMed] [Google Scholar]
- 21. Lindsay JA. Genomic variation and evolution of Staphylococcus aureus. Int J Med Microbiol 2010; 300:98–103. [DOI] [PubMed] [Google Scholar]
- 22. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B 1995; 57: 289–300. [Google Scholar]
- 23. Gualandi N, Mu Y, Bamberg WM, et al. Racial disparities in invasive methicillin-resistant Staphylococcus aureus infections, 2005–2014. Clin Infect Dis 2018; 67: 1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klevens RM, Morrison MA, Nadle J, et al. ; Active Bacterial Core surveillance (ABCs) MRSA Investigators . Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–71. [DOI] [PubMed] [Google Scholar]
- 25. See I, Wesson P, Gualandi N, et al. Socioeconomic factors explain racial disparities in invasive community-associated methicillin-resistant Staphylococcus aureus disease rates. Clin Infect Dis 2017; 64:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bagger JP, Zindrou D, Taylor KM. Postoperative infection with meticillin-resistant Staphylococcus aureus and socioeconomic background. Lancet 2004; 363:706–8. [DOI] [PubMed] [Google Scholar]
- 27. Tosas Auguet O, Betley JR, Stabler RA, et al. Evidence for community transmission of community-associated but not health-care-associated methicillin-resistant Staphylococcus Aureus strains linked to social and material deprivation: spatial analysis of cross-sectional data. PLoS Med 2016; 13:e1001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gorwitz RJ, Kruszon-Moran D, McAllister SK, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis 2008; 197: 1226–34. [DOI] [PubMed] [Google Scholar]
- 29. Neves FPG, Marlow MA, Rezende-Pereira G, et al. Differences in gram-positive bacterial colonization and antimicrobial resistance among children in a high income inequality setting. BMC Infect Dis 2019; 19:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freitas EA, Harris RM, Blake RK, Salgado CD. Prevalence of USA300 strain type of methicillin-resistant Staphylococcus aureus among patients with nasal colonization identified with active surveillance. Infect Control Hosp Epidemiol 2010; 31:469–75. [DOI] [PubMed] [Google Scholar]
- 31. Cyr DD, Allen AS, Du GJ, et al. Evaluating genetic susceptibility to Staphylococcus aureus bacteremia in African Americans using admixture mapping. Genes Immun 2017; 18:95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeLorenze GN, Nelson CL, Scott WK, et al. Polymorphisms in HLA class II genes are associated with susceptibility to Staphylococcus aureus infection in a white population. J Infect Dis 2016; 213:816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Albertus P, Morgenstern H, Robinson B, Saran R. Risk of ESRD in the United States. Am J Kidney Dis 2016; 68:862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tomson CR, Foley RN, Li Q, Gilbertson DT, Xue JL, Collins AJ. Race and end-stage renal disease in the United States Medicare population: the disparity persists. Nephrology (Carlton) 2008; 13:651–6. [DOI] [PubMed] [Google Scholar]
- 35. Saunders MR, Lee H, Maene C, Schuble T, Cagney KA. Proximity does not equal access: racial disparities in access to high quality dialysis facilities. J Racial Ethn Health Disparities 2014; 1:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Corl K, Levy M, Phillips G, Terry K, Friedrich M, Trivedi AN. Racial and ethnic disparities in care following the New York State sepsis initiative. Health Aff (Millwood) 2019; 38:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marzec NS, Bessesen MT. Risk and outcomes of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia among patients admitted with and without MRSA nares colonization. Am J Infect Control 2016; 44:405–8. [DOI] [PubMed] [Google Scholar]
- 38. Wertheim HF, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 2004; 364:703–5. [DOI] [PubMed] [Google Scholar]
- 39. von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 2001; 344:11–6. [DOI] [PubMed] [Google Scholar]
- 40. Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol 2001; 22:83–7. [DOI] [PubMed] [Google Scholar]
- 41. McNicholas S, Talento AF, O’Gorman J, et al. Cytokine responses to Staphylococcus aureus bloodstream infection differ between patient cohorts that have different clinical courses of infection. BMC Infect Dis 2014; 14:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.