Abstract
Background
The Infectious Diseases Society of America recommends either a fluoroquinolone or a macrolide as a first-line antibiotic treatment for Legionella pneumonia, but it is unclear which antibiotic leads to optimal clinical outcomes. We compared the effectiveness of fluoroquinolone versus macrolide monotherapy in Legionella pneumonia using a systematic review and meta-analysis.
Methods
We conducted a systematic search of literature in PubMed, Cochrane, Scopus, and Web of Science from inception to 1 June 2019. Randomized controlled trials and observational studies comparing macrolide with fluoroquinolone monotherapy using clinical outcomes in patients with Legionella pneumonia were included. Twenty-one publications out of an initial 2073 unique records met the selection criteria. Following PRISMA guidelines, 2 reviewers participated in data extraction. The primary outcome was mortality. Secondary outcomes included clinical cure, time to apyrexia, length of hospital stay (LOS), and the occurrence of complications. The review and meta-analysis was registered with PROSPERO (CRD42019132901).
Results
Twenty-one publications with 3525 patients met inclusion criteria. The mean age of the population was 60.9 years and 67.2% were men. The mortality rate for patients treated with fluoroquinolones was 6.9% (104/1512) compared with 7.4% (133/1790) among those treated with macrolides. The pooled odds ratio assessing risk of mortality for patients treated with fluoroquinolones versus macrolides was 0.94 (95% confidence interval, .71–1.25, I2 = 0%, P = .661). Clinical cure, time to apyrexia, LOS, and the occurrence of complications did not differ for patients treated with fluoroquinolones versus macrolides.
Conclusions
We found no difference in the effectiveness of fluoroquinolones versus macrolides in reducing mortality among patients with Legionella pneumonia.
Keywords: Legionella pneumonia, Legionnaire’s disease, macrolides, fluoroquinolones
In this systematic review and meta-analysis, we evaluated the effectiveness of fluoroquinolones in comparison to macrolides for first-line treatment of Legionella pneumonia. For the primary outcome of mortality, fluoroquinolones and macrolides had similar effectiveness.
(See the Editorial Commentary by Torres and Cillóniz on pages 1990–1.)
The incidence of Legionella pneumonia continues to increase worldwide. In the United States, 7500 cases were reported to the Centers for Disease Control and Prevention in 2017 [1], up from 6079 in 2015 [1] and 6141 in 2016 [2]. Mortality ranges from 9% to 25% [3–9] and is higher among intensive care unit (ICU) patients. Among primarily waterborne diseases, treatment of Legionella pneumonia has the highest cost per episode and totals $434 million per year in the United States, with the cost of a single episode of illness ranging from $26 741 to $38 363 [10].
The Infectious Diseases Society of America recommends either a fluoroquinolone or a macrolide as first-line treatment for Legionella pneumonia. Older macrolides such as erythromycin have been replaced by newer macrolides (eg, azithromycin) and fluoroquinolones including levofloxacin and moxifloxacin [11]. Appropriate antibiotic treatment improves clinical outcomes; however, it is unknown which antibiotic offers better outcomes. This is an important question considering potential drug side effects. Fluoroquinolones are associated with adverse effects such as increased risk of Clostridioides difficile infection, tendinopathy, and neurologic effects (eg, altered mental status) [12–14]. The frequent use of fluoroquinolones for treatment of Legionella pneumonia has implications for these side effects and antibiotic stewardship [15, 16].
Observational studies have compared macrolides and fluoroquinolones for the treatment of Legionella pneumonia [6–8, 17, 18], but high-quality evidence to support the choice of macrolides or fluoroquinolones is sparse due to the absence of large randomized controlled trials (RCTs) [19]. A 2014 systematic review of 879 patients in 12 studies compared macrolides and fluoroquinolones for treating Legionella pneumonia [20] and found that fluoroquinolones were associated with a shorter length of hospital stay (LOS), a trend towards reduced mortality, a greater likelihood of clinical cure, a shorter time to apyrexia, and a lower rate of complications from Legionella pneumonia [8, 18]. Since the publication of the 2014 systematic review, newer studies have compared fluoroquinolones and macrolides in Legionella pneumonia treatment. We thus undertook a systematic review and meta-analysis to compile data from more recent studies and a larger patient population to evaluate the effectiveness of fluoroquinolones in comparison to macrolides for treatment of Legionella pneumonia.
METHODS
Data Sources and Search Strategy
With the help of a research librarian, we searched Scopus, Cochrane Central Register of Controlled Trials, PubMed, and Web of Science from inception through 1 June 2019. Gray literature was excluded. We conducted a “Legionella pneumonia” query that included search terms to identify fluoroquinolones, macrolides, and individual drugs in each drug category, with other terms for Legionella pneumonia such as “legionnaires’ disease” and “legionellosis.” We also searched for all RCTs comparing fluoroquinolones and macrolides for the treatment of Legionella pneumonia, substituting “pneumonia” for legionellosis and other Legionella pneumonia–related terms to increase the sensitivity of the search. No language restriction was applied; for data retrieval from studies in a language other than English, we sought the help of an individual fluent in the relevant language. Records were identified by reviewing references of included articles. The complete search methodology is included as Supplementary Table 1. We conducted this review in conformity with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [21], and registered the protocol with PROSPERO (CRD42019132901) [22].
Study Selection
Two authors (A. S. J. and J. S. M) independently screened each article by reviewing titles and abstracts using an online tool, Rayyan [23]. We narrowed our search results to records that compared fluoroquinolones and macrolides for the treatment of pneumonia. The full text of the remaining records was reviewed to apply the inclusion and exclusion criteria and identify articles for the final qualitative synthesis and meta-analysis. Any conflicts were resolved through discussion and review by a third author (N. S.).
For the inclusion criteria, only RCTs, cluster-randomized trials, and quasi-experimental and observational human studies that compared fluoroquinolones versus macrolides for the treatment of pneumonia were included. Studies that did not compare the effects of both antibiotics were excluded. We included studies conducted in settings such as inpatient, outpatient, or ICU. For a study to be included, the diagnosis of Legionella had to be confirmed using urinary antigen testing, culture of lower respiratory tract secretions, serology, or polymerase chain reaction.
Data Extraction and Risk-of-Bias Assessment
We extracted data on the following: patient population, country of study, number of patients included, antimicrobial agents used, clinical outcomes, severity of pneumonia assessed by the Fine score [24], and adverse effects.
The primary outcome was mortality. Secondary outcomes were clinical cure; time to apyrexia; LOS; the occurrence of complications, including respiratory complications (pleural effusion, respiratory failure, and need for mechanical ventilation); need for vasopressor support in hemodynamic instability; and acute renal failure.
Risk of bias was assessed using the Modified Downs and Black risk-assessment scale [25]. This scale consists of 27 items assessing study characteristics such as internal validity (bias and confounding), statistical power. and external validity. Two authors (A. S. J. and J. S. T.) conducted the bias assessments independently and a third author (N. S.) adjudicated any disagreements. Publication bias was assessed using a funnel plot and Egger’s test.
Data Synthesis and Analysis
We performed a standard meta-analysis using “METAN” command in Stata software, version 14.0 (Stata Corp., College Station, TX). We used the DerSimonian and Laird method to obtain estimates of the average intervention effect and the heterogeneity of intervention effects across studies using a random-effects model [26]. Heterogeneity of the incidence rate ratio across studies was evaluated using the I2 statistic [26]. We calculated the risk of categorical outcomes—death, clinical cure, and complications—using odds ratios (ORs). Patients treated with fluoroquinolones or macrolides were stratified according to the presence or absence of the outcome of interest. In addition, we conducted subgroup analysis comparing mortality following use of fluoroquinolones versus 2 commonly used macrolides: azithromycin and clarithromycin. Other subgroup analyses—for example, comparing specific fluoroquinolones such as levofloxacin and moxifloxacin versus macrolides—were not conducted due to limited data in the original studies.
We used standard mean differences to compare the LOS and time to apyrexia between fluoroquinolones and macrolides. For each outcome, the average estimate was calculated only for studies that provided data. Meta-regression could not be performed for disease severity or ICU admission status as stratified mortality rates were not reported. However, we conducted analysis for 3 ICU studies that provided mortality data. We considered P values < .05 to be statistically significant. All analyses were conducted using Stata version 14.0 (StataCorp).
RESULTS
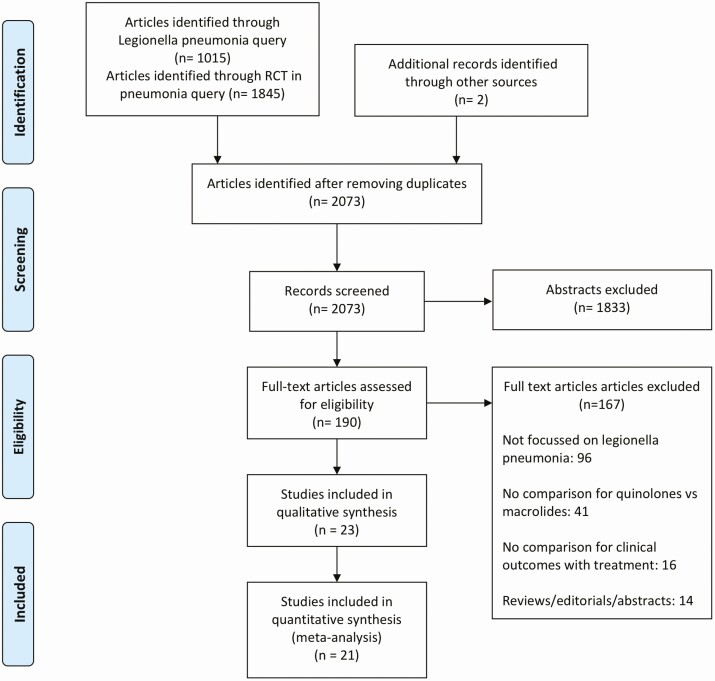
The search yielded 2861 records, 788 duplicates, and 2073 unique records. After review of titles and abstracts, 1872 were found to be ineligible. Full-text review was done for 201 articles; 103 did not have data on Legionella pneumonia and were excluded. Figure 1 shows a full list of reasons for exclusion. Ultimately, 21 studies met our inclusion criteria.
Figure 1.
Summary of evidence search and study selection. Abbreviation: RCT, randomized controlled trial.
Study characteristics for the 21 publications are shown in Table 1. Eighteen were observational studies and 3 were RCTs. Single-center studies represented 8 of 21 studies and the rest were multisite studies. Eleven studies reported the number of patients who were treated in the ICU; in 4 of these, all patients diagnosed with Legionella pneumonia presenting with severe symptoms were treated in the ICU. The rest did not provide details regarding ICU management. Six of 21 included studies were conducted among adult patients, the rest of the studies did not report the age of patients studied.
Table 1.
Main Characteristics of the Studies Included in the Analysis
| Number of Patients With Legionellosis | Agent(s) Used (n) With Dosage and Duration | Mean Age, years | Proportion of Men, % | Underlying Disease, n (%) | Fine Score ≥4, n (%) | Treatment in ICU, n (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Study Design | Place of Study | Setting | Q | M | Q | M | Q | M | Q | M | Q | M | Q | M | Q | M | Risk-of-Bias Score |
| Dournon 1990 [27] | Retrospective observational study | France | Multicenter | 7 | 20 | PEF, 0.8 g/day | ERY, 0.8 g/day | NR | 49.8 | NR | 70 | NR | NR | NR | NR | NR | NR | 22 |
| Lode 1995 [28] | Randomized controlled trial | France, Germany, Italy, UK, Belgium, Greece, Israel, Netherlands, and Spain | Multicenter | 1 | 7 | SPX, 400 mg loading followed by 200 mg every morning; range, 1–16 days | ERY, 1000 mg b.i.d; range, 1–16 days | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 25 |
| Gacouin 2002 [29] | Retrospective observational study | France | Single-center, ICU | 3 | 2 | OFX, 0.4 g/day | ERY, 3–4 g/day | NR | NR | NR | NR | NR | NR | NR | NR | 3 (100) | 2 (100) | 20 |
| Sokol 2002 [30] | Randomized controlled trial | USA | Multicenter | 7 | 7 | TVA, 200 mg q.d. for 7 days | CLR, 500 mg 2 tablets q.d for 7 days | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 25 |
| Fogarty 2004 [31] | Randomized controlled trial | USA | Multicenter | 5 | 11 | LVX, 500 mg iv every 24 hours for 7–14 days | ERY, 500–1000 mg q6h iv + ceftriaxone 1–2 g iv or im q24h and then switched to CLR 500 mg po b.i.d. + amoxiclav 875 mg po b.i.d. for 7–14 days | NR | NR | NR | NR | NR | NR | NR | NR | 5 (100) | 11 (100) | 24 |
| Blázquez Garrido 2005 [18] | Prospective observational study | Spain | Single-center | 143 | 65 | LVX, mean total dosage 4.5 g | AZM (35), mean total dosage 4.5 g; CLR (30), mean total dosage 4.5 g | NR | NR | NR | NR | NR | NR | 29 (20.3) | 11 (16.9) | NR | NR | 21 |
| Querol- Ribelles 2005 [32] | Prospective observational study | Spain | Single-center | 8 | 3 | LVX, 500 mg o.d (except first 24 hours—2 doses given) | Ceftrioxone 2 g iv q24h + CLR 500 mg q12h iv or orally | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 22 |
| Mykietiuk 2005 [33] | Prospective observational study | Spain | Single-center | 40 | 80 | LVX, 500 mg iv q.d. for 11.1 ± 6.39 days | ERY, 1000 mg iv q.i.d.; CLR, 500 mg iv b.i.d for 15.44 ± 7.83 days | 57.5 | 56 | 90 | 86.2 | NR | NR | 17 (42.5) | 38 (47.5) | 5 (12.5) | 2 (2.5) | 23 |
| Sabrià 2005 [34] | Prospective observational study | Spain and Andorra | Multicenter | 54 | 76 | LVX (50), 500 mg q12h till apyrexia; thereafter, 400 mg; 500 mg q.d., OFX, (4) 400 mg q12h for >14 days | ERY, 500 to 1000 mg q6h; CLR, 500 mg q12h for >14 days | 57.4 | 60 | 66.6 | 81.5 | 37 (68.5) | 59 (77.6) | NR | NR | 6 (11.1) | 9 (11.8) | 21 |
| Falcó 2006 [35] | Prospective observational study | Spain | Single-center | 18 | 95 | LVX for 10–14 days | CLR (52) for 14–21 days, AZM (43) for 5–10 days | 57.8 | 60.05 | 72.2 | 71.57 | NR | NR | 6 (37.5) | 37 (38.94) | 4 (22.2) | 12 (12.63) | 22 |
| Haranaga 2007 [36] | Retros pective observational study | Japan | Multicenter | 9 | 18 | CIP, 7 patients 300 mg b.i.d., 2 patients with renal failure, 300 mg q.d., 1 patient severe 600 mg b.i.d. for 15.3 days | ERY, 500 mg q6h/ q8h for 21.4 days | 69.7 | 62.8 | 67 | 78 | 8 (88.9) | 12 (66.7) | 6 (66.7) | 9 (50.0) | NR | NR | 21 |
| Nakamura 2009 [37] | Retros pective observational study | Japan | Multicenter | 12 | 4 | CIP (10), PAZ (2) | NR | NR | NR | NR | NR | NR | NR | 5 (41.7) | 2 (50.0) | NR | NR | 17 |
| Griffin 2010 [38] | Retros pective observational study | 14 countries (patients identified from CAPO international database) | Multicenter | 17 | 23 | LVX | AZM (13), CLR (10) | NR | NR | 81.3 | 73.9 | NR | NR | 7 (41.2) | 14 (60.9) | 3 (18.8) | 5 (21.7) | 21 |
| Viasus 2013 [39] | Prospective observational study | Spain | Multicenter | 111 | 74 | LVX 500 mg iv/day for 14 days (IQR, 21–28) | ERY (48) 500 mg iv/ day, CLR (24) 500 mg q.d, AZM (1), RXM (1) for 25 days (IQR, 21–28) | NR | NR | NR | NR | NR | NR | NR | NR | 38 (17.8) | 19 | |
| Rello 2013 [40] | Prospective observational study | Spain | Multicenter, ICU | 4 | 4 | LVX | CLR | NR | NR | NR | NR | NR | NR | NR | NR | 4 (100) | 4 (100) | 19 |
| Nagel 2014 [17] | Retrospective observational study | USA | Single-center | 21 | 20 | NR | AZM | 50.67 | 52.65 | 66.7 | 50 | NR | NR | NR | NR | 10 (47.6) | 5 (25) | 22 |
| Gershengorn 2015 [6] | Retrospective observational study | USA | Multicenter | 908 | 1073 | LVX (337), high dose 750 mg; others (571) standard dose for 7.2 ± 5.0 days | AZM for 6.8 ± 4.4 days | 61.46 | 61.8 | 61.2 | 66.1 | NR | NR | NR | NR | −45.9 | −37.8 | 22 |
| Cecchini 2017 [41] | Retrospective observational study | France | Multicenter, ICU | 43 | 45 | LVX, OFX, CIP | ERY, RXM, AZM, SPM | NR | NR | 73 | NR | NR | NR | NR | NR | 43 (100) | 45 (100) | 23 |
| Garcia-Vidal 2017 [42] | Retrospective observational study | Spain | Multicenter | 175 | 235 | LVX, 500 mg q.d. for 3 days (IQR, 2–5.25) | AZM (177) 500 mg q.d., for 4 days (IQR, 3–6); CLR (58) 500 mg b.i.d. for 5 days (IQR, 3–6.25) | 59.8 | 62.2 | 69.1 | 74.5 | 100 (57.1) | 102 (43.4) | 77 (44) | 91 (38.7) | 27 (15.4) | 20 (8.5) | 22 |
| Kao 2017 [43] | Retrospective observational study | Taiwan | Single-center | 12 | 16 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 21 |
| Hung 2018 [44] | Retrospective observational study | Taiwan | Single-center | 38 | 11 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 21 |
Abbreviations: AZM, azithromycin; b.i.d., twice a day; CIP, ciprofloxacin; CLR, clarithromycin; ERY, erythromycin; IQR, interquartile range; LVX, levofloxacin; M, macrolide monotherapy; NR, not reported (data for the presented variables were not reported in the respective studies); OFX, ofloxacin; PAZ, pazufloxacin; PEF, pefloxacin; q.d., once a day; q.i.d., four times a day; q6h, q8h, q12h, every 6, 8 or 12 hours respectively; Q, fluoroquinolone monotherapy; RXM, roxithromycin; SPM, spiramycin; SPX, sparfloxacin; TVA, trovafloxacin.
Risk-of-Bias Assessment
The risk-of-bias assessment is reported in Supplementary Figure 1. The average Downs and Black score was 21, with a range between 17 and 25. A higher score indicates less bias. Four studies had scores less than 21 generally due to confounding, selection bias, and deficits in reporting outcomes. The 3 RCTs had the lowest risk of bias (24–25).
Visual inspection of the funnel plot (Supplementary Figure 2) and Egger’s test (P = .946) did not demonstrate evidence of publication bias.
Patient Characteristics in Included Studies
Data from 3525 patients from the 21 studies were analyzed (see Table 1 for characteristics of patients in each individual study). The number of patients treated with fluoroquinolones was 1636 of 3525 (46.4%), whereas 1889 of 3525 patients (53.6%) were treated with macrolides. Seventeen studies reported the specific fluoroquinolones used for treatment, with levofloxacin being the most commonly administered (908 patients). Other fluoroquinolones used were ciprofloxacin (19 patients), pefloxacin (7 patients), ofloxacin (7 patients), trovafloxacin (7 patients), pazufloxacin (2 patients), and sparfloxacin (1 patient). Among the 16 studies that reported the use of specific macrolides, azithromycin was the most commonly prescribed (1362 patients) followed by clarithromycin (188 patients). Erythromycin was administered to 95 patients, and 1 patient received roxithromycin. The mean age was 60.9 years (for 2849 patients from 8 studies) and 67.2% were men (among 2925 patients from 10 studies). The prevalence of smoking was 52.2% (580/1111). Chronic obstructive pulmonary disease was reported in 17.3% of patients (107/620), and 7 studies reported immunosuppressed status in 23.4% (145/621) of the study population [17, 27, 35, 37, 40–42]. The Fine score was reported in 7 studies and, using this score, severe pneumonia was diagnosed in 38.8% of the subjects (436/1125). Overall mortality, recorded in 17 studies for patients treated with both fluoroquinolones and macrolides, was 7.18% (237/3302).
Outcomes
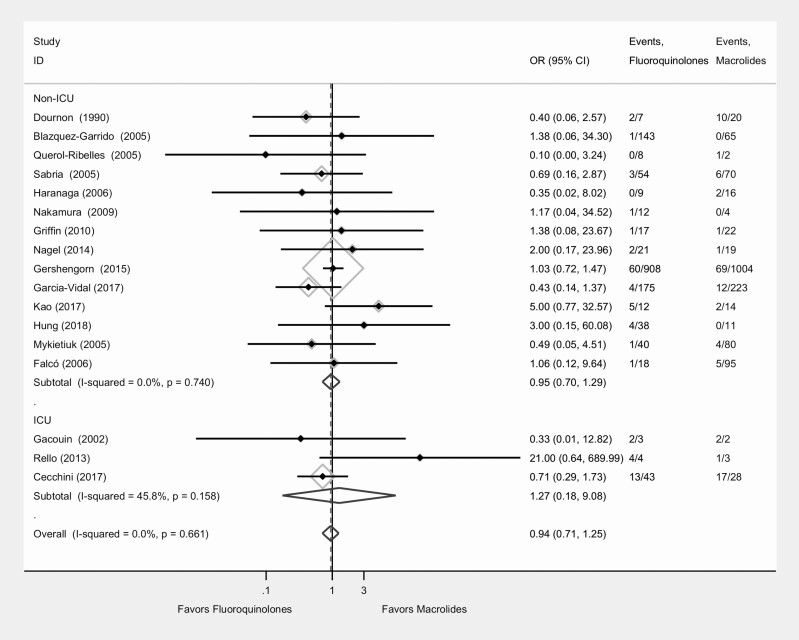
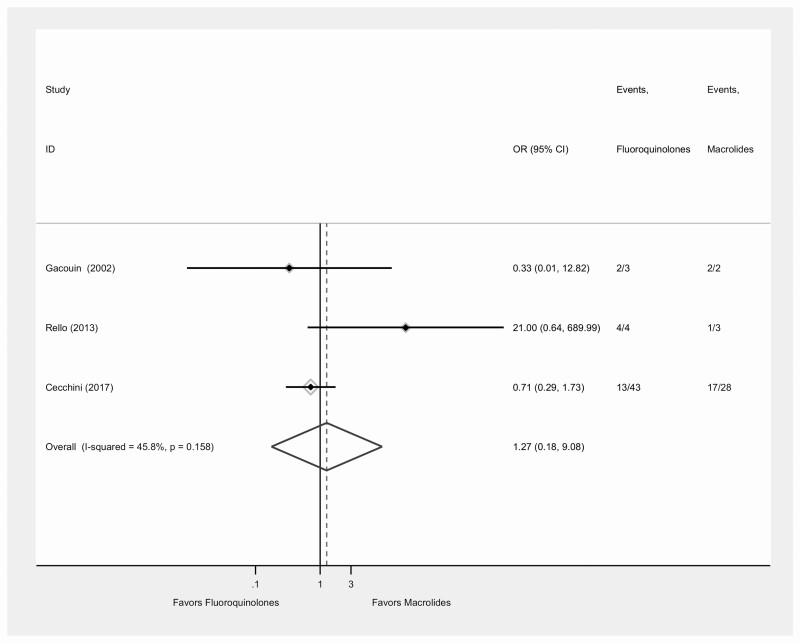
Clinical outcomes are displayed in Table 2. Seventeen of the 21 studies reported mortality data (Figure 2); the mortality rate was 6.88% (104/1512) for patients treated with fluoroquinolones and 7.43% (133/1790) for those treated with macrolides. The overall pooled OR for mortality for patients treated with fluoroquinolones versus macrolides was 0.94 (95% confidence interval [CI], .71–1.25; I2 = 0.0%; P = .66). The pooled OR for mortality comparing fluoroquinolones versus macrolides for 3 studies that were purely ICU-based and had complete data was 1.27 (95% CI, .18–9.01; I2 = 45%; P = .16) (Figure 3).
Table 2.
Clinical Outcomes of Studies Included in the Analysis Based on Treatment Groups
| Number of Patients With Legionellosis | Overall Mortality, n (%) | Mean (SD) Time to Apyrexia, hours | Mean (SD) Hospital Stay, days | Secondary Complications, n (%) | Clinical Cure, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Q | M | Q | M | Q | M | Q | M | Q | M | Q | M |
| Dournon1990 [27] | 7 | 20 | 2 (28.6) | 10 (50) | NR | NR | NR | NR | NR | NR | NR | NR |
| Lode 1995 [28] | 1 | 7 | NR | NR | NR | NR | NR | NR | NR | NR | 1 (100) | 7 (100) |
| Gacouin 2002 [29] | 3 | 2 | 2 (66.7) | 2 (100) | NR | NR | NR | NR | NR | NR | NR | NR |
| Sokol 2002 [30] | 7 | 7 | NR | NR | NR | NR | NR | NR | NR | NR | 7 (100) | 7 (100) |
| Fogarty 2004 [31] | 5 | 11 | NR | NR | NR | NR | NR | NR | NR | NR | 4 (80) | 5 (45.5) |
| Blázquez Garrido 2005 [18] | 143 | 65 | 1 (0.7) | 0(0) | 105.6(58.6) | 110.4(59.2) | 4.4 (1.8) | 7.2 (10.7) | 1 (0.7) | 3 (4.6) | 142 (99.3) | 65 (100) |
| Querol-Ribelles 2005 [32] | 8 | 3 | 0 (0) | 1 (33.3) | NR | NR | NR | NR | NR | NR | NR | NR |
| Mykietiuk 2005 [33] | 40 | 80 | 1 (2.5) | 4 (5) | 60 (45.6) | 146.4 (155.8) | 9.73 (5.7) | 14.48 (13.11) | 10 (25) | 20 (25) | NR | NR |
| Sabrià 2005 [34] | 54 | 76 | 3 (5.6) | 6 (7.9) | 48 | 77.1 | 7.6 | 9.9 | 9 (17.7) | 18 (23.7) | NR | NR |
| Haranaga 2006 [35] | 9 | 18 | 0 (0) | 2 (11.1) | 84 | 96 | 16.7 | 20 | NR | NR | NR | NR |
| Falcó 2006 [36] | 18 | 95 | 1 (5.6) | 5 (5.26) | 60 (43.2) | 59.14 (45.3) | 10.9 (8.7) | 8.55 (7.1) | NR | NR | NR | NR |
| Nakamura 2009 [37] | 12 | 4 | 1 (8.3) | 0 (0) | NR | NR | 29.6 (16.3) | 32.3 (21.7) | NR | NR | NR | NR |
| Griffin 2010 [38] | 17 | 23 | 1 (5.9) | 1 (4.3) | NR | NR | 8.9 (7.3) | 12.7 (8.3) | NR | NR | NR | NR |
| Viasus 2013 [39] | 111 | 74 | NR | NR | NR | NR | 7 | 10 | NR | NR | NR | NR |
| Rello 2013 [40] | 4 | 4 | 4 (100) | 1 (25) | NR | NR | NR | NR | NR | NR | NR | NR |
| Nagel 2014 [17] | 21 | 20 | 2 (9.5) | 1 (5) | NR | NR | 19.29 (16.6) | 11.35 (7.49) | 18 (85.7) | 18 (90) | NR | NR |
| Gershengorn 2015 [6] | 908 | 1073 | 60 (6.6) | 69 (6.4) | NR | NR | 10.2 | 9.3 | NR | NR | NR | NR |
| Cecchini 2017 [41] | 43 | 45 | 13 (30.2) | 17 (37.8) | NR | NR | NR | NR | NR | NR | NR | NR |
| Garcia-Vidal 2017 [42] | 175 | 235 | 4 (2.3) | 12 (5.1) | 48 | 48 | 7 | 6.74 | NR | NR | NR | NR |
| Kao 2017 [43] | 12 | 16 | 5 (41.6) | 2 (12.5) | NR | NR | NR | NR | NR | NR | NR | NR |
| Hung 2018 [44] | 38 | 11 | 4 (10.5) | 0 (0) | NR | NR | NR | NR | NR | NR | NR | NR |
Abbreviations: M, macrolide monotherapy; NR, not reported; Q, fluoroquinolone monotherapy.
Figure 2.
Forest plot for comparison of fluoroquinolone and macrolide effectiveness in treating Legionella pneumonia: analysis of mortality. Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
Figure 3.
Forest plot for comparison of mortality following treatment of Legionella pneumonia with fluoroquinolone versus macrolides among ICU patients. Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
The pooled OR for comparison of fluoroquinolones versus azithromycin was 0.97 (95% CI, .70–1.36; I2 = 0.0%; P = .70), while that of fluoroquinolones versus clarithromycin was 0.74 (95% CI, .19–2.84; I2 = 26.6%; P = .24) (Supplementary Figure 3).
Fourteen studies reported data for secondary outcomes. Four studies evaluated clinical cure, defined by resolution of signs and symptoms of Legionella pneumonia assessed at the test of cure visit conducted 1 to 21 days after completing antibiotic therapy [18, 28, 30, 31]. Two studies reported 100% clinical cure [28, 30]. Since there was no difference in the clinical cure between the 2 treatment groups, we did not use these data for meta-analysis. We used the 2 remaining studies [18, 31] to compare the clinical cure rates with a pooled OR of 2.36 (95% CI, .33–16.92) (Supplementary Figure 4A)
While 6 studies [18, 33–36, 42] reported the mean time to apyrexia, only 3 provided the standard deviations (SDs) needed for computation of the standardized mean difference [18, 33, 35] (Supplementary Figure 4B). There was no difference in mean time to apyrexia between fluoroquinolones and macrolides (0.0; 95% CI, −.21 to .21).
Of the 11 studies reporting mean LOS in days, 6 provided the data needed to calculate the standardized mean difference [17, 18, 33, 35, 37, 38] (Supplementary Figure 4C). Fluoroquinolones showed a mean reduction in LOS of 0.13 days (95% CI, −.50 to .24; I2= 67.2; P = .009).
Four studies reported on complications [17, 18, 33, 34] (Supplementary Figure 4D), defined as Legionella pneumonia with respiratory complications such as pleural effusion, respiratory failure, and need for mechanical ventilation. Acute renal failure was reported in 3 studies [17, 33, 34]. The other reported complications were empyema, septic shock, hepatotoxicity, hemodynamic instability requiring vasopressor therapy, and admission to the ICU for hemodynamic instability. One study [33] identified complications as any untoward circumstance occurring during hospitalization, with the exception of the side effects of the treatment. The most frequent complications in this study were respiratory failure and a worsening of comorbid conditions. The pooled OR for occurrence of complications was 0.80 (95% CI, .45–1.41), with fewer complications occurring among patients receiving fluoroquinolones.
Adverse Events
Three studies [6, 18, 33] compared the incidence of adverse effects between the 2 treatment groups. The most common adverse effects in 2 studies were gastrointestinal events, liver function abnormalities, and phlebitis. One study [18] observed a higher frequency in patients receiving clarithromycin than in those receiving levofloxacin therapy (P < .01), while the other [33] described a similar incidence of adverse effects in both treatment groups (8 of 40 patients in the fluoroquinolone treatment group, compared with 24 of 80 patients in the macrolide group). The occurrence of rash was also similar in the latter study ([38]; 2.5% vs 3.7%). The third retrospective cohort study [6] reported the rates of development of C. difficile colitis and found no difference after propensity matching (1.4% vs 2.1%; P = .25).
DISCUSSION
Fluoroquinolones and macrolides had similar effectiveness for reducing mortality in Legionella pneumonia. With 17 studies reporting mortality rates, the evidence for this outcome was high and risk of bias was low for comparison of the primary outcome of mortality. The risk-of-bias assessment was used to determine study limitations and support evidence for each clinical outcome.
For the secondary outcome measures, no statistically significant difference was found between macrolides and fluoroquinolones for clinical cure. The strength of evidence for clinical cure was downgraded as only 2 studies were included in the analysis, one favoring macrolides and the other favoring fluoroquinolones. Although 6 studies reported mean time to apyrexia, only 3 that provided the SD could be used for analysis. There was no difference in time to apyrexia between macrolides and fluoroquinolones. Six studies reporting the LOS showed a reduction of 0.13 days in hospital stay for patients treated with fluoroquinolones, but no statistically significant difference was observed compared with LOS for patients treated with macrolides. Among 4 studies that reported the incidence of complications, 3 were RCTs. The incidence of complications favored fluoroquinolones with an OR of .80 in the pooled analysis, but this was not statistically significant and was skewed heavily by the results of a single study that found fewer complications in the group receiving a fluoroquinolone compared with clarithromycin [18]. For secondary clinical outcomes, the overall quality of evidence was low due to risk of bias. Contributors to high risk of bias were primarily confounding, selection bias, and inconsistent reporting and unavailability of data for all clinical outcomes. There was also rare occurrence of secondary complications and adverse events in the included studies. Thus, no significant difference was found in the analysis of any secondary outcomes. Generally, the pooled results of our systematic review were similar to the individual studies included in the analysis.
Our findings contrast with a 2014 systematic review by Burdet et al [20] that compared the effectiveness of macrolides and fluoroquinolones for the treatment of Legionella pneumonia. We used the same measures for both primary and secondary outcomes as the previous systematic review; hence, we were able to make comparisons between our conclusions. Burdet et al included 12 studies with 879 patients in their analysis and found that mortality among patients receiving fluoroquinolone therapy was 4% (10/253) compared to 10.9% (23/211) among patients treated with macrolides. The pooled OR for mortality was 0.5 (95% CI, .2–1.3; 8 studies, 464 patients) in their study and favored fluoroquinolones in comparison to macrolides. However, this association was not statistically significant. In addition, unlike the previous review, we conducted subgroup analyses comparing fluoroquinolones with 2 commonly used macrolides versus azithromycin and clarithromycin, but this did not show a difference in mortality risk.
By including recent studies with more patients (879 vs 3525), we found that, overall, there were similar mortality rates in patients receiving macrolides and those receiving fluoroquinolones. Focusing only on ICU studies and comparing fluoroquinolones with macrolides, we found an OR of 1.27 (95% CI, .18–9.01; I2 = 45%; P = .16), but this was not statistically significant and was based on only 3 studies. The previous systematic review found a significant reduction in mean LOS of 3.0 days for LOS with fluoroquinolone versus macrolides (95% CI, 25.3–20.7 days) by assessing 3 studies with 263 patients. We included 6 studies with 537 patients and found a mean reduction of 0.13 days for fluoroquinolones with no statistically or clinically significant difference. To assess time to apyrexia and incidence of complications, we included 2 new studies and similarly found no difference between macrolides and fluoroquinolones. The number of studies comparing clinical cure remained the same (n = 2), and no difference was found between the treatment groups.
A key strength of this study is our use of comprehensive search strategies for identification of relevant studies on Legionella pneumonia and RCTs in pneumonia with the help of an experienced librarian. The search for RCTs in pneumonia made it possible for us to identify studies that did not mention Legionella pneumonia in the abstract but did include data on this in the publication. Using our search strategy, we were able to identify 7 studies published after the 2014 systematic review. It also helped us identify 2 additional studies that might not have been found using previously reported methods [20]. Since no language filters were used, we were able to include 1 additional article that was not published in English. Moreover, no evidence of publication bias was noted in our analysis.
Our systematic review had limitations, the most important being the lack of sufficient data for analysis of secondary outcomes. All studies published since the previous systematic review were observational studies and hence susceptible to bias and confounding. The 3 RCTs focused on community-acquired pneumonia in general, and only limited data on Legionella pneumonia were available. We could not perform a subgroup analysis based on subpopulations of patients, disease severity, and ICU versus non-ICU because of diverse subpopulations and the fact that stratified mortality rates were not reported in the included studies. The studies did not report sufficient data to analyze adverse effects of fluoroquinolones, such as occurrence of C. difficile infection, tendinopathy, and aortic aneurysms.
Future research should analyze clinical outcomes among patients documented to have confirmed Legionella pneumonia to compare fluoroquinolones with macrolides in a methodologically rigorous manner. Given the increasing incidence of Legionella pneumonia, a randomized, multicenter, controlled trial may be feasible to obtain a definitive answer to this question.
CONCLUSIONS
In this systematic review and meta-analysis of 21 studies and 3525 patients, we found that fluoroquinolones and macrolides had similar effectiveness in reducing mortality among patients with Legionella pneumonia.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions . Conception and design: A. S. J., N. S. Literature review and data abstraction from publications: A. S. J., J. S. M. Risk-of-bias assessment: A. S. J., J. S. T. Analysis and interpretation of the data: A. S. J., J. S. M., F. O., N. S. First draft of the manuscript: A. S. J., J. S. M., N. S. Critical revision for important intellectual content: A. S. J., J. S. M., J. S. T., V. W. S., S. D. G., F. O., N. S. Reading and final approval the manuscript: All authors. Obtaining of funding: N. S.
Acknowledgments. The authors thank Mary E. Hitchcock, MLS, senior academic librarian for her help with developing and executing the search strategy. They also thank Glen M. Jasper, MS, for technical assistance with this project. All data used in analysis of this manuscript are freely available by contacting the corresponding author.
Financial support . Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number DP2AI144244 (N. S.), and N. S. is supported by the President’s Early Career Award for Scientists and Engineers (PECASE) from the Department of Veterans Affairs. The content is solely the responsibility of the authors and does not necessarily represent the official views of either the National Institutes of Health or the Department of Veterans Affairs.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Centers for Disease Control and Prevention. Legionnaires Disease Surveillance Summary Report, United States, 2014–2015. Published 2018. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, CDC. Available at: https://www.cdc.gov/legionella/health-depts/surv-reporting/2014-15-report-tables/index.html. Accessed 2 September 2019. [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System, 2016 Annual Tables of Infectious Disease Data. Published 2017. Available at: https://wonder.cdc.gov/nndss/static/2016/annual/2016-table2h.html. Accessed 2 September 2019. [Google Scholar]
- 3. Dooling KL, Toews KA, Hicks LA, et al. Active bacterial core surveillance for Legionellosis—United States, 2011–2013. MMWR Morb Mortal Wkly Rep 2015; 64:1190–3. [DOI] [PubMed] [Google Scholar]
- 4. Soda EA, Barskey AE, Shah PP, et al. Vital signs: health care–associated Legionnaires’ disease surveillance data from 20 states and a large metropolitan area—United States, 2015. MMWR Morb Mortal Wkly Rep 2017; 66:584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benin AL, Benson RF, Besser RE. Trends in Legionnaires disease, 1980–1998: declining mortality and new patterns of diagnosis. Clin Infect Dis 2002; 35:1039–46. [DOI] [PubMed] [Google Scholar]
- 6. Gershengorn HB, Keene A, Dzierba AL, Wunsch H. The association of antibiotic treatment regimen and hospital mortality in patients hospitalized with Legionella pneumonia. Clin Infect Dis 2015; 60:e66–79. [DOI] [PubMed] [Google Scholar]
- 7. Gamage SD, Ambrose M, Kralovic SM, Simbartl LA, Roselle GA. Legionnaires disease surveillance in US Department of Veterans Affairs medical facilities and assessment of health care facility association. JAMA Netw Open 2018; 1:e180230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sabrià M, Campins M. Legionnaire’s disease: update on epidemiology and management options. Am J Respir Med 2003; 2:235–43. [DOI] [PubMed] [Google Scholar]
- 9. Hicks LA, Garrison LE, Nelson GE, Hampton LM. Legionellosis—United States, 2000–2009. Am J Transplant 2012; 12:250–3. [DOI] [PubMed] [Google Scholar]
- 10. Collier SA, Stockman LJ, Hicks LA, Garrison LE, Zhou FJ, Beach MJ. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol Infect 2012; 140:2003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dedicoat M, Venkatesan P. The treatment of Legionnaires’ disease. J Antimicrob Chemother 1999; 43:747–52. [DOI] [PubMed] [Google Scholar]
- 12. Singh S, Nautiyal A. Aortic dissection and aortic aneurysms associated with fluoroquinolones: a systematic review and meta-analysis. Am J Med 2017; 130:1449–57, e9. [DOI] [PubMed] [Google Scholar]
- 13. Leone R, Venegoni M, Motola D, et al. Adverse drug reactions related to the use of fluoroquinolone antimicrobials: an analysis of spontaneous reports and fluoroquinolone consumption data from three Italian regions. Drug Saf 2003; 26:109–20. [DOI] [PubMed] [Google Scholar]
- 14. McCusker ME, Harris AD, Perencevich E, Roghmann MC. Fluoroquinolone use and Clostridium difficile-associated diarrhea. Emerg Infect Dis 2003; 9:730–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edelstein PH. Antimicrobial chemotherapy for Legionnaires’ disease: a review. Clin Infect Dis 1995; 21(Suppl 3):S265–76. [DOI] [PubMed] [Google Scholar]
- 16. Roig J, Rello J. Legionnaires’ disease: a rational approach to therapy. J Antimicrob Chemother 2003; 51:1119–29. [DOI] [PubMed] [Google Scholar]
- 17. Nagel JL, Rarus RE, Crowley AW, Alaniz C. Retrospective analysis of azithromycin versus fluoroquinolones for the treatment of legionella pneumonia. Pharmacy and Therapeutics 2014; 39:203–5. [PMC free article] [PubMed] [Google Scholar]
- 18. Blázquez Garrido RM, Espinosa Parra FJ, Alemany Francés L, et al. Antimicrobial chemotherapy for Legionnaires disease: levofloxacin versus macrolides. Clin Infect Dis 2005; 40:800–6. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention. Legionella (Legionnaires’ disease and Pontiac Fever). Published 2018. Available at: https://www.cdc.gov/legionella/about/history.html. Accessed 12 February 2019.
- 20. Burdet C, Lepeule R, Duval X, et al. Quinolones versus macrolides in the treatment of legionellosis: a systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:2354–60. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg 2010; 8:336–41. [DOI] [PubMed] [Google Scholar]
- 22. Jasper AS, Musuuza JS, Tischendorf JS, et al. Are fluoroq uinolones or macrolides better for treating Legionella pneumonia? A systematic review and meta-analysis. PROSPERO: CRD42019132901. Available at: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=132901. Accessed 10 August 2019.
- 23. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016; 5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–50. [DOI] [PubMed] [Google Scholar]
- 25. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dournon E, Mayaud C, Wolff M, et al. Comparison of the activity of three antibiotic regimens in severe Legionnaires’ disease. J Antimicrob Chemother 1990; 26(Suppl B):129–39. [DOI] [PubMed] [Google Scholar]
- 28. Lode H, Garau J, Grassi C, et al. Treatment of community-acquired pneumonia: a randomized comparison of sparfloxacin, amoxycillin-clavulanic acid and erythromycin. Eur Respir J 1995; 8:1999–2007. [DOI] [PubMed] [Google Scholar]
- 29. Gacouin A, Le Tulzo Y, Lavoue S, et al. Severe pneumonia due to Legionella pneumophila: prognostic factors, impact of delayed appropriate antimicrobial therapy. Intens Care Med 2002; 28:686–91. [DOI] [PubMed] [Google Scholar]
- 30. Sokol WN Jr, Sullivan JG, Acampora MD, Busman TA, Notario GF. A prospective, double-blind, multicenter study comparing clarithromycin extended-release with trovafloxacin in patients with community-acquired pneumonia. Clin Ther 2002; 24:605–15. [DOI] [PubMed] [Google Scholar]
- 31. Fogarty C, Siami G, Kohler R, et al. Multicenter, open-label, randomized study to compare the safety and efficacy of levofloxacin versus ceftriaxone sodium and erythromycin followed by clarithromycin and amoxicillin-clavulanate in the treatment of serious community-acquired pneumonia in adults. Clin Infect Dis 2004; 38(Suppl 1):S16–23. [Google Scholar]
- 32. Querol-Ribelles JM, Tenías JM, Querol-Borrás JM, et al. Levofloxacin versus ceftriaxone plus clarithromycin in the treatment of adults with community-acquired pneumonia requiring hospitalization. Int J Antimicrob Agents 2005; 25:75–83. [DOI] [PubMed] [Google Scholar]
- 33. Mykietiuk A, Carratalà J, Fernández-Sabé N, et al. Clinical outcomes for hospitalized patients with Legionella pneumonia in the antigenuria era: the influence of levofloxacin therapy. Clin Infect Dis 2005; 40:794–9. [DOI] [PubMed] [Google Scholar]
- 34. Sabrià M, Pedro-Botet ML, Gómez J, et al. ; Legionnaires Disease Therapy Group . Fluoroquinolones vs macrolides in the treatment of Legionnaires disease. Chest 2005; 128:1401–5. [DOI] [PubMed] [Google Scholar]
- 35. Falcó V, Molina I, Juste C, et al. [Treatment for Legionnaires’ disease: macrolides or quinolones?] Enferm Infecc Microbiol Clin 2006; 24:360–4. [DOI] [PubMed] [Google Scholar]
- 36. Haranaga S, Tateyama M, Higa F, et al. Intravenous ciprofloxacin versus erythromycin in the treatment of Legionella pneumonia. Intern Med 2007; 46:352–6. [DOI] [PubMed] [Google Scholar]
- 37. Nakamura S, Yanagihara K, Izumikawa K, et al. The clinical efficacy of fluoroquinolone and macrolide combination therapy compared with single-agent therapy against community-acquired pneumonia caused by Legionella pneumophila. J Infect 2009; 59:222–4. [DOI] [PubMed] [Google Scholar]
- 38. Griffin AT, Peyrani P, Wiemken T, Arnold F. Macrolides versus quinolones in Legionella pneumonia: results from the community-acquired pneumonia organization international study. Int J Tuberc Lung Dis 2010; 14:495–9. [PubMed] [Google Scholar]
- 39. Viasus D, Di Yacovo S, Garcia-Vidal C, et al. Community-acquired Legionella pneumophila pneumonia: a single-center experience with 214 hospitalized sporadic cases over 15 years. Medicine (Baltimore) 2013; 92:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rello J, Gattarello S, Souto J, et al. ; Community-Acquired Pneumonia in Unidad de Cuidados Intensivos 2 (CAPUCI 2) Study Investigators . Community-acquired Legionella pneumonia in the intensive care unit: impact on survival of combined antibiotic therapy. Med Intensiva 2013; 37:320–6. [DOI] [PubMed] [Google Scholar]
- 41. Cecchini J, Tuffet S, Sonneville R, et al. Antimicrobial strategy for severe community-acquired Legionnaires’ disease: a multicentre retrospective observational study. J Antimicrob Chemother 2017; 72:1502–9. [DOI] [PubMed] [Google Scholar]
- 42. Garcia-Vidal C, Sanchez-Rodriguez I, Simonetti AF, et al. Levofloxacin versus azithromycin for treating legionella pneumonia: a propensity score analysis. Clin Microbiol Infect 2017; 23:653–8. [DOI] [PubMed] [Google Scholar]
- 43. Kao WF, Wang JT, Sheng WH, Chen YC. Community-acquired Legionnaires’ disease at a medical center in northern Taiwan. J Microbiol Immunol Infect 2019; 52:465–70. [DOI] [PubMed] [Google Scholar]
- 44. Hung TL, Li MC, Wang LR, et al. Legionnaires’ disease at a medical center in southern Taiwan. J Microbiol Immunol Infect 2018; 51:352–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.