Abstract
Background
Retreatment tuberculosis (TB) disease is common in high-prevalence settings. The risk of repeated episodes of recurrent TB is unknown. We calculated the rate of recurrent TB per subsequent episode by matching individual treatment episodes over a period of 13 years.
Methods
All recorded TB episodes in Cape Town between 2003 and 2016 were matched by probabilistic linkage of personal identifiers. Among individuals with a first episode notified in Cape Town and who completed their prior treatment successfully we estimated the recurrence rate stratified by subsequent episode and HIV status. We adjusted person-time to background mortality by age, sex, and HIV status.
Results
A total of 292 915 TB episodes among 263 848 individuals were included. The rate of recurrent TB was 16.4 per 1000 person-years (95% CI, 16.2–16.6), and increased per subsequent episode (8.4-fold increase, from 14.6 to 122.7 per 1000 from episode 2 to 6, respectively). These increases were similar stratified by HIV status. Rates among HIV positives were higher than among HIV negatives for episodes 2 and 3 (2- and 1.5-fold higher, respectively), and the same thereafter.
Conclusions
TB recurrence rates were high and increased per subsequent episode, independent of HIV status. This suggests that HIV infection is insufficient to explain the high burden of recurrence; it is more likely due to a high annual risk of infection combined with an increased risk of infection or progression to disease associated with a previous TB episode. The very high recurrence rates would justify increased TB surveillance of patients with >1 episode.
Keywords: antitubercular agents/therapeutic use, incidence, recurrence, South Africa, epidemiology
Tuberculosis treatment episodes over a period of 13 years were linked. Rates of recurrence increased 9-fold between episode 2 and 5 and did not differ by HIV status or previous treatment outcome, suggesting an underlying mechanism not mediated by HIV infection.
Tuberculosis (TB) has seen a worldwide resurgence since the advent of the human immunodeficiency virus (HIV) epidemic in the 1990s. The World Health Organization launched its End TB Strategy in 2014 with the aim to cut TB incidence by 90% by 2035 [1]. Globally, the burden of disease is still very high; TB notification rates are decreasing but much more slowly than needed to reach this aim. There is an urgent need for improved understanding of propagating factors of the epidemic.
Tuberculosis disease among previously treated individuals (recurrent TB) constitutes 5–30% of the TB burden, with higher proportions found in high-prevalence settings [2–4]. Recurrence may be due to endogenous relapse or exogenous reinfection. In high-prevalence settings reinfection is thought to drive the higher proportion of retreatment due to higher transmission rates [4, 5], and limited data have suggested the risk of recurrence due to reinfection is increased after a previous episode of TB disease [6]. Repeated recurrences in the same individual add to the TB burden, but the extent has not been quantified due to difficulties in identifying recurrence in routinely collected data because of a lack of longitudinal patient registration systems.
Cape Town has one of the highest burdens of TB disease worldwide. Annually, almost one-third of TB represents retreatment [7], but the risk of repeated episodes of recurrent TB and the impact of HIV on this burden are unknown.
We developed a stepwise probabilistic linkage protocol to identify TB treatment episodes in the same individual within Cape Town over a period of 13 years and validated it through comparison with a manually matched subset of the database. We calculated the incidence of recurrent TB disease stratified by the number of previous episodes and by previous treatment outcome. Among those who had completed their prior treatment, we calculated incidence stratified by year of follow-up, age, gender, and HIV status.
METHODS
Study Setting
The Cape Town metropolitan area encompassed an estimated population of 3.7 million people in 2011 [8]. Diagnosis and treatment of tuberculosis are provided free of charge by 101 primary care clinics. Only a small number of TB cases are treated in the private sector as drugs are difficult to access outside of the national TB-control program [9].
Diagnosis and treatment of TB follow national guidelines [10]. Diagnostic tests available were sputum smear microscopy and, after 2013, Xpert MTB/RIF. Empirical treatment (after negative or no tests performed) declined since the introduction of Xpert (32% to 19%) [11]. Drug-sensitive TB is treated with a 2-month intensive phase of isoniazid, rifampicin, ethambutol, and pyrazinamide, followed by 4 months of isoniazid and rifampicin. Patients with rifampicin-resistant TB are referred to multidrug-resistant TB (MDR-TB) units; these patients are recorded in a separate database.
Study Design and Population
In this population-based cohort study we included all recorded drug-sensitive TB treatment episodes in Cape Town from 1 January 2003 to 31 March 2016. Patients who failed treatment due to drug-resistant TB were excluded. We defined a TB treatment episode as a TB notification in the Electronic TB Register (ETR) [12]. We matched these individual episodes using a probabilistic matching protocol (see below) to create a cohort of persons with 1 or more episodes of TB.
We then generated a virtual cohort consisting of all persons whose first TB treatment episode was notified in Cape Town during these years. Persons who, at that episode, reported to have had TB treatment previously were excluded. This cohort formed the basis of the incidence analyses. Linkage to the South African vital registration system was not possible due to lack of identification numbers; we therefore adjusted for unmeasured mortality by applying estimates of mortality rates by age, sex, HIV status, and calendar year.
Data Sources
Tuberculosis notification data were abstracted from the ETR for Cape Town [12]. We sourced estimates of annual HIV prevalence, HIV incidence, and mortality rates by 5-year age group, gender, and HIV status from the Western Cape version of the Thembisa model, a mathematical model of the South African HIV epidemic, and a demographic projection model [13].
Data Linkage
The Provincial Health Data Centre (PHDC) of the Western Cape linked and collated all unique patient identifiers (PIDs) used across provincial health services [14]. The ETR database was linked and collated in the same process using the PID for the treatment episodes that had this recorded. For episodes without PID, probabilistic linkage using the recorded demographic information was performed to link to an existing PID. Among those that could not be linked, repeated episodes within individuals and with those with a PID were identified using stepwise probabilistic linkage (Supplementary Figure 1). These matches were designated “probable,” “possible,” and “potential” depending on the certainty of the match. After manual review of all potential matches and a sample of probable and possible matches, all categories were included in the final matched dataset. (See the online data supplement for detailed information regarding the data linkage procedures [Supplementary Table 1].) Linkage was performed using Microsoft SQL Server Management Studio (SSMS) 2014 (Microsoft Corporation, Redmond, WA).
Data Validation
We validated our linkage and matching algorithm by comparing with a manually matched research database of a peri-urban community in Cape Town and a subset of the final matched dataset for the same period [15]. Taking the research database as the gold standard, we calculated the sensitivity and specificity of the matching algorithm.
Statistical Methods
Within this cohort we calculated person-years at risk (PYAR) of a second episode of TB from the end of treatment until either a second episode or the end of study follow-up (March 2016), whichever came first. In those who developed a second episode, the PYAR of a third episode were calculated from the end of treatment of the second episode until the third episode or the end of follow-up. This was repeated for all subsequent TB treatment episodes.
We adjusted the person-years of participants after their last episode of TB (ie, until the end of study follow-up) to estimates of mortality in the general Cape Town population [13]. Mortality rates in strata of 5-year age groups, gender, HIV status, and calendar year (thereby taking into account the effect of antiretroviral therapy [ART]) were applied to the person-years per stratum to calculate the number of person-years to be subtracted. We did not adjust for increased mortality after TB treatment or mobility of participants outside of Cape Town as no reliable estimates were available.
Using these person-years we calculated the incidence rate and timing of recurrent TB per 1000 PYAR (with 95% confidence intervals [CIs]) by previous treatment outcome (treatment completion or cure vs default) and by the number of previous treatment episodes.
To avoid including recurrences that were continuations of the prior episode, all additional analyses were restricted to recurrences after treatment completion or cure. Among those, we further stratified rates by year after the end of treatment, sex, age, and HIV status. In case of HIV seroconversion between episodes, the accrued person-time was halved between the HIV-negative and HIV-positive person-time. We also performed a sensitivity analysis restricting to bacteriologically confirmed episodes and censoring on other episodes as participants received treatment for them.
All analyses were performed using Stata 16.0 SE (StataCorp, College Station, TX) and Microsoft Excel 2013 (Microsoft Corporation).
Regulatory Approval
This study was approved by the Human Research Ethics Committee at the University of Cape Town and by the Cape Town City Health Department.
RESULTS
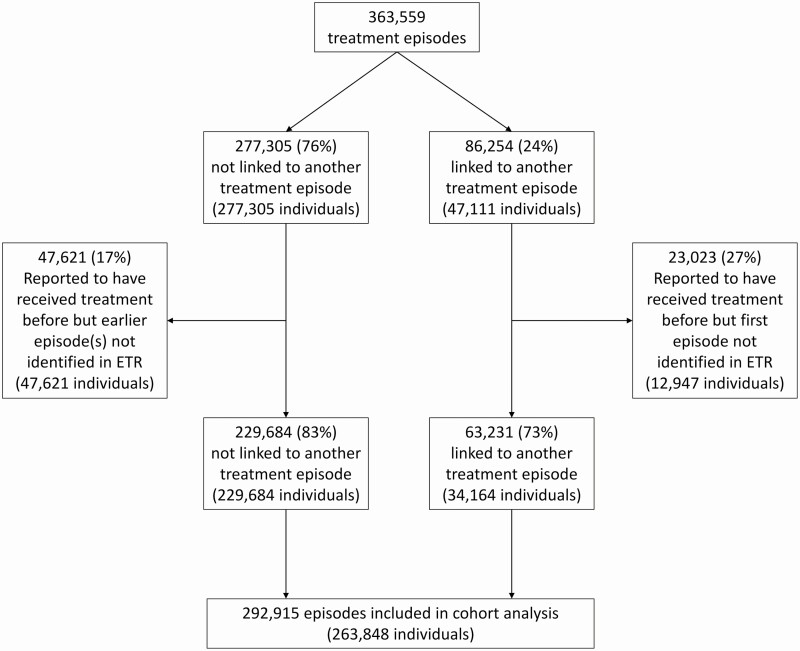
There were a total of 363 559 treatment episodes recorded in the Cape Town ETR between January 2003 and March 2016 (Supplementary Figure 2). Of these, 24% were linked to another episode (Figure 1). The proportion of recurrent cases increased per calendar year, reflecting accrual of person-time at risk over the study period (Supplementary Figure 3).
Figure 1.
Flow diagram of TB treatment episodes included in the analysis. Abbreviations: ETR, Electronic TB Register; TB, tuberculosis.
In the validation dataset, 834 out of 3243 (26%) notified TB episodes were linked to another TB episode. Of these, the matching algorithm identified 586 (sensitivity, 70%). Nine matches were false positive (specificity, 99%).
Our algorithm identified 324 416 individuals (Figure 1). At their first episode in the ETR, 60 568 (19%) were recorded as having been treated previously. These and any subsequent episodes in the same individual were excluded, to leave 292 915 episodes. These represented 263 848 individuals and formed the cohort for the remainder of the analysis.
Among these individuals, 23 422 (9%) experienced 2 TB treatment episodes, 4303 (2%) experienced 3, 978 (0.4%) experienced 4, and 366 (0.1%) experienced 5 or more episodes during follow-up. Table 1 shows their baseline characteristics by treatment episode. More men than women had TB, and this difference increased per subsequent episode. The average age at first episode was 30 years and increased to 34 years for subsequent episodes. Bacteriological confirmation increased from 68% to 75% and was much lower in children (8% increasing to 20%). The proportion of extrapulmonary TB decreased per episode (from 17% to 5%). Human immunodeficiency virus status was unknown in 25% of treatment episodes; this declined rapidly from 100% in 2003 to less than 10% from 2009 onwards (Supplementary Figure 4A). We were able to assess HIV seroconversion between episodes in 66% of recurrences; 3% of recurrences were in individuals who seroconverted between episodes (Supplementary Figure 4C).
Table 1.
Baseline Characteristics of Cohort Patients (at End of Treatment Episode), Stratified by Tuberculosis Episode (Up to Episode 5)
| Episode | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Total (n) | 263 848 | 23 422 | 4303 | 978 | 260 |
| Gender | |||||
| Female | 123 399 (46.8) | 9600 (41.0) | 1663 (38.6) | 363 (37.1) | 93 (35.8) |
| Male | 140 366 (53.2) | 13 402 (57.2) | 2528 (58.7) | 585 (59.8) | 156 (60) |
| Age (years) | |||||
| 0–14 | 43 470 (16.5) | 1625 (6.9) | 114 (2.6) | 18 (1.8) | 2 (0.8) |
| 15–24 | 49 347 (18.7) | 3419 (14.6) | 599 (13.9) | 136 (13.9) | 44 (16.9) |
| 25–34 | 75 803 (28.7) | 7819 (33.4) | 1542 (35.8) | 367 (37.5) | 93 (35.8) |
| 35–44 | 51 416 (19.5) | 6282 (26.8) | 1334 (31) | 319 (32.6) | 88 (33.8) |
| 45–54 | 26 778 (10.1) | 3126 (13.3) | 548 (12.7) | 111 (11.3) | 31 (11.9) |
| 55–64 | 11 547 (4.4) | 915 (3.9) | 142 (3.3) | 26 (2.7) | 2 (0.8) |
| ≥65 | 5487 (2.1) | 236 (1.0) | 24 (0.6) | 1 (0.1) | 0 (0.0) |
| Age, mean (SD), years | 30 (16.0) | 33 (13.0) | 34 (11.0) | 34 (10.0) | 33 (9.0) |
| Type of disease | |||||
| Extrapulmonary | 43 832 (16.6) | 2527 (10.8) | 363 (8.4) | 68 (7.0) | 13 (5.0) |
| Pulmonary | 220 014 (83.4) | 20 895 (89.2) | 3940 (91.6) | 910 (93.0) | 247 (95.0) |
| Type of diagnosis | |||||
| Test(s) positive | 149 221 (56.6) | 15 651 (66.8) | 3017 (70.1) | 685 (70.0) | 196 (75.4) |
| Test(s) negative | 50 814 (19.3) | 4669 (19.9) | 911 (21.2) | 212 (21.7) | 48 (18.5) |
| No tests done | 63 813 (24.2) | 3102 (13.2) | 375 (8.7) | 81 (8.3) | 16 (6.2) |
| HIV status | |||||
| Negative | 106 907 (40.5) | 8893 (38.0) | 1601 (37.2) | 381 (39.0) | 117 (45.0) |
| Positive | 86 401 (32.7) | 11 789 (50.3) | 2436 (56.6) | 562 (57.5) | 134 (51.5) |
| Unknown | 70 540 (26.7) | 2740 (11.7) | 266 (6.2) | 35 (3.6) | 9 (3.5) |
| Outcome of previous episode | |||||
| Completion or cure | N/A | 17 808 (76.0) | 2753 (64.0) | 560 (57.3) | 132 (50.8) |
| Default | N/A | 4478 (19.1) | 1353 (31.4) | 370 (37.8) | 110 (42.3) |
| Failure | N/A | 388 (1.7) | 73 (1.7) | 20 (2.0) | 9 (3.5) |
| Move or transfer out | N/A | 445 (1.9) | 75 (1.7) | 17 (1.7) | 4 (1.5) |
| Not evaluated | N/A | 298 (1.3) | 48 (1.1) | 11 (1.1) | 4 (1.6) |
| MDR or RIF resistance | N/A | 5 (0.0) | 1 (0.0) | 0 (0.0) | 1 (0.4) |
| Outcome of present episode | |||||
| Completion or cure | 220 017 (83.4) | 17 481 (74.6) | 2961 (68.8) | 589 (60.2) | 169 (65.0) |
| Default | 21 261 (8.1) | 3590 (15.3) | 869 (20.2) | 265 (27.1) | 63 (24.2) |
| Death | 9719 (3.7) | 1343 (5.7) | 277 (6.4) | 78 (8.0) | 16 (6.2) |
| Failed | 1578 (0.6) | 262 (1.1) | 67 (1.6) | 19 (1.9) | 6 (2.3) |
| Moved or transferred | 7660 (2.9) | 500 (2.1) | 73 (1.7) | 15 (1.5) | 4 (1.5) |
| Not evaluated | 3449 (1.3) | 214 (0.9) | 37 (0.9) | 8 (0.8) | 2 (0.8) |
| MDR or RIF resistance | 164 (0.1) | 32 (0.1) | 19 (0.4) | 4 (0.4) | 0 (0.0) |
| Matching certainty | |||||
| Probable | N/A | 21 892 (93.5) | 4192 (97.4) | 965 (98.7) | 257 (98.8) |
| Possible | N/A | 1242 (5.3) | 93 (2.2) | 12 (1.2) | 3 (1.2) |
| Potential | N/A | 288 (1.2) | 18 (0.4) | 1 (0.1) | 0 (0.0) |
Values are n (%) unless otherwise specified.
Abbreviations: HIV, human immunodeficiency virus; MDR, multidrug resistance; N/A, not applicable; RIF, rifampicin.
Stratified by previous treatment outcome, 17 808 out of 220 017 (8%) individuals who completed treatment and 4478 out of 21 261 (21%) individuals who did not complete treatment had a recurrent episode of TB. Baseline characteristics by previous treatment outcome are presented in Supplementary Tables 1 and 2.
These patients contributed a total of 1 525 314 PYAR. We removed 15 138 PYAR (1%; 8628 with HIV and 6510 without HIV) to adjust for unmeasured mortality, leading to a total of 1 510 176 PYAR (median, 6.1 years; maximum, 13.2 years).
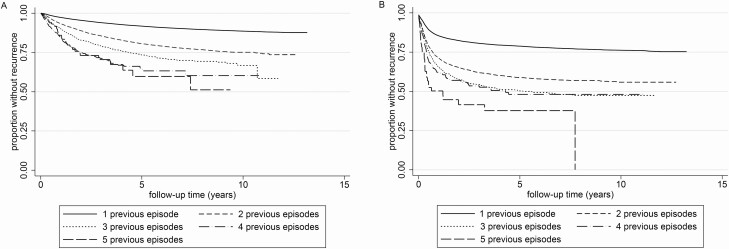
The overall incidence rate of recurrent TB was 19.1 per 1000 PYAR (95% CI, 18.9–19.4). Rates after previous treatment completion or cure were 16.4 (95% CI, 16.2–16.6) per 1000 PYAR and increased per subsequent episode: 8.4-fold from the second to the sixth episode (Figure 2A and Supplementary Table 3). Rates were highest in the first 2 years after treatment (27.2/1000; 95% CI, 6.7–27.7), after which they stabilized (12.2/1000; 95% CI, 11.6–13.0). The same patterns were seen among the patients who had not completed their previous treatment, with the latter difference being more extreme (130.5/1000 [95% CI, 127.0–134.1] vs 11.4/1000 [95% CI, 11.2–11.6]) (Figure 2B).
Figure 2.
A, Kaplan-Meier curves of recurrent TB stratified by number of prior TB treatment episodes among persons who were cured or completed their previous TB treatment episode. B, Kaplan-Meier curves of recurrent TB stratified by number of prior TB treatment episodes among persons who did not complete treatment for their previous TB treatment episode. Abbreviation: TB, tuberculosis.
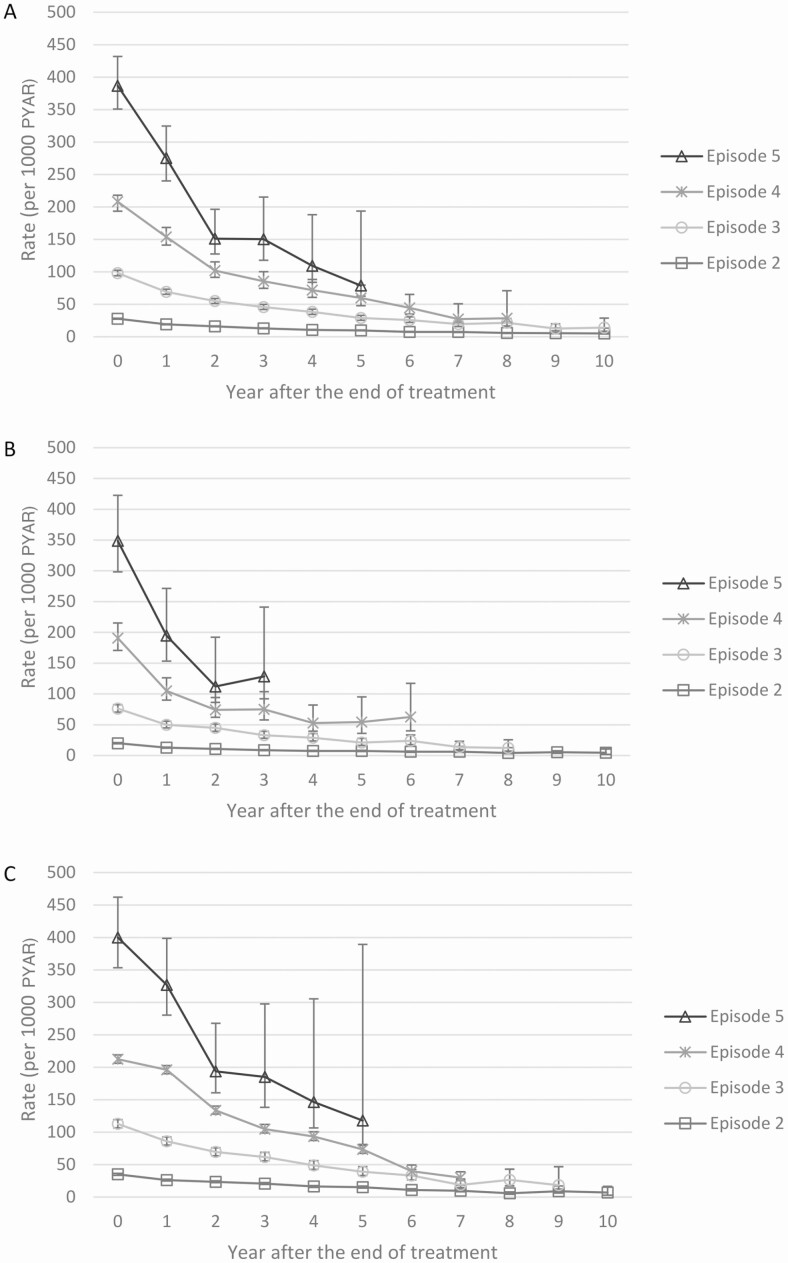
Recurrences and rates stratified by year since the end of treatment, age, sex, and HIV among those who had completed treatment are shown in Supplementary Table 4. Rates were highest in the first year after the end of treatment and decreased over time across all episodes and stratified by HIV status (Figure 3). Rates were higher among men than women in episodes 2 and 3; thereafter, rates were similar between the sexes. Recurrence was less frequent among children, and no major differences were found between younger and older adults. Rates of recurrent TB were higher among those with HIV than among those without HIV for episodes 2 and 3; thereafter, the rates were similar. Lower rates, but with the same patterns, were seen when restricting the analysis to only bacteriologically confirmed recurrences (Supplementary Table 5).
Figure 3.
A, Rates of recurrent TB per 1000 PYAR among patients who completed their previous treatment, by year since the end of treatment and by number of episode. The confidence intervals for episodes 2 and 3 are very narrow and therefore not visible. B, Rates of recurrent TB per 1000 PYAR among patients without HIV who completed their previous treatment, by year since the end of treatment and by number of episode. The confidence intervals for episodes 2 and 3 are very narrow and therefore not visible. C, Rates of recurrent TB per 1000 PYAR among patients with HIV who completed their previous treatment, by year since the end of treatment and by number of episode. The confidence intervals for episodes 2 and 3 are very narrow and therefore not visible. Abbreviations: PYAR, person-years at risk; TB, tuberculosis
Discussion
Our analysis of the notified TB disease episodes among the population of Cape Town over a 13-year period showed a very high incidence of recurrent TB. Eighteen percent of patients who had their first episode of TB disease in 2003 had at least 1 other drug-sensitive episode by 2016. Among those whose first episode was in Cape Town and who completed treatment, 8% had a recurrent episode during follow-up. Two smaller prospective studies with similar follow-up durations in southern Africa found similar proportions (10%) [16, 17]. Higher recurrence risks have only been described among goldminers in South Africa (20% over 3 years) [18].
This large population–based cohort allowed for calculation of incidence rates of recurrent TB per subsequent episode, which increased per number of previous episodes. In comparison with the average background annual TB notification rate in the general population for the study period, which we previously estimated at 876 per 100 000 population [19], the rate doubled after the first episode and was 17-fold higher at the fifth episode. Restricting to recurrences after previous successful treatment, the absolute rates were lower but the increase in incidence per subsequent episode was similar (19.1 vs 16.4 per 1000 PYAR and an 8.8- vs 8.4-fold increase from the second to fifth episode, respectively).
Our data do not allow distinction between recurrent disease caused by relapse of the previous infection or a new infection as this requires molecular epidemiological data on the strain of Mycobacterium tuberculosis (Mtb). Reinfection is more common than relapse in Southern Africa, with reinfection being strongly associated with HIV infection [15, 17, 20–22]. Relapse is more likely during the first year after treatment completion, after which reinfection becomes the predominant mechanism [16]. Rates of new infection were 4-fold higher among previously treated patients compared with the general population in a suburban area in Cape Town [6], and 3-fold higher in miners both with and without HIV in Johannesburg [23]. Our data support these earlier findings: over this long observation period it is highly likely that the majority of recurrences are due to reinfection. As recurrence rates 2 or more years after treatment were similar in patients who completed and who defaulted from their previous treatment, it is likely that the underlying mechanism is also similar and based on reinfection rather than on relapse.
A number of mechanisms could explain this high rate of reinfection disease. A subset of the population could be at higher risk of exposure, infection, or progression of disease. Social and environmental factors include factors that increase exposure to TB (eg, crowding), factors that reduce host immunity (eg, smoking), and their social determinants (eg, poverty) [24]. Genetic factors could determine predisposition or resistance to infection and disease [25]. Specific risk factors for reinfection disease in both high- and low-incidence settings have been inconsistent, with the exception of HIV infection [15, 17, 18, 20–22, 26–30]. Lung tissue damage after pulmonary TB has been associated with an increased risk of recurrent TB [31]. Ongoing inflammation after the end of treatment, as has been shown in the majority of patients with TB [32], could represent lack of cure predisposing for relapse, or a dysregulated immune response predisposing for reinfection and progression to disease [33]. Microbiological factors could also play a role: patients with reinfection disease more often have mixed infections [5, 34]. Last, there could be immunological differences post–TB treatment that mediate an increased risk. T-cell recognition of Mtb antigens and/or immune mechanisms necessary for containment of Mtb during latent infection could be perturbed by active TB disease and not fully restored following successful treatment. This hypothesis is supported by findings that T-cell responses to a set of Mtb antigens were lower in persons with a history of TB, compared with latently infected controls without TB [35]. Among infants, the magnitude of T-cell responses was associated with risk of TB disease, further supporting the hypothesis that T-cell responses are critical for control of Mtb [36].
In keeping with prior reports, HIV was associated with increased recurrence rates, but this difference disappeared after the third episode. Therefore, the underlying mechanism for increased rate of recurrence is unlikely due to HIV coinfection alone. Interestingly, rates of second and third episodes of TB disease among persons with HIV were lower than the estimated rate in the general population with HIV (average annual TB notification rate 7146/100 000 population between 2008 and 2013) [19]. This might be the effect of ART initiation, as an episode of TB disease is the main entry into HIV care in Cape Town [37]. Alternatively, the estimates of PYAR used to calculate the latter rate were too high, not taking into account higher mortality or mobility among patients with HIV. Nevertheless, the increase in incidence of recurrence per subsequent episode was similar between participants with and without HIV. This suggests that increasing ART coverage will not be sufficient to reduce the burden of recurrent TB in the population with HIV. Additional strategies are needed to address the high burden of recurrent TB, such as active screening—for example, using annual chest radiology, maybe in combination with a form of preventive treatment [38]—and their impact investigated.
Strengths of our analysis include the very large dataset with long follow-up. We applied conservative matching criteria and excluded recurrences notified outside of Cape Town, biasing towards an under- rather than overestimate of recurrences. Limitations include the large proportion of episodes with an unknown HIV status, especially in the early years of the cohort. This reduced the sample size to analyze the impact of HIV, in particular among participants with multiple recurrences (who enrolled in the cohort the earliest). Restriction to drug-sensitive TB notifications led to an underestimation of the total number of relapses, although their inclusion would have obscured the findings as their recurrence may reflect nonresponsiveness to therapy. We may have overestimated follow-up time due to survival bias: mortality rates after a TB episode are known to be increased, but this was not factored into the person-years adjustment as no reliable estimates from a similar population or setting were available [39–41]. This would have led to a further underestimate of the true recurrence rate. Additional limitations were the unavailability of population out-migration estimates to adjust the PYAR and that the mortality rates stratified by age and HIV status were drawn from estimates for the Western Cape Province, rather than Cape Town. However, the HIV prevalence is similar in the 2 populations (5.2% vs 5.0% in 2012) [42].
In conclusion, we found a high rate of TB disease recurrence increasing per subsequent episode, which was independent of previous treatment outcome and HIV status. These findings suggest that the HIV epidemic cannot fully explain the high burden of retreatment TB in Cape Town, and therefore that high ART coverage will not be sufficient to curb it. It is more likely explained by high transmission rates in combination with an increased risk of infection or progression to disease associated with previous TB treatment, calling for further research into the mechanisms underlying this association. The very high recurrence rates would justify increased TB surveillance of patients with more than 1 episode.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. S. M. H., F. G. J. C., A. B., and R. W. conceived and designed the study. S. M. H. and N. Z. analyzed the data. J. C. was involved in collecting the data. All authors interpreted the data and critically reviewed the manuscript. S. M. H. wrote the first draft.
Acknowledgments. The authors thank Keren Middelkoop, Carl Morrow, and Gareth Bowers for their assistance with the data linkage and the validation thereof.
Financial support. This work was supported by the European Union (Marie Curie International Outgoing Fellowship for Career Development grant number PIOF-GA-2012–332311; to S. M. H.), the South African Medical Research Council (grant number MRC-RFAUFSP-01-2013/CCAMP; to R. W.), the National Institutes of Health (grant numbers R01AI058736, R01AI093269; to R. W.), and the Bill & Melinda Gates Foundation (grant number OPP1116641; to R. W.).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. The End TB Strategy. 2014. Available at: http://www.who.int/tb/strategy/en/. Accessed 9 December 2016.
- 2. Mirsaeidi M, Sadikot RT. Patients at high risk of tuberculosis recurrence. Int J Mycobacteriol 2018; 7:1–6. [DOI] [PubMed] [Google Scholar]
- 3. Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors: adequately treated patients are still at high risk. Int J Tuberc Lung Dis 2007; 11:828–37. [PubMed] [Google Scholar]
- 4. Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis 2003; 3:282–7. [DOI] [PubMed] [Google Scholar]
- 5. McIvor A, Koornhof H, Kana BD. Relapse, re-infection and mixed infections in tuberculosis disease. Pathog Dis 2017; 75. Available at: https://academic.oup.com/femspd/article/75/3/ftx020/3003284 [DOI] [PubMed] [Google Scholar]
- 6. Verver S, Warren RM, Beyers N, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med 2005; 171:1430–5. [DOI] [PubMed] [Google Scholar]
- 7. Wood R, Lawn SD, Caldwell J, Kaplan R, Middelkoop K, Bekker LG. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One 2011; 6:e25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strategic Development Information and GIS Department, City of Cape Town. Cape Town overview—census 2011. 2011. Available at: http://www.capetown.gov.za/en/stats/Documents/2011%20Census/2011_Census_Cape_Town_Profile.pdf. Accessed 26 November 2014.
- 9. Stevens M, Sinanovic E, Regensberg L, Hislop M. South African Health Review, chapter 14: HIV and AIDS, STI and TB in the private sector, 2007. Available at: http://www.hst.org.za/uploads/files/chap14_07.pdf. Accessed 30 January 2017.
- 10. Department of Health, Republic of South Africa. National tuberculosis management guidelines. 2014. Available at: http://www.nicd.ac.za/assets/files/National%20TB%20management%20guidelines%202014.pdf. Accessed 23 March 2017.
- 11. Hermans S, Caldwell J, Kaplan R, Cobelens F, Wood R. The impact of the roll-out of rapid molecular diagnostic testing for tuberculosis on empirical treatment in Cape Town, South Africa. Bull World Health Organ 2017; 95:554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ETR.Net. The Electronic Tuberculosis Register. Available at: http://www.etrnet.info/WhatIsETR.aspx. Accessed 5 November 2018.
- 13. Johnson LF, Dorrington RE, Moolla H. Progress towards the 2020 targets for HIV diagnosis and antiretroviral treatment in South Africa. South Afr J HIV Med 2017; 18:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boulle A, Heekes A, Tiffin N, et al. Data centre profile: the Provincial Health Data Centre of the Western Cape Province, South Africa. Int J Pop Data Sci 2019; 4. Available at: https://ijpds.org/article/view/1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Middelkoop K, Bekker LG, Shashkina E, Kreiswirth B, Wood R. Retreatment tuberculosis in a South African community: the role of re-infection, HIV and antiretroviral treatment. Int J Tuberc Lung Dis 2012; 16:1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marx FM, Dunbar R, Enarson DA, et al. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin Infect Dis 2014; 58:1676–83. [DOI] [PubMed] [Google Scholar]
- 17. Guerra-Assunção JA, Houben RM, Crampin AC, et al. Recurrence due to relapse or reinfection with Mycobacterium tuberculosis: a whole-genome sequencing approach in a large, population-based cohort with a high HIV infection prevalence and active follow-up. J Infect Dis 2015; 211:1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 2001; 358:1687–93. [DOI] [PubMed] [Google Scholar]
- 19. Hermans S, Boulle A, Caldwell J, Pienaar D, Wood R. Temporal trends in TB notification rates during ART scale-up in Cape Town: an ecological analysis. J Int AIDS Soc 2015; 18:20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Rie A, Warren R, Richardson M, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med 1999; 341:1174–9. [DOI] [PubMed] [Google Scholar]
- 21. Charalambous S, Grant AD, Moloi V, et al. Contribution of reinfection to recurrent tuberculosis in South African gold miners. Int J Tuberc Lung Dis 2008; 12:942–8. [PubMed] [Google Scholar]
- 22. Crampin AC, Mwaungulu JN, Mwaungulu FD, et al. Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS 2010; 24:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glynn JR, Murray J, Bester A, Nelson G, Shearer S, Sonnenberg P. High rates of recurrence in HIV-infected and HIV-uninfected patients with tuberculosis. J Infect Dis 2010; 201:704–11. [DOI] [PubMed] [Google Scholar]
- 24. Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med 2009; 68:2240–6. [DOI] [PubMed] [Google Scholar]
- 25. Möller M, Kinnear CJ, Orlova M, et al. Genetic resistance to mycobacterium tuberculosis infection and disease. Front Immunol 2018; 9:2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bang D, Andersen AB, Thomsen VO, Lillebaek T. Recurrent tuberculosis in Denmark: relapse vs. re-infection. Int J Tuberc Lung Dis 2010; 14:447–53. [PubMed] [Google Scholar]
- 27. Luzze H, Johnson DF, Dickman K, et al. ; Tuberculosis Research Unit . Relapse more common than reinfection in recurrent tuberculosis 1-2 years post treatment in urban Uganda. Int J Tuberc Lung Dis 2013; 17:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schiroli C, Carugati M, Zanini F, et al. Exogenous reinfection of tuberculosis in a low-burden area. Infection 2015; 43:647–53. [DOI] [PubMed] [Google Scholar]
- 29. Interrante JD, Haddad MB, Kim L, Gandhi NR. Exogenous reinfection as a cause of late recurrent tuberculosis in the United States. Ann Am Thorac Soc 2015; 12:1619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen X, Yang C, Wu J, et al. Recurrent tuberculosis in an urban area in China: relapse or exogenous reinfection? Tuberculosis (Edinb) 2017; 103:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pettit AC, Kaltenbach LA, Maruri F, et al. Chronic lung disease and HIV infection are risk factors for recurrent tuberculosis in a low-incidence setting. Int J Tuberc Lung Dis 2011; 15:906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malherbe ST, Shenai S, Ronacher K, et al. ; Catalysis TB–Biomarker Consortium . Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med 2016; 22:1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petruccioli E, Scriba TJ, Petrone L, et al. Correlates of tuberculosis risk: predictive biomarkers for progression to active tuberculosis. Eur Respir J 2016; 48:1751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Warren RM, Victor TC, Streicher EM, et al. Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med 2004; 169:610–4. [DOI] [PubMed] [Google Scholar]
- 35. Scriba TJ, Carpenter C, Pro SC, et al. Differential recognition of Mycobacterium tuberculosis-specific epitopes as a function of tuberculosis disease history. Am J Respir Crit Care Med 2017; 196:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fletcher HA, Snowden MA, Landry B, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun 2016; 7:11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lawn SD, Fraenzel A, Kranzer K, Caldwell J, Bekker LG, Wood R. Provider-initiated HIV testing increases access of patients with HIV-associated tuberculosis to antiretroviral treatment. S Afr Med J 2011; 101:258–62. [DOI] [PubMed] [Google Scholar]
- 38. Marx FM, Yaesoubi R, Menzies NA, et al. Tuberculosis control interventions targeted to previously treated people in a high-incidence setting: a modelling study. Lancet Glob Health 2018; 6:e426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Christensen AS, Roed C, Andersen PH, Andersen AB, Obel N. Long-term mortality in patients with pulmonary and extrapulmonary tuberculosis: a Danish nationwide cohort study. Clin Epidemiol 2014; 6:405–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang W, Zhao Q, Yuan Z, et al. Tuberculosis-associated mortality in Shanghai, China: a longitudinal study. Bull World Health Organ 2015; 93:826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fox GJ, Nguyen VN, Dinh NS, et al. Post-treatment mortality among patients with tuberculosis: a prospective cohort study of 10 964 patients in Vietnam. Clin Infect Dis 2019; 68:1359–66. [DOI] [PubMed] [Google Scholar]
- 42. Shisana O, Rehle T, Simbayi L, et al. South African National HIV Prevalence, Incidence and Behaviour Survey. 2012. Available at: https://www.hsrcpress.ac.za/books/south-african-national-hiv-prevalence-incidence-and-behaviour-survey-2012. Accessed 2 December 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.