Abstract
Purpose of Review
There is evidence from epidemiologic studies that variability in cardiovascular risk factors influences risk of cardiovascular disease. We review new studies and novel findings in the relationship between visit-to-visit glycemic variability and blood pressure variability and risk of adverse outcomes.
Recent Findings
Visit-to-visit glycemic variability is consistently linked to macrovascular disease. This relationship has been observed in both clinical trials and retrospective studies of electronic health records. Long-term blood pressure variability also predicts cardiovascular outcomes, and the association appears stronger in those with lower levels of systolic and diastolic function.
Summary
As epidemiologic evidence increases in support of a role for metabolic risk factor variability in cardiovascular risk, there is a corresponding rise in interest in applying this information toward improving risk factor prediction and treatment. Future investigation of underlying mechanisms for these associations as well as implications for therapy is also warranted. The potential additive contribution of variability of multiple parameters also merits additional scrutiny. As our technology for capturing risk factor variability continues to improve, this will only enhance our understanding of its links with vascular disease and how to best utilize this information to reduce cardiovascular outcomes.
Keywords: Risk factor variability, Macrovascular disease, Clinical trials, Type 2 diabetes, Mechanisms, Coronary hypoperfusion
Introduction
Research efforts over the last 50+ years have focused on identifying key risk factors for vascular complications and developing strategies to reduce mean levels of these factors to safer ranges in higher risk individuals, such as those with prediabetes and diabetes. Although this strategy has brought substantial success, such as reducing cardiovascular disease (CVD) with lowering of LDL cholesterol, its limitations have also become apparent. This has been particularly apparent with attempts to reduce macrovascular disease in patients with more advanced type 2 diabetes (T2D). Benefits of intensive glucose-lowering therapy on rates of macrovascular complications and death for T2D patients by lowering mean HbA1c levels to near normal levels were modest at best, as demonstrated in several major clinical trials including ACCORD, ADVANCE and VADT [1–3]. Why reduction of glucose levels and HbA1c did not have the anticipated success in reducing vascular outcomes and the optimal glucose-control strategies remain unclear.
However, we and others have demonstrated that long-term glycemic variability, defined as visit-to-visit glucose variation, was associated with risk of CVD, [4] renal disease, and mortality [5], independent of traditional markers of glycemic control such as average glucose or HbA1c. Similarly, there is an abundance of research over the last decade demonstrating that blood pressure variability is an independent predictor of CVD, including stroke, after accounting for mean blood pressure levels [6, 7]. These relationships persist even in the setting of aggressive blood pressure lowering, and in fact may be more robust at lower levels of systolic and diastolic function [8, 9•]. Importantly, fluctuation in other risk factors, such as weight and lipids, has also been reported to be independent contributors to CVD [10•]. Thus, there is increasing recognition that variation in risk factors cannot be ignored as simply measurement error; it instead represents both physiologically and environmentally driven risk factor change. Importantly, variability in multiple risk factors is linked with vascular outcomes in ways not fully appreciated and that is not captured by standard clinical assessments of mean levels of these risk factors.
Progress in this field, however, has been slowed in part by the complexity of characterizing risk factor fluctuation in terms of both time and variability metrics. For risk factors such as glucose and blood pressure, meaningful variation in these measures can occur over minutes or months and consequences may vary as a result. For example, beat to beat variation in blood pressure may have different determinants and consequences than visit-to-visit variation in blood pressure over months to years. Moreover, the optimal metrics (e.g., standard deviation, coefficient of variation, change in amplitude) for capturing variability risk are still being established and may vary among risk factors [6, 11]. We are also limited by the technology available for capturing acute and chronic variability. The precision of instruments used for short- and long-term assessments at home has typically been lower than that of instruments used in the ambulatory setting. This more controlled and consistent collection of vital signs and laboratory markers during clinic visits may be one reason that visit-to-visit variability assessments have proven useful in predicting risk. Importantly, as our home-based technology has become more sophisticated and broadly available, as with continuous glucose monitoring, we are realizing that newer metrics of glucose variation such as “time in range” may be as useful, or more useful than, mean levels of glucose control in determining acute and chronic diabetes complications [12].
Despite these limitations, substantial advances in our understanding of the importance of risk factor variability have occurred. There is little doubt that as our ability to more precisely and continually capture variability in risk factors increases, our appreciation of the clinical importance of this variability will expand dramatically. The current review will consider some of the complexities of assessing risk factor variability and will highlight the growing body of evidence supporting the importance of risk factor variability in vascular complications. We will focus on variability, particularly more chronic visit-to-visit changes, of glucose and blood pressure, as studies in these areas provide the most developed examples of our increasing understanding of risk factor variability and vascular disease. Importantly, the rapid development of electronic health record systems over the last two decades will allow providers to readily track and calculate risk factor variability and potentially integrate this into efforts to provide more personalized treatment strategies for each patient.
Metrics and Analysis Approaches for Visit-to-Visit Variability
Different statistical metrics of variability have been used to assess fluctuation of risk factors in different studies; however, there is also currently no consensus on the best statistical metrics to use to capture the risk of either short-term or long-term measures of risk factor variation. However, for the reasons noted above, in this review, we focus on visit-to-visit long-term variability and highlight several commonly used definitions of variability, including standard deviation (SD), coefficient of variation (CV), average real variability (ARV), sequential variability (SV), and variation independent of mean (VIM) (Table 1) for glucose, HbA1c, and blood pressure. Importantly, most of these variability metrics usually were highly correlated and typically lead to similar conclusions, so most studies focused on a few key complementary metrics. In a few studies where mean levels of risk factors were trending up or down over time, residuals of these metrics were generated (to better estimate variation after accounting for the time trend in risk factors).
Table 1.
Definitions of measures of variability
| Name | Computation | Description of variability captured | |
|---|---|---|---|
| Mean | Mean | Average over time | |
| Var, SD | Variance, standard deviation | Var or SD captures how far a set of numbers is spread out from their average value. Var or SD may include variation over time from both trend and fluctuations | |
| CV | Coefficient of variation | CV shows the extent of variability measured by SD in relation to the mean of the population | |
| ARV | Average real variability | ARV measures average of absolute differences between visits over time | |
| SV | Sequential variability | SV has the same features as ARV | |
| VIM* | Variation independent of mean | VIM measures the same type of variation over time as CV but by adding a higher order of means in the denominator, it further removes correlation between mean and variation | |
| HVS or BVS | Blood pressure/HbAlc variability score (HVS or BVS) | The percentage of total HbAlc or blood pressure measures that vary by 0.5% | |
| RSD | Residual standard deviation | Using residuals can remove the variation that was introduce due the time trend, e.g., linearly increasing over time | |
| RARV | Residual average real variability | See above (for RSD) | |
Let Xij denote the glycemic value or blood pressure value for subject i at time j. Variability measures for subject i at time j are defined as above
VIM is calculated by fitting a curve of the form y = kxp through a plot of SD of glucose/HbA1c (y-axis) against mean of glucose/HbA1c (x-axis), for all individuals in the cohort. The parameter p is estimated from the data and k is a constant which can be chosen such that the values of VIM are on the same scale as values of SD; (, , …, ) are the fitted values from a statistical model where linear time effects are estimated. In these formulas, n is the total number of measures for an individual, while i indexing for each measure
When estimating the risk of glycemic and blood pressure measures of variability, the selected metrics are often included in Cox proportional hazard models [13] as continuous and time-dependent covariates that permit one to consider their effects right up to the time of an outcome. Using time-dependent estimate models typically permits inclusion of more (serial) measures of risk factors (given the longer period of risk factor monitoring) and presumably quantifies the full extent of risk factor variation more accurately up to the outcome of interest. In contrast, some long-term observational studies have used an initial period of time where risk factor variation was captured as “a landmark period,” and this was related to events during a subsequent observation period [14]. Although the latter approach is reasonable and straightforward, it shortens the period of variability monitoring and assumes that variation during the observational period remains similar to that during the landmark period or will not substantially influence the outcomes.
Glycemic Variability and Macrovascular Disease
There has been growing support for the possibility that visit-to-visit fasting glucose and/or HbA1c variability may add to standard glycemic measures for prediction of cardiovascular complication in patients with diabetes. To build on older reviews of glucose variability, we searched PubMed/Medline for recent high-quality large cohort studies published between 2018 and September 2020. Similar criteria were also used in a review and meta-analysis of earlier studies [15]. This search identified 8 studies that examined the risk of glycemic variability for macrovascular diseases with relatively large sample sizes. A summary of these studies is shown in Table 2. Of these 8 studies, 4 were post hoc secondary analysis of clinical trials [4, 16, 17••, 18] and 4 were retrospective studies utilizing electronic medical records data [19–22]. Five studies included participants with T2D only, with mean age ranges from 62 to 67; two studies included non-diabetes populations with mean ages of 40 and 64.9, respectively; and one study enrolled participants with and without diabetes with a mean age of 65 years. The follow-up ranged from 2 years [18] to 8 years [22] in these studies. The number of HbA1c or glucose measurements per patient ranged from as few as 3 [16] to 18 [4]. The definition of glycemic variability, the outcome evaluated, and study follow-up time and main results are shown in Table 2. Both time-varying and landmark methods were adopted in these studies to evaluate risk of glycemic variability.
Table 2.
Studies of glycemic variability and risk of macrovascular disease
| Studies of macrovascular disease | Study design, country | Sample size and cohort characteristics | Macrovascular outcome definitions | Analysis approach and glycemic variability definition | Adjustments for average glucose control | Major conclusions |
|---|---|---|---|---|---|---|
| Zhou et al. [4] | Secondary analysis of VADT, USA | ■ N = 1791 ■ T2D only ■ Mean age of 66 ■ Mean follow-up of 5.3 years |
■ Primary outcome: the composite CVD (documented myocardial infarction, stroke, death from cardiovascular causes, new or worsening congestive heart failure, surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease, inoperable coronary artery disease, and amputation for ischemic gangrene) ■ Secondary: MACE: cardiovascular death, myocardial infarction, and stroke |
■ Time varying: mean # of measures of FBG and HbA1c is 18.5 ■ ARV and CV |
Adjusted for time-varying mean HbA1c and glucose | ■ Variability of FBG was associated with development of CVD complications beyond the influence of standard fasting glucose measures. ■ The adverse consequences of fasting glucose variability on CVD appeared greatest in those receiving intensive glucose control. |
| Echouffo-Tcheugu et al. [16] | Secondary analysis of ALLHAT, USA | ■ N = 4982 ■ All participants are hypertensive. 35% of the cohort are diabetes patients ■ >55 years old ■ Median follow-up period of 5 years (range 4–8) |
■ CVD events defined as a composite of major cardiovascular events (including fatal and non-fatal CHD, stroke, and heart failure) and ■ All-cause mortality |
■ Landmark: FBG were measured at baseline, 24 months, and 48 months following randomization ■ Using the average FBG at each of these three visits, VVV SD of FBG calculated. The alternative VVV of FBG metrics include CV, VIM, and ARV |
Adjusted for average HbA1c/FBG | ■ Greater VVV of FBG was associated with increased mortality risk. ■ FBG glycemic variability was not significantly associated with incident CVD after adjusting for average FBG |
| Segar et al. [17••] | Secondary analysis of ACCORD trial, USA | ■ N = 8576 ■ T2D only ■ Mean age of 62 ■ Median follow-up of 6.4 years from the end of variability measurements at year 3 |
■ Primary outcome: heart failure (HF) defined as the first hospitalization event for HF or death due to HF; ■ Secondary outcome: acute ischemic heart disease event was defined as cardiovascular death, non-fatal myocardial infarction (MI), or unstable angina |
■ Landmark: from 8 months to 3 years with median # of measures = 8 (interquartile range 7–8) ■ ARV, CV, and SD for both HbA1c and FBG |
Adjusted for baseline HbA1c, FBG, change of HbA1c from baseline and 3 years | ■ Variability in HbA1c and fasting glucose was independently associated with risk of HF and CVD among patients with T2D independent of changes in HbA1c, variability in BP, BMI, and LDL-C, and hypoglycemia events |
| Zinman et al. [18] | Secondary analysis of DEVOTE trial | ■ N = 7637 ■ T2D only ■ mean age of 65 years old ■ Medium observation time is 1.99 years |
■ Primary outcome: first occurrence of death from cardiovascular causes, non-fatal myocardial infarction or non-fatal stroke | ■ Time varying: average number of blood glucose from SMBG during week 1, month 12, and month 24 is 2.8 ■ SD |
Adjusted for both baseline and time-varying most recent HbA1c | ■ Higher day-to-day fasting glucose variability was associated with increased risks of MACE, severe hypoglycemia and all-cause mortality ■ The association of fasting glycemic variability and MACE did not stay after adjusting for most recent HbA1c measurement on a continuous scale and baseline characteristics |
| Critchley et al. [19] | A retrospective matched cohort study | ■ N = 58,832, ■ T2D only ■ Mean age of 67.7 ■ Mean follow-up time of 4.1 years |
■ Primary outcomes: all-cause mortality and first emergency hospitalization ■ Secondary outcomes: (1) CVD death and non-cardiovascular death; (2) deaths related to CAD and ischemic stroke versus all other CVD codes; (3) emergency hospitalizations with infection-related CVD, and CAD+ ischemic stroke admissions |
■ Landmark: average HbA1c, CV in HbA1c; trajectory in HbA1c estimated from the individual patient annual slope from a linear regression model ■ Time-varying covariate was analyzed as sensitivity analysis |
Adjusted for average HbA1c and trajectory of HbA1c | ■ HbA1c variability was strongly associated with overall mortality and emergency hospitalization which was not explained by average HbA1c or hypoglycemic episodes ■ No association was found for CAD and ischemic stroke |
| Ghouse et al. [20] | Population-based retrospective cohort study from medical records | ■ N = 6756 ■ No diabetes and CVD ■ Mean age of 64.9 ■ Median follow-up period was 6.3 years (IQR 4.3–9.0) |
■ The end points of interest were incident MACE, death from all causes, and type 2 diabetes | ■ Landmark: three HbA1c measures from index date ■ RSD, intercept, and slope from index date |
Stratified within HbA1c levels | ■ High HbA1c variability was associated with increased risk of MACE and all-cause mortality, after the adjustment of average glycemic control |
| Yu et al. [22] | Population-based retrospective cohort study from medical records | ■ N=3,211,319 general population without diabetes and CVD, with mean age around 40, and average follow-up of 8.3 years | ■ Primary outcomes: myocardial infarction (MI), stroke, and all-cause mortality | ■ Landmark: observation period is from 2002 to 2006 with an average of 4.2 ± 1.2 glucose measurements (median 4) per participant ■ SD of FPG |
■ Stratified within impaired fasting glucose (i.e., FPG ≥6.1 mmol/L) and normal glucose tolerance ■ Adjusted for FBG change of FBG level between 2002 and 2006 |
■ Long-term FPG variation was independently associated with CVD and mortality in a general population without diabetes, after adjusting for FPG |
| Wan et al. [21] | Population-based prospective cohort study from electronic health records, Hong Kong | ■ N =14,781 ■ T2D only ■ Mean age 64.2. ■ Median follow-up of 7.4 years |
■ Primary outcome: incidence of a composite of CVD and all-cause mortality ■ Secondary outcome: individual CVD, subtypes of coronary heart disease, stroke, heart failure, all-cause mortality, CVD mortality, and non-CVD mortality |
■ Time varying: the HbA1c metric was the RSD ■ The mean (SD) number of HbA1c measurements was 3.2 (0.8) |
Adjusted for average HbA1c | ■ Age group specific analysis was performed ■ HbA1c variability was strongly related to CVD and mortality in patients with diabetes across all age groups |
ACCORD, The Action to Control Cardiovascular Risk in Diabetes; VADT, Veterans Affairs Diabetes Trial; ALLHAT, The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; DEVOTE, A Trial Comparing Cardiovascular Safety of Insulin Degludec Versus Insulin Glargine in Subjects With Type 2 Diabetes at High Risk of Cardiovascular Events; T2D, type 2 diabetes; CVD, cardiovascular disease; MACE, major adverse cardiovascular events; CHD, congenital heart disease; CAD, coronary artery disease; HbA1c, hemoglobin A1C; FBG, fasting blood glucose; LDL-C, low-density lipoprotein cholesterol; SMBG, self-measured blood glucose; ARV, average real variability; CV, coefficient of variability; RSD, residual standard deviation; SD, standard deviation; VIM, variation independent of mean; IQR, interquartile range; VVV, visit-to-visit variability
All studies were adjusted for relevant CVD baseline covariates and covariates reflecting mean glycemic control. Segar et al. [17••] additionally adjusted for BP, BMI, LDL-c variability, and time-dependent myocardial infarction (MI) incidence; yet, even after these adjustments, glycemic variability, including both HbA1c and glucose variability defined by average successive variability (ARV), SD, and coefficient of variation (CV), still showed an independent association with heart failure (HF). In the fully adjusted model in this study, the hazard ratio (HR) for the risk of HF was 1.24 (95% CI 1.12–1.37) per 1 SD higher in ARV. Results stayed the same with other metrics of glycemic variability and after excluding patients with hypoglycemic events. Within the VADT, variability measures (CV and ARV) of fasting glucose were significantly associated with a composite CVD after adjusting for other risk factors, including mean fasting glucose, as shown by Zhou et al. [4]. The HR was estimated to be 1.138 (1.038, 1.247) per 1 SD increase in ARV. When considering separate groups receiving intensive and standard glycemic control, this relationship was evident only in the intensive treatment group but not in the standard group. This raises the possibility that excessive variability in the setting of intensive glucose lowering may counter the CVD benefits of improved overall glucose control. Although this would lead one to suspect hypoglycemia as a confounder or mediator of glucose variability induced harm, additional adjustment for severe hypoglycemic episodes did not alter the relationship between fasting glucose variability and CVD in the VADT analysis. Interestingly, in this study, variability in HbA1c measures was not associated with CVD, after adjusting for multiple baseline risk factors [4]. In contrast, in ACCORD, fasting blood glucose variability was less strongly associated with heart failure than was HbA1c variability, although similar strength of risks for both fasting blood glucose and HbA1c was shown for CVD [17••]. All eight studies demonstrated a dose-response pattern between glycemic variability and adverse outcomes, including heart failure, all-cause mortality, and CVD mortality. However, in both the DEVOTE trial and ALLHAT, while fasting blood glucose variability was significantly associated with MACE and other CVD outcomes, statistical significance did not persist after adjusting for either baseline HbA1c, most recent HbA1c, or average fasting blood glucose. At least in the ALLHAT study, this may reflect the fact that only a portion of the participants had diabetes and therefore a broader range of glucose variability. Several of the recent studies have taken advantage of the enormous data stored in medical records to conduct glycemic variability analyses in very large populations. Using a national records database in Korea of 3,211,319 people, fasting blood glucose variability was also found associated with MI, stroke, and all-cause mortality [22]. Age-specific analyses demonstrated that glycemic variability was not only a risk factor for older individuals with longer durations of diabetes [4, 17••] but also a risk factor among younger people with diabetes in relatively good glycemic control [21]. Overall, 6 out of these 8 studies found significant associations between glycemic variability and CVD events even in fully adjusted models that included measures of average glycemic control.
The present results add to the previous findings of significant associations between HbA1c variability (SD and CV) and macrovascular disease nicely summarized in the 2015 systematic review and meta-analysis by Gorst et al. Although this link between HbA1c variability and macrovascular outcomes still holds in more recent publications (after 2015) [4, 17••, 19–22], more current publications have highlighted that these associations are as strong or stronger with variability in other measures of glycemic control, such as fasting glucose. Similarly, recent studies have demonstrated that glucose variability is also associated with less traditional cardiovascular outcomes, such as heart failure.
Factors Contributing to Glycemic Variability
Although much attention has been paid to the possibility that glycemic variability is a risk factor for the development of complications in subjects with or without diabetes, few studies have focused on identifying factors, particularly modifiable ones, associated with glycemic variability. One hypothesis put forward by Ceriello et al. is that risk factor variability in general is a function of less than ideal medical care. In support of this concept, Ceriello et al. [23] showed that overall quality of care at baseline, as summarized by the Q-score, was able to predict the variability of HbA1c, blood pressure, serum uric acid, and lipid profile in patients with T2D. The Q-score is a weighted sum of quality care indicators including HbA1c, blood pressure, LDL cholesterol, and microalbuminuria. The score ranges between 0 and 40, with a higher score indicating better quality of care. Whether this relationship of glycemic variability with the Q-score reflects in part inadequate medication use or poor medication adherence, it is clear that medications likely contribute to glycemic variation. Early studies found that the use of sulfonylurea agents was positively and independently associated with glucose variability measured as the mean amplitude of glycemic excursion [24]. In contrast, it was also shown that glucose variability is lower in those taking dipeptidyl peptidase-4 inhibitors [25]. There is also evidence in recent studies that administration of either sodium-glucose cotransporter 2 (SGLT2) inhibitors [26] or glucagon-like peptide-1 (GLP-1) receptor agonists [27, 28] results in reductions in glucose variability. These observed benefits in glycemic variability may contribute to the cardiovascular outcome benefits seen with these latter two therapies. Although further investigations are needed, selecting a medication that lowers overall glucose levels and variability may provide improved clinical outcomes.
These above findings suggest that variability in clinical parameters can be influenced by poor quality of care, poor compliance with medical recommendations, and/or medication selection. Importantly, these are modifiable factors and suggest that greater attention to these contributors may permit limiting the degree and impact of glucose variability.
Glycemic Variability: Mechanistic Studies and Clinical Implications
There are several mechanisms that may explain the association between visit-to-visit glycemic variability and cardiovascular adverse events. It has been shown that glucose variability leads to activation of vascular oxidative stress, which may be a major contributor to development of atherosclerosis [29, 30]. Other potential mechanisms include activation of monocytes and macrophages and enhanced production of inflammatory cytokines from these and other vascular cells [31, 32]. One could also speculate that as glucose variability is associated with more frequent hypoglycemic events, this might lead to increased cardiovascular events [33]. However, this latter possibility is currently unsupported as severe hypoglycemia did not appear to contribute to the association of glycemic variability with CVD in the VADT [4] or ACCORD [17••].
Several recent studies have used population-level data to address the link between glycemic variability and increased oxidative stress. The results and conclusions vary depending on the stage of diabetes, oxidative stress markers measured, and the treatment intervention adopted. Studying 90 T1D patients, Rodrigues et al. [34] found that glycemic variability correlated with oxidative stress (e.g., thiobarbituric acid reactive substances, TBARS, and glutathione reductase) and erythrocyte membrane stability variables. Among T2D patients (n=69), glycemic variability measured by mean amplitude of glycemic excursion (MAGE) and mean of daily differences (MODD) using continuous glucose monitoring (CGM) was significantly associated with increases in diacron-reactive oxygen metabolites (d-ROMs) in multivariate analysis adjusting for mean glycemic levels [35]. A growing body of evidence suggests that epigenetic modifications—changes to the genome that do not involve changes in DNA sequence—may significantly derail transcriptional programs implicated in angiogenesis, oxidative stress, and inflammation, thus fostering vascular damage in patients with diabetes [36]. Costantino et al. [37] investigated whether epigenetic regulation of the adaptor protein p66Shc, a key driver of mitochondrial oxidative stress, contributes to persistent vascular dysfunction in patients with T2D. In this study, thirty-nine patients with uncontrolled T2D (HbA1c >7.5%) and 24 age- and sex-matched healthy control subjects were consecutively enrolled. Intensive treatment was implemented for 6 months in the patients with T2D to achieve a target HbA1c of <7.0%. The p66Shc gene expression was significantly upregulated among patients with T2D compared with control subjects and the upregulation of p66Shc was not blunted by intensive glycemic control. p66Shc mRNA levels were also independently associated with 8-isoPGF urinary excretion and brachial artery flow-mediated dilation (FMD), regardless of adjustment for potential confounders, suggesting p66Shc expression may contribute to ongoing oxidative stress and vascular dysfunction. The effects of glycemic control on epigenetic remodeling of the p66Shc promoter were then investigated. Epigenetic changes of p66Shc promoter, i.e., DNA hypomethylation and H3 acetylation, promoted gene transcription in patients with T2D. Importantly, intensive glycemic control did not reverse these changes nor were they related to HbA1c values. In contrast, MAGE was independently associated with these same epigenetic signatures. Hence, glucose fluctuations may contribute to chromatin remodeling in this important gene which may account for persistent vascular dysfunction even in patients with T2D who achieve target HbA1c levels. Although this study indicates an exciting connection between glucose variability and signal pathways linked to vascular disease, these results need to be validated in a larger cohort and more direct causality remains to be established.
Blood Pressure Variability and Cardiovascular Risk
High blood pressure is a major risk factor for CVD and mortality worldwide [38]. The early view was that variations in blood pressure can be disregarded as meaningless fluctuation around the patient’s true blood pressure [39]. This perspective has given way in the last decade to the notion that visit-to-visit variability in BP (hereafter, BPv) is associated with mortality risk and risk of a range of unfavorable cardiovascular outcomes, including stroke, coronary heart disease, myocardial infarction, and heart failure [6, 9•, 11, 40–42].
These advances notwithstanding, there is much we do not understand about BPv that requires further scrutiny. In this section of the review, we discuss (i) the evolution of BPv as a predictor of cardiovascular risk; (ii) the evidence that BPv may be more important in persons with lower BP levels; (iii) recent work that posits a mechanistic explanation for the role of BPv in risk of CVD; and (iv) important gaps in our understanding of BPv.
Though we concentrate here on clinical studies of visit-to-visit BPv, we note that short-term variability by ambulatory monitoring and mid-term variability by more chronic home monitoring have also been implicated in CVD risk [6].
The work of Rothwell et al. in 2011 greatly expanded our understanding of the role of BPv as a cardiovascular risk factor. Using data from the large Anglo-Scandinavian Cardiac Outcomes Trial - Blood Pressure Lowering Arm (ASCOT-BPLA), this group showed that systolic BPv was a predictor of stroke (HR = 6.22, 95% CI = 4.16–9.29), independent of mean blood pressure level [40].
Since that time, data from multiple cohort studies have shown that BPv is a predictor of adverse events and mortality. For example, Muntner and colleagues, using data from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), expanded on these results to show that systolic and diastolic BPv predicted stroke, coronary heart disease, and mortality, even in a model adjusted for mean blood pressure level and other covariates such as medication adherence [41]. Analyses in ALLHAT, the Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) Trial [9•], the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial, and the Veterans’ Affairs Diabetes Trial (VADT) [43] have also linked BPv to risk of heart failure. Hazard ratios for increased visit-to-visit BPv in a recent meta-analysis range from 1.10 to 1.18 for CVD events and mortality [6].
Blood Pressure Variability in the Setting of Low Blood Pressure
One important refinement that is emerging as a potential theme in the literature is modification of the effect of BPv on cardiovascular risk in the setting of low blood pressure (Table 3). In the VALUE trial, Mehlum and colleagues reported that, while systolic BPv was linked to risk for CVD in the whole cohort, the association was stronger in patients with lower blood pressure during the treatment period (p for interaction < 0.0001) [9•]. Similarly, Poortvliet and colleagues reported using data from PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) study that diastolic BPv was more predictive of vascular mortality in those with SBP below the median (p for interaction = 0.028) [8]. Moreover, in ACCORD, Nuyujukian et al. showed that the influence of BPv on risk of heart failure increased with progressively lower levels of baseline blood pressure [43].
Table 3.
Studies indicating low blood pressure level enhances effect of BPv
| Author, year | Cohort | Cohort characteristics | Major conclusions |
|---|---|---|---|
| Poortvliet et al., 2012 [8] | PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) Trial | Men and women aged 70–82 with preexisting vascular disease in a placebo-controlled trial of pravastatin (n = 5804)* | In long-term follow-up, diastolic BPv was more predictiveof coronary events inparticipants with SBP below median (P for interaction = 0.028) |
| Mehlum et al., 2018 [9•] | Valsartan Antihypertensive Long-Term Use (VALUE) Trial | Randomized, double-masked trial to examine valsartan vs amlodipine in patients with hypertension and at least one additional CVD risk factor (n = 13,803)** | Association between BPv and cardiovascular events was significant both among patients with high and low BP level (P<0.0001) but was stronger among patients with BP below median of 137.8 mmHg (P for interaction <0.0001) |
| Nuyujukian et al., 2020 [43] | Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial | Randomized 2×2 trial to test intensive vs. standard glycemic control; also includes a BP treatment arm and lipid arm, in T2D patients with previous CVD or at high risk for CVD (n = 9383)† | Hazard ratio for heart failure with BPv increases as baseline SBP or DBP decreases, e.g., for CV-SBP, those with baseline SBP >140, <140, <130, and <120 mmHg had HRs, respectively, of 1.03, P = 0.67; 1.21, P = 0.002; 1.50, P < 0.001; 1.69, P < 0.001 |
Outcome in PROSPER: incidence of cardiovascular events, including definite or suspected death from CHD or non-fatal MI, fatal or non-fatal stroke, heart failure hospitalization, vascular mortality, and total mortality
Outcome in VALUE: composite cardiovascular endpoint of cardiac event or stroke. Cardiac events were sudden cardiac death, fatal MI, death during percutaneous coronary intervention or coronary bypass surgery, death due to heart failure, death associated with recent MI on autopsy, heart failure requiring hospital management, non-fatal MI, or emergency procedures to prevent MI
Outcome in ACCORD: congestive heart failure death or hospitalization due to HF, documented with clinical and radiologic evidence and adjudicated by endpoint committee
Potential Mechanisms Linking Blood Pressure Variability to Adverse Outcomes
There is evidence from animal studies that indicates a direct role of BPv in vascular disease. For example, Miao et al. showed that BPv is a more important determinant of cardiac damage, aortic hypertrophy, and renal lesions in rats than blood pressure levels [44], and in another study that blood pressure variability may lead to aortic and left-ventricular hypertrophy [45].
Recent work in human cohorts sheds further light on mechanisms by which BPv may contribute to cardiovascular risk. Notably, Nwabuo et al., in an examination of echocardiographic data of 2400 participants of the Coronary Artery Risk Development in Young Adults (CARDIA) study, showed that increased systolic BPv was associated with higher left-ventricular mass index, worse diastolic function, and higher LV filling pressures. Results were similar for diastolic BPv and were consistent across variability metrics (SD, ARV, and VIM) [46]. While heart failure events were not available for analysis in this younger cohort, these associations suggest strongly that BPv may have a role in changes in cardiac structure that underlie adverse events. Using data from the Multi-Ethnic Study of Atherosclerosis (MESA), Shimbo et al. showed that aortic distensibility decreased as BPv increased, providing further evidence that the role of BPv in risk of CVD has a physiological basis [47].
It is unclear why low BP levels may exacerbate the influence of BPv on cardiovascular risk. Yet, in the STABILITY trial, BPv troughs (i.e., drops in BP below the mean, especially for diastolic blood pressure) appeared to have a more marked influence on cardiovascular outcomes. For example, at the lower end of diastolic BP levels (<67 mmHg), the highest tertile of diastolic BPv was linked to a 48% increased risk of MACE outcome (p = 0.008). Vidal-Petiot and colleagues speculate that impaired autoregulation or coronary stenosis may account for the enhanced CVD risk due to blood pressure troughs in their cohort of patients with stable CHD [48]. In ACCORD, we also observed that dips in blood pressure, but not elevations, drove the association between BPv and risk of heart failure in this cohort of T2D patients [43]. Although reverse causality [49] as an explanation for increased risk of adverse outcomes in those with low blood pressure cannot be ruled out, McEvoy and colleagues reported recently an association between low diastolic BP levels and subclinical myocardial damage as estimated by high-sensitivity cardiac troponin-T (hs-cTnT) levels [50]. Coronary blood flow peaks in diastole, and repeated transient declines in diastolic BP over time may put cardiac tissue at increased risk of hypoperfusion. It has been hypothesized that this is exacerbated in the setting of greater variability—particularly if associated with excessive declines in diastolic BP [43, 50, 51]. It is imperative that future studies examine, where possible, the influence of BPv on adverse outcomes by levels of baseline or on-study blood pressure to shed further light on this emerging question of clinical relevance. The epidemiologic data presented thus far clearly support additional investigation to tease out further the potential mechanistic underpinnings of BPv in CVD risk.
Gaps and Future Directions in Studies of Risk Factor Variability
Several gaps exist in our approach to, and understanding of, risk factor variability and vascular complications. There is a need for greater consistency in statistical approaches and selection of variability metrics [6, 11, 15, 43, 52, 53] across studies of risk factor variation to improve our ability to compare reported findings and detect patterns of risk. Moreover, a transition from epidemiologic studies of association to mechanistic examination [46] of the function of BPv will provide important insight into development of therapeutic approaches. Results in the VADT pointed to a significant association between fasting glucose variability and CVD only observed in the intensive glucose-lowering arm [4]. Therefore variability may have different effects in those at high or low ends of mean risk factor values (e.g., those receiving intensive glucose lowering or with lower diastolic BP levels); this may, if confirmed in future analyses, provide more personalized risk assessment and targeted treatment strategies. Moreover, improvement in our technologies to enable tracking of variability over longer periods of time, as well as the increased use of electronic health records for patient surveillance, will serve to make analysis of visit-to-visit variability more feasible, comprehensive, and precise [54, 55].
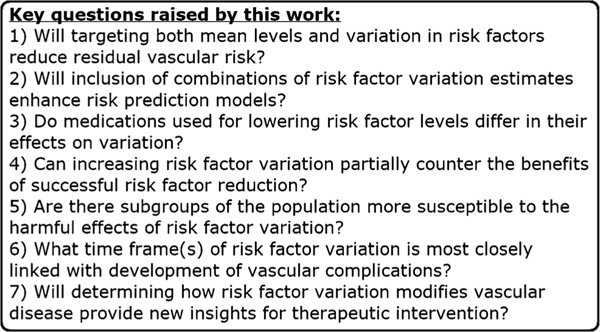
As research in this area continues to evolve, it will also be important to evaluate combined effects of variation in multiple risk factors. For instance, Kwon et al. showed, using a large Korean national registry, that high levels of variability of multiple metabolic parameters—SBP, BMI, FBG, and total cholesterol—have an additive effect on increasing incidence of heart failure [10•]. Although a very intriguing association, it remains unknown whether variability in these various risk factors may act independently or is interrelated in determining risk. Recent work by Segar et al. [17••] that assessed the role of HbA1c variability in risk of heart failure in ACCORD is a step forward in this effort, as they found that the association was independent of variability in BP, LDL cholesterol, and BMI. Future variability studies to assess the potential additive effects of variability of multiple risk parameters represent an exciting new direction of inquiry. These and other questions that are important to address in future studies to clarify the clinical implications of risk factor variability are summarized in Fig. 1.
Fig. 1.
Several important questions are raised by the work presented in this review for cardiologists and epidemiologic researchers
Conclusions
We have summarized in this review some of the major recent advances in the studies of visit-to-visit glucose variability and BPv. A growing body of evidence implicates glucose variability in risk of macrovascular disease. These findings have been observed in post hoc analyses of clinical trials as well as large retrospective analyses of electronic health record data. Similarly, there is increasing appreciation for BPv as a risk factor of adverse cardiovascular outcomes, and this association is possibly exacerbated at low levels of SBP and DBP. Exploring the role of variability in diverse subgroups of the population will be important for refining risk prediction. As the epidemiologic evidence accumulates, greater efforts to understand potential mechanisms by which risk factor variation contributes to vascular disease are needed. Nonetheless, it is becoming increasingly apparent that optimal control of cardiovascular risk factors, especially in high-risk populations, may entail reduction in levels of these factors and their variability.
Footnotes
Declarations
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358: 2545–59. 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 3.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 4.Zhou JJ, Schwenke DC, Bahn G, Reaven P. Glycemic variation and cardiovascular risk in the Veterans Affairs Diabetes trial. Diabetes Care. 2018;41:2187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou JJ, Koska J, Bahn G, Reaven P. Glycaemic variation is a predictor of all-cause mortality in the Veteran Affairs Diabetes Trial. Diab Vasc Dis Res. 2019;16:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ. 2016. 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Shi X, Ma C, Zheng H, Xiao J, Bian H, et al. Visit-to-visit blood pressure variability is a risk factor for all-cause mortality and cardiovascular disease: A systematic review and meta-analysis. J Hypertens. 2017;35:10–7. 10.1097/HJH.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 8.Poortvliet RKE, Ford I, Lloyd SM, Sattar N, Mooijaart SP, de Craen AJM, et al. Blood Pressure Variability and Cardiovascular Risk in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). PLoS One. 2012;7:e52438. 10.1371/journal.pone.0052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.•.Mehlum MH, Liestøl K, Kjeldsen SE, Julius S, Hua TA, Rothwell PM, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39:2243–51Results from this study strongly suggest that the role of blood pressure variability in cardiovascular risk is more pronounced in individuals with low baseline blood pressure levels. [DOI] [PubMed] [Google Scholar]
- 10.•.Kwon S, Lee SR, Choi EK, et al. Visit-to-visit variability of metabolic parameters and risk of heart failure: A nationwide population-based study. Int J Cardiol. 2019. 10.1016/j.ijcard.2019.06.035Kwon and colleagues use data from an ultra-large cohort to suggest that variabilities of multiple metabolic parameters have an additive effect on heart failure risk. [DOI] [PubMed] [Google Scholar]
- 11.Levitan EB, Kaciroti N, Oparil S, Julius S, Muntner P. Relationships between metrics of visit-to-visit variability of blood pressure. J Hum Hypertens. 2013;27:589–93. 10.1038/jhh.2013.19. [DOI] [PubMed] [Google Scholar]
- 12.Gabbay MAL, Rodacki M, Calliari LE, Vianna AGD, Krakauer M, Pinto MS, et al. Time in range: A new parameter to evaluate blood glucose control in patients with diabetes. Diabetol Metab Syndr. 2020;12:22. 10.1186/s13098-020-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lachin JM, Bebu I, Bergenstal RM, Pop-Busui R, Service FJ, Zinman B, et al. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care. 2017;40:777–83. 10.2337/dc16-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: The ADVANCE trial. Diabetes Care. 2014;37: 2359–65. 10.2337/dc14-0199. [DOI] [PubMed] [Google Scholar]
- 15.Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes: A systematic review and meta-analysis. Diabetes Care. 2015;38:2354–69. 10.2337/dc15-1188. [DOI] [PubMed] [Google Scholar]
- 16.Echouffo-Tcheugui JB, Zhao S, Brock G, Matsouaka RA, Kline D, Joseph JJ. Visit-to-visit glycemic variability and risks of cardiovascular events and all-cause mortality: The ALLHAT study. Diabetes Care. 2019;42:486–93. 10.2337/dc18-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.••.Segar MW, Patel KV, Vaduganathan M, Caughey MC, Butler J, Fonarow GC, et al. Association of long-term change and variability in glycemia with risk of incident heart failure among patients with type 2 diabetes: A secondary analysis of the ACCORD trial. Diabetes Care. 2020. 10.2337/dc19-2541A novel approach in this study is to adjust for variability of other metabolic risk factors (BMI, blood pressure, LDL-c) in the association between HbA1c variability and heart failure. The association was robust to these adjustments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zinman B, Marso SP, Poulter NR, et al. Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2). Diabetologia. 2018;61:48–57. 10.1007/s00125-017-4423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Critchley JA, Carey IM, Harris T, DeWilde S, Cook DG. Variability in glycated hemoglobin and risk of poor outcomes among people with type2diabetesinalargeprimary care cohort study. Diabetes Care. 2019;42:2237–46. 10.2337/dc19-0848. [DOI] [PubMed] [Google Scholar]
- 20.Ghouse J, Skov MW, Kanters JK, Lind B, Isaksen JL, Blanche P, et al. Visit-to-visit variability of hemoglobin A 1c in people without diabetes and risk of major adverse cardiovascular events and all-cause mortality. Diabetes Care. 2019;42:134–41. 10.2337/dc18-1396. [DOI] [PubMed] [Google Scholar]
- 21.Wan EYF, Yu EYT, Chin WY, Ng FTY, Chia SMC, Wong ICK, et al. Age-specific associations of glycated haemoglobin variability with cardiovascular disease and mortality in patients with type 2 diabetes mellitus: A 10- year cohort study. Diabetes Obes Metab. 2020;22:1316–27. 10.1111/dom.14034. [DOI] [PubMed] [Google Scholar]
- 22.Yu JH, Han K, Park S, Lee DY, Nam GE, Seo JA, et al. Effects of long-term glycemic variability on incident cardiovascular disease and mortality in subjects without diabetes: A nationwide population-based study. Medicine (Baltimore). 2019;98:e16317. 10.1097/MD.0000000000016317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceriello A, Rossi MC, De Cosmo S, et al. Overall quality of care predicts the variability of key risk factors for complications in type 2 diabetes: An observational, longitudinal retrospective study. Diabetes Care. 2019;42:514–9. 10.2337/dc18-1471. [DOI] [PubMed] [Google Scholar]
- 24.Kohnert KD, Augstein P, Zander E, Heinke P, Peterson K, Freyse EJ, et al. Glycemic variability correlates strongly with postprandial β-cell dysfunction in a segment of type 2 diabetic patients using oral hypoglycemic agents. Diabetes Care. 2009;32:1058–62. 10.2337/dc08-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo S, Chin SO, Lee SA, Koh G. Factors associated with glycemic variability in patients with type 2 diabetes: Focus on oral hypoglycemic agents and cardiovascular risk factors. Endocrinol Metab. 2015;30:352–60. 10.3803/EnM.2015.30.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebe K, Bando H, Menuta T, Bando M, Yonei Y. Remarkable improvement of glucose variability by sodium-glucose cotransporter 2 (SGLT2) inhibitors using continuous glucose monitoring. Diabetes Case Rep. 2019:4;139. 10.4172/2572-5629.1000139. [DOI] [Google Scholar]
- 27.Bajaj HS, Venn K, Ye C, Patrick A, Kalra S, Khandwala H, et al. Lowest glucose variability and hypoglycemia are observed with the combination of a GLP-1 receptor agonist and basal insulin (VARIATION Study). Diabetes Care. 2017;40:194–200. 10.2337/dc16-1582. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura R, Osonoi T, Kanada S, Jinnouchi H, Sugio K, Omiya H, et al. Effects of luseogliflozin, a sodium-glucose co-transporter 2 inhibitor, on 24-h glucose variability assessed by continuous glucose monitoring in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled, crossover study. Diabetes Obes Metab. 2015;17:800–4. 10.1111/dom.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niskanen L, Virkamäki A, Hansen JB, Saukkonen T. Fasting plasma glucose variability as a marker of nocturnal hypoglycemia in diabetes: Evidence from the PREDICTIVE™ study. Diabetes Res Clin Pract. 2009;86:e15–8. 10.1016/j.diabres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Monnier L, Wojtusciszyn A, Colette C, Owens D. The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther. 2011;13:813–8. 10.1089/dia.2011.0049. [DOI] [PubMed] [Google Scholar]
- 31.Ceriello A, Ihnat MA. “Glycaemic variability”: A new therapeutic challenge in diabetes and the critical care setting. Diabet Med. 2010;27:862–7. 10.1111/j.1464-5491.2010.02967.x. [DOI] [PubMed] [Google Scholar]
- 32.Keating ST, van Diepen JA, Riksen NP, El-Osta A. Epigenetics in diabetic nephropathy, immunity and metabolism. Diabetologia. 2018;61:6–20. 10.1007/s00125-017-4490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saremi A, Bahn GD, Reaven PD. A link between hypoglycemia and progression of atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care. 2016;39:448–54. 10.2337/dc15-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigues R, de Medeiros LA, Cunha LM, da Silva Garrote-Filho M, Bernardino Neto M, Jorge PT, et al. Correlations of the glycemic variability with oxidative stress and erythrocytes membrane stability in patients with type 1 diabetes under intensive treatment. Diabetes Res Clin Pract. 2018;144:153–60. 10.1016/j.diabres.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Ohara M, Fukui T, Ouchi M, Watanabe K, Suzuki T, Yamamoto S, et al. Relationship between daily and day-to-day glycemic variability and increased oxidative stress in type 2 diabetes. Diabetes Res Clin Pract. 2016;122:62–70. 10.1016/j.diabres.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15: 327–45. 10.1038/s41581-019-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costantino S, Paneni F, Battista R, Castello L, Capretti G, Chiandotto S, et al. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes. 2017;66:2472–82. 10.2337/db17-0294. [DOI] [PubMed] [Google Scholar]
- 38.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7. 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 39.MacMahon S, Peto R, Collins R, et al. Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990. 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 40.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 41.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality a cohort study. Ann Intern Med. 2015;163:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiriacò M, Pateras K, Virdis A, Charakida M, Kyriakopoulou D, Nannipieri M, et al. Association between blood pressure variability, cardiovascular disease and mortality in type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21: 2587–98. 10.1111/dom.13828. [DOI] [PubMed] [Google Scholar]
- 43.Nuyujukian DS, Koska J, Bahn G, Reaven PD, Zhou JJ. Blood pressure variability and risk of heart failure in ACCORD and the VADT. Diabates Care. 2020;43(7):1471–8. 10.2337/dc19-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miao CY, Xie HH, Zhan LS, Su DF. Blood pressure variability is more important than blood pressure level in determination of endorgan damage in rats. J Hypertens. 2006;24:1125–35. 10.1097/01.hjh.0000226203.57818.88. [DOI] [PubMed] [Google Scholar]
- 45.Miao CY, Su DF. The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens. 2002;20:1865–72. 10.1097/00004872-200209000-00033. [DOI] [PubMed] [Google Scholar]
- 46.Nwabuo CC, Yano Y, Moreira HT, Appiah D, Vasconcellos HD, Aghaji QN, et al. Association Between Visit-to-Visit Blood Pressure Variability in Early Adulthood and Myocardial Structure and Function in Later Life. JAMA Cardiol. 2020;5:795–801. 10.1001/jamacardio.2020.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimbo D, Shea S, McClelland RL, Viera AJ, Mann D, Newman J, et al. Associations of aortic distensibility and arterial elasticity with long-term visit-to-visit blood pressure variability: The multi-ethnic study of atherosclerosis (MESA). Am J Hypertens. 2013;26:896–902. 10.1093/ajh/hpt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidal-Petiot E, Stebbins A, Chiswell K, Ardissino D, Aylward PE, Cannon CP, et al. Visit-to-visit variability of blood pressure and cardiovascular outcomes in patients with stable coronary heart disease. Insights from the STABILITY trial. Eur Heart J. 2017;38: 2813–22. 10.1093/eurheartj/ehx250. [DOI] [PubMed] [Google Scholar]
- 49.Hansen TW, Li Y, Staessen JA. Blood pressure variability remains an elusive predictor of cardiovascular outcome. Am J Hypertens. 2009;22:3–4. 10.1038/ajh.2008.322. [DOI] [PubMed] [Google Scholar]
- 50.McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, et al. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J Am Coll Cardiol. 2016;68:1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danzi GB, Cuspidi C. Diastolic Blood Pressure and Myocardial Damage: What About Coronary Perfusion Time? J Am Coll Cardiol. 2017;69:1645–6. 10.1016/j.jacc.2016.11.086. [DOI] [PubMed] [Google Scholar]
- 52.De Courson H, Leffondré K, Tzourio C. Blood pressure variability and risk of cardiovascular event: Is it appropriate to use the future for predicting the present? Eur Heart J. 2018;39:4220. [DOI] [PubMed] [Google Scholar]
- 53.Monnier L, Colette C, Wojtusciszyn A, Dejager S, Renard E, Molinari N, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40:832–8. 10.2337/dc16-1769. [DOI] [PubMed] [Google Scholar]
- 54.Smith TR, Drozda JP, Vanslette JA, Hoeffken AS, Nicholson RA. Medication class effects on visit-to-visit variability of blood pressure measurements: Analysis of electronic health record data in the “real world.”. J Clin Hypertens. 2013;15:655–62. 10.1111/jch.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao J, Feng QP, Wu P, Lupu RA, Wilke RA, Wells QS, et al. Learning from Longitudinal Data in Electronic Health Record and Genetic Data to Improve Cardiovascular Event Prediction. Sci Rep. 2019;9:717. 10.1038/s41598-018-36745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]