Abstract
Background
Human adenoviruses (HAdVs) are commonly associated with acute respiratory illness. HAdV outbreaks are well documented in congregate military training settings, but less is known about outbreaks on college campuses. During fall 2018 and spring 2019, 5 United States (US) colleges reported increases in HAdV-associated respiratory illness. Investigations were performed to better understand HAdV epidemiology in this setting.
Methods
A case was defined as a student at one of the 5 colleges, with acute respiratory illness and laboratory-confirmed HAdV infection during October 2018–December 2018 or March–May 2019. Available respiratory specimens were typed by HAdV type-specific real-time polymerase chain reaction assays, and for a subset, whole genome sequencing was performed. We reviewed available medical records and cases were invited to complete a questionnaire, which included questions on symptom presentation, social history, and absenteeism.
Results
We identified 168 HAdV cases. Median age was 19 (range, 17–22) years and 102 cases (61%) were male. Eleven cases were hospitalized, 10 with pneumonia; 2 cases died. Among questionnaire respondents, 80% (75/94) missed ≥ 1 day of class because of their illness. Among those with a type identified (79%), HAdV types 4 and 7 were equally detected, with frequency of each varying by site. Genome types 4a1 and 7d were identified, respectively, by whole genome sequence analysis.
Conclusions
HAdV respiratory illness was associated with substantial morbidity and missed class time among young, generally healthy adults on 5 US college campuses. HAdVs should be considered a cause of respiratory illness outbreaks in congregate settings such as college campuses.
Keywords: adenovirus, respiratory illness, colleges, outbreak
Outbreaks of adenovirus types 4 and 7 among students at colleges during 2018–2019 were associated with severe acute respiratory illness and considerable missed class time. Adenovirus should be considered as a possible cause of respiratory illness among college students.
Human adenoviruses (HAdVs) are nonenveloped, double-stranded DNA viruses comprising 7 species (A–G) and > 100 types [1, 2]. HAdVs can cause a range of clinical manifestations, including acute respiratory illness (ARI), fever, gastroenteritis, and conjunctivitis [1]. HAdV types 4 (HAdV-4) and 7 (HAdV-7) are common circulating types associated with upper and lower respiratory tract disease and conjunctivitis [1, 3]. HAdV-associated ARI is typically self-limiting, but may be severe, causing pneumonia, disseminated disease, and death, more frequently among those with HAdV-7 infections and among individuals with immunocompromising conditions [1, 4–7].
HAdVs have no distinct seasonal pattern [5, 8]. Establishing transmission chains can be challenging; infections are often mild or asymptomatic and HAdVs can persist in the environment for prolonged periods [1]. Transmission is facilitated in congregate settings [9]. For example, HAdV-associated ARI outbreaks are well described among military personnel undergoing basic training, where young adult recruits train together and sleep in barracks [10–12]. In response to the documented burden in this population, an oral, live nonattenuated vaccine for HAdV-4 and HAdV-7 was implemented in 1971 and is available for use in military personnel [13]. HAdV-associated ARI outbreaks have also recently occurred in military populations that are not routinely vaccinated, including at the United States (US) Naval Academy, where students live in dormitory-style housing and congregate in shared spaces [14]. However, in colleges, where young adults share similar living and social environments, few HAdV-associated ARI outbreaks have been reported, and the extent of HAdV-associated ARI is not well understood [15–17].
During fall 2018, student health centers (SHCs) and state health departments identified increases in HAdV-associated ARI at colleges in Maryland (college A), Pennsylvania (college B), Virginia (college C), and Wisconsin (college D). The following spring, in March 2019, an SHC at a college in Michigan (college E) identified a cluster of HAdV-associated ARI primarily among members of an athletic team.
SHCs and state or local health departments, in collaboration with the Centers for Disease Control and Prevention (CDC), investigated to characterize HAdV-associated ARI among students at the 5 colleges and to better understand HAdV epidemiology associated with college campus settings. We describe the epidemiologic, clinical, and laboratory findings from our joint investigation.
METHODS
Case Definition and Ascertainment
A case was defined as a student at one of the 5 colleges with onset of acute respiratory symptoms and laboratory-confirmed HAdV infection during October 2018–December 2018 (colleges A–D) or March–May 2019 (college E). Colleges A, D, and E are large colleges with > 30 000 undergraduate students, while colleges B and C have approximately 5000 and 3000 undergraduate students, respectively. SHCs and health departments first detected the HAdV outbreaks from either increases in ARI visits or pneumonia diagnoses among students evaluated at SHCs or local healthcare facilities (colleges A and C), or from an increase in HAdV detections from routine public health influenza-like illness (ILI) surveillance (colleges B and D). At college E, the SHC detected a cluster of ARI among members of an athletic team. Cases were generally identified at SHCs, either through clinician-directed testing of students with ARI (colleges A, B, C, and E) or routine ILI surveillance (temperature ≥ 100°F [37.8°C] and cough or sore throat without a known cause other than influenza) (colleges B and D). Additional cases were identified when HAdV testing of students occurred at urgent care facilities, hospitals, or emergency departments (EDs) (colleges A and D; Supplementary Table 1). HAdV infection was confirmed by the detection of HAdV DNA in a respiratory specimen (nasopharyngeal swab, sputum, tracheal aspirate, or bronchoalveolar lavage), serum, or plasma using a polymerase chain reaction (PCR) assay. Molecular detection assays, including commercial multiplex tests for respiratory viruses, commercial or laboratory-developed tests for HAdV, or the CDC real-time reverse-transcription PCR assay for HAdV were performed at clinical, commercial, or state public health laboratories.
Data Collection and Analysis
Cases were invited to complete a structured questionnaire administered online using REDCap [18], by phone interview, or by email. Information collected included demographic characteristics (age, sex, year of study, housing), date of illness onset, symptom presentation, medical history, social history (smoking, e-cigarette use, sick contacts), and absenteeism. We identified available inpatient, outpatient, and ED medical records and collected information on date of illness onset, symptom presentation, clinical course, medical history, social history, diagnostic tests, and clinician diagnosis using a standardized chart abstraction tool (Supplementary Table 1). Data were entered into REDCap and Excel software and were analyzed with descriptive statistics using Stata version 14 software (StataCorp, College Station, Texas).
Adenovirus Type Determination and Molecular Characterization
To understand HAdV type circulation and transmission within outbreaks, available HAdV-positive specimens were sent to CDC for further characterization (n = 133). Total nucleic acid extracts from available specimens were tested by a pan-HAdV real-time PCR (rPCR) assay and typed by HAdV type-specific rPCR assays [19]. Twenty-eight HAdV-positive respiratory specimens, representing a sample of cases infected at the beginning, middle, and end of each college outbreak, were inoculated into A549 cells for virus isolation and further genetic characterization. For genome sequencing, DNA libraries were constructed directly from the virus isolate extracts using Nextera XT DNA Library Prep Kit (Illumina, San Diego, California), and paired-end sequencing was performed on the MiSeq using 500-cycle Miseq Reagent Kit V2 (Illumina). Reference-guided assembly was achieved using CLC Genomics Workbench version 11.0 (Qiagen, Redwood City, California). Complete genome sequences obtained from this study and representative sequences from GenBank were aligned using MAFFT in Geneious 10.0.9 (Biomatters, Auckland, New Zealand). Phylogenetic trees of the alignments were constructed using the maximum-likelihood method based on the Tamura 3-parameter model with 1000 bootstrap replicates implemented in MEGA7 [20]. In silico restriction enzyme analysis of the HAdV-4 and HAdV-7 genome sequences was performed using Geneious 10.0.9, and genome types were determined using established guidelines and reference fragment patterns [21, 22].
RESULTS
We identified 168 HAdV cases among students at 5 colleges in 5 states, including 42 (25%) from college A, 58 (35%) from college B, 8 (5%) from college C, 31 (18%) from college D, and 29 (17%) from college E. Median age was 19 (range, 17–22) years and 102 cases were male (61%) (Table 1).
Table 1.
Characteristics of Human Adenovirus Cases From 5 College Campuses, United States (N = 168)
| Characteristic | Total (N = 168) | College A | College B | College C | College D | College E | ||
|---|---|---|---|---|---|---|---|---|
| (n = 42) | (n = 58) | (n = 8) | (n = 31) | (n = 29) | ||||
| Sex | ||||||||
| Male | 102 (61) | 26 (62) | 40 (69) | 3 (38) | 15 (48) | 18 (62) | ||
| Female | 66 (39) | 16 (38) | 18 (31) | 5 (63) | 16 (52) | 11 (38) | ||
| Age, y, median (range) | 19 (17–22) | 19 (17–22) | 18 (17–22) | 19 (18–20) | 19 (17–20) | 19 (18–22) | ||
| HAdV type | ||||||||
| HAdV-4 | 65 (39) | 2 (5) | 47 (81) | 8 (100) | 7 (23) | 1 (3) | ||
| HAdV-7 | 65 (39) | 14 (33) | 7 (12) | 0 (0) | 18 (58) | 26 (90) | ||
| HAdV-4/HAdV-7 | 3 (2) | 0 (0) | 0 (0) | 0 (0) | 3 (10) | 0 (0) | ||
| Not typeda | 35 (21) | 26 (62) | 4 (7) | 0 (0) | 3 (10) | 2 (7) | ||
| Healthcare utilization | ||||||||
| Outpatient clinicb | 145 (86) | 19 (45) | 58 (100) | 8 (100) | 31 (100) | 29 (100) | ||
| Emergency department | 27 (16) | 15 (36) | 2 (3) | 1 (13) | 3 (10) | 6 (21) | ||
| Hospitalized | 11 (7) | 9 (21) | 0 (0) | 1 (13) | 1 (3) | 0 (0) | ||
| Unknown | 7 (4) | 7 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Died | 2 (1) | 1 (2) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | ||
| Medications or medical conditionsc | ||||||||
| Asthma | 19/139 (14) | 2/20 (10) | 7/51 (14) | 2 (25) | 5 (16) | 3 (10) | ||
| Immunosuppressive medicationd | 2/137 (1) | 2/20 (10) | 0/49 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Diabetes mellitus | 2/139 (1) | 0/20 (0) | 2/51 (4) | 0 (0) | 0 (0) | 0 (0) | ||
| Social historyc | ||||||||
| Smokes cigarettes (in past 30 d) | 10/138 (7) | 0/20 (0) | 2/52 (4) | 0 (0) | 7/30 (23) | 1/28 (4) | ||
| Uses electronic cigarettes (in past 30 d) | 13/100 (13) | 1/18 (6) | 6/52 (12) | 1/8 (13) | 2/7 (29) | 3/15 (20) | ||
| Any sick contacts | 94/131 (72) | 8/18 (44) | 43/52 (83) | 4/7 (57) | 17/26 (65) | 22/28 (79) |
Data are presented as no. (%) unless otherwise indicated. Where shown, denominators are total No.
Abbreviation: HAdV, human adenovirus.
aSpecimens not available.
bIncludes student health center, urgent care, primary care outside of university health system, or other outpatient clinic.
cBased on responses to chart abstraction and/or student questionnaire.
dMedications include tumor necrosis factorα inhibitors (n = 2). Use of systemic corticosteroids at or prior to illness onset for limited duration (≤1 week) (n = 3) not included.
At the 4 colleges with outbreaks in fall semester 2018, onset of illness occurred during October–December at colleges A and B, during November at college C, and during November–December at college D (Supplementary Figure 1). At college E, illness onset occurred during March–May 2019. Among the 168 cases, 133 (79%) had an HAdV type identified. HAdV-4 (n = 65 [39%]) and HAdV-7 (n = 65 [39%]) were equally identified, and for 3 (2%) specimens, both HAdV-4 and HAdV-7 were detected. HAdV types varied by college. Only HAdV-4 was identified at college C. Both HAdV-4 and HAdV-7 were detected at the 4 other colleges; HAdV-7 was the most common type detected at college A, college D, and college E, whereas HAdV-4 was the predominant type at college B (Table 1).
All cases received medical attention; 145 (86%) had ≥ 1 outpatient clinic visit (eg, SHC, urgent care, primary care outside of university health system, or other outpatient clinic), 27 (16%) had an ED visit, and 11 (7%) were hospitalized (Table 1). Two cases (1%) died. Among cases with available medical history information, 19 of 139 (14%) had asthma, 2 of 139 (1%) had diabetes, and 2 of 137 (1%) were taking an immunosuppressive medication. The majority (72%) reported at least 1 sick contact before illness onset, such as roommates, housemates, friends, teammates, or partners. Among those with symptom information available (n = 145), the most common symptoms were fever (97%), sore throat (90%), chills (84%), and cough (84%) (Table 2). Eighty-eight cases (63%) reported ≥ 1 gastrointestinal symptom (nausea, vomiting, and/or diarrhea).
Table 2.
Reported Symptoms Among Human Adenovirus Cases From 5 College Campuses, United States (n = 145)
| Symptom | No./Total | (%) |
|---|---|---|
| Fevera | 141/145 | 97 |
| Sore throat | 126/140 | 90 |
| Chills | 118/140 | 84 |
| Cough | 118/140 | 84 |
| Headache | 115/145 | 79 |
| Body aches | 113/145 | 78 |
| Fatigue | 113/145 | 78 |
| Nasal congestion/runny nose | 109/140 | 78 |
| Nausea | 35/77 | 45 |
| Shortness of breath | 58/140 | 41 |
| Diarrhea | 57/140 | 41 |
| Vomitingb | 51/140 | 36 |
| Red eyes | 37/145 | 26 |
| Loss of appetite | 19/77 | 25 |
| Wheezing | 28/140 | 20 |
| Earache | 20/100 | 20 |
Denominators are based on responses to chart abstraction, student questionnaire, and/or influenza-like illness surveillance data.
aSubjective or reported measured fever.
bn = 2 documented as posttussive vomiting.
Clinical Course
Outpatient
Medical records from an outpatient clinic or ED visit were available for 67 of 168 (40%) cases. The most common findings were pharyngitis/tonsillitis (n = 45 [67%]) and cervical lymphadenopathy (n = 35 [52%]); 57% (n = 38) had measured fever (range, 100°F–103°F [37.8°C–39.4°C]) (Table 3). Most (93%) had normal lung auscultation. Seventeen had a chest radiograph performed and 4 had radiograph-confirmed pneumonia. Half of the cases who had a medical record available had > 1 visit to an outpatient clinic or ED (n = 34 [51%]; range, 1–5 visits).
Table 3.
Clinical Characteristics and Course of Human Adenovirus Cases With Outpatient or Emergency Department Visits (n = 67)
| Characteristic | No. (%) |
|---|---|
| Age, y, median (range) |
19 (17–22) |
| Sex | |
| Male |
35 (52) |
| Female |
32 (48) |
| Vital signs and physical exam findings (on any visit during illness) | |
| Fever (≥100.0 °F [37.8 °C]) |
38 (57) |
| Respiratory rate, breaths/min, median (range)a |
16 (14–22) |
| Oxygen saturation, %, median (range)b |
97 (95–100) |
| Tachycardiac |
25 (37) |
| Cervical lymphadenopathy |
35 (52) |
| Pharyngitis/tonsillitis |
45 (67) |
| Exudative |
16 (36) |
| Abnormal lung auscultationd |
5 (7) |
| Chest radiograph performed |
17 (25) |
| Normal |
13 (76) |
| Single lobar consolidation |
3 (18) |
| Bilateral interstitial infiltrate |
1 (6) |
| Additional positive diagnostic tests (on any visit during illness) | |
| Respiratory codetections | |
| Rhinovirus/enterovirus |
6 (9) |
| Chlamydia pneumoniae |
2 (3) |
| Influenza A |
1 (1) |
| Human coronavirus |
1 (1) |
| Rapid group A streptococcus test or throat culture |
4 (6) |
| Mononucleosis spot test |
1 (1) |
| Visit diagnosis (on any visit during illness)e | |
| Influenza-like illness/syndrome |
41 (61) |
| Pharyngitis/tonsillitis |
25 (37) |
| Fever |
21 (31) |
| Sore throat |
18 (27) |
| Upper respiratory infection/respiratory illness |
16 (24) |
| Cough |
8 (12) |
| No. of outpatient or emergency department visits, median (range) |
2 (1–5) |
| 1 |
33 (49) |
| 2 |
19 (28) |
| ≥ 3 |
15 (22) |
Data are presented as no. (%) unless otherwise indicated.
aIncludes some participants with multiple measurements: n = 25 across 30 outpatient visits.
bIncludes some participants with multiple measurements: n = 35 across 61 outpatient visits.
cDefined as measured heart rate > 100 beats per minute or noted on the physical examination.
dIncludes rhonchi/coarse breath sounds, wheezing, rales/crackles, and decreased breath sounds.
eVisit diagnoses accounting for < 10% included adenovirus, pneumonia, influenza, asthma, cervical lymphadenopathy, mononucleosis, sinusitis, gastroenteritis, nausea, vomiting, malaise, viral syndrome/viral illness/viral infection, shortness of breath, infectious disease exposure, bronchitis, bronchospasm, posttussive emesis, nasal congestion, body aches, nonintractable headache, vasovagal syncope, orthostatic hypertension, diarrhea, coronavirus infection, and muscle pain.
Inpatient
Of the 11 hospitalized cases, the majority were male (n = 9 [82%]) and had no underlying conditions (n = 9 [82%]) (Table 4). HAdV-7 was identified in 6 cases (55%) and HAdV-4 in 3 cases (27%), and specimens from 2 were not available for typing. Ten cases (91%) had radiograph-confirmed pneumonia and 1 case (9%) had febrile illness without pneumonia. Two cases were critically ill, requiring intensive care unit admission, and both died. Among 9 hospitalized cases without critical illness, 2 had documented hypoxia and received supplemental oxygen. Ten cases were treated with antibiotics and 4 with antivirals, including cidofovir in the 2 critically ill patients. The median length of hospital stay was 4 (range, 1–79) days.
Table 4.
Clinical Characteristics and Course of Human Adenovirus Cases With Hospital Admission (n = 11)
| Characteristic | No. (%) |
|---|---|
| Age, y, median (range) |
19 (18–22) |
| Sex | |
| Male |
9 (82) |
| Female |
2 (18) |
| HAdV type | |
| HAdV-4 |
3 (27) |
| HAdV-7 |
6 (55) |
| Not typed |
2 (18) |
| Medical history | |
| None |
9 (82) |
| Asthma |
1 (9) |
| Inflammatory bowel disease |
1 (9) |
| Hospital course | |
| Intensive care unit admission |
2 (18) |
| Radiograph-confirmed pneumonia |
10 (91) |
| Initial radiographic findings | |
| Single lobar consolidation |
7 (70) |
| Multilobar consolidation |
2 (20) |
| Bilateral interstitial infiltrate |
1 (10) |
| Laboratory findings on admission, median (range) | |
| WBC count, K/mm3 (n = 8) |
6.9 (2.4–13) |
| Platelet count, K/mm3 (n = 10) |
183.5 (88–198) |
| AST level, IU/L (n = 8) |
49 (20–221) |
| ALT level, IU/L (n = 8) |
28 (15–422) |
| Treatment | |
| Antiviral(s) |
4 (36) |
| Cidofovir |
2 (18) |
| Oseltamivir |
4 (36) |
| Antibiotic(s) |
10 (91) |
| Intravenous immunoglobulin |
1 (9) |
| Intravenous rehydration |
7 (64) |
| Highest level of respiratory support | |
| None |
7 (64) |
| Supplemental oxygen by nasal cannula |
2 (18) |
| Extracorporeal membrane oxygenation |
2 (18) |
| Concurrent pathogen detections | |
| Mycoplasma pneumoniae |
1 (9) |
| Epstein-Barr virus |
1 (9) |
| No. of days from symptom onset to admission, median (range) |
6 (0–15) |
| No. of days hospitalized, median (range) |
4 (1–79) |
| Required transfer to a tertiary care center |
3 (27) |
| Outcome | |
| Died |
2 (18) |
| Discharged home |
9 (82) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HAdV, human adenovirus; WBC, white blood cell.
The 2 decedents (A and B) were college freshmen who developed pneumonia, multiorgan failure, and acute respiratory distress syndrome requiring extracorporeal membrane oxygenation. Both had disseminated HAdV-7 infection with high viral loads in serum or plasma and received cidofovir after the onset of respiratory failure. Decedent A had inflammatory bowel disease, treated with a tumor necrosis factor–α inhibitor. Decedent B had no known underlying medical conditions but was prescribed a short course (3 days) of oral corticosteroids for Epstein-Barr virus mononucleosis approximately 8–10 days before the onset of respiratory symptoms. Decedent A died on hospital day 9. Decedent B had a complicated hospital course, including possible hemophagocytic lymphohistiocytosis, and died on hospital day 79.
Student Questionnaire
Ninety-eight of 168 (58%) cases responded to the questionnaire (Table 5) within an average of approximately 2 months after illness onset. Of respondents, 57 (58%) were first-year undergraduate students, and the majority reported seeking care for their illness at the SHC (91%). Most respondents lived in dormitories (n = 81 [83%]) and shared either a bedroom or common space with others (n = 94 [97%]). Among those who were recovered from illness at the time of the questionnaire (n = 79), the median illness duration was 10 (range, 3–30) days. Eighty percent of respondents (75/94) missed 1 or more days of classes due to illness. The median number of class days missed was 3 (range, 1–20) days.
Table 5.
Student Questionnaire: Characteristics, Healthcare Seeking, and Missed Obligations of Human Adenovirus Cases From 5 College Campuses, United States (n = 98)
| Characteristic | No. (%) |
|---|---|
| Demographic characteristics | |
| Age, y, median (range) |
19 (17–22) |
| Sex | |
| Male |
64 (65) |
| Female |
34 (35) |
| Undergraduate year of study | |
| 1st year |
57 (58) |
| 2nd year |
26 (27) |
| 3rd year |
10 (10) |
| 4th year |
5 (5) |
| Housing | |
| Residence hall/dormitory |
81 (83) |
| Fraternity/sorority house |
6 (6) |
| Off-campus housing |
11 (11) |
| Shares a bedroom with others |
83/97 (86) |
| Shares bedroom or common spaces with others |
94/97 (97) |
| Healthcare seeking | |
| Healthcare type | |
| Student health center |
86/95 (91) |
| Urgent care |
13/95 (14) |
| Emergency department |
11/95 (12) |
| Primary care provider |
10/95 (11) |
| Other |
4/95 (4) |
| No. of visits to any healthcare provider | |
| 1 |
30/93 (32) |
| 2 |
40/93 (43) |
| 3 |
14/93 (15) |
| ≥ 4 |
9/93 (10) |
| Recovered from illness |
79/93 (85) |
| Illness duration, d, median (range) |
10 (3–30) |
| Missed obligations | |
| Missed any classes |
75/94 (80) |
| 1 d |
11 (15) |
| 2–4 d |
44 (59) |
| 5–9 d |
14 (19) |
| ≥10 d |
6 (8) |
| No. of days of missed classes, median (range) |
3 1–20 |
| Missed collegiate athletic practice/games |
14/93 (15) |
| No. of days missed, median (range) |
4 (1–12) |
| Missed full or part-time job |
20/95 (21) |
| No. of days missed, median (range) |
2 (1–7) |
| Missed other obligations (volunteer work, clubs, intramurals, other) |
39/95 (41) |
Data are presented as no. (%) unless otherwise indicated. Where shown, denominators are total No.
Among cases who reported living in a dormitory or fraternity/sorority house (n = 87), 80 specified the name of the residence. Of these 80, 58 lived in 16 dormitories or fraternity/sorority houses that had ≥ 2 cases, with a median of 2 cases per residence (range, 2–12). Twenty-four cases were linked to 5 athletic teams. Notably, at college E, 48% (14/29) of the cases had an epidemiologic link to 1 athletic team.
Molecular Characterization
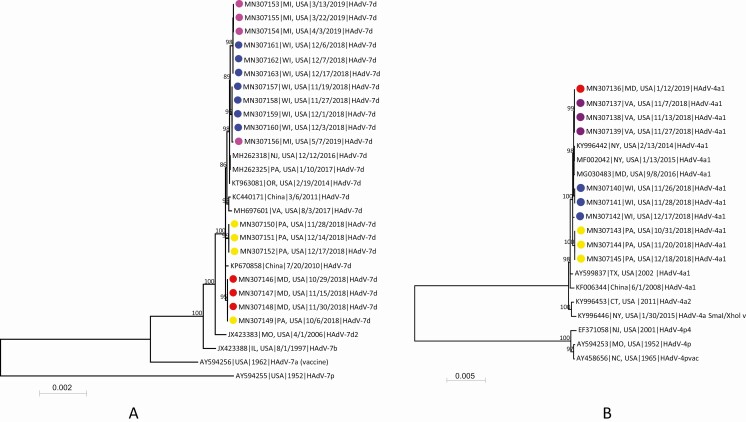
Complete genome sequences were obtained from 18 HAdV-7 isolates and 10 HAdV-4 isolates (GenBank accession numbers MN307136–MN307163). All were identified as genome type 7d or 4a1 and shared > 99.9% nucleotide sequence identity within each type. The genomic sequences of HAdV-7 clustered together with HAdV-7d strains from the US from 2014 to 2017 [6, 16, 23] and China in 2010 and 2011 [7, 24] (Figure 1A). The HAdV-4 genomes showed highest sequence identity to HAdV-4a1 strains from the US from 2011 to 2016 [14, 25] and China in 2008 [26] (Figure 1B). Within each college outbreak, genome sequences were within 0–15 nucleotide differences of each other for both HAdV-7 and HAdV-4. Among the 5 colleges, genome sequences differed by 0–20 nucleotides (Supplementary Table 2).
Figure 1.
Phylogenetic analysis of human adenovirus 7 (A) and human adenovirus 4 (B) genomic sequences obtained from students associated with respiratory illness outbreaks in 5 college campuses and reference sequences available in GenBank. Genomic sequences were aligned by using MAFFT implemented in Geneious version 10.0.9, and the maximum-likelihood phylogenetic trees were constructed by using MEGA7 software [20] based on the Tamura 3-parameter nucleotide substitution model. Numbers at selected nodes indicate level of support using 1000 bootstrap replicates. Genomic sequences with colored dots were obtained in this study, and different colors represent sequences from different college outbreaks: red, college A; yellow, college B; purple, college C; blue, college D; pink, college E. Sequences are identified by GenBank accession number, geographic location, sample collection date or year, and virus genome type. Scale bar indicates estimated number of nucleotide substitutions per site. Abbreviations: CT, Connecticut; HAdV, human adenovirus; IL, Illinois; MD, Maryland; MI, Michigan; MO, Missouri; NC, North Carolina; NJ, New Jersey; NY, New York; OR, Oregon; PA, Pennsylvania; TX, Texas; USA, United States; VA, Virginia; WI, Wisconsin.
DISCUSSION
We describe a multistate investigation of HAdV-4 and HAdV-7 respiratory illness outbreaks associated with severe illness and death among students at 5 US college campuses during fall 2018 and spring 2019. HAdV ARI was associated with substantial morbidity among generally healthy, undergraduate college students. Eleven students required hospitalization, 10 of whom had pneumonia; 9 recovered with supportive therapy, and 2 died. The illnesses resulted in considerable missed class time, time to recovery, and healthcare utilization.
Outbreaks of HAdV-associated ARI have not been well documented in nonmilitary congregate settings such as college campuses. Although recent reports have described HAdV detections among college students using ILI surveillance at SHCs, these accounts were primarily laboratory characterizations, with limited or no epidemiologic data [15–17]. HAdV is likely an underrecognized cause of acute respiratory illness; it is often not routinely tested for, and outbreak notification to CDC is voluntary. Additional studies are needed to understand the burden and risk of HAdV-associated respiratory illness among student populations in college settings.
In the US, HAdV-associated ARI outbreaks are well described among military recruits in training facilities, with published reports as early as the 1950s [10, 11, 27]. An oral, live, nonattenuated vaccine for HAdV-4 and HAdV-7 was first implemented for military use in 1971. The vaccine is safe, effective, and immunogenic in military populations and is currently US Food and Drug Administration (FDA) approved for use in healthy military personnel aged 17–50 years [13, 28]. An interruption in vaccine supply during 1999–2011 resulted in a surge of HAdV infections and 8 documented deaths among military recruits [12, 29, 30]. After reintroduction of the vaccine, dramatic declines in ARI and HAdV infections among vaccinated military populations were demonstrated [29, 31]. Recent reports documented HAdV outbreaks among unvaccinated military populations, including at the US Naval Academy (HAdV-4) and at the US Marine Corps Officer Candidates School (OCS) in Quantico, Virginia (HAdV-7) [14, 23]. As a result of the OCS outbreak, the vaccine is now administered to all incoming officer candidates in Quantico under a temporary vaccine protocol to assess effectiveness in this population [23]. The vaccine has not been evaluated in the general civilian population. Because live virus can be shed in stool of vaccinated persons for up to 28 days, transmission of vaccine-strain virus is possible, and avoiding close contact with vulnerable populations, including pregnant women, young children, and immunocompromised individuals, during this period is recommended [13]. Consideration of broader prevention strategies targeting HAdV in civilian populations, including college students, could require the development of other vaccine constructs, for example, attenuated vaccine.
In this report, cases were commonly freshmen living in dormitories and more frequently male. Similarly, in the 1990s, freshmen in college dormitories were noted to be at moderately increased risk for meningococcal disease compared with other college students [32, 33]. This led to a change in US vaccine policy, recommending that college freshmen living in dormitories receive the meningococcal vaccine [33, 34]. For HAdV-associated respiratory illness, however, more research is needed on the risk and burden of disease specific to college settings to understand the potential benefit of specific intervention strategies.
While most students in this investigation presented with self-limiting upper respiratory tract infection, 11 were hospitalized with severe illness, and 2 died. Although HAdV can also cause severe disease in otherwise healthy individuals [35, 36], immunocompromised individuals, particularly solid organ (SOT) or stem cell transplant (SCT) recipients, are at increased risk of severe and disseminated HAdV infection [1, 5]. One death occurred in a student who was taking an immunosuppressive medication, and the second death occurred in a student with no known underlying medical conditions but with recent history of mononucleosis and brief steroid treatment.
Although there is currently no FDA-approved treatment for HAdV infection, both decedents were treated with cidofovir, a nucleotide analogue with in vitro activity against HAdV that has been used to treat severe HAdV infections [37]. Data on cidofovir use for HAdV infection are limited to observational studies and case reports, almost exclusively among patients who are severely immunocompromised, such as SOT and SCT recipients [36, 38–40]. Studies in these populations have had mixed results on clinical improvement and mortality [1, 41].
Molecular analysis of 28 genome sequences identified HAdV-7 strains as genotype 7d and HAdV-4 strains as 4a1. HAdV-7d was detected in China in 2009 after a 21-year apparent absence and was first reported in the US in a 2014 community outbreak in Oregon [6, 7]. Recent outbreaks in college and military settings have also identified HAdV-7d [16, 23]. During nonvaccine years, HAdV-4 was the most common cause of HAdV outbreaks in military recruits [12, 29], and 4a1 was identified in a recent outbreak at the US Naval Academy and among cases of ILI at 2 colleges in New York [14, 25]. At 4 of the 5 colleges, both HAdV-4 and HAdV-7 were detected during the investigation period. Co-circulation of HAdV types is common and has been described in other investigations [11, 15]. While we identified genome sequence similarities both within and between the outbreaks, this is expected given the single base genetic stability of double-stranded DNA viruses, such as adenovirus [42]. The genomic data are informative in understanding HAdV circulation, but do not allow for development of detailed transmission chains or conclusions about directionality of transmission in this investigation.
Our investigation has limitations. First, testing practices likely influenced the number and type of cases identified. Students presenting to SHCs met certain criteria for testing based on ILI or ARI symptom presentation, and only a subset were tested for HAdV. Severity of illness may have influenced students’ health-seeking behaviors and clinician-directed testing. Thus, this report reflects a convenience sample of students tested for HAdV, and cases were likely missed. Relatedly, testing based on ILI case definitions, at the 2 colleges that performed ILI surveillance, could have influenced the proportion of symptoms presented here. For example, the most common symptoms included fever, cough, and sore throat, all of which are part of the ILI case definition. Second, the case definition included laboratory-confirmed HAdV cases only. Information on students presenting with ARI but who were not tested for HAdV was not collected. Future studies using active surveillance or standardized approaches to testing are needed to document HAdV burden. Third, available data varied by site, and our results were limited by response rates and access to medical records and specimens for molecular characterization. Specifically, this restricted our ability to draw conclusions about differences in disease presentation and clinical course by HAdV type. Data were combined across sources, where possible. However, our findings should be interpreted in light of these limitations.
This investigation describes HAdV-associated ARI outbreaks among young adults on 5 US college campuses. HAdVs should be considered as a possible cause of respiratory illness among college students, with the potential to cause severe illness and, occasionally, death. Understanding overall disease burden, risk, and risk factors for HAdV illness are necessary to inform prevention strategies and to evaluate the potential utility of a vaccine in these settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Shifaq Kamili, Kia Crocker, Angela Myrick-West, Lynne Deane, and Sarah Fisher, for their contributions to this public health investigation.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. Specimen testing at the Pennsylvania Department of Health was partially supported by the CDC’s Epidemiology and Laboratory Capacity for Infectious Diseases (cooperative agreement 6 NU50CK000375-05).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 2014; 27:441–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Human Adenovirus Working Group. Homepage. Available at: http://hadvwg.gmu.edu/. Accessed 20 December 2019.
- 3. Binder AM, Biggs HM, Haynes AK, et al. Human adenovirus surveillance—United States, 2003–2016. MMWR Morb Mortal Wkly Rep 2017; 66:1039–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cui X, Wen L, Wu Z, et al. Human adenovirus type 7 infection associated with severe and fatal acute lower respiratory illness and nosocomial transmission. J Clin Microbiol 2015; 53:746–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis 2006; 43:331–9. [DOI] [PubMed] [Google Scholar]
- 6. Scott MK, Chommanard C, Lu X, et al. Human adenovirus associated with severe respiratory infection, Oregon, USA, 2013–2014. Emerg Infect Dis 2016; 22:1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao S, Wan C, Ke C, et al. Re-emergent human adenovirus genome type 7d caused an acute respiratory disease outbreak in southern China after a twenty-one year absence. Sci Rep 2014; 4:7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao L-H, Wang C, Wei T-L, Wang H, Ma F-L, Zheng L-S. Human adenovirus among hospitalized children with respiratory tract infections in Beijing, China, 2017–2018. Virol J 2019; 16:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Russell KL, Broderick MP, Franklin SE, et al. Transmission dynamics and prospective environmental sampling of adenovirus in a military recruit setting. J Infect Dis 2006; 194:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dudding BA, Top FH Jr, Winter PE, Buescher EL, Lamson TH, Leibovitz A. Acute respiratory disease in military trainees: the adenovirus surveillance program, 1966–1971. Am J Epidemiol 1973; 97:187–98. [DOI] [PubMed] [Google Scholar]
- 11. Ryan MA, Gray GC, Smith B, McKeehan JA, Hawksworth AW, Malasig MD. Large epidemic of respiratory illness due to adenovirus types 7 and 3 in healthy young adults. Clin Infect Dis 2002; 34:577–82. [DOI] [PubMed] [Google Scholar]
- 12. Russell KL, Hawksworth AW, Ryan MA, et al. Vaccine-preventable adenoviral respiratory illness in US military recruits, 1999–2004. Vaccine 2006; 24:2835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. US Food and Drug Administration. Package insert—adenovirus type 4 and type 7 vaccine, live, oral. Available at: https://www.fda.gov/media/80211/download. Accessed 3 July 2019.
- 14. Rogers AE, Lu X, Killerby ME, et al. Outbreak of acute respiratory illness associated with adenovirus type 4 at the U.S. Naval Academy, 2016. MSMR 2019; 26:21–7. [PubMed] [Google Scholar]
- 15. Biggs HM, Lu X, Dettinger L, Sakthivel S, Watson JT, Boktor SW. Adenovirus-associated influenza-like illness among college students, Pennsylvania, USA. Emerg Infect Dis 2018; 24:2117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Killerby ME, Rozwadowski F, Lu X, et al. Respiratory illness associated with emergent human adenovirus genome type 7d, New Jersey, 2016–2017. Open Forum Infect Dis 2019; 6:ofz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamson DM, Kajon A, Shudt M, Girouard G, St George K. Detection and genetic characterization of adenovirus type 14 strain in students with influenza-like illness, New York, USA, 2014–2015. Emerg Infect Dis 2017; 23:1194–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu X, Trujillo-Lopez E, Lott L, Erdman DD. Quantitative real-time PCR assay panel for detection and type-specific identification of epidemic respiratory human adenoviruses. J Clin Microbiol 2013; 51:1089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li QG, Wadell G. Analysis of 15 different genome types of adenovirus type 7 isolated on five continents. J Virol 1986; 60:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li QG, Wadell G. The degree of genetic variability among adenovirus type 4 strains isolated from man and chimpanzee. Arch Virol 1988; 101:65–77. [DOI] [PubMed] [Google Scholar]
- 23. Bautista-Gogel J, Madsen CM, Lu X, et al. Outbreak of respiratory illness associated with human adenovirus type 7 among persons attending Officer Candidates School, Quantico, Virginia, 2017. J Infect Dis 2020; 221:697–700. [DOI] [PubMed] [Google Scholar]
- 24. Yu Z, Zeng Z, Zhang J, et al. Fatal community-acquired pneumonia in children caused by re-emergent human adenovirus 7d associated with higher severity of illness and fatality rate. Sci Rep 2016; 6:37216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kajon AE, Lamson DM, Bair CR, et al. Adenovirus type 4 respiratory infections among civilian adults, northeastern United States, 2011-20151. Emerg Infect Dis 2018; 24:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tian X, Chen Y, Li H, et al. Characterization of a replication-competent vector encoding DsRed based on a human adenovirus type 4 a-like strain. Virus Res 2019; 270:197662. [DOI] [PubMed] [Google Scholar]
- 27. Hilleman MR. Acute respiratory illness caused by adenoviruses; a military problem. U S Armed Forces Med J 1956; 7:1717–25. [PubMed] [Google Scholar]
- 28. Kuschner RA, Russell KL, Abuja M, et al. Adenovirus Vaccine Efficacy Trial Consortium . A phase 3, randomized, double-blind, placebo-controlled study of the safety and efficacy of the live, oral adenovirus type 4 and type 7 vaccine, in U.S. military recruits. Vaccine 2013; 31:2963–71. [DOI] [PubMed] [Google Scholar]
- 29. Radin JM, Hawksworth AW, Blair PJ, et al. Dramatic decline of respiratory illness among US military recruits after the renewed use of adenovirus vaccines. Clin Infect Dis 2014; 59:962–8. [DOI] [PubMed] [Google Scholar]
- 30. Potter RN, Cantrell JA, Mallak CT, Gaydos JC. Adenovirus-associated deaths in US military during postvaccination period, 1999–2010. Emerg Infect Dis 2012; 18:507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clemmons NS, McCormic ZD, Gaydos JC, Hawksworth AW, Jordan NN. Acute respiratory disease in US army trainees 3 years after reintroduction of adenovirus vaccine. Emerg Infect Dis 2017; 23:95–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruce MG, Rosenstein NE, Capparella JM, Shutt KA, Perkins BA, Collins M. Risk factors for meningococcal disease in college students. JAMA 2001; 286:688–93. [DOI] [PubMed] [Google Scholar]
- 33. Meningococcal Disease and College Students. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000; 49:13– 20. [PubMed] [Google Scholar]
- 34. Bilukha OO, Rosenstein N; National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC) . Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005; 54:1–21. [PubMed] [Google Scholar]
- 35. Clark TW, Fleet DH, Wiselka MJ. Severe community-acquired adenovirus pneumonia in an immunocompetent 44-year-old woman: a case report and review of the literature. J Med Case Rep 2011; 5:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim SJ, Kim K, Park SB, Hong DJ, Jhun BW. Outcomes of early administration of cidofovir in non-immunocompromised patients with severe adenovirus pneumonia. PLoS One 2015; 10:e0122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chemaly RF, Hill JA, Voigt S, Peggs KS. In vitro comparison of currently available and investigational antiviral agents against pathogenic human double-stranded DNA viruses: a systematic literature review. Antiviral Res 2019; 163:50–8. [DOI] [PubMed] [Google Scholar]
- 38. Lindemans CA, Leen AM, Boelens JJ. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood 2010; 116:5476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neofytos D, Ojha A, Mookerjee B, et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant 2007; 13:74–81. [DOI] [PubMed] [Google Scholar]
- 40. Ljungman P, Ribaud P, Eyrich M, et al. Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation . Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2003; 31:481–6. [DOI] [PubMed] [Google Scholar]
- 41. Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis 2012; 14:555–63. [DOI] [PubMed] [Google Scholar]
- 42. Seto J, Walsh MP, Metzgar D, Seto D. Computational analysis of adenovirus serotype 5 (HAdV-C5) from an HAdV coinfection shows genome stability after 45 years of circulation. Virology 2010; 404:180–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.