Abstract
Background
Persons living with human immunodeficiency virus (HIV; PLWH) experience a high burden of cancer. It remains unknown which cancer types are reduced in PLWH with earlier initiation of antiretroviral therapy (ART).
Methods
We evaluated AIDS-free, ART-naive PLWH during 1996–2014 from 22 cohorts participating in the North American AIDS Cohort Collaboration on Research and Design. PLWH were followed from first observed CD4 of 350–500 cells/µL (baseline) until incident cancer, death, lost-to-follow-up, or December 2014. Outcomes included 6 cancer groups and 5 individual cancers that were confirmed by chart review or cancer registry linkage. We evaluated the effect of earlier (in the first 6 months after baseline) versus deferred ART initiation on cancer risk. Marginal structural models were used with inverse probability weighting to account for time-dependent confounding and informative right-censoring, with weights informed by subject’s age, sex, cohort, baseline year, race/ethnicity, HIV transmission risk, smoking, viral hepatitis, CD4, and AIDS diagnoses.
Results
Protective results for earlier ART were found for any cancer (adjusted hazard ratio [HR] 0.57; 95% confidence interval [CI], .37–.86), AIDS-defining cancers (HR 0.23; 95% CI, .11–.49), any virus-related cancer (HR 0.30; 95% CI, .16–.54), Kaposi sarcoma (HR 0.25; 95% CI, .10–.61), and non-Hodgkin lymphoma (HR 0.22; 95% CI, .06–.73). By 15 years, there was also an observed reduced risk with earlier ART for virus-related NADCs (0.6% vs 2.3%; adjusted risk difference −1.6; 95% CI, −2.8, −.5).
Conclusions
Earlier ART initiation has potential to reduce the burden of virus-related cancers in PLWH but not non-AIDS-defining cancers (NADCs) without known or suspected viral etiology.
Keywords: HIV, cancer, epidemiology, antiretroviral therapy, causal inference
Earlier antiretroviral therapy initiation has potential to reduce the burden of virus-related cancers in people living with Human Immunodeficiency Virus, but not cancers without known or suspected viral etiology.
Although life expectancy has dramatically increased with the introduction of combination antiretroviral therapy (ART) [1], persons living with human immunodeficiency virus (HIV; PLWH) now experience an increasing burden of age-associated morbidity, including cancers. Although the burden of AIDS-defining cancers (ADCs) has decreased, it is still elevated, and the incidence rates for a number of non-AIDS-defining cancers (NADCs), especially those with a known or suspected viral etiology, are considerably higher than in the general population [2].
Given the established link between immunosuppression, prolonged viral suppression, and many cancer types [3–10], especially those with a known or suspected viral etiology, it follows that earlier initiation of ART may reduce the risk of cancer in PLWH. Virus-related cancers have been shown to be especially increased in PLWH [10], which may be due to general deficits in immune surveillance or an impaired ability of the immune system to suppress oncogenic viruses [11]. Prior research [12] has demonstrated that the strongest associations of oncogenic viruses and cancer in PLWH were Kaposi sarcoma herpes virus with Kaposi sarcoma (KS); Epstein-Barr virus with non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL); human papillomavirus (HPV) with cervical, anal, vulvar, vaginal, penile, and oropharyngeal cancer; and hepatitis B and C with liver cancer. However, only 1 observational study [13] and 1 trial [14] have directly evaluated the association of timing of ART and cancer risk.
The goal of the current analysis was to evaluate whether the timing of ART initiation (ie, how long it takes to initiate ART once linked to care) is associated with the risk of specific cancers. The study will be conducted within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) and will use causal statistical methodologies [15] to overcome the inherent biases using observational data. The large sample size and extended follow-up of NA-ACCORD [16] enable the evaluation of more cancer types than previous studies, and the setting has greater generalizability to PLWH in routine clinical care. Results may help inform cancer prevention and screening efforts among PLWH, many of whom may not have linked to care or initiated ART until many years after infection. Evaluating timing of ART initiation and cancer risk can also provide further insights to etiology of specific cancers, because any effect of deferred ART initiation on cancer risk is likely mediated by prolonged exposure to immunodeficiency and inflammation.
METHODS
Study Design and Population
The source population for the study sample is the NA-ACCORD, a consortium of 22 single-site and multisite cohorts of HIV-seropositive adults (≥18 years) from >200 clinical sites in the United States and Canada [16]. All contributing cohorts annually submit a standard set of demographic, treatment, clinical, laboratory, and vital status data, with complete data available for analysis through 2014. The human subjects research activities of the NA-ACCORD have been reviewed and approved by the individual cohorts local institutional review boards and by the Johns Hopkins School of Medicine.
Here, our study design aimed to use observational data to emulate [17] inferences from a hypothetical randomized controlled trial that would be conducted to inform the decision when to initiate ART to prevent cancer risk after PLWH first present to care. Eligible PLWH for the target trial would be randomized to one of >200 treatment arms corresponding with the timing of ART initiation in monthly (ie, 30-day) intervals for up to 20 years after first entering care. Although PLWH could theoretically start ART in any month after first presenting to care, we were specifically interested in comparing those who started ART early (ie, initiated ART within the first 6 months) with those who deferred ART (ie, initiated ART at 7 months or later). See Supplementary Appendix Figure 1 for more details about the target trial we are emulating. To identify the study population from observational data that best represented the target population for the hypothetical trial, we identified PLWH who were AIDS-free (because an AIDS diagnosis has long been an indication for earlier ART initiation), ART-naive, no prior cancer diagnosis, and who presented to care during 1996–2014 with CD4 between 350 and 500 cells/µL, to answer the focused clinical question of whether cancer risk varied by the timing of ART initiation in this narrow CD4 range. We also chose the 350–500 cells/µL range because CD4 threshold guidelines varied during the study period but for the most part were at or below this threshold [18]. An advantage of the 2014 end date is that the follow-up was prior to the release of the START trial findings which informed ART guidelines recommending immediate initiation of ART [19]. Time zero (ie, baseline) was the date of the first clinically observed CD4 in the target range while also meeting all other criteria. We also identified a secondary study population with a broader CD4 range at presentation (ie, ≥350 cells/µL) to provide greater generalizability to PLWH in care.
Study Endpoints and Follow-up
As described previously, NA-ACCORD developed a standardized process for case finding and validation for all invasive cancers by cancer registry linkage or manual review of medical records and pathology reports [20]. The following 6 cancer groups were analyzed: (1) any cancer (excluding non-melanoma skin cancer); (2) ADCs, which included KS, NHL, and cervical cancer; (3) NADCs; (4) virus-related NADCs, which included anal, liver, oropharyngeal, penile, vaginal, vulvar cancers, and HL; (5) virus-unrelated NADCs, which included all NADCs not included in virus-related NADCs group; and (6) virus-related, which combined ADCs and virus-related NADCs. Based on a priori power calculations, we also evaluated the 5 most common individual cancers: KS, NHL, HL, and lung and prostate cancers. PLWH were followed from baseline until the earliest of a cancer diagnosis, death, administrative end of follow-up (31 December 2014 or end of an NA-ACCORD cohort’s data collection window), or loss-to-follow-up. For those with more than 1 cancer type during follow-up, we did not censor follow-up for a given cancer at the time of the first cancer.
Study Exposure
The primary exposure of interest was timing of initiation of ART, defined as earlier ART (started ART within 6 months of baseline) and deferred ART (started ART after 6 months, which includes those who never started ART during follow-up). The units of observation were consecutive monthly (ie, 30-day) intervals following each participant’s baseline for up to 20 years (>200 monthly intervals), and for each month we determined whether a person had first started ART by the end of the month. Because we did not aim to evaluate cancer risks under each of the >200 possible treatment initiation patterns, we created a single dichotomous time-updated exposure status variable summarizing, at each time point, earlier versus deferred ART. Before month 6, this binary exposure was defined as the indicator of prior ART initiation; after 6 months, the binary exposure was defined as the indicator of ART initiation in the first 6 months of follow-up. Six months was chosen a priori to define earlier ART for consistency with prior studies using similar methods [21, 22]. Analogous to emulation [17] of an intention-to-treat (ITT) trial, we assumed that once a person started ART they remained on ART until the end of follow-up. In real-world settings, however, patients may not be perfectly adherent to ART after initiation, thus ITT results reflect the anticipated cancer burden that might occur in routine clinical care. We therefore also performed a per-protocol analysis [23], for which we censored follow-up once there was evidence of ART discontinuation (ie, no ART for ≥3 months) after first initiating ART. Results for the per-protocol analysis reflect the cancer burden if all subjects remained on ART once initiated for the remainder of follow-up.
Confounders
Time-independent variables considered were baseline age, sex, cohort, entry year, race/ethnicity, HIV transmission risk, ever smoked, ever hepatitis B virus (HBV) infected, and ever hepatitis C virus (HCV) infected. Time-dependent variables considered were CD4, time since last measured CD4, time-dependent AIDS diagnoses, and time since baseline date. We did not consider HIV RNA as a confounder because becoming undetectable during follow-up was collinear with recent ART initiation. We had missing data on race/ethnicity, HIV transmission risk, and smoking for some subjects, and partially observed data for the time-varying covariate CD4 because it was not measured at each monthly interval. Details about the statistical handling of missing or partially missing covariate data using the missing indicator approach [24–28] are provided in the Supplementary Appendix.
Statistical Analysis
We first present baseline characteristics for the study population, as well as a comparison of baseline characteristics between those that started earlier versus deferred ART. Next, we present the percentage of person-years contributed to the earlier and deferred ART groups, and mean CD4 over time. We also present crude cancer incidence rates per 1000 person-years for each cancer outcome.
For each cancer outcome we then estimated adjusted hazard ratios (HRs), by fitting working [29] logistic marginal structural models (MSM) [30] for the counterfactual discrete-time hazard functions associated with the >200 ART initiation regimens. Inverse probability weighting [15] estimation was implemented to account for both baseline and time-dependent confounding [31] and informative right-censoring [32]. Our ITT analyses incorporated weights for right-censoring due to death or loss to follow-up, and our per-protocol analyses additionally incorporated weights for right-censoring due to ART discontinuation. Next, for ITT analyses only, we used more complex working models that included interaction terms between time and the exposure to derive estimates of cumulative incidence curves, risk differences (RD), and risk ratios (RR).
To investigate the potential impact of unmeasured confounding, we calculated E-values [33], which quantify the strength of an unmeasured confounder with both the exposure and outcome that would fully explain away the observed findings. We also performed sensitivity analyses excluding potential prevalent cancers (ie, cancers diagnosed within 6 months after baseline). Additional details on all statistical methods are provided in the Supplementary Appendix. Analyses were conducted using SAS, version 9.4 (Cary, NC, USA).
RESULTS
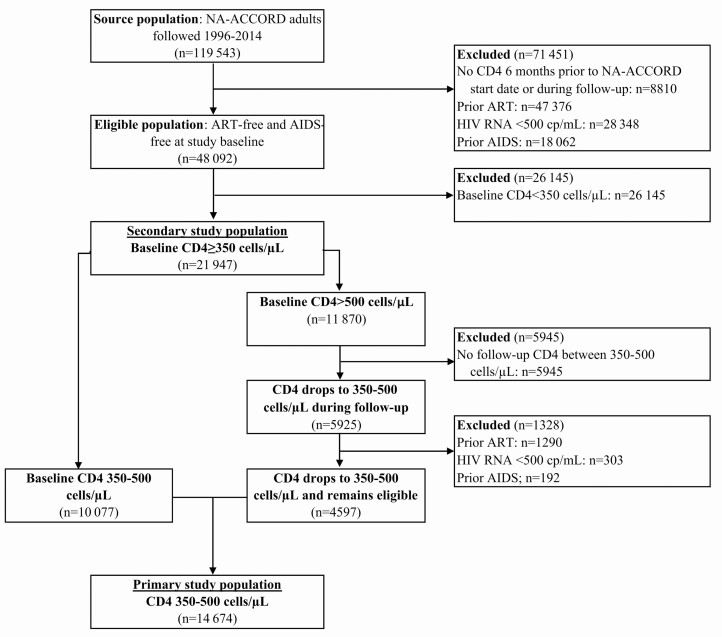
Of 119 543 PLWH in NA-ACCORD for years 1996–2014, our primary study population consisted of 14 674 PLWH who were ART-naive, AIDS-free, and with CD4 350–500 cells/µL at baseline (see Figure 1 for exclusions and Table 1 for baseline characteristics). The secondary study population included 21 947 PLWH with baseline CD4 ≥350 cells/µL. Those who started ART earlier had a later entry year, a higher proportion of Blacks, and greater percentage HCV infection compared with the deferred ART group (Table 1). Those in the earlier ART group initiated ART 0.1 years (1.5 months) after index on average, and the deferred ART group initiated ART 1.7 years (20 months) after index.
Figure 1.
Flow diagram. Depiction of criteria used to identify eligible population and primary and secondary study populations from the source population. Primary study population consists of AIDS-free, ART-free adult persons living with HIV with baseline CD4 between 350 and 500 cells/µL and secondary study population consists of those with CD4 ≥350 cells/µL at baseline. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; NA-ACCORD, North American AIDS Cohorts Collaboration on Research and Design.
Table 1.
Baseline Characteristics for All Persons Living With HIV in the Primary Study Population (N = 14 674)
| Characteristic | All PLWH (N = 14 674) | Earlier ARTa (N = 4353) | Deferred ARTa (N = 9916) |
|---|---|---|---|
| Median entry year (IQR) | 2004 (1999, 2008) | 2006 (1999, 2010) | 2003 (1999, 2007) |
| Median follow-up years (IQR) | 3.8 (2.0, 7.3) | 3.8 (2.1, 7.5) | 4.0 (2.1, 7.5) |
| Median years to first ART (IQR) | 0.6 (0.1, 1.8) | 0.1 (0.1, 0.3) | 1.7 (1.0, 3.1) |
| Median age (IQR), years | 39 (31, 47) | 39 (30, 47) | 39 (30, 47) |
| Men, N (%) | 12 553 (86) | 3811 (88) | 8376 (84) |
| HIV risk, N (%) | |||
| Men who have sex with men | 5612 (38) | 1767 (41) | 3720 (38) |
| Injection drug use | 1896 (13) | 599 (14) | 1248 (13) |
| Heterosexual | 2236 (15) | 612 (14) | 1578 (16) |
| Other | 259 (2) | 61 (1) | 188 (2) |
| Unknown | 4671 (32) | 1314 (30) | 3182 (32) |
| Imputed HIV risk, N (%) | 837 (6) | 286 (7) | 505 (5) |
| Race/ethnicity, % | |||
| White | 6699 (46) | 2137 (49) | 4391 (44) |
| Black | 6197 (42) | 1630 (37) | 4385 (44) |
| Hispanic | 973 (7) | 297 (7) | 649 (7) |
| Other | 805 (5) | 289 (7) | 491 (5) |
| Imputed HIV race, N (%) | 871 (6) | 366 (8) | 445 (4) |
| Median CD4 (IQR), cells/µL | 429 (390, 466) | 415 (380, 454) | 434 (396, 469) |
| Median log HIV RNA (IQR), copies/mL | 4.3 (3.8, 4.8) | 4.6 (4.0, 5.0) | 4.2 (3.7, 4.7) |
| Ever smoked, N (%) | 9476 (65) | 2876 (66) | 6396 (65) |
| Imputed smoking, N (%) | 4165 (28) | 1155 (27) | 2829 (29) |
| Ever hepatitis B infection, N (%) | 824 (6) | 228 (5) | 574 (6) |
| Ever hepatitis C infection, N (%) | 2915 (20) | 705 (16) | 2148 (22) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; PLWH, persons living with HIV.
aAmong PLWH with at least 6 months follow-up (N = 649 excluded with <6 months follow-up).
For the ITT analysis, 14 674 PLWH contributed 73 670 person-years; 46% of participants were administratively censored (at end of 2014), 42% were lost-to-follow up, 8% died, and 4% had any cancer. Thirty percent of person-years for the primary study population was classified as earlier ART and 70% as deferred ART, with consistent distributions over time (Supplementary Appendix Figure 2). From baseline to 15.5 years, mean CD4 increased from 418 to 700 cells/µL for the earlier ART group and from 428 to 634 cells/µL for the deferred ART group, with an average 96 cells/µL higher in the earlier ART group. Incidence rates for all cancers are shown in Table 2 and indicated the earlier ART group had reduced incidence rates compared with the deferred ART group for any cancer, ADC and any virus-related cancer, and similar rates comparing earlier and deferred ART for NADC, virus-related NADC, and virus-unrelated NADC. For individual cancers, we noted reduced cancer incidence rates with earlier ART for KS and NHL, higher rates for prostate cancer and similar rates for HL and lung cancer.
Table 2.
Crude Cancer Incidence Rates, by Earlier or Deferred ART, Among the Primary Study Population
| Earlier ART | Deferred ART | |||||
|---|---|---|---|---|---|---|
| N | PY | Incidence Rate per 1000 PY | N | PY | Incidence Rate per 1000 PY | |
| Cancer group | ||||||
| Any cancer | 158 | 21 792 | 725.0 | 471 | 51 878 | 907.9 |
| ADC | 34 | 22 374 | 152.0 | 166 | 53 163 | 312.2 |
| NADC | 127 | 21 979 | 577.8 | 320 | 52 334 | 611.5 |
| Virus-related NADC | 17 | 22 473 | 75.6 | 63 | 53 459 | 117.8 |
| Virus-unrelated NADC | 111 | 22 071 | 502.9 | 261 | 52 546 | 496.7 |
| Any virus-related | 50 | 22 282 | 224.4 | 225 | 52 962 | 424.8 |
| Individual cancer | ||||||
| KS | 16 | 22 482 | 71.2 | 73 | 53 408 | 136.7 |
| NHL | 17 | 22 470 | 75.7 | 92 | 53 459 | 172.1 |
| HL | 7 | 22 517 | 31.1 | 19 | 53 607 | 35.4 |
| Lung | 31 | 22 544 | 137.5 | 64 | 53 592 | 119.4 |
| Prostate | 37 | 22 402 | 165.2 | 44 | 53 425 | 82.4 |
Abbreviations: ADC, AIDS-defining cancer; ART, antiretroviral therapy; HL, Hodgkin lymphoma; KS, Kaposi sarcoma; NADC, non-AIDS-defining cancer; NHL, non-Hodgkin lymphoma; PY, person-years.
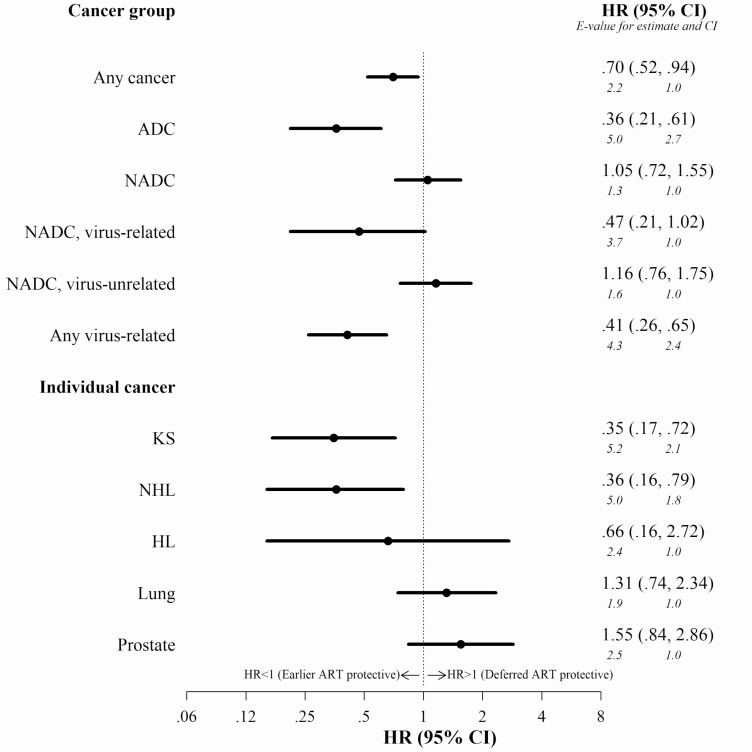
Adjusted HRs for ITT results shown in Figure 2, which ignore ART discontinuations, indicate a reduced risk with earlier ART compared with deferred ART for any cancer (hazard ratio [HR] 0.70, 95% confidence interval [CI], .52–.94), ADC (HR 0.36, 95% CI, .21–.61), any virus-related cancer (HR 0.41, 95% CI, .26–.65), KS (HR 0.35, 95% CI, .17–.72), and NHL (HR 0.36, 95% CI, .16–.79). No statistically significant differences between earlier and deferred ART were noted for any NADC, virus-unrelated NADC, HL, lung cancer or prostate cancer, and there was a trend toward a protective effect for virus-related NADCs (HR 0.47, 95% CI, .21–1.02). Adjusted HRs for per-protocol results (Supplementary Appendix Figure 3), which accounted for ART discontinuations, were similar and in general stronger compared with ITT, except for virus-related NADC, which no longer was reduced for earlier ART.
Figure 2.
Adjusted hazard ratios for intention-to-treat analysis of earlier versus deferred antiretroviral therapy initiation and risk of cancer among the primary study population (baseline CD4 350–500 cells/µL). HR and 95% CI for earlier versus deferred (reference) ART and risk for cancer are shown. Results from intention-to-treat marginal structural working models, with weights for ART initiation, and right-censoring due to death and loss to follow-up. With deferred ART as reference and as indicated on the figure, an HR < 1 indicates earlier ART is protective and an HR > 1 indicates deferred ART is protective. E-values presented in italics below HR estimates and CIs and interpreted as the minimum strength of an unmeasured confounder with both the exposure and outcome (on a risk ratio scale) that would account for the observed HR or CI above and beyond what is already accounted for in the models. Abbreviations: ADC, AIDS-defining cancer; CI, confidence interval; HL, Hodgkin lymphoma; HR, hazard ratio; KS, Kaposi sarcoma; NADC, non-AIDS-defining cancer; NHL, non-Hodgkin lymphoma.
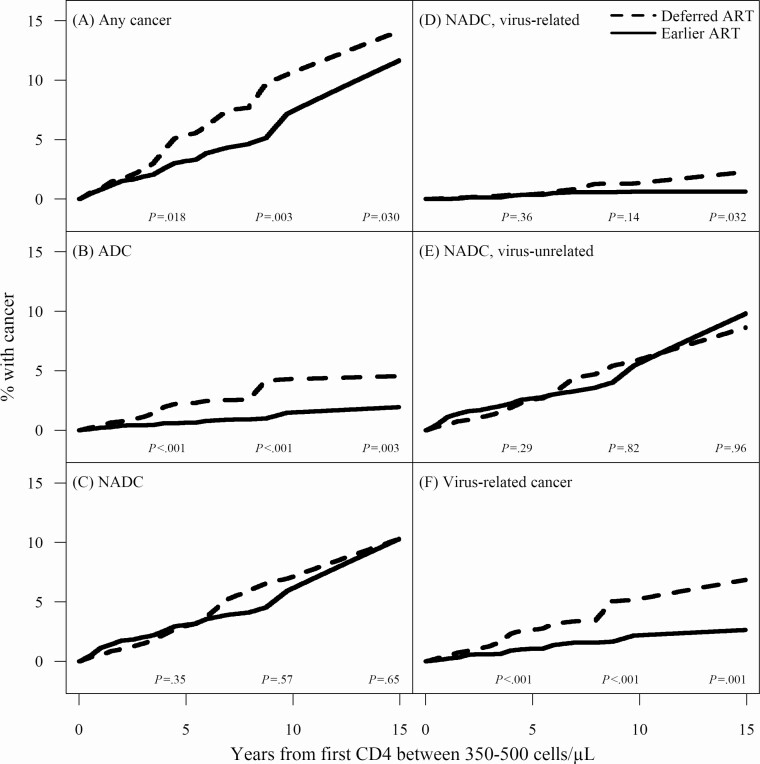
As shown in Figure 3, starting ART earlier resulted in a lower cumulative incidence of cancer at all time points (5, 10, and 15 years after baseline) for any cancer, ADC, and any virus-related cancer, and for virus-related NADCs by 15 years only (P < .001). As shown in Table 3, by 15 years the cumulative incidence of cancer was lower with earlier ART for ADC (1.9% vs 4.5%), virus-related NADC (0.6% vs 2.3%) and any virus-related cancer (2.6% vs 6.9%).
Figure 3.
Cumulative incidence curves for earlier versus deferred ART initiation and risk of cancer among the primary study population (baseline CD4 350–500 cells/µL). Cumulative incidence curves for each of 6 cancer groups, including (A) any cancer; (B) ADC; (C) NADC; (D) NADC, virus-related; (E) NADC, virus-unrelated; and (F) any virus-related cancers. The time scale is years from baseline, defined as date of the first clinically observed CD4 between 350 and 500 cells/µL. Solid lines correspond with cumulative incidence curves for those that started earlier and dashed lines correspond with cumulative incidence curves for those that deferred ART. Cumulative incidence curves were mapped from adjusted estimates of the hazards from intention-to-treat analysis. P-values on plot are for tests that the differences between the areas under the 2 curves at 5, 10, and 15 years after baseline are 0. Abbreviations: ADC, AIDS-defining cancers; ART, antiretroviral therapy; NADC, non-AIDS-defining cancers.
Table 3.
Cumulative Incidence of Cancer by 5, 10, and 15 Years After Baseline, With Adjusted Risk Differences and Adjusted Risk Ratios Comparing Earlier and Deferred ART
| Cumulative Incidence, % | Adjusted RDa (95% CI) | Adjusted RRa (95% CI) | E-valueb for: RR (95% CI) | ||
|---|---|---|---|---|---|
| Earlier ART | Deferred ART (ref) | ||||
| 5 years | |||||
| Any cancer | 3.2 | 5.4 | −2.2 (−3.8, −.6) | .59 (.37, .82) | 2.8 (1.7) |
| ADC | 0.6 | 2.2 | −1.6 (−2.3, −.9) | .28 (.12, .44) | 6.6 (4.0) |
| NADC | 3.1 | 3.0 | .1 (−1.2, 1.4) | 1.03 (.61, 1.46) | 1.2 (1.0) |
| Virus-related NADC | 0.4 | 0.4 | 0 (−.4, .3) | .90 (.02, 1.78) | 1.5 (1.0) |
| Virus-unrelated NADC | 2.7 | 2.6 | .1 (−1.1, 1.3) | 1.04 (.57, 1.51) | 1.2 (1.0) |
| Any virus-related | 1.1 | 2.6 | −1.6 (−2.5, −.7) | .40 (.19, .61) | 4.4 (2.7) |
| 10 years | |||||
| Any cancer | 7.4 | 10.7 | −3.3 (−7.0, .4) | .69 (.42, .97) | 2.3 (1.2) |
| ADC | 1.5 | 4.3 | −2.8 (−5.4, −.3) | .34 (.07, .62) | 5.3 (2.6) |
| NADC | 6.1 | 7.1 | −1.0 (−3.7, 1.8) | .86 (.50, 1.22) | 1.6 (1.0) |
| Virus-related NADC | 0.6 | 1.4 | −.7 (−1.6, .1) | .46 (.05, .88) | 3.8 (1.5) |
| Virus-unrelated NADC | 5.6 | 5.9 | −.3 (−3.0, 2.4) | .94 (.50, 1.39) | 1.3 (1.0) |
| Any virus-related | 2.2 | 5.2 | −3.0 (−5.7, −.4) | .42 (.15, .69) | 4.2 (2.3) |
| 15 years | |||||
| Any cancer | 11.7 | 14.1 | −2.5 (−7.3, 2.3) | .82 (.51, 1.13) | 1.7 (1.0) |
| ADC | 1.9 | 4.5 | −2.6 (−5.3, 0) | .42 (.10, .75) | 4.2 (2.0) |
| NADC | 10.3 | 10.3 | 0 (−4.0, 4.0) | 1.00 (.61, 1.39) | 1.6 (1.0) |
| Virus-related NADC | 0.6 | 2.3 | −1.6 (−2.8, −.5) | .27 (.03, .52) | 6.9 (3.3) |
| Virus-unrelated NADC | 9.8 | 8.6 | 1.1 (−2.8, 5.1) | 1.13 (.65, 1.62) | 1.5 (1.0) |
| Any virus-related | 2.6 | 6.9 | −4.2 (−7.3, −1.1) | .39 (.15, .62) | 4.6 (2.6) |
Bolding indicates P < .05.
Abbreviations: ADC, AIDS-defining cancer; ART, antiretroviral therapy; CI, confidence interval; NADC, non-AIDS-defining cancer; RD, risk difference; RR, risk ratio.
aResults from intention-to-treat working marginal structural models with interaction terms between time and the exposure, and weights for ART initiation, and right-censoring due to death and loss to follow-up.
bE-values interpreted as the minimum strength of an unmeasured confounder with both the exposure and outcome (on an RR scale) that would account for the observed RR or CI beyond what is already accounted for in the models.
Results for the secondary study population (Supplementary Appendix Figure 4) were similar with minor differences (eg, earlier ART was protective for NHL in ITT but not per-protocol analyses). Sensitivity analyses that excluded those with a cancer event during the first 6 months of follow-up had minimal influence on adjusted HRs with changes in inferences (Supplementary Appendix Figure 5).
DISCUSSION
In this large North American HIV cohort study, we found that PLWH who started ART earlier compared with those who deferred ART had significantly lower risks for any cancer (30% lower overall), ADC (64% lower), and any virus-related cancer (59% lower). Of individual cancers evaluated, we estimated lower overall risks with earlier ART for KS and NHL but not other cancer types. By 15 years, the cumulative incidence of cancer was lower with earlier ART for ADC, virus-related NADC, and any virus-related cancer.
Our results support the results of the START trial, which provided the most definitive evidence to date regarding effects of timing of ART initiation and risk of cancer [14]. Among 2326 PLWH randomized to initiate ART immediately and 2359 randomized to defer ART, the authors reported a 74% reduction in virus-related cancers and a nonsignificant 51% reduction in virus-unrelated cancers. Here, with more than 10 times as many events and longer follow-up we also demonstrated a reduction in virus-related cancers but not virus-unrelated cancers. A subsequent pooled analysis of the Strategies for Management of Antiretroviral Therapy (SMART) and START trials [34] did observe a higher risk of virus-unrelated cancers with deferred/intermittent ART compared with immediate/continuous ART. Unique findings in our study compared with the results from the trial data included the protection for individual cancers including KS and NHL, and the reduced cumulative incidence of virus-related NADCs for earlier ART by 15 years (0.6% vs 2.3%) but not virus-unrelated NADCs (9.8% vs 8.6%).
Few observational studies have evaluated the association of timing of ART initiation and cancer risk. Among >11 000 PLWH in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) cohort, of which some sites are also included in NA-ACCORD, Yanik et al [13] noted a lower risk of KS, lymphomas (HL or NHL combined) and HPV-related ADCs and NADCs but not for liver and virus-unrelated NADCs, with increasing CD4 at ART initiation. Consistent with our results here, among PLWH in the US Veterans Administration, Park et al [5] noted the highest risk of virus-related cancers among those with unsuppressed virus, and no association of virus suppression for NADCs.
The mechanism for the lower risk of virus-associated cancers for the earlier ART group is likely via improved maintenance of higher CD4. In our study, the overall unadjusted mean difference in CD4 was 96 cells/µL. Many prior studies have documented associations between low CD4 and increased risk of specific cancers, but the studies were not designed specifically to evaluate the timing of ART use and cancer risk [3–10]. The protective effect for earlier ART may also be a result of reduced exposure to chronic inflammation [35, 36] but few studies have directly evaluated immune activation markers and cancer risk in PLWH [37]. Our findings of stronger effects with earlier ART for ADCs and virus-related NADCs than observed for other NADCs support the concept that immunodeficiency and inflammation may have substantial effects on the long-term ability to suppress oncogenic viruses [11]. It should be noted that our definition of virus-related cancers was based on associations reported in the literature [12] and not actual infections. Future research is needed to further clarify the precise role of delayed ART and prolonged immunodeficiency on viral-induced oncogenesis in PLWH.
Our study’s main limitation is the potential for residual confounding due to unmeasured confounding (eg, body mass index, diet, HPV) or imperfect measurement of smoking. However, as described in detail in the Supplementary Appendix, except for any cancer, the large E-values computed in sensitivity analyses suggest our results are robust to unmeasured confounders. An additional limitation was that the primary findings based on ITT analyses may be attenuated with greater levels of nonadherence to ART. The per-protocol results that censored at first evidence of ART discontinuation did have stronger results although inferences were similar. We believe the ITT results provide greater generalizability to routine clinical care. Next, inferences rely on the assumption of correct specification of the logistic models that were used to estimate propensity scores, although the distribution of the inverse probability weights did not raise concerns over practical violations of the positivity assumption due to strong confounding. Our study also included a low percentage of females and Hispanics and only US and Canadian populations in care, thus limiting generalizability to other populations.
In summary, our results further support test and treat all strategies because earlier ART initiation should substantially reduce the burden of virus-related cancers in PLWH. However, given that many PLWH are linked to care long after acquiring HIV, PLWH will continue to face a higher risk of these types of cancer, such that efforts to optimize cancer screening, HPV and HBV vaccination, and treatment of coinfections such as HCV are needed. Results also indicate that the risks of virus-unrelated cancers may not be substantially reduced with earlier ART initiation, providing evidence of limited protection against these cancers with a reconstituted immune system. Thus, preventive efforts for virus-unrelated NADCs should focus on risk mitigation including screening and reduction in known cancer risk factors such as smoking, obesity, and unhealthy alcohol use.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. NA-ACCORD collaborating cohorts and representatives.
AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch; AIDS Link to the IntraVenous Experience*: Gregory D. Kirk; Emory-Grady HIV Clinical Cohort: Vincent Marconi and Jonathan Colasanti; Fenway Health HIV Cohort*: Kenneth H. Mayer and Chris Grasso; HAART Observational Medical Evaluation and Research*: Robert S. Hogg, P. Richard Harrigan, Julio S. G. Montaner, Benita Yip, Julia Zhu, Kate Salters, and Karyn Gabler; HIV Outpatient Study*: Kate Buchacz and Jun Li; HIV Research Network*: Kelly A. Gebo and Richard D. Moore; Johns Hopkins HIV Clinical Cohort*: Richard D. Moore; John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University*: Benigno Rodriguez; Kaiser Permanente Mid-Atlantic States*: Michael A. Horberg; Kaiser Permanente Northern California*: Michael J. Silverberg; Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne; MACS/WIHS Combined Cohort Study: Todd Brown, Phyllis Tien, and Gypsyamber D’Souza; Maple Leaf Medical Clinic: Frederic Crouzat, Mona Loutfy, Graham Smith, and Meenakshi Gupta; The McGill University Health Centre, Chronic Viral Illness Service Cohort: Marina B. Klein; Multicenter Hemophilia Cohort Study–II*: Charles Rabkin; Ontario HIV Treatment Network Cohort Study*: Abigail Kroch, Ann Burchell, Adrian Betts, and Joanne Lindsay; Parkland/UT Southwestern Cohort: Ank Nijhawan; Retrovirus Research Center, Bayamon Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor; Southern Alberta Clinic Cohort*: M. John Gill; Study of the Consequences of the Protease Inhibitor Era*: Jeffrey N. Martin; Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Jun Li and John T. Brooks; University of Alabama at Birmingham 1917 Clinic Cohort*: Michael S. Saag, Michael J. Mugavero, and James Willig; University of California at San Diego*: William C. Mathews; University of North Carolina at Chapel Hill HIV Clinic Cohort*: Joseph J. Eron and Sonia Napravnik; University of Washington HIV Cohort*: Mari M. Kitahata and Heidi M. Crane; Vanderbilt Comprehensive Care Clinic HIV Cohort*: Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner; Veterans Aging Cohort Study*: Janet Tate, Robert Dubrow, and David Fiellin. NA-ACCORD STUDY ADMINISTRATION. Executive Committee: Richard D. Moore, Keri N. Althoff, Stephen J. Gange, Mari M. Kitahata, Jennifer S. Lee, Michael S. Saag, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman; Administrative Core: Richard D. Moore, Keri N. Althoff, and Aimee M. Freeman; Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Liz Morton, Justin McReynolds, and William B. Lober; Epidemiology and Biostatistics Core: Stephen J. Gange, Jennifer S. Lee, Bin You, Brenna Hogan, Elizabeth Humes, Cameron Stewart, Lucas Gerace, and Sally Coburn.
* Indicates inclusion of data in current analysis.
Financial support. This work was supported by National Institutes of Health (U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K08CA230170, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01CA165937, R01DA011602, R01DA012568, R01AG053100, R24AI067039, R34DA045592, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794, U54MD007587, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR002378, Z01CP010214 and Z01CP010176); contracts CDC-200-2006-18797, and CDC-200-2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; The Grady Health System; grants CBR-86906, CBR-94036, HCP-97105, and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute (NCI), National Heart, Lung, and Blood Institute (NHLBI), Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute for Mental Health (NIMH) and National Institute on Drug Abuse (NIDA), National Institute on Aging (NIA), National Institute of Dental & Craniofacial Research (NIDCR), National Institute of Neurological Disorders and Stroke (NINDS), National Institute of Nursing Research (NINR), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and other Communication Disorders (NIDCD), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Johns Hopkins University and the University of Calgary were subgrant recipients from NIH. The data used for analyses include data collected by cancer registries participating in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interests. M. J. S. and M. S. S. report grants from Gilead Sciences, Inc., outside the submitted work. K. N. A. reports personal fees outside the submitted work from Trio Health, All of Us Study, MedIQ. M. J. G. reports personal fees from ad hoc membership of the National HIV Advisory Boards to Merck, Gilead, and ViiV. H. M. C. reports grants from AHRQ and ViiV, outside the submitted work. R. B. reports grant support from NIH/NIAID, outside the submitted work. H. C. reports grants from NIH, AHRQ, and Viiv, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: This work was previously presented at the 18th Conference on Retroviruses and Opportunistic Infections, Boston, MA, February 2011.
References
- 1. Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017; 4:e495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D; Clinical Epidemiology Group of the FHDH-ANRS CO4 cohort . Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 2009; 10:1152–9. [DOI] [PubMed] [Google Scholar]
- 4. Reekie J, Kosa C, Engsig F, et al. ; EuroSIDA Study Group . Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer 2010; 116:5306–15. [DOI] [PubMed] [Google Scholar]
- 5. Park LS, Tate JP, Sigel K, et al. Association of viral suppression with lower AIDS-defining and non-AIDS-defining cancer incidence in HIV-infected veterans: a prospective cohort study. Ann Intern Med 2018; 169:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dubrow R, Qin L, Lin H, et al. ; North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS . Association of CD4+ T-cell count, HIV-1 RNA viral load, and antiretroviral therapy with Kaposi sarcoma risk among HIV-infected persons in the United States and Canada. J Acquir Immune Defic Syndr 2017; 75:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernández-Ramírez RU, Qin L, Lin H, et al. ; North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS . Association of immunosuppression and HIV viraemia with non-Hodgkin lymphoma risk overall and by subtype in people living with HIV in Canada and the USA: a multicentre cohort study. Lancet HIV 2019; 6:e240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hernandez-Ramirez RU, Qin L, Lin H, et al. Association of immunosuppression and HIV viremia with anal cancer risk in persons living with HIV in the United States and Canada. Clin Infect Dis 2019. doi: 10.1093/cid/ciz329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yanik EL, Hernández-Ramírez RU, Qin L, et al. Brief report: cutaneous melanoma risk among people with HIV in the United States and Canada. J Acquir Immune Defic Syndr 2018; 78:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011; 20:2551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. International Agency for Research on Cancer (IARC) Working Group. IARC monographs on the evaluation of carcinogenic risks to humans: Biological Agents. Lyon, France: IARC; 2012. [PMC free article] [PubMed] [Google Scholar]
- 12. de Martel C, Shiels MS, Franceschi S, et al. Cancers attributable to infections among adults with HIV in the United States. AIDS 2015; 29:2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yanik EL, Napravnik S, Cole SR, et al. Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clin Infect Dis 2013; 57:756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borges ÁH, Neuhaus J, Babiker AG, et al. ; INSIGHT START Study Group . Immediate antiretroviral therapy reduces risk of infection-related cancer during early HIV infection. Clin Infect Dis 2016; 63:1668–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robins JM. Association, causation and marginal structural models. Synthese 1999; 121:151–79. [Google Scholar]
- 16. Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016; 183:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eholié SP, Badje A, Kouame GM, et al. Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question. AIDS Res Ther 2016; 13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundgren JD, Babiker AG, Gordin F, et al; INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. NEJM 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abraham AG, Althoff KN, Jing Y, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) . End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis 2015; 60:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cole SR, Hernán MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol 2003; 158:687–94. [DOI] [PubMed] [Google Scholar]
- 22. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000; 11:561–70. [DOI] [PubMed] [Google Scholar]
- 23. Hernán MA, Hernández-Díaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials 2012; 9:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kreif N, Sofrygin O, Schmittdiel J, et al. Evaluation of adaptive treatment strategies in an observational study where time-varying covariates are not monitored systematically. Available at: https://arxiv.org/abs/1806.11153. Accessed 21 February 2020.
- 25. Hernán MA, McAdams M, McGrath N, Lanoy E, Costagliola D. Observation plans in longitudinal studies with time-varying treatments. Stat Methods Med Res 2009; 18:27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci 2010; 25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattei A. Estimating and using propensity score in presence of missing background data: an application to assess the impact of childbearing on wellbeing. Stat Methods Appl 2009; 18:257–73. [Google Scholar]
- 28. D’Agostino RB Jr, Rubin DB. Estimating and using propensity scores with partially missing data. JASA 2000; 95: 749–59. [Google Scholar]
- 29. Neugebauer RS, van der Laan M. Nonparametric causal effects based on marginal structural models. J Stat Plan Inference 2007; 137:419–34. [Google Scholar]
- 30. Robins JM Marginal structural models. Anaheim, CA: American Statistical Association, Section on Bayesian Statistical Science, 1997. [Google Scholar]
- 31. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–60. [DOI] [PubMed] [Google Scholar]
- 32. Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology 2004; 15:615–25. [DOI] [PubMed] [Google Scholar]
- 33. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167:268–74. [DOI] [PubMed] [Google Scholar]
- 34. Borges ÁH, Neuhaus J, Sharma S, et al. ; INSIGHT SMART; START Study Groups . The effect of interrupted/deferred antiretroviral therapy on disease risk: a SMART and START combined analysis. J Infect Dis 2019; 219:254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA 2004; 291:585–90. [DOI] [PubMed] [Google Scholar]
- 36. Heikkilä K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control 2009; 20:15–26. [DOI] [PubMed] [Google Scholar]
- 37. Borges ÁH, Silverberg MJ, Wentworth D, et al. ; INSIGHT SMART; ESPRIT; SILCAAT Study Groups . Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013; 27:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.