Abstract
Natural deep eutectic systems (NADES) are emerging as potential cryoprotective agents (CPA) for cell preservation. In this investigation, we develop an optimized CPA formulation using trehalose-glycerol NADES (T:G) diluted in Normosol-R and supplemented with isoleucine. Differential scanning calorimetry (DSC) is used to define the thermophysical properties of NADES-based solutions, and Raman spectroscopy is used to characterize the effect of NADES on ice formation and hydrogen bonding. Jurkat cells are cryopreserved in each solution, and post-thaw cell recovery, apoptosis, and growth are quantified. Raman spectra and heat maps show that NADES suppresses both ice formation and dehydration of the nonfrozen region. Supplementing NADES with isoleucine does not affect the solution’s thermophysical properties but significantly improves the cells’ survival and proliferation post-thaw. The study indicates that thermophysical properties of CPA solutions alone cannot predict optimal cell survival, suggesting that stabilization of biological structures by CPAs may play a role in successful cryopreservation.
Keywords: amino acids, cryopreservation, differential scanning calorimetry, natural deep eutectic solvents, Raman spectroscopy
1 |. INTRODUCTION
Natural deep eutectic systems or natural deep eutectic solvents (NADES) are solutions composed of primary metabolites including sugars, sugar alcohols, and amino acids at specific molar ratios that influence the freezing behavior of water.1 Intermolecular hydrogen bonding between the NADES components and water results in strong depression of the melting point of the solution. Organisms that routinely survive below freezing temperatures, such as wood frogs, earthworms, and tardigrades, protect themselves naturally from freezing damage by synthesizing metabolites that likely form NADES, resulting in melting point depression within the organism, reduced ice crystal formation, and protection from osmotic stress.2,3
NADES are emerging as alternative nontoxic solutions for a variety of applications, most commonly as drug carriers or nontoxic extraction media to replace organic solvents.4 NADES have been recently explored as potential cryoprotective agents (CPA) for the preservation of mouse fibroblast cells5 and lactobacillus.6 Cells are commonly cryopreserved in 5–10% dimethyl sulfoxide (DMSO),7 which is cytotoxic and unsuitable for cells intended to be administered to patients as cell therapies.8 Thus, there is great interest in the development of DMSO-free CPAs for the cryopreservation of cells. Pure NADES typically have high viscosities, making them unsuitable for cell preservation, but previous studies have shown that NADES can be added to water to decrease their viscosity.5,9–12 In particular, a NADES composed of trehalose and glycerol (T:G) has been shown to successfully preserve mouse fibroblast cells when added to water (10% T:G wt/vol).5
In our previous studies, we used naturally occurring metabolites to develop an optimized multicomponent osmolyte (MCO) solution for the cryopreservation of Jurkat cells, an immortalized cell line used as a T lymphocyte model, via a differential evolution algorithm-driven optimization process. The optimized MCO solution composed of trehalose, glycerol, and isoleucine (TGI) resulted in 84% post-thaw recovery of Jurkat cells.13
In this investigation, we study Jurkat cell cryopreservation using T:G NADES diluted in Normosol-R and supplemented with isoleucine to explore the capability of NADES as a CPA and the benefit of isoleucine as an additional CPA. Freezing behavior is characterized for all the dilute NADES solutions experimented in this study in comparison with the preexisting MCO solution and a standard DMSO solution. Differential scanning calorimetry (DSC) is used to define the thermophysical properties of the various CPA solutions. Raman spectroscopy is used to characterize the effect of T:G NADES on hydrogen bonding as well as the microscopic behavior of water at low temperatures. Cells are also frozen in NADES, MCO and DMSO solutions using a controlled rate freezing method, and post-thaw apoptosis and proliferation are used to quantify the freezing responses of the cells.
2 |. MATERIALS AND METHODS
2.1 |. Preparation of CPA solutions
NADES were prepared by mixing trehalose (Sigma-Aldrich) and glycerol (Humco) at a molar ratio of 1:30. NADES were mixed with a magnetic stir rod in a water bath at 70°C on top of a magnetic stirring hot plate until a clear viscous liquid was formed. To obtain dilute NADES-based CPA solutions, NADES were diluted in Normosol-R (Hospira, Lake Forest, IL) and supplemented with isoleucine (Sigma-Aldrich) at varying concentrations. The MCO solution was prepared by adding trehalose, glycerol, and isoleucine directly to Normosol-R and mixed using a vortex mixer until a clear liquid was formed. The concentration of each component of the TGI MCO has been published previously.14 A 10% vol/vol. DMSO solution in Normosol-R was used as control.
2.2 |. Light microscopy
Mixtures of trehalose and glycerol at a molar ratio of 1:10 and 1:30 were imaged using a Zeiss TIRF imaging system in its phase contrast configuration with a ×20 air objective (Plan-Apochromat, NA 0.8, Zeiss) and a Zeiss AxioCam MRm 12-bit monochrome camera. Microscope slides containing 2–3 drops of each solution were observed under the microscope. Sample space was surveyed to verify the absence of sugar crystals or obtain the microscopic images of identified sugar crystals. Fibrous debris and dust particles in the samples were excluded based on their morphology.
2.3 |. Low-temperature Raman spectroscopy
Low-temperature Raman spectroscopy was performed on a temperature-controlled sample holder with a confocal Raman microscope. The temperature-controlled stage consisted of a four-stage Peltier (Thermonamic Electronics) and a series 800 temperature controller (Alpha Omega Instruments). A 1 μl sample of the respective CPA solution was placed on the stage and covered by a piece of mica (Ted Pella). The cooling rate was −10°C/min. Ice nucleation was induced at −8°C by briefly touching the sample with a liquid nitrogen-chilled needle. Independent experiments were performed with final sample temperature stabilized respectively at 20, −10, and − 50°C to collect Raman data. Raman spectroscopic measurements were made using a WITec Alpha 300R Confocal Raman Microscope with UHTS spectrometer and DV401 CCD detector with 600/mm grating. A 532 nm Nd:YAG laser was used as the excitation source. A ×100 air objective (NA 0.90, Nikon Instruments) was used to focus the laser. Laser power at the objective was measured at 10 mW using an optical power meter (Thorlabs). Spatial resolution of the microscope was measured by WITec (unpublished) at 305 nm lateral and 790 nm axial.
Brightfield images of frozen samples were taken using the confocal Raman microscope. Raman heat maps of ice, trehalose and glycerol were rendered by integrating the Raman spectra that was acquired at each pixel in the frozen sample of their respective characteristic wavenumbers36–38 (Table S1). An integration time of 0.2 s was used to scan each pixel. The size of a pixel was 333 by 333 nm. Raman heat maps in the lateral direction were acquired over a 15-by-15 μm area, and those in axial direction over a 15-by-5 μm area. Raman spectra line graphs were each acquired using constant laser power, over an integration time of 5 s, and averaged over 10 collections. Before acquiring the Raman heat maps or spectra, the focal point of the laser was positioned near the center of the sample feature of interest and adjusted in the axial direction, so that the highest signal-to-noise ratio was observed. Raman images were analyzed using FIJI via automated thresholding and boundary recognition.
2.4 |. Differential scanning calorimetry
DSC was performed on a TA Differential Scanning Calorimeter Q1000. CPA solutions were loaded into T zero pans and hermetically sealed. Samples were frozen and thawed using the following protocol: (a) start at 20°C, (b) cool to −150°C at 10°C/min, (c) hold for 3 min at −150°C, and (d) warm to 20°C at 10°C/min. An empty pan was used as a reference, and samples were run in independent triplicate.
DSC results were analyzed using TA Universal Analysis version 4.5A. The endothermic peak for each sample was used to calculate enthalpy of melting and melting temperature. Enthalpy of melting was defined as the area under the endothermic peak, and melting temperature was defined as the onset of melting of the endothermic peak. Glass transition temperature and softening temperature, both second-order phase transitions, were determined by plotting the first derivative of heat flow over time against temperature. Two local maxima were found for each CPA solution except for DMSO, representing the glass transition and softening temperatures of each solution.
2.5 |. Osmolality
Osmolality of solutions was measured based on freezing point depression using an OSMETTE osmometer (Precisions Systems). All measurements were repeated in independent triplicate. A dilution curve (Figure S1) showed a linear relationship between the dilution ratio of NADES in water and the measurement of osmolality and was used to extrapolate the osmolality of solutions that were out of range of the osmometer.
2.6 |. Cell culture maintenance
Jurkat cells (ATCC) were cultured in high-glucose RPMI-1640 (Gibco) supplemented with 10% fetal bovine serum (FBS; Qualified, Gibco). The cell concentration was maintained between 1 × 105 and 3 × 106 cells/ml. Passage number was maintained between 8 and 25. Cell line identification was done by short tandem repeat (STR) through ATCC using the FTA Sample Collection Kit (ATCC).
2.7 |. Cell processing for cryopreservation
Cells were cultured to 3 × 106 cells/ml on the day of cryopreservation. After centrifugation, cells were resuspended in normosol-R to 1.2 × 107 cells/ml. The CPA solutions were prepared at twice (2X) the final working concentration and added stepwise to the cell suspension at 1:1 volume ratio, so that the cell freezing concentration was 6 × 106 cells/ml. The mixture was incubated at room temperature for 1 hr before freezing to allow sufficient internalization of intracellular CPAs.
2.8 |. Controlled-rate freezing
Cells were frozen in cryogenic vials (Nunc CryoTubes, Thermo Scientific) at 1 ml/vial using a liquid nitrogen-based controlled-rate freezer (Kryo 560–16, Planer). Cells were frozen using the following cooling profile: (1) start at 20°C, (2) −10°C/min to 0°C, (3) hold at 0°C for 15 min, (4) −1°C/min to −8°C, (5) −50°C/min to −45°C, (6) +15°C/min to −12°C, (7) −1°C/min to −60°C, and (8) −10°C/min to −100°C. The rapid cooling and rewarming (steps 5 and 6) were used to induce nucleation in the extracellular solution. After the freezing protocol was complete, cells were stored in the vapor phase of liquid nitrogen for at least 24 hr.
2.9 |. Thawing and high-throughput post-thaw cell counting
Post-thaw recovery was measured for cells cryopreserved in the NADES, MCO, and DMSO solutions. Frozen vials were thawed rapidly in a 37°C water bath for 2 min 30 s until only a miniscule amount of ice remained. Pre-warmed Jurkat cell growth media (10% FBS in high-glucose RPMI-1640) was added dropwise to the thawed cell suspension at 1:6 dilution ratio. After gentle mixing and centrifugation, cells were resuspended in 1 ml pre-warmed media per sample and distributed at 50 μl/well using multi-channel pipettes and reservoirs into a 96-well clear-bottom black microplate (Corning). Cells were then stained for viability (i.e., esterase activity) using calcein AM (Invitrogen) upon 30 min incubation at 37°C. Fluorescence intensity of the stained cells was measured using a BioTek Synergy HT microplate reader using a 485/20 nm excitation filter, a 528/20 nm emission filter and the top probes with sensitivity of 35. The fluorescence measurement of each well was correlated to a cell count via a pre-established control curve. Fresh Jurkat cells were resuspended in media and distributed into a 96-well plate at varying concentrations via serial dilution to establish the correlation between live cell count per well and fluorescence measurement. The calculated live cell count of thawed cell samples was divided by the cell freezing concentration of 6 × 106 cells/ml to produce the post-thaw cell recovery rate. Six biological replicates were used per sample condition.
2.10 |. Post-thaw Jurkat cell culture
Cells were thawed as described above. Each cryogenic vial of cells was resuspended and cultured in 12 ml 10% FBS in high-glucose RPMI-1640. Cell growth was monitored daily for the post-thaw culture of cells cryopreserved in the NADES, MCO, and DMSO solutions. At 0, 24, 48, 72 hr post-thaw, cell cultures were each sampled to measure the live cell concentration. Cell viability was determined based on membrane integrity using a solution of 8.2 mg/ml acridine orange and 20 mg/ml propidium iodide (Sigma-Aldrich) in PBS. Cell count was measured using a hemocytometer. Three independent biological replicates were used per sample conditions.
2.11 |. Flow cytometric characterization of apoptosis
Post-thaw apoptosis of Jurkat cells cryopreserved in NADES, MCO, and DMSO solutions was assessed immediately post-thaw. Cells were thawed as described above and then stained for activated caspases according to manufacturer’s instructions (Vybrant FAM Poly Caspases Assay Kit, Invitrogen). Briefly, cells were incubated with FLICA reagent in the dark for 60 min at 37°C and 5% CO2. Cells were washed twice with apoptosis wash buffer, stained for CD3 (Human Anti-CD3, PE-Cy7, BioLegend), and incubated in the dark at room temperature for 15 min. After a final wash step, cells were stained with a membrane integrity-based viability dye for 10 min (ViaProbe, 7-AAD, BD Biosciences). Samples were run in biological triplicate on a BD FACS Canto (BD Biosciences) and the data analysis was performed using FlowJo 10.7 software (Tree Star, Ashland, OR).
The gating strategy identified Jurkat cells using forward scattering channel (FSC) and side scattering channel (SSC) to eliminate debris. Compensation was performed using single-antibody stained fresh Jurkat cell controls for each dye (FLICA, CD3, and 7-AAD). A gate was applied to FSC-W and FSC-H to select only single cells. CD3+ cells were identified from this plot and gated to select only CD3+ cells. FLICA was plotted against 7-AAD to divide CD3+ cells into viable, necrotic, early apoptotic, and late apoptotic sub-populations. Viable, healthy cells stained negative for both caspases and 7-AAD. Necrotic cells stained negative for caspases but positive for 7-AAD. Cells in early apoptosis stained positive for caspases but negative for 7-AAD, while cells in late apoptosis stained positive for both caspases and 7-AAD (Figure S2).
2.12 |. Statistics
Power analysis was performed for the experiments in this study to ensure sufficient sample size with a power of 0.95. One-sample hypothesis testing was performed using a T distribution on the DSC measurements, post-thaw cell recovery rates and proportions of post-thaw apoptosis subpopulations, and 95% confidence intervals were calculated. Two-sample t test was performed for comparisons between two samples. ANOVA was corrected for multiple comparison using Bonferroni method and performed for comparisons between multiple samples. Null hypothesis was defined as no statistically significant difference between any pair of samples. p-value less than .05 was used to reject the null hypothesis and determine the statistical significance.
3 |. RESULTS
3.1 |. T:G NADES formation
The T:G NADES was prepared at a molar ratio of 1:30,5 which resulted in the formation of a clear viscous liquid after continuous mixing at 70°C (Figure 1a). Phase contrast microscopic imaging at room temperature showed that no trehalose crystals were formed in the T:G 1:30 NADES, while sugar crystals were easily found in the mixture of trehalose and glycerol at a molar ratio of 1:10, as exemplified in Figure 1b.
FIGURE 1.

NADES preparation. (a) From left to right: trehalose, glycerol, and T:G 1:30. (b) Confocal microscope image of T:G 1:30 shows no undissolved crystals (left) while trehalose and glycerol at a molar ratio of 1:10 shows undissolved crystal (right)
3.2 |. Low-temperature Raman spectroscopy of NADES
To demonstrate the effect of NADES formation on the freezing behaviors of water, aqueous solutions containing only trehalose, only glycerol or T:G NADES of equivalent concentrations of trehalose and glycerol were frozen and analyzed using low-temperature Raman spectroscopy. Both ice crystal morphology and hydrogen bonding were characterized.
As shown by the brightfield images in Figure 2, ice morphology was different among the three solutions of interest. Ice crystals were fitted to ellipses, and ellipticity was defined by the first flattening:
FIGURE 2.

(a–c) Brightfield images (scale bar: 10 μm) and Raman spectral heat maps (scale bar: 3 μm) of ice, trehalose and glycerol acquired in both lateral and axial planes at −50°C for (A) 1.1% wt/vol trehalose in normosol-R, (b) 8.9% wt/vol glycerol in normosol-R and (C) 10% wt/vol. T: G NADES in normosol-R, showing difference in ice morphology and width of the nonfrozen channel between ice crystals, the quantified results of which are given in Table 1. (d–f) Overlaid Raman spectra independently acquired at 20, −10, and − 50°C from (d) 1.1% wt/vol trehalose in normosol-R, (E) 8.9% wt/vol. glycerol in normosol-R and (F) 10% wt/vol. T:G NADES in normosol-R, showing difference in shift of the OH stretching band (solid arrows) in response to temperature decrease
f, ellipticity (or first flattening); a, semimajor axis; b, semiminor axis.
As quantified from Raman heat maps in Figure 2 (Table 1), the ellipticity of ice crystals that formed in 10% T:G NADES solution at −50°C was significantly lower than that in the solution composed of trehalose alone but significantly higher than that in the solution composed of glycerol alone. However, the distance between adjacent ice crystals in the NADES solution was significantly greater than that in the trehalose or glycerol solution.
TABLE 1.
Quantified morphology of ice crystals in Raman heat mapsa
| CPA solutions | Ellipticity of ice crystals | Distance between adjacent ice crystals (μm) |
|---|---|---|
| 1.1% wt/vol trehalose | 0.617 ± 0.096 | 0.956 ± 0.504 |
| 8.9% wt/vol glycerol | 0.212 ± 0.071 | 1.607 ± 0.288 |
| 10% wt/vol T:G | 0.436 ± 0.073b,c | 2.328 ± 0.417b,c |
Abbreviations: CPA, cryoprotective agents; T:G, NADES composed of trehalose and glycerol at 1:30 M ratio and equivalent concentrations to the single component solution.
95% confidence intervals each calculated from a sample size greater than 8 using a T distribution.
p < .05 compared with the trehalose solution using ANOVA with Bonferroni correction.
p < .05 compared with the glycerol solution using ANOVA with Bonferroni correction.
To characterize hydrogen bonding during freezing, the trehalose, glycerol and T:G NADES solutions were also cooled to different final temperatures using a constant cooling rate. Raman spectra were acquired in the middle of a nonfrozen region. All three compositions of interest remained in solution as temperature decreased (no precipitation was observed). As shown in Figure 2d–f, a red shift of the broad OH stretching band was observed in all samples as temperature decreased, indicating lower-energy vibration of the chemical bonds and therefore, strengthened hydrogen bond network. For the sample containing only trehalose, the O H stretching peak of ice was clearly visible at −50°C in the Raman spectra at the visually based middle of the nonfrozen region, indicating that the space between adjacent ice crystals was less than 333 nm (the spatial resolution of the Raman microscope) apart (Figure 2d). In comparison, the sample containing only glycerol was found with an upshift of the ice region of the broad OH stretching band (Figure 2e), whereas the NADES sample was found with a downshift of entire OH stretching band (Figure 2f) as temperature decreased. This result suggests suppression of ice formation by NADES in comparison to its two components separately.
3.3 |. DSC
The thermophysical properties of varying NADES compositions, a multicomponent osmolyte (MCO) solution containing trehalose, glycerol, and isoleucine (TGI), and a DMSO solution were measured using DSC. Melting temperature, enthalpy of melting, glass transition temperature, and softening temperature were calculated and are listed in Table 2. T:G NADES diluted in Normosol-R showed lower melting temperature and enthalpy of melting as percentage of NADES increased (Figure 3a, p < .05). Lower melting temperature indicates depressed ice crystallization, and lower enthalpy of melting indicates reduced amount of ice crystal formation. As 5% T:G was determined to be the optimal dilution for post-thaw survival of Jurkat cells, the thermophysical properties of 5% T:G supplemented with different concentrations of isoleucine were determined. DSC results of 5% T:G with varying amounts of isoleucine showed no significant change in melting temperature or enthalpy of melting (Figure 3b), indicating that adding isoleucine did not significantly depress or suppress ice formation. Figure 3c compares the melting profiles of the optimized NADES (i.e., 5% T:G supplemented with 21.5 mM isoleucine), MCO, and 10% DMSO solutions. The optimized NADES solution has the highest melting temperature and enthalpy of melting, while 10% DMSO has the lowest.
TABLE 2.
DSC measurements of melting temperatures, enthalpy of melting, glass transition, and softening temperatures for NADES, MCO, and DMSO solutionsa
| CPA solutions | Tm (°C) | ΔHm (J/g) | Tg (°C) | Ts (°C) | |
|---|---|---|---|---|---|
| NADES solutions | 5% T:G | −5.407 ± 1.709 | 248.867 ± 17.497 | −88.823 ± 1.322 | −64.047 ± 3.384 |
| 10% T:G | −8.660 ± 0.197b | 201.133 ± 5.601b | −93.917 ± 0.291b | −65.813 ± 4.143 | |
| 15% T:G | −10.347 ± 1.963b | 199.633 ± 28.799b | −95.353 ± 0.619b | −66.253 ± 2.758 | |
| 5% T:G + 10.75 mM isoleucine | −5.820 ± 0.323 | 249.167 ± 10.231 | −89.700 ± 1.193 | −63.983 ± 3.253 | |
| 5% T:G + 21.5 mM isoleucine | −5.527 ± 1.491 | 249.767 ± 12.740 | −87.597 ± 0.436 | −63.707 ± 0.274 | |
| 5% T:G + 43 mM isoleucine | −5.367 ± 0.188 | 250.333 ± 16.807 | −86.463 ± 0.911b | −63.243 ± 3.098 | |
| TGI (MCO) | −7.853 ± 0.438c | 206.600 ± 2.370c | −91.490 ± 2.035 | −63.220 ± 1.334 | |
| 10% DMSO | −10.270 ± 0.422c | 188.467 ± 11.578c | −121.150 ± 0.675c | (N/A) |
Abbreviations: ΔHm, enthalpy of melting; CPA, cryoprotective agents; DMSO, dimethyl sulfoxide; DSC, differential scanning calorimetry; NADES, natural deep eutectic system; T:G, NADES composed of trehalose and glycerol at 1:30 M ratio; Tg, glass transition temperature; TGI or MCO, a multicomponent osmolyte solution containing trehalose, glycerol, and isoleucine; Tm, melting temperature; Ts, softening temperature.
95% confidence interval calculated from 3 independent replicates for each sample using a T distribution.
p < .05 in two-sample comparison with 5% T:G.
p < .05 in two-sample comparison with 5% T:G + 21.5 mM isoleucine.
FIGURE 3.
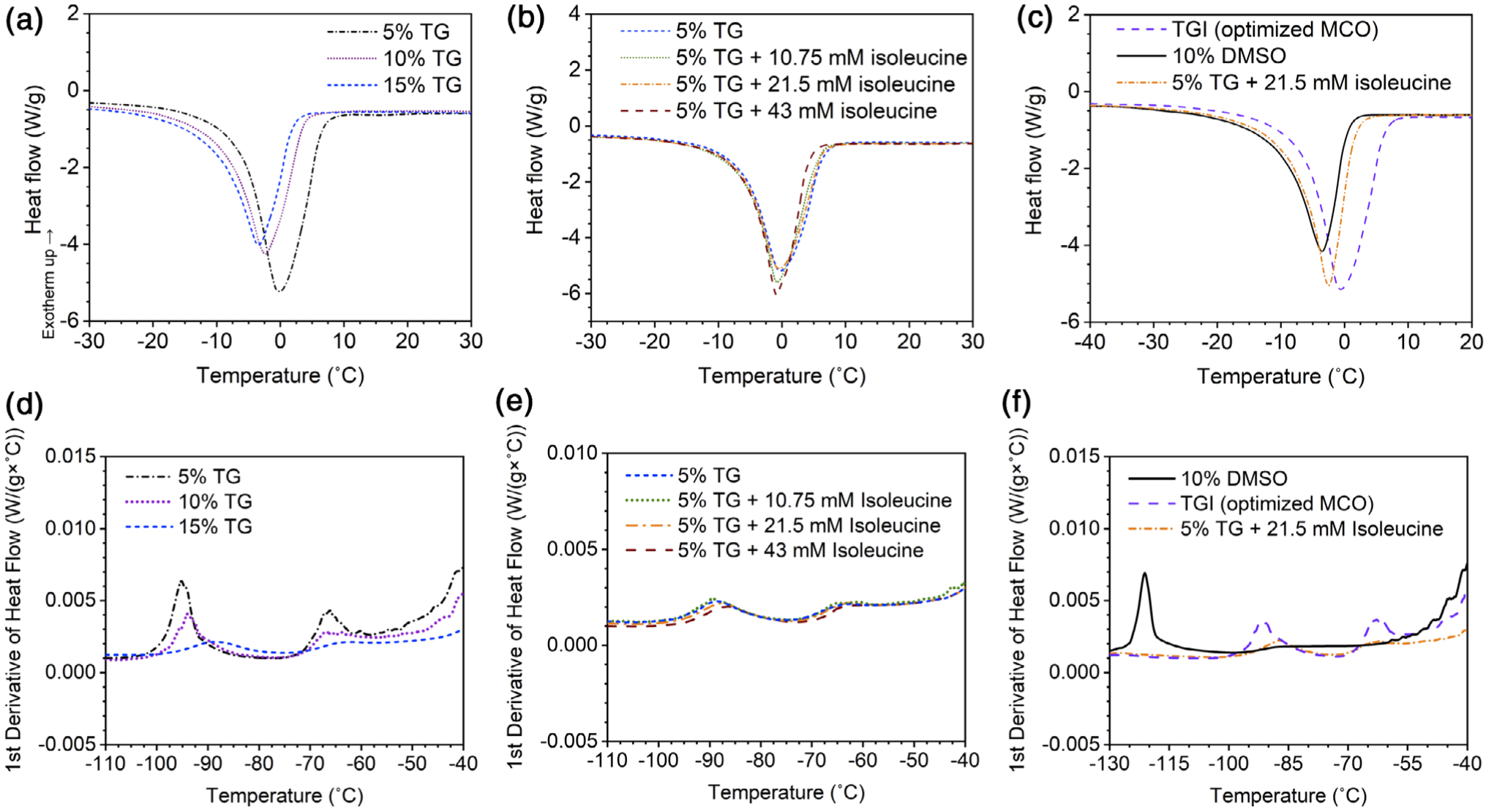
DSC melting profiles (a–c) and glass transitions (d–f) of (a, d) 5, 10, and 15% (wt/vol) T:G in Normosol-R, (b, e) 5% T:G with increasing concentration of isoleucine, and (c, f) 5% T:G with 21.5 mM isoleucine (optimized NADES), TGI (optimized MCO), and 10% DMSO (control)
The glass transition, consisting of glass transition temperature and softening temperature, was also measured for each solution. The glass transition is defined as the transition of a cooling material from a viscoelastic to a glassy state, and softening temperature is when the warming material becomes viscoelastic, collapsing under its own weight.15–17 As the percentage of NADES increased, the glass transition (p < .05) and softening (p > .05) temperatures decreased (Figure 3d). Comparing the three isoleucine-containing solutions shows that the addition of isoleucine gradually increased the glass transition and softening temperatures (Figure 3e). This increase in glass transition temperature increases the stability of the solution in cryogenic storage, since transient warming above this temperature could damage the cells. Both glass transition peaks were identified for all the tested solutions except for 10% DMSO (Figure 3f).
3.4 |. Osmolality
Cryopreservation solutions are typically hypertonic, and the cells must respond to the changes in osmolarity (or osmolality) in their environment both at introduction of the CPA solution pre-freeze and removal of the solution post-thaw. In order to characterize the solutions, the osmolality of the T:G NADES and MCO were measured and are shown in Table 3. A higher percentage of NADES resulted in higher osmolality, and the TGI MCO had comparable osmolality to the 10% T:G NADES. Significant cell losses were not observed for a single step introduction of the NADES solutions for NADES compositions<20% (data not shown).
TABLE 3.
Osmolality measurements of increasing NADES concentration (%wt/vol) in normosol-R and MCOa
| CPA solution | Osmolality (mOsm/kg) |
|---|---|
| 5% T:G | 640 ± 10 |
| 10% T:G | 1,350 ± 60 |
| 15% T:G | 1710 ± 10b |
| TGI (MCO) | 1,340 ± 10 |
NADES: natural deep eutectic system; MCO or TGI: a multicomponent osmolyte solution containing trehalose, glycerol and isoleucine; CPA: cryoprotective agent; T:G: NADES composed of trehalose and glycerol at 1:30 M ratio. 95% confidence interval calculated from 3 independent replicates for each sample using a T distribution.
Estimated osmolality extrapolated using a dilution curve (Figure S1).
3.5 |. Post-thaw recovery
Jurkat cells were frozen using a controlled-rate freezer at a cooling rate of 1°C/min as determined previously.18 Post-thaw recovery was measured for samples of Jurkat cells cryopreserved in NADES, MCO, and DMSO solutions immediately after thawing, based on cell esterase activity determined using calcein AM. Cell loss due to lysis as well as intact, dead cells were accounted. As shown in Table 4, there was no statistically significant difference among the post-thaw recovery rates for all conditions of interest. Despite the varying thermophysical properties displayed by the different solutions, their different effect on ice formation did not result in significantly different post-thaw recovery of the Jurkat cells. This suggests that CPA components in each solution may not only act upon altering the freezing behavior of water but interact with cells stabilizing them, and immediate post-thaw recovery may not be reflective of the complete cell response to cryopreservation or the longer-term manifestation of freezing damage.
TABLE 4.
Post-thaw recovery of Jurkat cells cryopreserved in NADES, MCO, and DMSO solutionsa
| CPA solutions | Post-thaw recovery (%)n.s. | CPA solutions | Post-thaw recovery (%)n.s. |
|---|---|---|---|
| 5% T:G | 75.8 ± 6.3 | 5% T:G + 10.75 mM isoleucine | 86.1 ± 16.7 |
| 10% T:G | 77.2 ± 5.5 | 5% T:G +21.5 mM isoleucine | 76.0 ± 13.0 |
| 15% T:G | 81.9 ± 6.4 | 5% T:G + 43 mM isoleucine | 78.7 ± 15.8 |
| TGI (MCO) | 79.8 ± 5.7 | 10% DMSO | 82.2 ± 13.9 |
Abbreviations: CPA, cryoprotective agents; DMSO, dimethyl sulfoxide; n. s., not significant; NADES, natural deep eutectic system; T:G, NADES composed of trehalose and glycerol at 1:30 M ratio; TGI or MCO, a multicomponent osmolyte solution containing trehalose, glycerol, and isoleucine.
95% confidence interval calculated from six biological replicates for each sample using a T distribution. p > .05 in ANOVA with Bonferroni correction for all samples.
3.6 |. Post-thaw stability of samples
Cryopreserved samples of Jurkat cells in NADES, MCO, and DMSO solutions were thawed and immediately stained. While viability can determine cell loss that occurred during freezing and thawing, cell losses over time post-thaw can also be significant. Therefore, post-thaw apoptosis was quantified in order to determine the extent of those losses (Table 5). For each of the solution compositions tested, the sample had minimal cells in late apoptosis or necrosis (<3%). Cells frozen in a high percentage of T:G (10%, 15%) experienced a high percentage of early apoptosis (>40%), while cells frozen in 5% T:G had less than 10% early apoptosis. Adding 10.75 mM isoleucine to 5% T:G resulted in the highest fraction of healthy, viable cells, outperforming all other NADES, MCO, and 10% DMSO. In general, as percentage of T:G or isoleucine concentration increased, the percentage of healthy, viable cells decreased.
TABLE 5.
Post-thaw apoptosis of Jurkat cells cryopreserved in NADES, MCO and DMSO solutionsa
| CPA solutions | Live (%) | Early apoptotic (%) | Late apoptotic (%) | Necrotic (%) | |
|---|---|---|---|---|---|
| NADES solutions | 5% T:G | 89.97 ± 0.29 | 9.03 ± 0.39 | 0.34 ± 0.17 | 0.65 ± 0.48 |
| 10% T:G | 52.37 ± 2.01b | 46.40 ± 2.15b | 0.58 ± 0.15b | 0.68 ± 0.73 | |
| 15% T:G | 47.07 ± 7.52b | 50.83 ± 8.61b | 1.29 ± 0.16b | 0.80 ± 0.89 | |
| 5% T:G + 10.75 mM isoleucine | 94.97 ± 0.52b | 4.45 ± 0.33b | 0.22 ± 0.087 | 0.37 ± 0.18 | |
| 5% T:G + 21.5 mM isoleucine | 88.70 ± 0.25b | 10.43 ± 0.14b | 0.42 ± 0.20 | 0.42 ± 0.11 | |
| 5% T:G + 43 mM isoleucine | 80.80 ± 0.90b | 18.03 ± 0.76b | 0.62 ± 0.38b | 0.50 ± 0.13 | |
| TGI (MCO) | 90.30 ± 1.14b | 8.91 ± 1.29b | 0.36 ± 0.029 | 0.42 ± 0.19 | |
| 10% DMSO | 92.87 ± 0.14b | 5.90 ± 3.19b | 0.27 ± 0.12 | 0.32 ± 0.28 |
Abbreviations: CPA, cryoprotective agents; DMSO, dimethyl sulfoxide; NADES, natural deep eutectic system; T:G, NADES composed of trehalose and glycerol at 1:30 M ratio; TGI or MCO, a multicomponent osmolyte solution containing trehalose, glycerol, and isoleucine.
95% confidence interval calculated from three independent replicates for each sample using a T distribution. Fresh Jurkat cells in culture were 99.1% live, 0.29% early apoptotic, 0.03% late apoptotic, and 0.56% necrotic.
p < .05 in two-sample comparison with 5% T:G.
Post-thaw function of the cells was determined by measuring cell growth in normal culture conditions over a 72-hr period. Among the different T:G concentrations tested, cells frozen in 5% T:G showed the highest proliferation as well as total cell number (p < .05) at the end of a 3-day culture period (Figure 4a,d) compared with 10 and 15% T:G. Both 10 and 15% T:G samples displayed cell loss in the initial 24 hr post-thaw that was consistent with post-thaw early apoptosis measurements (Table 5). Among the 5% T:G solutions supplemented with amino acids, 5% T:G with 21.5 mM isoleucine showed optimal cell growth (highest proliferation with statistical significance, p < .05, and highest mean of final cell number, p > .05) in post-thaw culture compared with lower or higher amount of isoleucine (Figure 4b,e). Interestingly, the solution with the lowest immediate post-thaw apoptosis and highest percentage of healthy cells (5% T:G with 10.75 mM isoleucine) displayed the lowest cell proliferation (p < .05) in the initial 24 hr after thawing and ultimately lower than optimal cell yield by 72 hr. The proliferation of 5% TG with 21.5 mM exceeded that of all other NADES solutions and was comparable to DMSO (p > .05) throughout the entire 72-hr culture period (Figure 4c, f). Cell concentration of the optimal NADES and 10% DMSO grew to 2.14 × 106 and 2.19 × 106 cells/ml, respectively, compared with 1.36 × 106 cells/ml for the MCO at the end of 72 hr in culture.
FIGURE 4.

Cell growth in post-thaw culture of Jurkat cells cryopreserved in various NADES, MCO, and DMSO solutions. (a–c) Growth curves visualized in the form of growth rate, with all measurements normalized to 0 hr of each sample, emphasizing on the cell proliferation. (d–f) Growth curves visualized in the form of absolute cell concentration at each point of post-thaw culture encompassing both the cell proliferation and the small differences in post-thaw cell recovery. (a, d) showing the effect of increasing concentration of T:G on post-thaw cell growth. *: p < .05 in comparison between three sample means using ANOVA with Bonferroni correction at 72 hr. (b, e) showing the effect of increasing concentration of isoleucine in 5% T:G on post-thaw cell growth. *: p < .05; n.s.: p > .05 in comparison between four sample means using ANOVA with Bonferroni correction at 72 hr. (c, f) showing the effect of 5% TG + 21.5 mM isoleucine (optimized NADES) versus TGI (optimized MCO) versus 10% DMSO on post-thaw cell growth. n.s.: p > .05 in two-sample comparisons between the optimized NADES and 10% DMSO at 0, 24, 48, and 72 hr
4 |. DISCUSSION
This study investigated the capability of NADES as a nontoxic, alternative CPA to DMSO for the cryopreservation of Jurkat cells. Existing literature of NADES5,9–12 has contributed its unique properties to the establishment of hydrogen bonding between its molecular components as well as with water, which has been observed using nuclear magnetic resonance (NMR) methods. Strengthened hydrogen bonding has been associated with cryoprotective properties of CPAs in recent studies.5,19 Here, low-temperature Raman spectroscopy interrogated the role of hydrogen bonding in NADES for cryopreservation applications from both microscopic and spectroscopic perspectives.
Controlling ice formation has been a focus of cryopreservation research. While literature has presented mathematical and empirical evidence connecting different extents of hydrogen bonding to different water lattice structures and ice modifications,20–22 our Raman studies14,19 including this one show that morphology of ice crystals can be a macroscopic reflection of the molecular interactions between water molecules and solutes. In addition, low-temperature Raman spectroscopy-based analysis of molecular vibrations in NADES expands upon the capabilities of NMR-based methods in literature of NADES by investigating the hydrogen bonding in frozen samples.
Previous studies have demonstrated that changes in solidification patterns can influence post thaw viability.23,24 More recent studies have demonstrated that specific microstructures (lower ellipticity14 and greater distance between adjacent ice crystals18,22) are associated improved post thaw viability. Yu and colleagues have elucidated a mechanism for the differences in post thaw viability with microstructure/solidification patterns. Specifically, they demonstrated that proximity of the ice phase to cells correlated with the location intracellular ice formation and consequently freezing damage to cells.25 These studies taken as a whole suggest that changes in the freezing behavior of water resulting from intermolecular interactions with CPA can, in turn, influence post-thaw viability.
Based on the quantitative analysis of Raman heat maps described in this study, T:G NADES had a relatively neutral effect on ice morphology resulting in ellipticity of ice crystals lower than trehalose alone and higher than glycerol alone. However, the T:G NADES resulted in significantly wider channels of nonfrozen solution between ice crystals than both trehalose and glycerol alone. As cells are located in the nonfrozen channels, wider channels will decrease the likelihood of lethal intracellular ice formation due to close proximity to extracellular ice.23 This effect on the nonfrozen channels will likely contribute to the cryoprotective benefits of NADES-based solutions.
Red shift of OH stretching band of Raman spectra during freezing indicates that hydrogen bonds between trehalose, glycerol, and water, all of which contain O H covalent bonds, were strengthened in the nonfrozen regions surrounding ice crystals. This effect was observed in solutions of both T:G NADES and its individual components. In contrast, the downshift of OH stretching band from −10°C to −50°C was observed only in the NADES solution. This downshift cannot be explained by the different molecular composition of NADES than its individual components, as the greater abundance of solutes containing O H bonds would, on the contrary, result in an upshift of OH stretching band. In combination with the lower CH stretching band (2,800–3,000 cm−1) in the NADES sample, this likely suggests that NADES formation resulted in resulted reduced ice content and higher levels of unfrozen solution, which is consistent with both the Raman studies finding a wider distance between two adjacent ice crystals and observation using DSC that there is less ice formation. Further studies using spectral deconvolution of the Raman spectra can be used to measure the CPA concentration and water content respectively in these nonfrozen regions to validate this hypothesis further.
Thermophysical behavior of the CPA solutions were determined using DSC. Higher concentrations of NADES had lower melting temperatures and enthalpies of melting, which indicates less ice crystal formation.5,6,24 However, higher NADES concentration has higher osmolality, and higher osmotic stress can lead to greater cell loss. The addition of isoleucine to 5% T:G did not have a significant impact on the melting properties of the solution, but it did marginally increase the glass transition and softening temperatures. A lower glass transition temperature results in a less stable CPA, since in the event of a slight rewarming (i.e., when transferring from a controlled rate freezer to liquid nitrogen storage) the sample would transition from glassy to rubbery and could suffer damage.25
Conventional wisdom indicates that CPAs work by controlling the freezing of water to reduce ice crystal formation and protect the cells from mechanical damage.26 In this study, however, solutions with more favorable thermophysical properties (lower melting temperature, lower enthalpy of melting) did not result in improved post-thaw cell recovery and proliferation. Although immediate post-thaw recovery showed no significant differences between the MCO and varying NADES, post-thaw proliferation showed that 5% T:G with 21.5 mM isoleucine had the highest growth rate over 72 hr compared with the MCO and all other NADES, with a comparable growth curve to 10% DMSO. These results suggest that reducing ice formation is not the only factor that contributes to cryoprotection of cells. In addition to controlling the freezing of water, biological interactions between CPA molecules and cells likely play a major role in the success of cryopreservation. Sugars have been shown to interact with and stabilize the cell membrane during freezing,19 glycerol penetrates the cell and can stabilize proteins,27 and isoleucine interacts with sugars and may help to keep them from precipitating out of solution.28
In the development of alternatives to DMSO-based cryopreservation, post-thaw apoptosis is a critical concern for lymphocytes29–32 and was included in the post-thaw analysis. The role of caspase activation in post-thaw apoptosis has been well-established in the literature. In particular, caspase-3 has been found to be activated post-thaw in T cells frozen in DMSO and likely contributes to cell death.30,31 For suboptimal conditions (i.e., 10–15% T:G), evidence of early apoptosis was found in both post-thaw growth and caspase activation. In contrast, using a lower concentration of T:G resulted in improved preservation of healthy cells (<10% of cells in early apoptosis). When supplemented with a small amount of isoleucine (10.75 mM), the live cell fraction increased, but larger amounts of isoleucine increased the percentage of cells in early apoptosis. This suggests that there is a “sweet spot” for the optimal concentration of isoleucine in T:G NADES. The importance of optimizing each component of a CPA is consistent with findings in our previous studies of MCO solutions.13,33 While post-thaw caspase activation is a direct indicator of apoptosis immediately post-thaw, cell stress could manifest later, as seen in the discrepancy between immediate post-thaw apoptosis and cell growth over 72 hr. This highlights the importance of direct measurement of cell survival at the functional timepoint of interest.
In this study, the proliferation of Jurkat cells cryopreserved in the respective solutions were compared using optimized NADES and MCO solution compositions. It should be noted that the molar ratio of trehalose and glycerol in the MCO do not form a NADES. Despite containing the same molecules (trehalose, glycerol and isoleucine), the optimized NADES solution outperforms the optimized MCO solution, resulting in faster cell growth in the post-thaw culture. As temperature decreases during freezing, the concentration of CPA molecules in the unfrozen fraction of solution concentrate increases. A possible interpretation of our Raman spectral results is that the NADES retains more water in the nonfrozen solution than trehalose or glycerol alone, which can translate to reduced cell dehydration/decrease anti-proliferative signaling.34
T:G NADES demonstrated the potential to act as a highly effective CPA for Jurkat cells when supplemented with isoleucine. In addition, this study challenges the conventional wisdom that minimizing ice is the major factor in optimizing CPAs, suggesting that biological interactions between the CPA and cells play a significant role in post-thaw cell outcomes. This study highlights the importance of tuning each component of a CPA to identify a sweet spot for optimal cell survival. This objective is especially important for adapting a cryopreservation method to different cell types or heterogeneous populations. Our recent study found that subsets of peripheral blood mononuclear cells (PBMCs) responded differently to MCOs made up of sugars, glycerol, and isoleucine.35 The interactions between CPAs and cells may be cell-type specific, indicating a need for custom CPA formulations for different cell types. CPA solutions composed of sugars, glycerol, and amino acids have demonstrated their use for a variety of cell types. With these same components, NADES present a novel formulation strategy to enhance the capability of these CPAs. As a result, NADES can be a powerful tool to advance the DMSO-free cryopreservation of valuable cell types for both scientific and clinical purposes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R01EB023880, R25HL128372), National Science Foundation Graduate Research Fellowship (CON-75851, Project 00074041) and ERC-2016-CoG 725034 (ERC Consolidator Grant Des.solve). The confocal light microscopy in this work was carried out using a Zeiss Scope with the assistance of Guillermo Marques at the University Imaging Centers, University of Minnesota. The flow cytometry in this work was carried out at the University of Minnesota Flow Cytometry Resource. The Raman spectroscopy in this work was carried out in the Characterization Facility, University of Minnesota, which receives partial support from the NSF through the MRSEC program.
Funding information
European Research Council, Grant/Award Number: ERC-2016-CoG 725034; National Institutes of Health, Grant/Award Numbers: R01EB023880, R25HL128372; National Science Foundation, Grant/Award Number: CON-75851; Project 00074041
Footnotes
DISCLOSURE OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Craveiro R, Aroso I, Flammia V, et al. Properties and thermal behavior of natural deep eutectic solvents. J Mol Liq. 2016;215:534–540. 10.1016/j.molliq.2016.01.038. [DOI] [Google Scholar]
- 2.Hubálek Z Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003;46(3):205–229. 10.1016/S0011-2240(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 3.Gertrudes A, Craveiro R, Eltayari Z, Reis RL, Paiva A, Duarte ARC. How do animals survive extreme temperature amplitudes? The role of natural deep eutectic solvents. ACS Sustain Chem Eng. 2017;5(11):9542–9553. 10.1021/acssuschemeng.7b01707. [DOI] [Google Scholar]
- 4.Liu Y, Friesen JB, Mcalpine JB, et al. Natural deep eutectic solvents: properties, applications, and perspectives. J Nat Prod. 2019;81(3): 679–690. 10.1021/acs.jnatprod.7b00945.Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro VIB, Craveiro R, Silva JM, Reis RL, Paiva A, Duarte AR. Natural deep eutectic systems as alternative nontoxic cryoprotective agents. Cryobiology. 2018;83(April):15–26. 10.1016/j.cryobiol.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Qiao Y, Cai HL, Yang X, Zang YY, Chen ZG. Effects of natural deep eutectic solvents on lactic acid bacteria viability during cryopreservation. Appl Microbiol Biotechnol. 2018;102(13):5695–5705. 10.1007/s00253-018-8996-3. [DOI] [PubMed] [Google Scholar]
- 7.Luzar A, Chandler D. Structure and hydrogen bond dynamics of water-dimethyl sulfoxide mixtures by computer simulations. J Chem Phys. 1993;98(10):8160–8173. 10.1063/1.464521. [DOI] [Google Scholar]
- 8.Cox MA, Kastrup J, Hrubiško M. Historical perspectives and the future of adverse reactions associated with haemopoietic stem cells cryopreserved with dimethyl sulfoxide. Cell Tissue Bank. 2012;13(2): 203–215. 10.1007/s10561-011-9248-2. [DOI] [PubMed] [Google Scholar]
- 9.Dai Y, Witkamp GJ, Verpoorte R, Choi YH. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015;187:14–19. 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- 10.Dai Y, van Spronsen J, Witkamp GJ, Verpoorte R, Choi YH. Natural deep eutectic solvents as new potential media for green technology. Anal Chim Acta. 2013;766:61–68. 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Esquembre R, Sanz JM, Wall JG, Del Monte F, Mateo CR, Ferrer ML. Thermal unfolding and refolding of lysozyme in deep eutectic solvents and their aqueous dilutions. Phys Chem Chem Phys. 2013;15 (27):11248–11256. 10.1039/c3cp44299c. [DOI] [PubMed] [Google Scholar]
- 12.Hayyan M, Mbous YP, Looi CY, et al. Natural deep eutectic solvents: cytotoxic profile. Springerplus. 2016;5(1):913. 10.1186/s40064-016-2575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pi C-H, Yu G, Petersen A, Hubel A. Characterizing the “sweet spot” for the preservation of a T-cell line using osmolytes. Sci Rep. 2018;8: 16223. 10.1038/s41598-018-34638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pi C, Yu G, Dosa PI, Hubel A. Characterizing modes of action and interaction for multicomponent osmolyte solutions on Jurkat cells. Biotechnol Bioeng. 2019;116(3):631–643. 10.1002/bit.26880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baboo J, Kilbride P, Delahaye M, et al. The impact of varying cooling and thawing rates on the quality of cryopreserved human peripheral blood T cells. Sci Rep. 2019;9(1):1–13. 10.1038/s41598-019-39957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angell ACA, Angell CA. Formation of glasses from liquids and biopolymers. Science (80-). 1995;267(5206):1924–1935. [DOI] [PubMed] [Google Scholar]
- 17.Angell CA. Liquid Fragility and the Glass Transition in Water and Aqueous Solutions. 2002. doi: 10.1021/cr000689q [DOI] [PubMed]
- 18.Pollock K, Budenske JW, McKenna DH, Dosa PI, Hubel A. Algorithm-driven optimization of cryopreservation protocols for transfusion model cell types including Jurkat cells and mesenchymal stem cells. J Tissue Eng Regen Med. 2017;11(10):2806–2815. 10.1002/term.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu G, Li R, Hubel A. Interfacial interactions of sucrose during cryopreservation detected by Raman spectroscopy. Langmuir. 2019;35 (23):7388–7395. 10.1021/acs.langmuir.8b01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Chen S, Li J. Hydrogen-bond potential for ice VIII-X phase transition. Sci Rep. 2016;6(1):37161. 10.1038/srep37161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Ross DK. Evidence for two kinds of hydrogen bond in ice. Nature. 1993;365(6444):327–329. 10.1038/365327a0. [DOI] [Google Scholar]
- 22.Ignatov I, Mosin O. Nature of hydrogen bonds in liquids and crystals. Ice Crystal Modifications and their Physical Characteristics. Gen Physiol Biophys. 2014;4:58–80. [Google Scholar]
- 23.Li R, Hornberger K, Dutton JR, Hubel A. Cryopreservation of human iPS cell aggregates in a DMSO-free solution—an optimization and comparative study. Front Bioeng Biotechnol. 2020;8(January):1–13. 10.3389/fbioe.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haque E, Kikuchi T, Kanemitsu K, Tsuda Y. Effect of Cryoprotectants on the eutectic crystallization of NaCl in frozen solutions studied by differential scanning calorimetry (DSC) and broad-line pulsed NMR. Chem Pharm Bull. 1995;34(1):430–433. [Google Scholar]
- 25.Meneghel J, Kilbride P, Morris JG, Fonseca F. Physical events occurring during the cryopreservation of immortalized human T cells. PLoS One. 2019;14(5):1–14. 10.1371/journal.pone.0217304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahy GM, Wowk B. Principles of cryopreservation by vitrification. In: Wolkers WF, Oldenhof H (Eds.), Cryopreservation and freeze-drying protocols. New York: Springer, 2015:21–82. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa T, Kita Y, Carpenter JF. Protein-solvent interactions in pharmaceutical formulations. Pharm Res. 1991;8(3):285–291. [DOI] [PubMed] [Google Scholar]
- 28.Wen X, Wang S, Duman JG, et al. Antifreeze proteins govern the precipitation of trehalose in a freezing-avoiding insect at low temperature. Proc Natl Acad Sci U S A. 2016;113(24):6683–6688. 10.1073/pnas.1601519113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baust JM, Van Buskirk R, Baust JG. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cell Dev Biol Anim. 2000;36(4):262–270. . [DOI] [PubMed] [Google Scholar]
- 30.Sarkar S, Kalia V, Montelaro RC. Caspase-mediated apoptosis and cell death of rhesus macaque CD4 + T-cells due to cryopreservation of peripheral blood mononuclear cells can be rescued by cytokine treatment after thawing. Cryobiology. 2003;47(1):44–58. 10.1016/S0011-2240(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 31.Stroh C, Cassens U, Samraj AK, Sibrowski W, Schulze-Osthoff K, Los M. The role of caspases in cryoinjury: caspase inhibition strongly improves the recovery of cryopreserved hematopoietic and other cells. FASEB J. 2002;16(12):1651–1653. 10.1096/fj.02-0034fje. [DOI] [PubMed] [Google Scholar]
- 32.Chong EA, Levine BL, Grupp SA, et al. CD19-directed CAR T-cell (CTL019) product viability and clinical outcomes in non-Hodgkin lymphomas and B-cell acute lymphoblastic Leukemia. Blood. 2018;132 (Supplement 1):197.29784641 [Google Scholar]
- 33.Pollock K, Yu G, Moller-Trane R, et al. Combinations of osmolytes, including monosaccharides, disaccharides, and sugar alcohols act in concert during cryopreservation to improve mesenchymal stromal cell survival. Tissue Eng Part C Methods. 2016;22(11):999–1008. 10.1089/ten.tec.2016.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okotrub KA, Surovtsev NV. Raman scattering evidence of hydrohalite formation on frozen yeast cells. Cryobiology. 2013;66(1):47–51. 10.1016/j.cryobiol.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Pi CH, Hornberger K, Dosa P, Hubel A. Understanding the freezing responses of T cells and other subsets of human peripheral blood mononuclear cells using DSMO-free cryoprotectants. Cytotherapy. 2020;In Press;22:291–300. 10.1016/j.jcyt.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendelovici E, Frost RL, Kloprogge T. Cryogenic Raman spectroscopy of glycerol. J Raman Spectrosc. 2000;31(12):1121–1126. [Google Scholar]
- 37.Häussinger D, Gerok W, Roth E, Lang F. Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet. 1993;341(8856):1330–1332. 10.1016/0140-6736(93)90828-5. [DOI] [PubMed] [Google Scholar]
- 38.Abazari A, Chakraborty N, Hand S, Aksan A, Toner M. A Raman microspectroscopy study of water and trehalose in spin-dried cells. Biophys J. 2014;107(10):2253–2262. 10.1016/j.bpj.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


