Abstract
The aim of this review was to examine the existing evidence about interventions proposed for the treatment of clinical chorioamnionitis, with the goal of developing an evidence-based contemporary approach for the management of this condition. Most trials that assessed the use of antibiotics in clinical chorioamnionitis included patients with a gestational age ≥34 weeks and in labor. The first-line antimicrobial regimen for the treatment of clinical chorioamnionitis is ampicillin combined with gentamicin, which should be initiated during the intrapartum period. In the event of a cesarean delivery, patients should receive clindamycin at the time of umbilical cord clamping. The administration of additional antibiotic therapy does not appear to be necessary after vaginal or cesarean delivery. However, if post-delivery antibiotics are prescribed, there is support for the administration of an additional dose. Patients should receive antipyretics, mainly acetaminophen, even though there is no clear evidence of their benefits. Current evidence suggests that the administration of antenatal corticosteroids for fetal lung maturation and of magnesium sulfate for fetal neuroprotection to patients with clinical chorioamnionitis between 24 0/7 and 33 6/7 weeks of gestation, and possibly between 23 0/7 and 23 6/7 weeks, has an overall beneficial effect on the infant. However, delivery should not be delayed in order to complete the full course of corticosteroids and magnesium sulfate. Once the diagnosis of clinical chorioamnionitis has been established, delivery should be considered, regardless of the gestational age. Vaginal delivery is the safer option and cesarean delivery should be reserved for standard obstetric indications. The time interval between the diagnosis of clinical chorioamnionitis and delivery is not related to most adverse maternal and neonatal outcomes. Patients may require a higher dose of oxytocin to achieve adequate uterine activity and/or greater uterine activity to effect a given change in cervical dilation. The benefit of using continuous electronic fetal heart rate monitoring in these patients is unclear. We identified the following promising interventions for the management of clinical chorioamnionitis: (1) an antibiotic regimen including ceftriaxone, clarithromycin, and metronidazole that provides coverage against the most commonly identified microorganisms in patients with clinical chorioamnionitis; (2) vaginal cleansing with antiseptic solutions before cesarean delivery with the aim of decreasing the risk of endometritis and, possibly, postoperative wound infection; and (3) antenatal administration of N-acetylcysteine, an antioxidant and anti-inflammatory agent, to reduce neonatal morbidity and mortality. Well-powered randomized controlled trials are needed to assess these interventions in patients with clinical chorioamnionitis.
Keywords: abnormal fetal heart rate patterns, abnormal labor progression, adverse maternal outcomes, adverse neonatal outcomes, antenatal corticosteroids, antibiotics, antipyretics, cerebral palsy, cesarean delivery, continuous electronic fetal heart rate monitoring, fever, intra-amniotic infection, intra-amniotic inflammation, magnesium sulfate, management of labor, maternal infection, N-acetylcysteine, neonatal sepsis, neuroprotection, postpartum hemorrhage, prolonged labor, vaginal cleansing
INTRODUCTION
Clinical chorioamnionitis is the most common infection-related complication in labor and delivery units worldwide, affecting 1–6% of pregnancies in the United States.1–7 This syndrome is a well-known risk factor for adverse maternal outcomes such as postpartum hemorrhage secondary to uterine atony,8,9 uterine rupture,5 unplanned hysterectomy,7 blood transfusion,5–8 postoperative wound infection,6,10 endometritis,6,11 pelvic abscess,8 septic pelvic thrombophlebitis,8,12 sepsis,13–15 and intensive care unit admission,5–7 among others.
Neonates born to mothers diagnosed with clinical chorioamnionitis are at higher risk for low Apgar scores at 5 minutes,7,8,16 neonatal seizures,7,8,16,17 neonatal sepsis,8,16–22 bronchopulmonary dysplasia,23,24 intraventricular hemorrhage (IVH),18,25,26 periventricular leukomalacia,24,26–28 use of mechanical ventilation,7,17 admission to the neonatal intensive care unit (NICU),17 neonatal death,2,7,20 and long-term infectious morbidity29 compared to neonates born to women without this syndrome. Evidence regarding the association between clinical chorioamnionitis and the risk of cerebral palsy and long-term adverse neurodevelopmental outcomes is conflicting: some studies reported a positive association,20,30–35 whereas others did not.36–40
Clinical chorioamnionitis has been traditionally diagnosed by the presence of maternal fever (temperature ≥37.8°C or ≥38.0°C) plus two or more of the five following clinical signs: maternal tachycardia (heart rate >100 beats/min), fetal tachycardia (heart rate >160 beats/min), uterine tenderness, purulent or foul-smelling amniotic fluid or vaginal discharge, and maternal leukocytosis (white blood cell count >15,000/mm3).41–43 The diagnostic accuracy of these criteria to identify patients with proven intra-amniotic infection is about 50%.44
Fifteen percent of cases of clinical chorioamnionitis are diagnosed in the antepartum period and 85% in the intrapartum period.6 The most frequent microorganisms identified in the amniotic fluid of women with clinical chorioamnionitis include Ureaplasma urealyticum, Gardnerella vaginalis, Mycoplasma hominis, Streptococcus agalactiae, Lactobacillus species, and Bacteroides species.45–59 Polymicrobial invasion of the amniotic cavity is present in approximately 50% of cases.45,46,51,52
Ascending microbial invasion from the lower genital tract appears to be the most frequent pathway for intra-amniotic infection.49,52,60–62 However, hematogenous dissemination of microorganisms from the oral cavity or intestine, retrograde seeding from the peritoneal cavity through the fallopian tubes, and accidental introduction at the time of an invasive medical procedure have also been proposed as potential pathways for intra-amniotic infection.49,52,62–69
Clinical chorioamnionitis is typically thought to occur as a result of microbial invasion of the amniotic cavity, which can elicit systemic and local inflammatory responses.49,62,70–74 However, recent studies have shown that microbial invasion of the amniotic cavity is present in only 61% of women with clinical chorioamnionitis at term45 and in 34% of those with preterm clinical chorioamnionitis.46 Intra-amniotic inflammation (amniotic fluid interleukin-6 concentration ≥ 2.6 ng/mL) is detected in ~77% of patients with preterm or term clinical chorioamnionitis.45,70 Overall, 24% of patients with preterm clinical chorioamnionitis46 and 15% of patients with clinical chorioamnionitis at term45,70 have no evidence of either intra-amniotic infection or intra-amniotic inflammation. Recent studies in women with clinical chorioamnionitis at term have characterized the nature of the maternal and fetal inflammatory response through identification of profiles of cytokines and leukocytes in amniotic fluid,70,72,73,75,76 maternal plasma,77 and umbilical cord plasma.78
The standard treatment for clinical chorioamnionitis has been administration of antibiotics and antipyretics and expedited delivery.79–82 However, the management of patients with this condition presents several clinical challenges. A survey conducted among US obstetricians revealed a wide variation in practice patterns for the management of clinical chorioamnionitis, including the use of more than 25 different primary antibiotic regimens and postpartum antibiotic duration ranging from no treatment to 48 hours of postpartum treatment.83 Therefore, a rigorous, up-to-date evaluation of the interventions proposed for the management of clinical chorioamnionitis is necessary. The objectives of this review were (1) to examine and summarize the existing evidence regarding interventions proposed for treating clinical chorioamnionitis; (2) to develop an evidence-based approach for the contemporary management of this condition; and (3) to identify promising interventions in this field.
In 2015, an expert panel proposed to replace the term clinical chorioamnionitis with the term “intrauterine inflammation or infection or both”, abbreviated as “Triple I”.84 However, this proposal has not gained popularity because it implies that the inflammatory status of the amniotic cavity and the presence of microorganisms have been established, and this is rarely the case. Therefore, we continue to use the term “clinical chorioamnionitis” to refer to this syndrome.
A literature search for articles related to the treatment of clinical chorioamnionitis was conducted in MEDLINE, EMBASE, POPLINE, LILACS, CINAHL, the Cochrane Central Register of Controlled Trials, clinical trial registries (all from their inception to June 30, 2020), and Google Scholar using the terms chorioamnionitis, intra-amniotic infection, intra-amniotic inflammation, amniotic fluid infection, amnionitis, and intrauterine infection. There were no language restrictions. We prioritized data from randomized controlled trials and systematic reviews and meta-analyses of randomized controlled trials. Case series, observational studies, systematic reviews and meta-analyses of observational studies, review articles, and guidelines of major professional societies were also reviewed. Selected articles were mutually agreed upon by the authors. We updated the meta-analyses if we located eligible studies that had been published after the latest literature search date.
Antibiotics
There is a broad consensus that women with a diagnosis of clinical chorioamnionitis should receive antibiotic therapy to prevent adverse maternal and perinatal outcomes.84–98 Based on information about amniotic fluid microbiology of patients with clinical chorioamnionitis, several antibiotic regimens have been proposed. Nevertheless, most microbiological studies were performed before the introduction of molecular techniques and did not include specific cultures for genital Mycoplasmas. Therefore, the antibiotic regimens that have been assessed in randomized controlled trials pre-date the modern understanding of the microbiology of the amniotic cavity in clinical chorioamnionitis and intra-amniotic infection.45–53,58,59,62
We identified 14 randomized controlled trials99–112 (Table 1) and one systematic review and meta-analysis113 that assessed the use of antibiotics in women with clinical chorioamnionitis. Most trials included patients with a gestational age ≥34 weeks and in labor. No study reported results separately for patients with clinical chorioamnionitis before 34 weeks of gestation and/or those not in labor. Therefore, the findings of these trials might not apply to patients with these characteristics.
TABLE 1.
Randomized controlled trials that assessed the use of antibiotics in women with clinical chorioamnionitis
| First author, year (country) | Interventions (sample size) | Main outcome (definition) | Main findings |
|---|---|---|---|
| Intrapartum vs. postpartum treatment | |||
| Gibbs, 198899 (United States) | (1) Intrapartum treatment: ampicillin 2 g IV every 6 hours and gentamicin 1.5 mg/kg IV every 8 hours, beginning at the time of diagnosis of chorioamnionitis, until afebrile for approximately 48 hours (N=26) (2) Postpartum treatment: ampicillin 2 g IV every 6 hours and gentamicin 1.5 mg/kg IV every 8 hours immediately after cord clamping, until afebrile for approximately 48 hours (N=19) Patients delivered by cesarean section also received clindamycin 900 mg IV every 8 hours, beginning after cord clamping |
Neonatal sepsis (bacteremia or death with a clinical diagnosis of sepsis and positive peripheral cultures) and pneumonia | Intrapartum treatment, as compared with immediate postpartum treatment, was associated with a significant decrease in the risk of neonatal pneumonia or sepsis (0.0% vs. 31.6%; RR 0.06, 95% CI 0.000.95; P = 0.046), mean neonatal hospital stay (3.8 days vs. 5.7 days; MD −1.9 days, 95% CI −0.4 to −3.4 days; P = 0.02), and mean maternal postpartum hospital stay (4.0 days vs. 5.0 days; MD −1.0 days, 95% CI 0.1 to −1.9 days; P = 0.04), and a non-significant reduction in the risk of neonatal sepsis (0.0% vs. 21.1%; RR 0.08, 95% CI 0.00–1.44; P = 0.09) |
| Intrapartum treatment | |||
| Scalambrino, 1989100 (Italy) | (1) Ampicillin/sulbactam 3 g IV every 6 hours for at least 72 h (N=11) (2) Cefotetan 2 g IV every 12 hours for at least 72 h (N=8) |
Ineffective treatment (signs and symptoms and/or temperature curve remained unchanged or rose during the first 72 hours of treatment) | Treatment was effective in 100% of women in both antibiotic regimens |
| Maberry, 1991101 (United States) | (1) Ampicillin plus gentamicin (dual antibiotic regimen) (N=69) (2) Ampicillin plus gentamicin plus clindamycin (triple antibiotic regimen) (N=64) Dosage not reported. Antibiotics were administered for 24–48 hours after delivery and stopped if the patient remained afebrile |
Endometritis | There were no significant differences between the dual antibiotic and triple antibiotic regimens in the risk of endometritis (14.5% vs. 7.8%; RR 1.86, 95% CI 0.67–5.14; P = 0.23) and neonatal sepsis (1.5% vs. 3.1%; RR 0.46, 95% CI 0.04–4.99; P = 0.53). The frequencies of other adverse neonatal outcomes did not significantly differ between the study groups |
| Locksmith, 2005102 (United States) | (1) Ampicillin 2 g IV every 6 hours plus gentamicin 5.1 mg/kg IV every 24 hours (once-daily dosing) (N=18) (2) Ampicillin 2 g IV every 6 hours plus gentamicin 120 mg IV followed by 80 mg IV every 8 hours (thrice-daily dosing) (N=20) | Maternal and umbilical cord serum peak gentamicin concentrations | Median maternal and umbilical cord serum peak gentamicin concentrations were higher with once-daily dosing compared with thrice-daily dosing. There were no significant differences between the once-daily and thrice-daily dosing groups in the risk of puerperal metritis (5.6% vs. 5.0%; RR 1.11, 95% CI 0.07–16.49; P = 0.94) and suspected neonatal sepsis (16.7% vs. 25.0%, RR 0.67, 95% CI 0.19–2.40; P = 0.54). The frequencies of other adverse maternal and neonatal outcomes did not significantly differ between the study groups |
| Lyell, 2010103 (United States) | (1) Ampicillin 2 grams IV every 6 hours for 4 doses total plus oncedaily gentamicin (5 mg/kg IV, then 2 placebo doses IV after 8 and 16 hours) (N=62) (2) Ampicillin 2 grams IV every 6 hours for 4 doses total plus thrice-daily gentamicin (2 mg/kg IV, then 1.5 mg/kg IV after 8 and 16 hours) (N=63) Patients delivered by cesarean section also received clindamycin 900 mg IV every 8 hours, for 3 doses total |
Treatment success (resolution of chorioamnionitis after 16 hours of treatment without development of endometritis) | The frequency of treatment success did not significantly differ between the once-daily and thrice-daily gentamicin groups (93.6% vs. 88.9%; RR 1.05, 95% CI 0.94–1.17: P = 0.36). Once-daily gentamicin was noninferior to thrice-daily gentamicin because the range of risk difference (5.2% to 14.5%) fell within the predefined margin (15%). There were no significant differences between the study groups in the risk of endometritis (6.5% vs. 7.9%; RR 0.81, 95% CI 0.23–2.89; P = 0.75), neonatal sepsis (6.5% vs. 3.2%; RR 2.03, 95% CI 0.39–10.70; P = 0.40), and other adverse maternal and neonatal outcomes |
| Greenberg, 2015104 (United States) | (1) Ampicillin/sulbactam 3 g IV every 6 hours plus IV normal saline placebo dose every 8 hours until 24 hours postdelivery (N=43) (1) Ampicillin 2 g IV every 6 hours plus gentamicin 1.5 mg/kg IV every 8 hours until 24 hours postdelivery (N=49) Patients delivered by cesarean section also received clindamycin IV (dosage not reported) at the time of umbilical cord clamping, which was continued as part of the antibiotic regimen |
Postpartum composite morbidity (any of the following: endometritis, sepsis, pneumonia, blood transfusion or ileus); postpartum infectious morbidity (any of the following: endometritis, sepsis, or pneumonia); and neonatal sepsis | There were no significant differences between the ampicillin/sulbactam and ampicillin plus gentamicin groups in the frequency of postpartum composite morbidity (0.0% vs. 12.2%; RR 0.09, 95% CI 0.01–1.51; P = 0.09), postpartum infectious morbidity (0.0% vs. 8.2%; RR 0.13, 95% CI 0.01–2.28; P = 0.16), and neonatal sepsis (2.3% vs. 4.1%; RR 0.57, 95% CI 0.05–6.07; P = 0.64) |
| Postpartum treatment | |||
| Berry, 1994105 (United States) | (1) Ampicillin 2 g IV plus gentamicin 2.0 mg/kg IV at the time of diagnosis of clinical chorioamnionitis. After vaginal delivery, ampicillin 2 g IV every 6 hours for 8 doses plus gentamicin 2.0 mg/kg IV every 8 hours for 6 doses (N=21) (2) Ampicillin 2 g IV plus gentamicin 2.0 mg/kg IV at the time of diagnosis of clinical chorioamnionitis. After vaginal delivery, normal saline on an identical dosing schedule (placebo) (N=17) |
Treatment failure (temperature >38 °C after the first postpartum antibiotic or placebo dose) | There was no significant difference between the antibiotic and placebo groups in the frequency of treatment failure (4.8% vs. 5.9%, RR 0.81, 95% CI 0.05–12.01; P = 0.88). There were no cases of endometritis, wound infection, or sepsis |
| Chapman, 1997106 (United States) | (1) Ampicillin plus gentamicin during the intrapartum period (dosage not reported). After vaginal delivery, cefotetan 2 g IV single dose (N=55) (2) Ampicillin plus gentamicin during the intrapartum period (dosage not reported). After vaginal delivery, cefotetan 2 g IV every 12 hours for at least 48 h (N=54) |
Interval from delivery to discharge and failed therapy (any of the following: (1) two temperatures ≥38 °C [single dose group] or ≥38.9 °C [multiple dose group] ≥4 hours apart; or (2) a single temperature ≥38.9 °C >4 hours after delivery [single dose group] or ≥38 °C >24 hours after delivery [multiple dose group]; or (3) postpartum readmission for endometritis) | There was no significant difference between the study groups in the frequency of failed therapy (10.9% vs. 3.7%; RR 2.95, 95% CI 0.62–13.96; P = 0.17). The median interval from delivery to discharge was 24 hours shorter in the single dose group (33 hours, range I6–190) than in the multiple dose group (57 hours, range 36–190) (P = 0.0001). |
| Mitra, 1997107 (United States) | (1) Gentamicin 4 mg/kg IV every 24 hours plus clindamycin 1200 mg IV every 12 hours after delivery (N=65) (2) Gentamicin 1.33 mg/kg IV plus clindamycin 800 mg IV every 8 hours after delivery (N=66) There was no report on antibiotics used in the intrapartum period |
Cure (an average temperature of ≤37.2 °C for 24 hours and the resolution of symptoms); failure (a persistently elevated temperature 72 hours after the initiation of antibiotic therapy, clinical deterioration, or the need for additional antibiotic or heparin therapy); and duration and cost of treatment | There were no significant differences between the two treatment groups in the frequency of cure (93.9% vs. 93.9%; RR 1.00, 95% CI 0.92–1.09; P = 0.98) and failure (6.1% vs. 6.1%, RR 1.02, 95% CI 0.27–3.89; P = 0.98). The group receiving once-daily gentamicin dosing with twice-daily clindamycin dosing had a shorter mean treatment duration (2.0 days) and a lower mean treatment cost (US $251) than the group receiving thrice-daily dosing of gentamicin and clindamycin (2.3 days and US $442, respectively; P = 0.04 and 0.0001, respectively) |
| Adashek, 1998108 (United States) | (1) Gentamicin plus clindamycin after vaginal delivery. Dosage and duration of treatment were not reported (N=127) (2) Placebo after vaginal delivery (N=123) There was no report on antibiotics used in the intrapartum period |
Treatment failure (persistent fever after the third dose of the study drug or readmission for endomyometritis) | There was no significant difference between the antibiotic and placebo groups in the frequency of treatment failure (1.6% vs. 3.3%; RR 0.48, 95% CI 0.09–2.60; P = 0.40) |
| Turnquest, 1998109 (United States) | (1) Ampicillin 2 g IV every 6 hours. Preoperative gentamicin 2 mg/kg IV plus clindamycin 900 mg IV. After cesarean delivery, gentamicin 1.5 mg/kg IV plus clindamycin 900 mg IV every 8 hours until afebrile for a minimum of 24 hours (N=55) (2) Ampicillin 2 g IV every 6 hours. Preoperative gentamicin 2 mg/kg IV plus clindamycin 900 mg IV. No antibiotics after cesarean delivery (N=61) |
Endometritis | There was no significant difference between the postoperative antibiotic and no postoperative antibiotic groups in the risk of endometritis (21.8% vs. 14.8%; RR 1.48, 95% CI 0.68–3.24; P=0.33). The frequencies of other adverse maternal and neonatal outcomes did not significantly differ between the study groups. |
| Edwards, 2003110 (United States) | (1) Ampicillin 2 g IV every 6 hours and gentamicin 1.5 mg/kg IV every 8 hours at the time of diagnosis of chorioamnionitis. After delivery, ampicillin 2 g IV plus gentamicin 1.5 mg/kg IV, single additional dose. Patients delivered by cesarean section received clindamycin 900 mg IV single dose at the time of umbilical cord clamping (N=151) (2) Ampicillin 2 g IV every 6 hours and gentamicin 1.5 mg/kg IV every 8 hours at the time of diagnosis of chorioamnionitis. After delivery, ampicillin 2 g IV every 6 hours plus gentamicin 1.5 mg/kg IV every 8 hours until afebrile and asymptomatic for 24 hours. Patients delivered by cesarean section received clindamycin 900 mg IV at the time of umbilical cord clamping, then every 8 hours until antibiotics were discontinued (N=141) |
Treatment failure (a single temperature after the first postpartum dose of antibiotics ≥39.0 °C, or two temperatures ≥38.4 °C at least 4 hours apart) | There was no significant difference between the single antibiotic dose and the continued use of antibiotics groups continued antibiotic regimen and the single additional dose groups in the frequency of treatment failure (4.6% vs 3.5%; RR 1.31, 95% CI 0.42–4.02; P = 0.64). The frequencies of wound infection (0.7% vs. 1.3%) and pelvic abscess (0.0% vs. 0.7%) did not significantly differ between the study groups |
| Shanks, 2016111 (United States) | (1) Ampicillin 2 g IV every 6 hours and gentamicin 1.5 mg/kg IV every 8 hours until cesarean delivery, plus preoperative clindamycin 900 mg IV. After cesarean delivery, one additional dose of gentamicin 1.5 mg/kg IV and clindamycin 900 mg IV (N=41) (2) Ampicillin 2 g IV every 6 hours and gentamicin 1.5 mg/kg IV every 8 hours until cesarean delivery, plus preoperative clindamycin 900 mg IV. No antibiotics after cesarean delivery (N=39) |
Endometritis | There was no significant difference in the frequency of endometritis between women who received one additional dose of antibiotics after cesarean delivery and those who did not receive postoperative antibiotics (9.8% vs. 7.7%; RR 1.27, 95% CI 0.30–5.31; P = .74). The frequency of wound infection (17.1% vs. 5.1%, P = .12) and median length of hospital stay (4 days vs. 4 days, P = .88) did not significantly differ between the study groups. Neonatal outcomes were similar between the two study groups |
| Goldberg, 2017112 (United States) | (1) Single antibiotic dose after vaginal delivery (N=23) (2) Antibiotics until afebrile for 24 hours after vaginal delivery (N=23) There was no report on antibiotics used in both intrapartum and postpartum periods |
Endometritis | “There were no significant differences for length of stay and no participants experienced treatment failures requiring resumption of antibiotics for endometritis or fevers” |
CI, confidence interval; IV, intravenously; MD, mean difference; RR, relative risk
Timing of antibiotic therapy initiation
Evidence suggests that antibiotic administration should be initiated in the intrapartum period when the diagnosis of clinical chorioamnionitis is made. This recommendation is based on the findings of a randomized controlled trial that compared intrapartum (N=26) versus postpartum (immediately after umbilical cord clamping; N=19) treatment with antibiotics in patients with a gestational age >34 weeks and a diagnosis of “intra-amniotic infection”, which was based on clinical criteria.99 The antimicrobial agents used were ampicillin and gentamicin. In both study groups, patients who underwent cesarean delivery also received clindamycin after cord clamping to extend coverage for anaerobic organisms.
Intrapartum antibiotic treatment was associated with a significant reduction in the frequency of neonatal pneumonia or sepsis (0.0% vs. 31.6%, P = 0.046) and a decrease in neonatal hospital stay (3.8 days vs. 5.7 days; P = 0.02), maternal postpartum hospital stay (4.0 days vs. 5.0 days, P = 0.04), and maternal febrile days (0.4 days vs. 1.5 days, P = 0.03). Similar results were reported in two nonrandomized studies that compared antibiotic administration during labor versus immediately postpartum.114,115
Selection of antibiotics and regimens
Thirteen trials compared two antibiotic regimens during the intrapartum (5 trials)100–104 and intrapartum/postpartum (8 trials)105–112 periods. Overall, there were no significant differences in maternal and neonatal infectious morbidity between the antibiotic regimens assessed in the individual trials (Table 1). Nine of these trials101–106,109–111 used ampicillin 2 g intravenously (IV) every 6 hours combined with gentamicin 1.5–2.0 mg/kg IV every 8 hours or 4.0–5.0 mg/kg IV every 24 hours. In five trials, patients who underwent cesarean delivery also received clindamycin at the time of umbilical cord clamping (usually 900 mg IV single dose).103,104,109–111 The second most frequently used antibiotic regimen during the intrapartum period was ampicillin/sulbactam 3 g IV every 6 hours.100,104 A trial that compared ampicillin combined with gentamicin (N=49) versus ampicillin/sulbactam (N=43) for 24 hours after delivery reported no significant differences in the frequency of maternal postpartum infection (0.0% vs. 8.2%, P = 0.16) and neonatal sepsis (2.3% vs. 4.1%, P = 0.64) between the two antibiotic regimens.104
Two trials compared the combination of ampicillin and gentamicin once-daily versus ampicillin and gentamicin thrice-daily.102,103 A meta-analysis of the two trials (N=163) showed no significant differences between the two antibiotic regimens in the risk of endometritis (relative risk [RR] 0.86, 95% confidence interval [CI] 0.27–2.70) and neonatal sepsis (RR 1.07, 95% CI 0.40–2.86),113 which suggests that the once-daily dosing regimen of gentamicin is as effective as the thrice-daily dosing. A recent nonrandomized study reported that gentamicin once-daily significantly reduced the risk of postpartum endometritis in patients with clinical chorioamnionitis, as compared to gentamicin thrice-daily.116
In summary, although there is insufficient data to demonstrate the most appropriate antimicrobial regimen for the treatment of this obstetric condition, current available evidence indicates that women with clinical chorioamnionitis, mainly those with a gestational age ≥34 weeks and in labor, can be treated with ampicillin 2 g IV every 6 hours combined with gentamicin 5 mg/kg every 24 hours or 1.5 mg/kg every 8 hours, or ampicillin/sulbactam 3 g IV every 6 hours. In the event of cesarean delivery, patients should receive clindamycin 900 mg IV at the time of umbilical cord clamping. Based on expert opinion, metronidazole 500 mg IV has been proposed as an alternative to clindamycin in the event of cesarean delivery.84,85,87,93,95,96 In penicillin-allergic patients, clindamycin 900 mg IV every 8 hours or vancomycin 1 g IV every 12 hours or erythromycin 500 mg-1 g IV every 6 hours can be used instead of ampicillin.85,87,93,94,96 Studies assessing the use of antibiotics among women with clinical chorioamnionitis before 34 weeks of gestation and those not in labor are needed to determine the most appropriate regimen in this subset of patients.
Ureaplasma species are the most common microorganisms isolated from the amniotic fluid of patients with clinical chorioamnionitis.45–53,58,59,62,117–119 The antibiotics that were used in the randomized controlled trials shown in Table 1 do not provide coverage against Ureaplasma species and mycoplasma species.120 Recently, the successful use of an antibiotic regimen, i.e., ceftriaxone 1 g IV every 24 hours, clarithromycin 500 mg orally every 12 hours, and metronidazole 500 mg IV every 8 hours, has been reported among women with preterm prelabor rupture of membranes (PROM),121,122 and a subset of patients with confirmed intra-amniotic infection/inflammation and preterm labor with intact membranes123 or cervical insufficiency.124,125 The rationale for using this antibiotic regimen was as follows: clarithromycin for its much higher rate of transplacental passage than erythromycin or azithromycin and its effectiveness against Ureaplasma species and mycoplasma species; ceftriaxone for its enhanced coverage of aerobic bacteria and high rate of transplacental passage; and metronidazole for its optimal coverage of anaerobic microorganisms. We believe that this new antibiotic regimen, using clarithromycin 500 mg IV (instead of orally) every 12 hours, should be the subject of study in patients with clinical chorioamnionitis given the high concordance between microorganisms associated with clinical chorioamnionitis44–59 and those associated with confirmed infection/inflammation and preterm PROM, preterm labor with intact membranes, and cervical insufficiency.121–124 These studies should determine whether clarithromycin eradicates Ureaplasma species and mycoplasma species in patients with clinical chorioamnionitis in whom these microorganisms are identified, and whether this eradication is associated with an improvement in maternal and neonatal outcomes.
Alternative antibiotic regimens that have been proposed for the treatment of clinical chorioamnionitis are shown in Table 2.10,87,91,96,97,100,126–129 None of these regimens have been tested in randomized controlled trials and most of them have been recommended based on expert opinion.
TABLE 2.
Alternative antibiotic regimens proposed for the treatment of clinical chorioamnionitis
| Antibiotic regimen |
|---|
| Cefotetan 2 g IV every 12 hours87,100,126 |
| Cefoxitin 2 g IV every 6 to 8 hours87,96,97,126,127 |
| Ceftizoxime 2 g IV every 12 hours126 |
| Cefotaxime 2 g IV every 8 to 12 hours126 |
| Cefuroxime 1.5 g IV every 8 hours96 |
| Cefazolin 1 g IV every 8 hours plus gentamicin 5 mg/kg IV every 24 hours or 1.5 mg/kg IV every 8 hours96 |
| Cefuroxime 750 mg IV every 8 hours plus metronidazole 500 mg IV every 8 hours128 |
| Mezlocillin 3–4 g IV every 6 hours126,127 |
| Piperacillin-Tazobactam 3.375 g IV every 6 hours87,91,96,126 |
| Piperacillin-Tazobactam 4 g IV every 6 hours plus clarithromycin 500 mg orally every 12 hours129 |
| Ticarcillin-clavulanic acid 3.1 g IV every 6 hours96,97,126,127 |
| Ertapenem 1 g IV every 24 hours10,87 |
| Meropenem 1 g IV every 12 hours126 |
| Imipenem-cilastatin 500 mg IV every 6 hours126 |
| IV, intravenously |
Use of antibiotics after delivery
Two trials compared the use of antibiotics versus placebo in the postpartum period after vaginal delivery.105,108 One trial (N=38) compared ampicillin combined with gentamicin for 48 hours versus placebo,105 and the other (N=250) compared gentamicin combined with clindamycin versus placebo (duration was not reported).108 Both studies reported non-significant differences between the antibiotic and placebo groups in the frequency of “treatment failure”, defined as a temperature >38 °C after the first postpartum antibiotic or placebo dose in one trial,105 and persistent fever after the third dose of the study drug or readmission for endomyometritis in the other trial.108 A meta-analysis of the two studies (N=288) showed no significant difference in the frequency of “treatment failure” between the antibiotic and placebo groups (2.0% vs 3.6%; RR 0.55, 95% CI 0.13–2.29; P = 0.41).
Two trials assessed the use of antibiotics versus non-use of antibiotics after cesarean delivery.109,111 Both trials included only laboring patients with preterm or term gestations and clinical chorioamnionitis. One study (N=116) compared gentamicin combined with clindamycin until afebrile for a minimum of 24 hours versus no antibiotics;109 another (N=80) compared one additional postpartum dose of gentamicin and clindamycin versus no antibiotics.111 There were no significant differences in the frequency of endometritis and wound infection between the study groups in both trials. A meta-analysis of the two trials (N=196) showed no significant differences in the risk of endometritis (16.7% vs 12.0%; RR 1.42, 95% CI 0.72–2.83; P = 0.31) and wound infection (8.3% vs 5.0%; RR 1.30, 95% CI 0.15–10.98; P = 0.81) between the antibiotic and no-antibiotic groups.
Two trials, one among patients who delivered vaginally (N=46)112 and another among patients who delivered either vaginally or by cesarean section (N=292),110 compared a single antibiotic dose after delivery versus continued use of antibiotics until afebrile for 24 hours. In both studies, there were no significant differences between the single antibiotic dose group and the continued use of antibiotics group in the frequency of “treatment failure”, defined as a single temperature ≥39.0 °C after the first postpartum dose of antibiotics or two temperatures ≥38.4 °C at least 4 hours apart in one study,110 and endometritis and postpartum fever in the other study.112
In summary, even though there is limited information to guide the appropriate use of antibiotics after delivery in patients with clinical chorioamnionitis, current evidence suggests that antibiotic administration may not be necessary after vaginal or cesarean delivery. However, if post-delivery antibiotics are prescribed, one additional dose of the antibiotic regimen appears to be as effective as continued use of antibiotics to reduce the risk of maternal infection. A longer duration of antibiotic therapy may be required in patients with persistent fever, bacteremia, or sepsis in the postpartum period.
Antipyretics
Maternal intrapartum fever has been associated with a higher frequency of fetal tachycardia,130,131 intervention for non-reassuring electronic fetal monitoring,132 operative vaginal delivery,133–135 cesarean delivery,130,133–136 neonatal depression,130,133,135–141 neonatal encephalopathy,137,138,140,142–144 perinatal arterial ischemic stroke,144,145 neonatal seizures,137,138,139,144,146–148 and NICU admission.130,132,133,135,136 The extent to which these complications are a result of maternal fever is uncertain. It is possible that antipyretic administration to patients with intrapartum fever can reduce adverse obstetric and neonatal outcomes.
Acetaminophen has been the most recommended antipyretic in patients with clinical chorioamnionitis.88,92,94–97 It can be administered orally, rectally, or IV. Serum peak levels (~12 μg/ml) and half-life (~1.5 hours) of acetaminophen in pregnant women are similar to those in non-pregnant adults.149 The conventional oral dose of acetaminophen is 325–650 mg every 4–6 hours; total daily doses should not exceed 4 g. Unfortunately, studies on the effect of acetaminophen on maternal and fetal temperatures during labor, as well as on adverse obstetric and neonatal outcomes are sparse and show conflicting results. In 1989, a case series study reported on the effects of acetaminophen administration (650 mg rectally) in eight febrile patients with clinical chorioamnionitis.150 When the temperature remained >38.3°C, the dose was repeated in one to two hours. Acetaminophen administration resulted in a mean decrease in temperature of 1.2°C. In addition, the fetal heart rate tracings at the peak of the maternal fever, characterized by tachycardia, decreased variability and late decelerations, changed to a normal heart rate pattern without decelerations when the mother’s fever was reduced. Moreover, significant improvements in acid-base status were noted at birth as compared to that of the fetal scalp blood at the peak of the maternal fever.
A nonrandomized study reported that acetaminophen administration in women with intrapartum fever was associated with a significant decrease in the frequency of failure to progress in labor in comparison to no administration of acetaminophen (16% [20/122] vs 32% [12/38], P = 0.04).132 There was no evidence of an effect of acetaminophen on the presence of meconium in the amniotic fluid, intervention for non-reassuring electronic fetal monitoring, or NICU admission. In 2013, a study reported that the administration of acetaminophen 1000 mg orally to women with intrapartum fever ≥38.0°C (N=18) decreased neither maternal axillary nor fetal scalp temperatures; however, acetaminophen halted ongoing increases in fetal temperatures.151 A reanalysis of study data demonstrated that both maternal and fetal temperatures decreased after acetaminophen administration.152 A more recent nonrandomized study, including 54 patients with intrapartum fever ≥ 38°C of which only three had a diagnosis of clinical chorioamnionitis, reported no significant differences between patients who received acetaminophen 650 mg orally (N=41) and those who did not (N=13) in the frequency of cesarean delivery, presence of meconium, requirement for neonatal bag/mask ventilation, requirement for continuous positive pressure ventilation, and NICU admission.130
Intravenous acetaminophen might be useful when patients are unable to tolerate oral administration or when an earlier onset of action is desirable. Indeed, intravenous acetaminophen has increased bioavailability and more rapid onset of action.153 Recently, it was reported that two patients with intrapartum fever and fetal tachycardia had a reduction of maternal temperature and resolution of fetal tachycardia 20 minutes after administration of acetaminophen 1 g IV.154 A randomized controlled trial comparing intravenous acetaminophen versus oral acetaminophen in women in active labor with a fever >38.0°C is ongoing (NCT02625454).155
In conclusion, although there is no clear evidence that the treatment of intrapartum fever reduces the risk of adverse obstetric and neonatal outcomes, antipyretics, mainly acetaminophen, have been used to treat hyperthermia in patients with clinical chorioamnionitis. Randomized controlled trials evaluating the effects of acetaminophen on intrapartum fever and both obstetric and neonatal outcomes in women with clinical chorioamnionitis are necessary.
Antenatal corticosteroids
Currently, there is a broad consensus for administering a single course of antenatal corticosteroids (ACS) between 24 0/7 and 33 6/7 weeks of gestation to pregnant women at risk of preterm delivery within 7 days.156–165 Some professional organizations recommend that ACS may also be considered for women between 23 0/7 and 23 6/7 weeks of gestation156,157,159,161,162,166 and between 34 0/7 and 36 6/7 weeks of gestation156,161,167 who are at risk of preterm delivery within 7 days.
The use of ACS among women with clinical chorioamnionitis remains controversial given the immunosuppressive effects, which, theoretically, could exacerbate systemic infections or activate latent infections in the mother, or increase the risk of neonatal infection. Some clinical guidelines advise against the use of ACS for fetal lung maturation in patients with clinical chorioamnionitis,160,163 others advise caution in its use,162,164 and one openly recommends its use in these patients.158 To date, there are no published randomized controlled trials evaluating the efficacy and safety of ACS in women with clinical chorioamnionitis. Only four of 30 trials included in the Cochrane review on ACS in women at risk for preterm birth reported that they included a proportion of women (2–15%) who had a diagnosis of clinical chorioamnionitis at trial entry.168 Nevertheless, no studies reported results for this subset of pregnant women.
Two meta-analyses of nonrandomized studies of interventions, one169 including seven studies (1335 women)170–176 and another177 including eight studies (1424 women),170–176,178 evaluated the effects of ACS administration to women with clinical or histologic chorioamnionitis before 34 weeks of gestation on adverse neonatal outcomes. Overall, ACS administration was associated with (1) significant decrease in the risk of neonatal death and other adverse neonatal outcomes among infants born to women diagnosed with histologic chorioamnionitis, and (2) significant reduction in a few adverse neonatal outcomes among neonates born to women with clinical chorioamnionitis.
We updated these meta-analyses by incorporating three nonrandomized studies that assessed the effect of ACS administration in women with histologic chorioamnionitis on neonatal outcomes179,180 and on mortality and neurodevelopmental outcomes at 3 years of age181 (Table 3). Compared to infants born to mothers with histologic chorioamnionitis who did not receive ACS, infants born to mothers with histologic chorioamnionitis who received any ACS (≥1 dose) had a significantly lower risk of neonatal morbidity and mortality. Importantly, ACS administration significantly decreased the risk of neonatal sepsis (odds ratio [OR] 0.76, 95% CI 0.63–0.93). Among infants born to mothers with clinical chorioamnionitis, the administration of ACS was associated with a significant reduction in the risk of any IVH and periventricular leukomalacia. There were no significant differences between the ACS and non-ACS groups in other adverse neonatal outcomes as well as in neurodevelopmental outcomes.
TABLE 3.
Updated meta-analysis of nonrandomized studies assessing the effect of antenatal corticosteroids on adverse neonatal and child outcomes in women with chorioamnionitis
| Outcome | No. of studies | Any exposure to antenatal corticosteroids | Non-exposure to antenatal corticosteroids | Pooled odds ratio (95% CI) | P value | I2,a % |
|---|---|---|---|---|---|---|
| Neonatal death | ||||||
| Histological chorioamnionitis | 8171,173–176,178-180 | 150/2071 | 259/1794 | 0.45 (0.36–0.56) | <0.00001 | 18 |
| Clinical chorioamnionitis | 3172,175,176 | 17/149 | 15/98 | 0.77 (0.36–1.65) | 0.50 | 0 |
| Respiratory distress syndrome | ||||||
| Histological chorioamnionitis | 7171,173,175,176,178–180 | 991/2013 | 1051/1780 | 0.66 (0.58–0.76) | <0.00001 | 0 |
| Clinical chorioamnionitis | 4170,172,175,176 |
99/209 |
99/208 | 0.73 (0.48–1.12) | 0.15 | 0 |
| Bronchopulmonary dysplasia/ chronic lung disease | ||||||
| Histological chorioamnionitis | 5175,176,178–180 | 802/1676 | 651/1238 | 0.78 (0.47–1.31) | 0.35 | 52 |
| Clinical chorioamnionitis | 3172,175,176 | 25/142 | 16/90 | 0.91 (0.44–1.86) | 0.80 | 0 |
| Neonatal sepsis | ||||||
| Histological chorioamnionitis | 7171,173,175,176,178–180 | 247/2013 | 266/1780 | 0.76 (0.63–0.93) | 0.006 | 32 |
| Clinical chorioamnionitis | 2175,176 | 26/104 | 12/46 | 0.94 (0.40–2.18) | 0.88 | 34 |
| Necrotizing enterocolitis | ||||||
| Histological chorioamnionitis | 7171,173,175,176,178–180 | 90/2013 | 56/1780 | 1.13 (0.77-1.65) | 0.53 | 0 |
| Clinical chorioamnionitis | 2175,176 | 16/104 | 3/46 | 2.63 (0.72–9.68) | 0.15 | 0 |
| Any intraventricular hemorrhage | ||||||
| Histological chorioamnionitis | 7173-176,178–180 | 281/1896 | 328/1434 | 0.62 (0.52–0.75) | <0.00001 | 2 |
| Clinical chorioamnionitis | 3170,175,176 | 13/164 | 20/156 | 0.37 (0.16–0.83) | 0.02 | 49 |
| Grade III/IV intraventricular hemorrhage | ||||||
| Histological chorioamnionitis | 5174–176,178,180 | 32/472 | 18/128 | 0.37 (0.20–0.71) | 0.003 | 0 |
| Clinical chorioamnionitis | 3170,175,176 | 5/164 | 14/156 | 0.32 (0.03–3.29) | 0.34 | 55 |
| Periventricular leukomalacia | ||||||
| Histological chorioamnionitis | 5175,176,178–180 | 74/1750 | 72/1378 | 0.80 (0.57–1.12) | 0.19 | 0 |
| Clinical chorioamnionitis | 3170,175,176 | 8/163 | 24/155 | 0.35 (0.14–0.85) | 0.02 | 42 |
| Neonatal seizure | ||||||
| Histological chorioamnionitis | 1179 | 49/1336 | 80/1264 | 0.56 (0.39–0.81) | 0.002 | NA |
| Patent ductus arteriosus | ||||||
| Histological chorioamnionitis | 4171,178–180 | 597/1654 | 563/1670 | 0.92 (0.60–1.41) | 0.70 | 56 |
| Death before 3 years of age | ||||||
| Histological chorioamnionitis | 1181 | 33/438 | 66/402 | 0.41 (0.27–0.65) | <0.0001 | NA |
| Cerebral palsy at 3 years of age | ||||||
| Histological chorioamnionitis | 2174,181 | 26/307 | 18/208 | 0.93 (0.50–1.75) | 0.83 | 43 |
| Developmental quotient <70 | ||||||
| Histological chorioamnionitis | 1181 | 27/189 | 25/161 | 0.91 (0.50–1.64) | 0.74 | NA |
| Severe hearing impairment | ||||||
| Histological chorioamnionitis | 1181 | 3/247 | 2/195 | 1.19 (0.20–7.17) | 0.85 | NA |
| Visual impairment | ||||||
| Histological chorioamnionitis | 1181 | 2/246 | 3/192 | 0.52 (0.09–3.12) | 0.47 | NA |
| Neurodevelopmental impairment | ||||||
| Histological chorioamnionitis | 1181 | 46/194 | 37/160 | 1.03 (0.63–1.69) | 0.90 | NA |
CI, confidence interval; NA, not applicable
Heterogeneity measure. Fixed-effect model used if I2 <50%; random-effects model used if I2 ≥50 Legend for Figures
The most important limitation of this updated meta-analysis is the lack of information in the included studies about the timing of ACS administration relative to the diagnosis of clinical chorioamnionitis. It is noteworthy that none of the primary studies provided data on adverse maternal outcomes. However, the use of ACS in women with clinical chorioamnionitis was not associated with a significant increase in any adverse neonatal outcome, and this was also the case in retrospective studies comparing patients with and without histologic chorioamnionitis.
In a Cochrane review, ACS administration to women with preterm PROM significantly decreased the risk of neonatal death (RR 0.61, 95% CI 0.46–0.83), RDS (RR 0.70, 95% CI 0.55–0.90), and IVH (RR 0.47, 95% CI 0.28–0.79), with no evidence of an effect on the risk of chorioamnionitis (RR 0.98, 95% CI 0.69–1.40), endometritis (RR 1.02, 95% CI 0.35–2.97), or puerperal sepsis (RR 1.11, 95% CI 0.55–2.25).168 Given that the frequency of microbial invasion of the amniotic cavity in women with preterm PROM ranges from 20–50%,182–189 it seems logical that ACS administration may be beneficial in patients with clinical chorioamnionitis.
Considering >90% of patients with clinical chorioamnionitis are expected to deliver within 12 hours of diagnosis,6,8,79,99,102,114,190–193 most will receive only one dose of ACS. Nonetheless, there is evidence from observational studies showing that infants exposed to an incomplete course of ACS had a significantly lower risk of death and/or other adverse neonatal or neurodevelopmental outcomes compared to infants not exposed to ACS.194–200 In addition, a subgroup analysis of the Cochrane review showed that ACS administration reduces the risk of neonatal death in infants who are born less than 24 hours after the first dose has been administered (RR 0.53, 95% CI 0.29–0.96).201 Noticeably, the authors of a recent population-based prospective cohort study reported that if patients received ACS at least 3 hours before delivery, there was a 26% decrease in neonatal mortality. If they received ACS 3 to 5 hours before delivery, there was a 37% decrease in neonatal mortality, and if patients received ACS 6 to 12 hours before delivery, there was a 51% decrease in neonatal mortality.198
In summary, current available evidence suggests that the administration of at least one single dose of ACS to patients with clinical chorioamnionitis has an overall beneficial effect on the neonate without increasing the risk of sepsis or other adverse neonatal outcomes. Thus, it appears reasonable to administer ACS to women with clinical chorioamnionitis between 24 0/7 and 33 6/7 weeks of gestation and to consider its administration to those with a gestational age between 23 0/7 and 23 6/7 weeks. Delivery should not be delayed in order to complete the full course of ACS.
Magnesium sulfate for fetal neuroprotection
Currently, there is strong evidence from several systematic reviews and meta-analyses that magnesium sulfate administered to women at risk of imminent preterm delivery reduces the risk of cerebral palsy in their children by about 32%.202–205 Antenatal magnesium sulfate is also associated with a significant reduction in the risk of moderate or severe cerebral palsy and substantial gross motor dysfunction. Although most clinical guidelines recommend the administration of magnesium sulfate for fetal neuroprotection to women at risk of imminent preterm delivery (expected within 2–24 hours) regardless of the reason for preterm birth, there is controversy with respect to gestational age at treatment (from “viability” or 24 0/7 weeks to 29 6/7 weeks,206 31 6/7 weeks160,207 or 33 6/7 weeks167,208). Some guidelines recommend that magnesium sulfate administration should also be considered for women at risk of imminent delivery between 23 0/7 and 23 6/7 weeks.157,166 An individual patient data (IPD) meta-analysis205 of five trials209–215 of magnesium sulfate for fetal neuroprotection reported no significant differences in the beneficial effect of this intervention on cerebral palsy among subgroups based on gestational age at trial entry (<28, 28–31, and ≥32 weeks; P for interaction = 0.85).
Thus far, no specific randomized controlled trial has assessed the efficacy of magnesium sulfate in patients with clinical chorioamnionitis. Four210,211,213,215 of the five trials included in the previously mentioned meta-analyses included a proportion of women with the diagnosis of clinical chorioamnionitis (11–51%); however, results were not reported separately for these women. A subgroup analysis of the IPD meta-analysis showed no clear differences in treatment effects on cerebral palsy among the subgroups of women according to the reason for imminent preterm delivery (preeclampsia, preterm labor, chorioamnionitis, antepartum hemorrhage, and preterm PROM ≥24 hours; P for interaction = 0.48).205 More recently, a secondary analysis of the BEAM trial,215 which included 1944 women with live, non-anomalous, singleton gestations, assessed separately the effects of antenatal administration of magnesium sulfate on the risk of cerebral palsy among women with (N=228) and without (N=1716) clinical chorioamnionitis.216 Magnesium sulfate reduced the odds of cerebral palsy in children born to mothers with clinical chorioamnionitis (OR 0.76, 95% CI 0.19–2.76) and in those born to mothers without clinical chorioamnionitis (OR 0.52, 95% CI 0.31–0.86). However, the odds reduction was statistically significant only among children born to mothers without clinical chorioamnionitis. The authors of this study concluded that “antenatal magnesium did not show a clear neuroprotective effect in the setting of chorioamnionitis”.
These results, representing a post-hoc subgroup analysis of the BEAM trial,215 were not correctly interpreted. Rather, the appropriate question in this study216 is to determine whether the results in the two subgroups differed significantly from each other. We re-analyzed the data reported in this secondary analysis and calculated a test for interaction to examine whether intervention effects on cerebral palsy and moderate/severe cerebral palsy differ between women with and without clinical chorioamnionitis (Figure 1). The beneficial effect of magnesium sulfate on both cerebral palsy and moderate/severe cerebral palsy did not differ significantly between patients with a diagnosis of clinical chorioamnionitis and those without such diagnosis (P for interaction = 0.58 for cerebral palsy and 0.81 for moderate/severe cerebral palsy).
Figure 1.
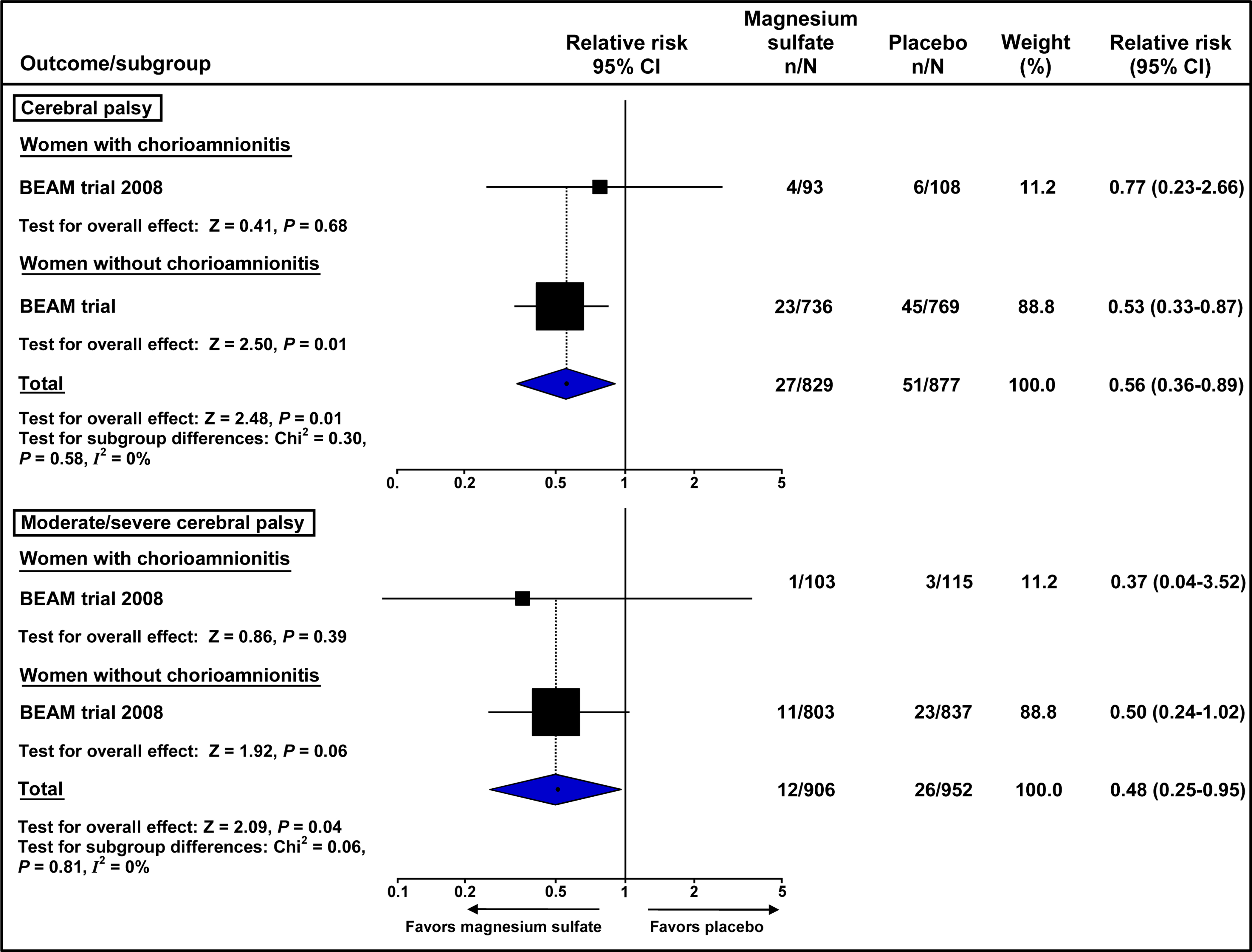
Effect of antenatal magnesium sulfate on the risk of cerebral palsy and moderate/severe cerebral palsy according to the presence of clinical chorioamnionitis in the BEAM trial215
Given that more than 90% of patients with clinical chorioamnionitis deliver within 12 hours of diagnosis with a mean diagnosis-to-delivery interval ranging from 4–8 hours,6,8,79,99,102,114,190–193 this short interval may cause some clinicians to doubt the efficacy of magnesium sulfate for fetal neuroprotection. However, magnesium sulfate readily crosses the placenta217–219 and achieves high fetal serum concentrations within 1 hour after the initiation of maternal intravenous administration,217 which remain elevated to 24 hours in the neonate.218 In two of the trials that assessed the neuroprotective effects of magnesium sulfate, the median time from magnesium sulfate initiation to birth was 3.7 hours in one trial211 and 1.6 hours in the other trial,213 suggesting that magnesium sulfate crosses rapidly to the fetal compartment and that this may confer a neuroprotective effect. Importantly, a subgroup analysis according to the time interval from the first magnesium sulfate dose to delivery in the IPD meta-analysis205 revealed that the beneficial effect of magnesium sulfate on cerebral palsy did not significantly differ between women with an interval <4 hours and those with intervals 4–11 hours and ≥12 hours (P for interaction = 0.77).
In summary, the current evidence supports the administration of antenatal magnesium sulfate to women with clinical chorioamnionitis between 24 0/7 and 33 6/7 weeks of gestation for preventing cerebral palsy in their offspring. It also may be considered for women with a gestational age between 23 0/7 and 23 6/7 weeks. Delivery should not be delayed in order to administer the full course of antenatal magnesium sulfate for fetal neuroprotection.
Management of labor
Mode of delivery
Once a diagnosis of clinical chorioamnionitis has been established, delivery should be considered, regardless of the gestational age. Clinical chorioamnionitis alone is not an indication for cesarean delivery. Unless contraindicated, induction and trial of labor can be considered. Vaginal delivery is the safer option and cesarean delivery should be reserved for standard obstetric indications. This recommendation is strongly supported by the findings of a large multicenter retrospective cohort study, which evaluated the effect of clinical chorioamnionitis on the risk of adverse maternal outcomes according to mode of delivery.6 The study included 216,467 women without clinical chorioamnionitis and 4807 women with clinical chorioamnionitis of which 2794 delivered vaginally and 2013 underwent cesarean delivery. Clinical chorioamnionitis, regardless of antibiotic therapy type and duration, was associated with a significantly increased risk of adverse maternal outcomes among women who had a cesarean delivery (adjusted OR 2.31, 95% CI 1.97–2.71), but not among women who had a vaginal delivery (adjusted OR 1.15, 95% CI 0.93–1.43).
Duration of chorioamnionitis and adverse maternal and neonatal outcomes
The time interval between the diagnosis of clinical chorioamnionitis and delivery is not related to the risk of most adverse maternal and neonatal outcomes.6,8,79,190,192,193,220 In 1994, a study reported that there was no association between the time elapsed from diagnosis of clinical chorioamnionitis to delivery and several adverse neonatal outcomes such as umbilical artery pH <7.20, low Apgar scores at 5 minutes, oxygen requirement, and sepsis.193 A large prospective cohort study among women who underwent primary cesarean delivery assessed the relationship between duration of clinical chorioamnionitis (diagnosis-to-delivery interval) and adverse maternal and neonatal outcomes.8 Unadjusted analyses showed that only 3 of 18 outcomes assessed were marginally (uterine atony and Apgar ≤3 at 5 minutes) or significantly (use of mechanical ventilation within 24 hours of birth) associated with the duration of clinical chorioamnionitis. Nevertheless, the absolute increase of these outcomes by each additional hour of chorioamnionitis was very low. A more recent cohort study evaluated the impact of the estimated duration of clinical chorioamnionitis and found that a longer duration did not appear to significantly increase the risk of adverse maternal outcomes.6 In summary, there is no evidence supporting that immediate delivery after the diagnosis of clinical chorioamnionitis prevents adverse maternal and neonatal outcomes, or long-term neurodevelopmental outcomes. On the contrary, such an approach would lead to an increase in the frequency of cesarean delivery and, therefore, to an increased risk of adverse maternal outcomes.
Labor progression
Women with clinical chorioamnionitis are more likely to have abnormal labor progression or prolonged labor5,191,221–223 and cesarean delivery for failure to progress or non-reassuring fetal heart rate tracing,4,5,8,191,192,221–224 and to receive oxytocin for induction or augmentation of labor191,192,222–224 than those without clinical chorioamnionitis. A large nation-based study showed that women with clinical chorioamnionitis were 40 percent more likely to have a cesarean delivery than those without clinical chorioamnionitis, after controlling for obstetric and medical confounding variables.4 It is controversial as to whether chorioamnionitis is the cause or the ultimate effect of dysfunctional labor. Recently, a retrospective cohort study examined the temporal association between the diagnosis of maternal fever in women with suspected clinical chorioamnionitis (N=100) and uterine contractility, measured by an intrauterine pressure catheter, which was placed at least one hour prior to the time of first temperature ≥38°C.225 This study reported that uterine contractility was maintained for 2 hours after the onset of maternal fever but significantly and steadily declined thereafter, despite no changes in oxytocin dosage. Patients who delivered vaginally (32%) maintained contractility, while those who delivered by cesarean (68%) had diminishing contractility following the onset of fever (P = 0.01). Most cesarean deliveries were attributable to arrest of dilatation. Moreover, the responsiveness to oxytocin significantly decreased after the diagnosis of clinical chorioamnionitis. In support of these findings, an in vitro study performed in the early 80s showed that bacteria causing chorioamnionitis, such as anaerobic streptococcus species, Veillonella species, Bacteroides species, and enterococcus faecalis, reduce the contractility of human myometrial tissue and its responsiveness to oxytocin during the period of decreased contractility.226
In summary, the available evidence supports the hypothesis that clinical chorioamnionitis is associated with reduced uterine contractility. Thus, patients with clinical chorioamnionitis may require higher doses of oxytocin to achieve adequate uterine activity and/or greater uterine activity to effect a given change in cervical dilation.
Continuous electronic fetal heart rate monitoring
Abnormal cardiotocography patterns during labor are significantly more frequent among patients with clinical chorioamnionitis than among those without clinical chorioamnionitis.191,224,227–229 The most common fetal heart rate (FHR) patterns observed in clinical chorioamnionitis include tachycardia, absence of accelerations, presence of variable and late decelerations, persistently reduced variability, and absence of cycling.193,228–230 However, none of these patterns have been associated with a significant increase in the risk of adverse neonatal or infant outcomes in pregnancies complicated with clinical chorioamnionitis. A study of 197 women with clinical chorioamnionitis found no association between umbilical artery pH <7.20 and several FHR patterns including loss of variability, absence of accelerations and tachycardia.193 Another study of 139 patients with intrauterine bacterial infection (defined as clinical chorioamnionitis plus a positive bacterial amniotic fluid culture or neonatal infection) reported that FHR deceleration patterns, decreased variability, and absence of accelerations were not significantly associated with the risk of cerebral palsy at 2 years of age.228
Clinical chorioamnionitis has been considered a cause of non-hypoxic fetal compromise. Given that electronic FHR monitoring is a test for fetal hypoxia, its role in clinical chorioamnionitis is less clear.231 In addition, the benefit of continuous electronic FHR monitoring during labor either in low- or high-risk pregnancies has not been clearly demonstrated.232 To date, the usefulness of continuous electronic FHR monitoring in the setting of clinical chorioamnionitis has not been assessed in randomized controlled trials. Despite these issues, most professional and scientific organizations recommend using continuous electronic FHR monitoring during labor in patients with clinical chorioamnionitis.233–235 This recommendation is largely based upon expert opinion and medicolegal experience. Overall, the management of intrapartum fetal heart rate tracings in patients with clinical chorioamnionitis does not differ from that in patients without clinical chorioamnionitis. It is worth noting that isolated fetal tachycardia is a poor predictor of fetal hypoxemia or acidemia, unless accompanied by minimal or absent FHR variability or recurrent decelerations or both, and is not an indication for immediate operative delivery.
In summary, external continuous electronic FHR monitoring is generally used once the diagnosis of clinical chorioamnionitis has been made and may be used in identifying fetal hypoxic insults so that timely and appropriate action could be instituted to improve perinatal outcome.
Delivery and immediate postpartum period
Women with clinical chorioamnionitis are more likely to have uterine atony, postpartum hemorrhage, and blood transfusion than those without clinical chorioamnionitis.5–9,223,224,236 Increased frequencies of uterine atony and postpartum hemorrhage appear to be directly related to impairment in myometrial contractility caused by intra-amniotic infection/inflammation.225 Health professionals who provide obstetric care should be aware that chorioamnionitis is a well-established risk factor for the development of postpartum hemorrhage and should be prepared to manage patients with clinical chorioamnionitis who experience this complication. In addition, interventions that have been shown to prevent and treat postpartum hemorrhage should be readily available in both delivery and operating rooms.
Promising interventions
Vaginal cleansing with antiseptic solutions before cesarean delivery
Evidence from three recent meta-analyses supports that vaginal cleansing with antiseptic solutions before cesarean delivery reduces postoperative infectious morbidity.237–239 The most recent and comprehensive meta-analysis reported that use of vaginal antiseptic solutions before cesarean delivery significantly reduced the frequency of endometritis, wound infection, and fever when compared to saline solution or no treatment.239 A subgroup analysis found that vaginal cleansing with antiseptic solutions before cesarean delivery significantly reduced the risk of endometritis in women with ruptured membranes (OR 0.21, 95% CI 0.10–0.44). Subgroup analyses according to the preoperative presence of clinical chorioamnionitis were not reported. A network meta-analysis showed that povidone-iodine 1% had the highest probability of being the most effective treatment for the prevention of endometritis and chlorhexidine had the highest probability for the best agent for the prevention of wound infection.239–241
It appears that vaginal antiseptic solutions decrease the risk of endometritis by reducing ascending infection through a reduction of vaginal bacterial load.242–245 Cleansing the vagina with antiseptic solutions reduces the frequency of endometritis in patients with ruptured membranes even though bacteria may have already ascended and colonized in the uterus prior to cleansing.182–189 Because most patients with clinical chorioamnionitis who undergo cesarean delivery have ruptured membranes and are in labor, the use of vaginal cleansing with antiseptic solutions in these women appears to be logical. Further trials are warranted to determine the efficacy of vaginal cleansing with antiseptic solutions before cesarean delivery to reduce the risk of postoperative infectious morbidity in patients with clinical chorioamnionitis.
N-acetylcysteine
N-acetylcysteine (NAC), an antioxidant and anti-inflammatory agent, has been shown to provide substantial neuroprotection against perinatal brain injury in newborn rats.246–248 A study conducted by our team demonstrated that postnatal dendrimer-based NAC therapy for brain injury suppressed neuroinflammation and led to a significant improvement in motor function of newborn rabbits with cerebral palsy.249
Administration of NAC to patients with clinical chorioamnionitis results in rapid placental transfer and predictable NAC plasma concentrations in the fetus.250 In 2016, the results of a small randomized, placebo-controlled trial that assessed the fetal and neonatal effects of NAC administered antenatally to 22 patients (12 preterm, 10 term) with clinical chorioamnionitis and postnatally to their infants (N=24) were reported.251 Compared to infants who received saline, infants who received NAC showed beneficial effects such as preserved cerebrovascular regulation, decreased proinflammatory vascular endothelial growth factor, and increased anti-inflammatory interleukin-1 receptor antagonist with no adverse events related to NAC administration.
Recently, the main results of a randomized controlled trial of NAC to prevent adverse neonatal outcome in patients with intra-amniotic infection/inflammation were reported in abstract form.252 In this study, women with intra-amniotic infection or inflammation diagnosed by transabdominal amniocentesis at 23–33 weeks of gestation were randomized either to NAC 150 mg/kg IV loading dose (60 min), followed by 50 mg/kg IV continuous infusion rate for 4 hours, and followed by 100 mg/kg IV continuous infusion rate until delivery (N=34) or to placebo (N=34). The primary outcome was a composite of mortality and severe short-term neonatal morbidities (grade III/IV IVH, necrotizing enterocolitis grades 2–4, retinopathy of prematurity “grades 2–4”, bronchopulmonary dysplasia, or death). NAC administration was associated with a significant reduction in the frequency of the primary outcome (4/34 [11.8%] vs 13/34 [38.2%]; RR 0.31, 95% CI 0.11–0.85; P = 0.02), mainly as a consequence of a reduction in bronchopulmonary dysplasia (1/34 [2.9%] vs 11/34 [32.4%]; RR 0.09, 95% CI 0.01–0.67; P = 0.02). There were no significant differences between the study groups in the risk of neonatal sepsis (9/34 [26.5%] vs 12/34 [35.3%]; RR 0.75, 95% CI 0.36–1.54; P = 0.43) and newborn death (2/34 [5.9%] vs 6/34 [17.6%]; RR 0.33, 95% CI 0.07–1.54; P = 0.16). In summary, the antenatal administration of NAC in patients with chorioamnionitis aiming to reduce neonatal morbidity and mortality is promising, and the beneficial effects reported by these small trials need to be confirmed in future studies.
Algorithm for the management of clinical chorioamnionitis
Based upon the presented evidence, we developed an approach for the management of patients with clinical chorioamnionitis (Figure 2). This approach can be modified as new evidence arises.
Figure 2.
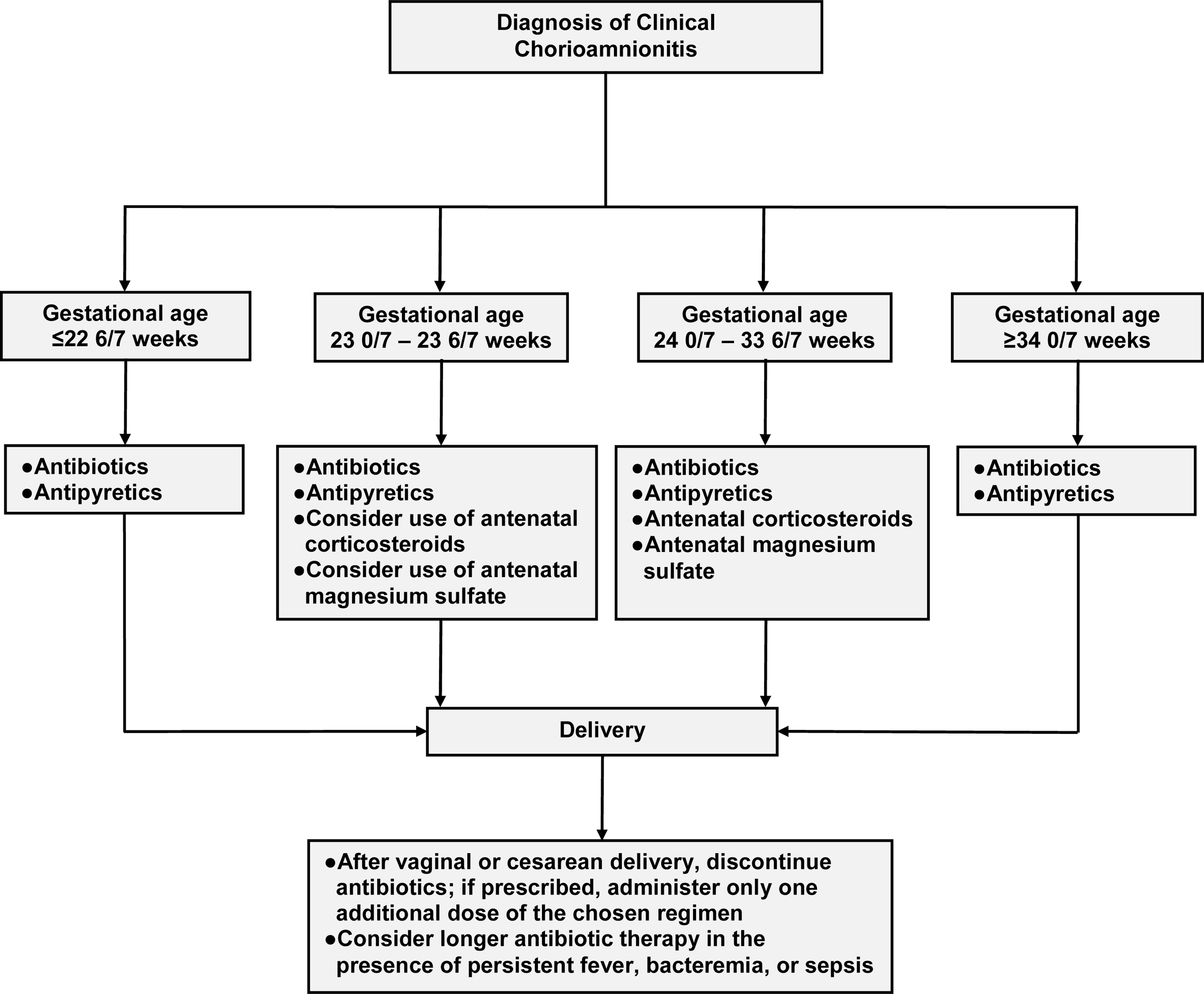
Proposed approach for the management of clinical chorioamnionitis with a live fetus
Supplementary Material
Condensation.
We examined the available evidence supporting interventions proposed for the treatment of clinical chorioamnionitis, developed a contemporary approach for managing this condition, and identified promising interventions.
Acknowledgments
Financial support: This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Role of the funding source: The funder had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Disclosure: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fassett MJ, Wing DA, Getahun D. Temporal trends in chorioamnionitis by maternal race/ethnicity and gestational age (1995–2010). Int J Reprod Med 2013;2013:906467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malloy MH. Chorioamnionitis: epidemiology of newborn management and outcome United States 2008. J Perinatol 2014;34:611–5. [DOI] [PubMed] [Google Scholar]
- 3.Braun D, Bromberger P, Ho NJ, Getahun D. Low rate of perinatal sepsis in term Infants of mothers with chorioamnionitis. Am J Perinatol 2016;33:143–50. [DOI] [PubMed] [Google Scholar]
- 4.Bommarito KM, Gross GA, Willers DM, Fraser VJ, Olsen MA. The effect of clinical chorioamnionitis on cesarean delivery in the United States. Health Serv Res 2016;51:1879–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry AK, Rossi RM, DeFranco EA. Severe adverse maternal outcomes associated with chorioamnionitis. Am J Obstet Gynecol MFM 2019;1:100027. [DOI] [PubMed] [Google Scholar]
- 6.Venkatesh KK, Glover AV, Vladutiu CJ, Stamilio DM. Association of chorioamnionitis and its duration with adverse maternal outcomes by mode of delivery: a cohort study. BJOG 2019;126:719–27. [DOI] [PubMed] [Google Scholar]
- 7.Wiley RL, Racusin D, Chen HY, Chauhan SP. Chorioamnionitis and adverse outcomes in low-risk pregnancies: a population based study. Am J Obstet Gynecol 2020;222(Suppl):S244–S245. [DOI] [PubMed] [Google Scholar]
- 8.Rouse DJ, Landon M, Leveno KJ, et al. The Maternal-Fetal Medicine Units cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol 2004;191:211–6. [DOI] [PubMed] [Google Scholar]
- 9.Rouse DJ, Leindecker S, Landon M, et al. The MFMU Cesarean Registry: uterine atony after primary cesarean delivery. Am J Obstet Gynecol 2005;193:1056–60. [DOI] [PubMed] [Google Scholar]
- 10.Dotters-Katz SK, Feldman C, Puechl A, Grotegut CA, Heine RP. Risk factors for post-operative wound infection in the setting of chorioamnionitis and cesarean delivery. J Matern Fetal Neonatal Med 2016;29:1541–5. [DOI] [PubMed] [Google Scholar]
- 11.Casey BM, Cox SM. Chorioamnionitis and endometritis. Infect Dis Clin North Am 1997;11:203–22. [DOI] [PubMed] [Google Scholar]
- 12.Witlin AG, Mercer BM, Sibai BM. Septic pelvic thrombophlebitis or refractory postpartum fever of undetermined etiology. J Matern Fetal Med 1996;5:355–8. [DOI] [PubMed] [Google Scholar]
- 13.Koh KS, Chan FH, Monfared AH, Ledger WJ, Paul RH. The changing perinatal and maternal outcome in chorioamnionitis. Obstet Gynecol 1979;53:730–4. [PubMed] [Google Scholar]
- 14.Romero R, Kadar N, Vaisbuch E, Hassan SS. Maternal death following cardiopulmonary collapse after delivery: amniotic fluid embolism or septic shock due to intrauterine infection? Am J Reprod Immunol 2010;64:113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chebbo A, Tan S, Kassis C, Tamura L, Carlson RW. Maternal sepsis and septic shock. Crit Care Clin 2016;32:119–35. [DOI] [PubMed] [Google Scholar]
- 16.Randis TM, Rice MM, Myatt L, et al. Incidence of early-onset sepsis in infants born to women with clinical chorioamnionitis. J Perinat Med 2018;46:926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkatesh KK, Jackson W, Hughes BL, Laughon MM, Thorp JM, Stamilio DM. Association of chorioamnionitis and its duration with neonatal morbidity and mortality. J Perinatol 2019;39:673–82. Erratum in: J Perinatol 2019;39:761. [DOI] [PubMed] [Google Scholar]
- 18.Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK; Canadian Neonatal Network. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol 2009;200:372.e1–6. [DOI] [PubMed] [Google Scholar]
- 19.Klinger G, Levy I, Sirota L, Boyko V, Reichman B, Lerner-Geva L; Israel Neonatal Network. Epidemiology and risk factors for early onset sepsis among very-low-birthweight infants. Am J Obstet Gynecol 2009;201:38.e1–6. [DOI] [PubMed] [Google Scholar]
- 20.Pappas A, Kendrick DE, Shankaran S, et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr 2014;168:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ericson JE, Laughon MM. Chorioamnionitis: implications for the neonate. Clin Perinatol 2015;42:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gowda H, Norton R, White A, Kandasamy Y. Late-onset neonatal sepsis-a 10-year review from North Queensland, Australia. Pediatr Infect Dis J 2017;36:883–88. [DOI] [PubMed] [Google Scholar]
- 23.Villamor-Martinez E, Álvarez-Fuente M, Ghazi AMT, et al. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and metaregression. JAMA Netw Open 2019;2:e1914611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LW, Lin YC, Wang ST, Huang CC; on behalf of the Taiwan Premature Infant Developmental Collaborative Study Group. Identifying risk factors shared by bronchopulmonary dysplasia, severe retinopathy, and cystic periventricular leukomalacia in very preterm infants for targeted intervention. Neonatology 2018;114:17–24. [DOI] [PubMed] [Google Scholar]
- 25.Villamor-Martinez E, Fumagalli M, Mohammed Rahim O, et al. Chorioamnionitis is a risk factor for intraventricular hemorrhage in preterm infants: a systematic review and meta-analysis. Front Physiol 2018;9:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegel AM, Li J, Oehlert JW, et al. A genome-wide analysis of clinical chorioamnionitis among preterm infants. Am J Perinatol 2019;36:1453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Muñoz Rodrigo F, Galán Henríquez GM, Ospina CG. Morbidity and mortality among very-low-birth-weight infants born to mothers with clinical chorioamnionitis. Pediatr Neonatol 2014;55:381–6. [DOI] [PubMed] [Google Scholar]
- 28.Romero-Guzman GJ, Lopez-Munoz F. Prevalence and risk factors for periventricular leukomalacia in preterm infants. A systematic review [In Spanish]. Rev Neurol 2017;65:57–62. [PubMed] [Google Scholar]
- 29.Gutvirtz G, Walfisch A, Wainstock T, Beloosesky R, Landau D, Sheiner E. Chorioamnionitis as a risk factor for long-term infectious morbidity of the offspring. Am J Obstet Gynecol 2019;220(Suppl):S419–20. [Google Scholar]
- 30.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA 2003;290:2677–84. [DOI] [PubMed] [Google Scholar]
- 31.Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol 2010;116:387–92. [DOI] [PubMed] [Google Scholar]
- 32.Bear JJ, Wu YW. Maternal infections during pregnancy and cerebral palsy in the child. Pediatr Neurol 2016;57:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao D, Zhu T, Qu Y, et al. Maternal chorioamnionitis and neurodevelopmental outcomes in preterm and very preterm neonates: A meta-analysis. PLoS One 2018;13:e0208302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freud A, Wainstock T, Sheiner E, et al. Maternal chorioamnionitis & long term neurological morbidity in the offspring. Eur J Paediatr Neurol 2019;23:484–90. [DOI] [PubMed] [Google Scholar]
- 35.Xing L, Wang G, Chen R, Ren J, Qian J, Huang Y. Is chorioamnionitis associated with neurodevelopmental outcomes in preterm infants? A systematic review and meta-analysis following PRISMA. Medicine (Baltimore) 2019;98:e18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vander Haar E, Gyamfi-Bannerman C. Chorioamnionitis and neurocognitive development at age 2 years. Obstet Gynecol 2016;127:437–41. [DOI] [PubMed] [Google Scholar]
- 37.Maisonneuve E, Ancel PY, Foix-L’Hélias L, Marret S, Kayem G. Impact of clinical and/or histological chorioamnionitis on neurodevelopmental outcomes in preterm infants: A literature review. J Gynecol Obstet Hum Reprod 2017;46:307–16. [DOI] [PubMed] [Google Scholar]
- 38.Shi Z, Ma L, Luo K, et al. Chorioamnionitis in the development of cerebral palsy: a meta-analysis and systematic review. Pediatrics 2017;139(6). pii: e20163781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yee LM. Infant and child neurodevelopmental outcomes after exposure to chorioamnionitis. Am J Obstet Gynecol 2019;220(Suppl):S429. [Google Scholar]
- 40.Ylijoki MK, Ekholm E, Ekblad M, Lehtonen L. Prenatal risk factors for adverse developmental outcome in preterm infants-systematic review. Front Psychol 2019;10:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbs RS. Diagnosis of intra-amniotic infection. Semin Perinatol 1977;1:71–7. [PubMed] [Google Scholar]
- 42.Hollander D. Diagnosis of chorioamnionitis. Clin Obstet Gynecol 1986;29:816–25. [DOI] [PubMed] [Google Scholar]
- 43.Newton ER. Preterm labor, preterm premature rupture of membranes, and chorioamnionitis. Clin Perinatol 2005;32:571–600. [DOI] [PubMed] [Google Scholar]
- 44.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection?. J Perinat Med. 2016;44(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2015;43:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh KJ, Kim SM, Hong JS, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol 2017;216:604.e1–604.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kikhney J, von Schöning D, Steding I, et al. Is Ureaplasma spp. the leading causative agent of acute chorioamnionitis in women with preterm birth? Clin Microbiol Infect 2017;23:119.e1–119.e7. [DOI] [PubMed] [Google Scholar]
- 48.Oh KJ, Romero R, Park JY, Hong JS, Yoon BH. The earlier the gestational age, the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species. J Perinat Med 2019;47:516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero R, Gomez-Lopez N, Kusanovic JP, et al. Clinical chorioamnionitis at term: new insights into the etiology, microbiology, and the fetal, maternal and amniotic cavity inflammatory responses. Nogyogyaszati Szuleszeti Tovabbkepzo Szemle 2018;20:103–12. [PMC free article] [PubMed] [Google Scholar]
- 50.Sweeney EL, Dando SJ, Kallapur SG, Knox CL. The human ureaplasma species as causative agents of chorioamnionitis. Clin Microbiol Rev 2016;30:349–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125.e1–125.e15. [DOI] [PubMed] [Google Scholar]
- 52.Mendz GL, Kaakoush NO, Quinlivan JA. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front Cell Infect Microbiol 2013;3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh KJ, Lee KA, Sohn YK, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2010;203:211.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krohn MA, Hillier SL, Nugent RP, Cotch MF, Carey JC, Gibbs RS, Eschenbach DA. The genital flora of women with intraamniotic infection. Vaginal Infection and Prematurity Study Group. J Infect Dis 1995;171:1475–80. [DOI] [PubMed] [Google Scholar]
- 55.Gibbs RS. Chorioamnionitis and bacterial vaginosis. Am J Obstet Gynecol 1993;169:460–2. [DOI] [PubMed] [Google Scholar]
- 56.Silver HM, Sperling RS, St Clair PJ, Gibbs RS. Evidence relating bacterial vaginosis to intraamniotic infection. Am J Obstet Gynecol 1989;161:808–12. [DOI] [PubMed] [Google Scholar]
- 57.Gibbs RS, Weiner MH, Walmer K, St Clair PJ. Microbiologic and serologic studies of Gardnerella vaginalis in intra-amniotic infection. Obstet Gynecol 1987;70:187–90. [PubMed] [Google Scholar]
- 58.Blanco JD, Gibbs RS, Malherbe H, Strickland-Cholmley M, St Clair PJ, Castaneda YS. A controlled study of genital mycoplasmas in amniotic fluid from patients with intra-amniotic infection. J Infect Dis 1983;147:650–3. [DOI] [PubMed] [Google Scholar]
- 59.Gibbs RS, Blanco JD, St. Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis 1982;145:1–8. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Gomez-Lopez N, Winters AD, et al. Evidence that intra-amniotic infections are often the result of an ascending invasion - a molecular microbiological study. J Perinat Med 2019. 26;47:915–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MJ, Romero R, Gervasi MT, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest 2009;89:924–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213(Suppl 4):S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robischon K, Amstey MS. Evidence for in utero hematogenous transmission of group B beta-hemolytic streptococcus. Infect Dis Obstet Gynecol 1994;2:184–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sorano S, Goto M, Matsuoka S, et al. Chorioamnionitis caused by Staphylococcus aureus with intact membranes in a term pregnancy: A case of maternal and fetal septic shock. J Infect Chemother. 2016;22:261–4. [DOI] [PubMed] [Google Scholar]
- 65.Jadhav AR, Belfort MA, Dildy GA 3rd. Eikenella corrodens chorioamnionitis: modes of infection? Am J Obstet Gynecol 2009;200:e4–5. [DOI] [PubMed] [Google Scholar]
- 66.Felix L, Rosenberg A, Caraballo KA, Taborga DP, Hamula C. Capnocytophaga spp. infection causing chorioamnionitis: an unusual suspect. Anaerobe 2019;59:115–7. [DOI] [PubMed] [Google Scholar]
- 67.Radochova V, Kacerovska Musilova I, Stepan M, et al. Periodontal disease and intra-amniotic complications in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2018;31:2852–61. [DOI] [PubMed] [Google Scholar]
- 68.Tongsong T, Wanapirak C, Kunavikatikul C, Sirirchotiyakul S, Piyamongkol W, Chanprapaph P. Cordocentesis at 16–24 weeks of gestation: experience of 1,320 cases. Prenat Diagn 2000;20:224–8. [PubMed] [Google Scholar]
- 69.Thadepalli H, Appleman MD, Chan WH, Maidman JC, Davidson EC Jr. Amniotic fluid contamination during internal fetal monitoring. J Reprod Med 1978;20:93–6. [PubMed] [Google Scholar]
- 70.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med 2016;44:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med 2016;44:33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez-Varea A, Romero R, Xu Y, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med 2017;45:523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gomez-Lopez N, Romero R, Maymon E, et al. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. J Perinat Med 2019;47:276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galaz J, Romero R, Xu Y, et al. Cellular immune responses in amniotic fluid of women with preterm clinical chorioamnionitis. Inflamm Res 2020;69:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gomez-Lopez N, Romero R, Xu Y, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017;217:693.e1–693.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gomez-Lopez N, Romero R, Xu Y, et al. Neutrophil extracellular traps in the amniotic cavity of women with intra-amniotic infection: a new mechanism of host defense. Reprod Sci 2017;24:1139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med 2016;44:77–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med 2016;44:53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol 1980;136:709–13. [DOI] [PubMed] [Google Scholar]
- 80.Looff JD, Hager WD. Management of chorioamnionitis. Surg Gynecol Obstet 1984;158:161–6. [PubMed] [Google Scholar]
- 81.Gilstrap LC 3rd, Cox SM. Acute chorioamnionitis. Obstet Gynecol Clin North Am 1989;16:373–9. [PubMed] [Google Scholar]
- 82.Gibbs RS, Duff P. Progress in pathogenesis and management of clinical intraamniotic infection. Am J Obstet Gynecol 1991;164:1317–26. [DOI] [PubMed] [Google Scholar]
- 83.Greenberg MB, Anderson BL, Schulkin J, Norton ME, Aziz N. A first look at chorioamnionitis management practice variation among US obstetricians. Infect Dis Obstet Gynecol 2012;2012:628362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Higgins RD, Saade G, Polin RA, et al. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol 2016;127:426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beucher G, Charlier C, Cazanave C. Diagnosis and management of intra-uterine infection: CNGOF preterm premature rupture of membranes guidelines [in French]. Gynecol Obstet Fertil Senol 2018;46:1054–67. [DOI] [PubMed] [Google Scholar]
- 86.Wisner K. Intrapartum management of chorioamnionitis. MCN Am J Matern Child Nurs 2018;43:52. [DOI] [PubMed] [Google Scholar]
- 87.Committee on Obstetric Practice. Committee Opinion No. 712: Intrapartum management of intraamniotic infection. Obstet Gynecol 2017;130:e95–e101. [DOI] [PubMed] [Google Scholar]
- 88.Johnson CT, Adami RR, Farzin A. Antibiotic therapy for chorioamnionitis to reduce the global burden of associated disease. Front Pharmacol 2017;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burke C, Chin EG. Chorioamnionitis at term: definition, diagnosis, and implications for practice. J Perinat Neonatal Nurs 2016;30:106–14. [DOI] [PubMed] [Google Scholar]
- 90.WHO recommendations for the prevention and treatment of maternal peripartum infections. World Health Organization, Geneva: 2015. Available at: http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/peripartum-infections-guidelines. Accessed June 20, 2020 [PubMed] [Google Scholar]
- 91.Johnson CT, Farzin A, Burd I. Current management and long-term outcomes following chorioamnionitis. Obstet Gynecol Clin North Am 2014;41:649–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hastings-Tolsma M, Bernard R, Brody MG, Hensley J, Koschoreck K, Patterson E. Chorioamnionitis: prevention and management. MCN Am J Matern Child Nurs 2013;38:206–12. [DOI] [PubMed] [Google Scholar]
- 93.Fishman SG, Gelber SE. Evidence for the clinical management of chorioamnionitis. Semin Fetal Neonatal Med 2012;17:46–50. [DOI] [PubMed] [Google Scholar]
- 94.Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: from pathogenesis to treatment. Clin Microbiol Infect 2011;17:1304–11. [DOI] [PubMed] [Google Scholar]
- 95.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 2010;37:339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fahey JO. Clinical management of intra-amniotic infection and chorioamnionitis: a review of the literature. J Midwifery Womens Health 2008;53:227–35. [DOI] [PubMed] [Google Scholar]
- 97.Newton ER. Preterm labor, preterm premature rupture of membranes, and chorioamnionitis. Clin Perinatol 2005;32:571–600. [DOI] [PubMed] [Google Scholar]
- 98.Gibbs RS. Management of clinical chorioamnionitis at term. Am J Obstet Gynecol 2004;191:1–2. [DOI] [PubMed] [Google Scholar]
- 99.Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol 1988;72:823–8. [DOI] [PubMed] [Google Scholar]
- 100.Scalambrino S, Mangioni C, Milani R, et al. Sulbactam/ampicillin versus cefotetan in the treatment of obstetric and gynecologic infections. Int J Gynecol Obstet 1989;(Suppl 2):21–7. [DOI] [PubMed] [Google Scholar]
- 101.Maberry MC, Gilstrap LC 3rd, Bawdon R, Little BB, Dax J. Anaerobic coverage for intra-amnionic infection: maternal and perinatal impact. Am J Perinatol 1991;8:338–41. [DOI] [PubMed] [Google Scholar]
- 102.Locksmith GJ, Chin A, Vu T, Shattuck KE, Hankins GD. High compared with standard gentamicin dosing for chorioamnionitis: a comparison of maternal and fetal serum drug levels. Obstet Gynecol 2005;105:473–9. [DOI] [PubMed] [Google Scholar]
- 103.Lyell DJ, Pullen K, Fuh K, et al. Daily compared with 8-hour gentamicin for the treatment of intrapartum chorioamnionitis: a randomized controlled trial. Obstet Gynecol 2010;115:344–9. [DOI] [PubMed] [Google Scholar]
- 104.Greenberg M, Yeaton-Massey A, Hazard K, et al. Comparison of ampicillin/sulbactam versus ampicillin/gentamicin for treatment of intrapartum chorioamnionitis: a randomized controlled trial. Am J Obstet Gynecol 2015;212(Suppl):S145. [Google Scholar]
- 105.Berry C, Hansen KA, McCaul JF. Abbreviated antibiotic therapy for clinical chorioamnionitis: a randomized trial. J Matern Fetal Med 1994;3:216–8. [Google Scholar]
- 106.Chapman SJ, Owen J. Randomized trial of single-dose versus multiple-dose cefotetan for the postpartum treatment of intrapartum chorioamnionitis. Am J Obstet Gynecol 1997;177:831–4. [DOI] [PubMed] [Google Scholar]
- 107.Mitra AG, Whitten MK, Laurent SL, Anderson WE. A randomized, prospective study comparing once-daily gentamicin versus thrice-daily gentamicin in the treatment of puerperal infection. Am J Obstet Gynecol 1997;177:786–92. [DOI] [PubMed] [Google Scholar]
- 108.Adashek JA, Asarat T, Lagrew DC, et al. The effect of antibiotics after a vaginal delivery complicated by chorioamnionitis in preventing the development of postpartum endomyometritis: a prospectively randomized double-blind placebo-controlled trial. Am J Obstet Gynecol 1998;178:S212. [Google Scholar]
- 109.Turnquest MA, How HY, Cook CR, O’Rourke TP, Cureton AC, Spinnato JA, Brown HL. Chorioamnionitis: is continuation of antibiotic therapy necessary after cesarean section? Am J Obstet Gynecol 1998;179:1261–6. [DOI] [PubMed] [Google Scholar]
- 110.Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol 2003;102:957–61. [DOI] [PubMed] [Google Scholar]
- 111.Shanks AL, Mehra S, Gross G, Colvin R, Harper LM, Tuuli MG. Treatment utility of postpartum antibiotics in chorioamnionitis study. Am J Perinatol 2016;33:732–7. [DOI] [PubMed] [Google Scholar]
- 112.Goldberg A. Post-partum management of chorioamnionitis: efficacy of continuation of antibiotics after delivery. Obstet Gynecol 2017;129(Suppl):5S. [Google Scholar]
- 113.Chapman E, Reveiz L, Illanes E, Bonfill Cosp X. Antibiotic regimens for management of intra-amniotic infection. Cochrane Database Syst Rev 2014;12:CD010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sperling RS, Ramamurthy RS, Gibbs RS. A comparison of intrapartum versus immediate postpartum treatment of intra-amniotic infection. Obstet Gynecol 1987;70:861–5. [PubMed] [Google Scholar]
- 115.Gilstrap LC 3rd, Leveno KJ, Cox SM, Burris JS, Mashburn M, Rosenfeld CR. Intrapartum treatment of acute chorioamnionitis: impact on neonatal sepsis. Am J Obstet Gynecol 1988;159:579–83. [DOI] [PubMed] [Google Scholar]
- 116.Martingano D, Renson A, Rogoff S, Singh S, Kesavan Nasir M, Kim J, Carey J. Daily gentamicin using ideal body weight demonstrates lower risk of postpartum endometritis and increased chance of successful outcome compared with traditional 8-hour dosing for the treatment of intrapartum chorioamnionitis. J Matern Fetal Neonatal Med 2019;32:3204–8. [DOI] [PubMed] [Google Scholar]
- 117.Sweeney EL, Dando SJ, Kallapur SG, Knox CL. The human ureaplasma species as causative agents of chorioamnionitis. Clin Microbiol Rev 2016;30:349–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Silwedel C, Speer CP, Glaser K. Ureaplasma-associated prenatal, perinatal, and neonatal morbidities. Expert Rev Clin Immunol 2017;13:1073–87. [DOI] [PubMed] [Google Scholar]
- 119.Murtha AP, Edwards JM. The role of Mycoplasma and Ureaplasma in adverse pregnancy outcomes. Obstet Gynecol Clin North Am 2014;41:615–27. [DOI] [PubMed] [Google Scholar]
- 120.Tantengco OAG, Yanagihara I. Current understanding and treatment of intraamniotic infection with Ureaplasma spp. J Obstet Gynaecol Res 2019;45:1796–1808. [DOI] [PubMed] [Google Scholar]
- 121.Lee J, Romero R, Kim SM, et al. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J Matern Fetal Neonatal Med 2016;29:707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee J, Romero R, Kim SM, Chaemsaithong P, Yoon BH. A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM. J Matern Fetal Neonatal Med 2016;29:2727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoon BH, Romero R, Park JY, et al. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol 2019;221:142.e1–142.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oh KJ, Romero R, Park JY, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol 2019;221:140.e1–140.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gravett MG. Successful treatment of intraamniotic infection/inflammation: a paradigm shift. Am J Obstet Gynecol 2019;221:83–5. [DOI] [PubMed] [Google Scholar]
- 126.Chorioamnionitis. Edmonton Zone Women’s Health Program Clinical Practice Guidelines. Available at: http://extcontent.covenanthealth.ca/Policy/Chorioamnionitis_Final_June_2014.pdf. Accessed June 20, 2020 [Google Scholar]
- 127.Maberry MC, Gilstrap LC 3rd. Intrapartum antibiotic therapy for suspected intraamniotic infection: impact on the fetus and neonate. Clin Obstet Gynecol 1991;34:345–51. [DOI] [PubMed] [Google Scholar]
- 128.Guideline for management of suspected chorioamnionitis. Worcestershire Acute Hospitals NHS Trust. Available at: http://www2.worcsacute.nhs.uk/search/?q=chorioamnionitis. Accessed on June 20, 2020 [Google Scholar]
- 129.Sospecha de Corioamnionitis y corioamnionitis clínica. Protocols Medicina Materno fetal Hospital Clinic – Hospital Sant Joan de Deu-Universitat de Barcelona. Available at: https://medicinafetalbarcelona.org/protocolos/es/patologia-materna-obstetrica/corioamnionitis.pdf. Accessed June 20, 2020 [Google Scholar]
- 130.Burgess APH, Katz JE, Moretti M, Lakhi N. Risk factors for intrapartum fever in term gestations and associated maternal and neonatal sequelae. Gynecol Obstet Invest 2017;82:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang C, Sirluck Schroeder I, Sosa Cazales A, et al. Management of fever in labor after institution of a standardized order set at a maternity quaternary care center. Am J Obstet Gynecol 2019;221:687. [Google Scholar]
- 132.Reilly DR, Oppenheimer LW. Fever in term labour. J Obstet Gynaecol Can 2005;27:218–23. [DOI] [PubMed] [Google Scholar]
- 133.Ashwal E, Salman L, Tzur Y, et al. Intrapartum fever and the risk for perinatal complications - the effect of fever duration and positive cultures. J Matern Fetal Neonatal Med 2018;31:1418–25. [DOI] [PubMed] [Google Scholar]
- 134.Lieberman E, Cohen A, Lang J, Frigoletto F, Goetzl L. Maternal intrapartum temperature elevation as a risk factor for cesarean delivery and assisted vaginal delivery. Am J Public Health 1999;89:506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dior UP, Kogan L, Eventov-Friedman S, et al. Very high intrapartum fever in term pregnancies and adverse obstetric and neonatal outcomes. Neonatology 2016;109:62–8. [DOI] [PubMed] [Google Scholar]
- 136.Lange EMS, Segal S, Pancaro C, et al. Association between intrapartum magnesium administration and the incidence of maternal fever: a retrospective cross-sectional study. Anesthesiology 2017;127:942–52. [DOI] [PubMed] [Google Scholar]
- 137.Perlman JM. Hyperthermia in the delivery: potential impact on neonatal mortality and morbidity. Clin Perinatol 2006;33:55–63. [DOI] [PubMed] [Google Scholar]
- 138.Blume HK, Li CI, Loch CM, Koepsell TD. Intrapartum fever and chorioamnionitis as risks for encephalopathy in term newborns: a case-control study. Dev Med Child Neurol 2008;50:19–24. [DOI] [PubMed] [Google Scholar]
- 139.Greenwell EA, Wyshak G, Ringer SA, Johnson LC, Rivkin MJ, Lieberman E. Intrapartum temperature elevation, epidural use, and adverse outcome in term infants. Pediatrics 2012;129:e447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Törnell S, Ekéus C, Hultin M, Håkansson S, Thunberg J, Högberg U. Low Apgar score, neonatal encephalopathy and epidural analgesia during labour: a Swedish registry-based study. Acta Anaesthesiol Scand 2015;59:486–95. [DOI] [PubMed] [Google Scholar]
- 141.Torres Yordán NC, Valentin EE, Lewis AG, Gordon DA, Robinson JN, Greenberg J. Effects of intrapartum fever on umbilical artery pH, lactate and base excess. Am J Obstet Gynecol 2020;222(Suppl 1):S227. [Google Scholar]
- 142.Badawi N, Kurinczuk JJ, Keogh JM, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ 1998;317:1554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Impey LW, Greenwood CE, Black RS, Yeh PS, Sheil O, Doyle P. The relationship between intrapartum maternal fever and neonatal acidosis as risk factors for neonatal encephalopathy. Am J Obstet Gynecol 2008;198:49.e1–6. [DOI] [PubMed] [Google Scholar]
- 144.Kasdorf E, Perlman JM. Hyperthermia, inflammation, and perinatal brain injury. Pediatr Neurol 2013;49:8–14. [DOI] [PubMed] [Google Scholar]
- 145.Li C, Miao JK, Xu Y, et al. Prenatal, perinatal and neonatal risk factors for perinatal arterial ischaemic stroke: a systematic review and meta-analysis. Eur J Neurol 2017;24:1006–15. [DOI] [PubMed] [Google Scholar]
- 146.Petrova A, Demissie K, Rhoads GG, Smulian JC, Marcella S, Ananth CV. Association of maternal fever during labor with neonatal and infant morbidity and mortality. Obstet Gynecol 2001;98:20–7. [DOI] [PubMed] [Google Scholar]
- 147.Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998–2002. J Pediatr 2009;154:24–28.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spain JE, Tuuli MG, Macones GA, Roehl KA, Odibo AO, Cahill AG. Risk factors for serious morbidity in term nonanomalous neonates. Am J Obstet Gynecol 2015;212:799.e1–7. [DOI] [PubMed] [Google Scholar]
- 149.Nitsche JF, Patil AS, Langman LJ, et al. Transplacental passage of acetaminophen in term pregnancy. Am J Perinatol 2017;34:541–3. [DOI] [PubMed] [Google Scholar]
- 150.Kirshon B, Moise KJ Jr, Wasserstrum N. Effect of acetaminophen on fetal acid-base balance in chorioamnionitis. J Reprod Med 1989;34:955–9. [PubMed] [Google Scholar]
- 151.Lavesson T, Åkerman F, Källén K, Olofsson P. Effects on fetal and maternal temperatures of paracetamol administration during labour: a case-control study. Eur J Obstet Gynecol Reprod Biol 2013;168:138–44. [DOI] [PubMed] [Google Scholar]
- 152.Morrison CL, Glenn LL. Statistical significance of paracetamol administration in fetal and maternal body temperatures. Eur J Obstet Gynecol Reprod Biol 2013;171:191. [DOI] [PubMed] [Google Scholar]
- 153.Holmér Pettersson P, Owall A, Jakobsson J. Early bioavailability of paracetamol after oral or intravenous administration. Acta Anaesthesiol Scand 2004;48:867–70. [DOI] [PubMed] [Google Scholar]
- 154.Ope-Adenuga S, Burgess APH, Reilly JG, Moretti M, Lakhi N. Intravenous acetaminophen for the treatment of intrapartum fever and resolution of fetal tachycardia: a novel use for an old medication. Clin Exp Obstet Gynecol 2017;44:166–8. [PubMed] [Google Scholar]
- 155.Reduction of intrapartum fever with intravenous acetaminophen (RIFIVA). Available at: https://clinicaltrials.gov/ct2/show/NCT02625454. Accessed June 25, 2020.
- 156.Committee on Obstetric Practice. Committee opinion No. 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol 2017;130:e102–e109. [DOI] [PubMed] [Google Scholar]
- 157.National Institute for Health and Care Excellence. Preterm labor and birth. NICE guideline 25. 2015. Available at: https://www.nice.org.uk/guidance/ng25/evidence/full-guideline-2176838029. Accessed June 25, 2020. [PubMed] [Google Scholar]
- 158.Antenatal Corticosteroid Clinical Practice Guidelines Panel. Antenatal corticosteroids given to women prior to birth to improve fetal, infant, child and adult health: Clinical Practice Guidelines. 2015. Liggins Institute, The University of Auckland, Auckland. New Zealand. [Google Scholar]
- 159.Skoll A, Boutin A, Bujold E, et al. No. 364-Antenatal Corticosteroid Therapy for Improving Neonatal Outcomes. J Obstet Gynaecol Can 2018;40:1219–39. [DOI] [PubMed] [Google Scholar]
- 160.WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. Geneva: World Health Organization; 2015. Available at: https://apps.who.int/iris/bitstream/handle/10665/183037/9789241508988_eng.pdf;jsessionid=87E3C5054297C41C337B19C9CA0F660F?sequence=1. Accessed June 25, 2020. [PubMed] [Google Scholar]
- 161.FIGO Working Group on Good Clinical Practice in Maternal-Fetal Medicine. Good clinical practice advice: antenatal corticosteroids for fetal lung maturation. Int J Gynaecol Obstet 2019;144:352–5.30710360 [Google Scholar]
- 162.Royal College of Obstetricians and Gynaecologists. Green—top guideline No. 7: antenatal corticosteroids to reduce neonatal morbidity and mortality. London: RCOG, 2010. Available at: https://www.glowm.com/pdf/Antenatal%20Corticosteroids%20to%20Reduce%20Neonatal%20Morbidity.pdf. Accessed June 25, 2020. [DOI] [PubMed] [Google Scholar]
- 163.Child Health Division, Ministry of Health and Family Welfare, Government of India. Use of antenatal corticosteroids in preterm labour. (Under specific conditions by ANM). Operational guidelines, 2014. Available at: http://www.nrhmorissa.gov.in/writereaddata/Upload/Documents/Operational%20Guidelines-Use%20of%20Antenatal%20Corticosteroids%20in%20Preterm%20Labour.pdf. Accessed June 25, 2020. [Google Scholar]
- 164.Miracle X, Di Renzo GC, Stark A, Fanaroff A, Carbonell-Estrany X, Saling E; Coordinators of World Association of Perinatal Medicine Prematurity Working Group. Guideline for the use of antenatal corticosteroids for fetal maturation. J Perinat Med 2008;36:191–6. [DOI] [PubMed] [Google Scholar]
- 165.Jobe AH, Goldenberg RL. Antenatal corticosteroids: an assessment of anticipated benefits and potential risks. Am J Obstet Gynecol 2018;219:62–74. [DOI] [PubMed] [Google Scholar]
- 166.American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus No. 6: periviable birth. Obstet Gynecol 2017;130:e187–e199. [DOI] [PubMed] [Google Scholar]
- 167.Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol 2016;215:B13–5. [DOI] [PubMed] [Google Scholar]
- 168.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Been JV, Degraeuwe PL, Kramer BW, Zimmermann LJ. Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis. BJOG 2011;118:113–22. [DOI] [PubMed] [Google Scholar]
- 170.Baud O, Zupan V, Lacaze-Masmonteil T, et al. The relationships between antenatal management, the cause of delivery and neonatal outcome in a large cohort of very preterm singleton infants. BJOG 2000;107:877–84. [DOI] [PubMed] [Google Scholar]
- 171.Elimian A, Verma U, Beneck D, Cipriano R, Visintainer P, Tejani N. Histologic chorioamnionitis, antenatal steroids, and perinatal outcomes. Obstet Gynecol 2000;96:333–6. [DOI] [PubMed] [Google Scholar]
- 172.Foix-L’helias L, Baud O, Lenclen R, Kaminski M, Lacaze-Masmonteil T. Benefit of antenatal glucocorticoids according to the cause of very premature birth. Arch Dis Child Fetal Neonatal Ed 2005;90:F46–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dempsey E, Chen MF, Kokottis T, Vallerand D, Usher R. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am J Perinatol 2005;22:155–9. [DOI] [PubMed] [Google Scholar]
- 174.Kent A, Lomas F, Hurrion E, Dahlstrom JE. Antenatal steroids may reduce adverse neurological outcome following chorioamnionitis: neurodevelopmental outcome and chorioamnionitis in premature infants. J Paediatr Child Health 2005;41:186–90. [DOI] [PubMed] [Google Scholar]
- 175.Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol 2006;195:1020–4. [DOI] [PubMed] [Google Scholar]
- 176.Been JV, Rours IG, Kornelisse RF, et al. Histologic chorioamnionitis, fetal involvement, and antenatal steroids: effects on neonatal outcome in preterm infants. Am J Obstet Gynecol 2009;201:587.e1–8. [DOI] [PubMed] [Google Scholar]
- 177.Amiya RM, Mlunde LB, Ota E, Swa T, Oladapo OT, Mori R. Antenatal corticosteroids for reducing adverse maternal and child outcomes in special populations of women at risk of imminent preterm birth: a systematic review and meta-analysis. PLoS One 2016;11:e0147604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ahn HM, Park EA, Cho SJ, Kim YJ, Park HS. The association of histological chorioamnionitis and antenatal steroids on neonatal outcome in preterm infants born at less than thirty-four weeks’ gestation. Neonatology 2012;102:259–64. [DOI] [PubMed] [Google Scholar]
- 179.Miyazaki K, Furuhashi M, Ishikawa K, et al. The effects of antenatal corticosteroids therapy on very preterm infants after chorioamnionitis. Arch Gynecol Obstet 2014;289:1185–90. [DOI] [PubMed] [Google Scholar]
- 180.Ryu YH, Oh S, Sohn J, Lee J. The associations between antenatal corticosteroids and in-hospital outcomes of preterm singleton appropriate for gestational age neonates according to the presence of maternal histologic chorioamnionitis. Neonatology 2019;116:369–75. [DOI] [PubMed] [Google Scholar]
- 181.Miyazaki K, Furuhashi M, Ishikawa K, et al. Long-term outcomes of antenatal corticosteroids treatment in very preterm infants after chorioamnionitis. Arch Gynecol Obstet 2015;292:1239–46. [DOI] [PubMed] [Google Scholar]
- 182.Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–6. [DOI] [PubMed] [Google Scholar]
- 183.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51. [DOI] [PubMed] [Google Scholar]
- 184.Asrat T. Intra-amniotic infection in patients with preterm prelabor rupture of membranes. Pathophysiology, detection, and management. Clin Perinatol 2001;28:735–51. [DOI] [PubMed] [Google Scholar]
- 185.Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol 2007;197:292.e1–5. [DOI] [PubMed] [Google Scholar]
- 186.DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Musilova I, Kutová R, Pliskova L, et al. Intraamniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One 2015;10:e0133929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Oh KJ, Lee JH, Romero R, Park HS, Joon-Seok H, Yoon BH. A new rapid bedside test to diagnose and monitor intra-amniotic inflammation in preterm PROM using transcervically collected fluid. Am J Obstet Gynecol. 2020. February 27. pii: S0002–9378(20)30225–8. doi: 10.1016/j.ajog.2020.02.037. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol 1982;59:539–45. [PubMed] [Google Scholar]
- 191.Duff P, Sanders R, Gibbs RS. The course of labor in term patients with chorioamnionitis. Am J Obstet Gynecol 1983;147:391–5. [DOI] [PubMed] [Google Scholar]
- 192.Hauth JC, Gilstrap LC 3rd, Hankins GD, Connor KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol 1985;66:59–62. [PubMed] [Google Scholar]
- 193.Wendel PJ, Cox SM, Roberts SW, Dax J, Gilstrap LC. Chorioamnionitis: association of nonreassuring fetal heart-rate patterns and interval from diagnosis to delivery on neonatal outcome. Infect Dis Obstet Gynecol 1994;2:162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wright LL, Verter J, Younes N, et al. Antenatal corticosteroid administration and neonatal outcome in very low birth weight infants: the NICHD Neonatal Research Network. Am J Obstet Gynecol 1995;173:269–74. [DOI] [PubMed] [Google Scholar]
- 195.Sen S, Reghu A, Ferguson SD. Efficacy of a single dose of antenatal steroid in surfactant-treated babies under 31 weeks’ gestation. J Matern Fetal Neonatal Med 2002;12:298–303. [DOI] [PubMed] [Google Scholar]
- 196.Elimian A, Figueroa R, Spitzer AR, Ogburn PL, Wiencek V, Quirk JG. Antenatal corticosteroids: are incomplete courses beneficial? Obstet Gynecol 2003;102:352–5. [DOI] [PubMed] [Google Scholar]
- 197.Chawla S, Natarajan G, Shankaran S, et al. Association of neurodevelopmental outcomes and neonatal morbidities of extremely premature infants with differential exposure to antenatal steroids. JAMA Pediatr 2016;170:1164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Norman M, Piedvache A, Børch K, et al. Association of short antenatal corticosteroid administration-to-birth intervals with survival and morbidity among very preterm infants: results from the EPICE cohort. JAMA Pediatr 2017;171:678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Norberg H, Kowalski J, Maršál K, Norman M. Timing of antenatal corticosteroid administration and survival in extremely preterm infants: a national population-based cohort study. BJOG 2017;124:1567–74. [DOI] [PubMed] [Google Scholar]
- 200.Travers CP, Carlo WA, McDonald SA, et al. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am J Obstet Gynecol 2018;218:130.e1–130.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2010;9:CD004454. [DOI] [PubMed] [Google Scholar]
- 202.Conde-Agudelo A, Romero R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: a systematic review and metaanalysis. Am J Obstet Gynecol 2009;200:595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev 2009;1:CD004661. [DOI] [PubMed] [Google Scholar]
- 204.Costantine MM, Weiner SJ. Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: a meta-analysis. Obstet Gynecol 2009;114:354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, Marret S, Doyle LW; AMICABLE Group. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis. PLoS Med 2017;14:e1002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.The Antenatal Magnesium Sulphate for Neuroprotection Guideline Development Panel. Antenatal magnesium sulphate prior to preterm birth for neuroprotection of the fetus, infant and child: national clinical practice guidelines. Adelaide: The University of Adelaide, 2010. [Google Scholar]
- 207.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 171: management of preterm labor. Obstet Gynecol 2016;128:e155–64. [DOI] [PubMed] [Google Scholar]
- 208.Magee LA, De Silva DA, Sawchuck D, Synnes A, von Dadelszen P. No. 376-Magnesium sulphate for fetal neuroprotection. J Obstet Gynaecol Can 2019;41:505–22. [DOI] [PubMed] [Google Scholar]
- 209.Mittendorf R, Covert R, Boman J, Khoshnood B, Lee KS, Siegler M. Is tocolytic magnesium sulphate associated with increased total paediatric mortality? Lancet 1997;350:1517–8. [DOI] [PubMed] [Google Scholar]
- 210.Mittendorf R, Dambrosia J, Pryde PG, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol 2002;186:1111–8. [DOI] [PubMed] [Google Scholar]
- 211.Crowther CA, Hiller JE, Doyle LW, Haslam RR; Australasian Collaborative Trial of Magnesium Sulphate (ACTOMg SO4) Collaborative Group. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA 2003;290:2669–76. [DOI] [PubMed] [Google Scholar]
- 212.Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 2002;359:1877–90. [DOI] [PubMed] [Google Scholar]
- 213.Marret S, Marpeau L, Zupan-Simunek V, et al. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial*. BJOG 2007;114:310–8. [DOI] [PubMed] [Google Scholar]
- 214.Marret S, Marpeau L, Bénichou J. Benefit of magnesium sulfate given before very preterm birth to protect infant brain. Pediatrics 2008;121:225–6. [DOI] [PubMed] [Google Scholar]
- 215.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med 2008;359:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Edwards JM, Edwards LE, Swamy GK, Grotegut CA. Magnesium sulfate for neuroprotection in the setting of chorioamnionitis. J Matern Fetal Neonatal Med 2018;31:1156–60. [DOI] [PubMed] [Google Scholar]
- 217.Hallak M, Berry SM, Madincea F, Romero R, Evans MI, Cotton DB. Fetal serum and amniotic fluid magnesium concentrations with maternal treatment. Obstet Gynecol 1993;81:185–8. [PubMed] [Google Scholar]
- 218.Smith CA, Crowther CA, Willson K, Hiller JE, Doyle LW. Placental transfer of magnesium sulphate: a randomised placebo controlled trial. Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9–12; Tasmania, Australia. 2003:P48. [Google Scholar]
- 219.Brookfield KF, Su F, Elkomy MH, Drover DR, Lyell DJ, Carvalho B. Pharmacokinetics and placental transfer of magnesium sulfate in pregnant women. Am J Obstet Gynecol 2016;214:737.e1–9. [DOI] [PubMed] [Google Scholar]
- 220.Locatelli A, Vergani P, Ghidini A, et al. Duration of labor and risk of cerebral white-matter damage in very preterm infants who are delivered with intrauterine infection. Am J Obstet Gynecol 2005;193:928–32. [DOI] [PubMed] [Google Scholar]
- 221.Satin AJ, Maberry MC, Leveno KJ, Sherman ML, Kline DM. Chorioamnionitis: a harbinger of dystocia. Obstet Gynecol 1992;79:913–5. [PubMed] [Google Scholar]
- 222.Alexander JM, McIntire DM, Leveno KJ. Chorioamnionitis and the prognosis for term infants. Obstet Gynecol 1999;94:274–8. [DOI] [PubMed] [Google Scholar]
- 223.Mark SP, Croughan-Minihane MS, Kilpatrick SJ. Chorioamnionitis and uterine function. Obstet Gynecol 2000;95:909–12. [PubMed] [Google Scholar]
- 224.Maberry MC, Ramin SM, Gilstrap LC 3rd, Leveno KJ, Dax JS. Intrapartum asphyxia in pregnancies complicated by intra-amniotic infection. Obstet Gynecol 1990;76:351–4. [PubMed] [Google Scholar]
- 225.Zackler A, Flood P, Dajao R, Maramara L, Goetzl L. Suspected chorioamnionitis and myometrial contractility: mechanisms for increased risk of cesarean delivery and postpartum hemorrhage. Reprod Sci 2019;26:178–83. [DOI] [PubMed] [Google Scholar]
- 226.Leek BF, McDonald D, Vaughan J. The spontaneous motility of human myometrial strips in vitro and its modification by some bacterial extracts. J Physiol 1982;324(Suppl):42P. [Google Scholar]
- 227.Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use of the nonstress test in patients with premature rupture of the membranes. Am J Obstet Gynecol 1986;155:149–53. [DOI] [PubMed] [Google Scholar]
- 228.Sameshima H, Ikenoue T, Ikeda T, Kamitomo M, Ibara S. Association of nonreassuring fetal heart rate patterns and subsequent cerebral palsy in pregnancies with intrauterine bacterial infection. Am J Perinatol 2005;22:181–7. [DOI] [PubMed] [Google Scholar]
- 229.Galli L, Dall’Asta A, Whelehan V, Archer A, Chandraharan E. Intrapartum cardiotocography patterns observed in suspected clinical and subclinical chorioamnionitis in term fetuses. J Obstet Gynaecol Res 2019;45:2343–50. [DOI] [PubMed] [Google Scholar]
- 230.Preti M, Chandraharan E. Importance of fetal heart rate cycling during the interpretation of the cardiotocograph (CTG). Int J Gynecol and Reprod Sci 2018;1:10–2. [Google Scholar]
- 231.Moore J, Chandraharan E. Role of chorioamnionitis and infection. In Chandraharan E, ed. Handbook of CTG interpretation: from patterns to physiology. Cambridge: Cambridge University Press; 2017:71–7. [Google Scholar]
- 232.Alfirevic Z, Devane D, Gyte GM, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev 2017;2:CD006066. [DOI] [PubMed] [Google Scholar]
- 233.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol 2009;114:192–202. [DOI] [PubMed] [Google Scholar]
- 234.Ayres-de-Campos D, Spong CY, Chandraharan E; FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int J Gynaecol Obstet 2015;131:13–24. [DOI] [PubMed] [Google Scholar]
- 235.National Institute for Health and Care Excellence. NICE Pathways. Fetal monitoring during labour. 2020. Available at: https://pathways.nice.org.uk/pathways/intrapartum-care/fetal-monitoring-during-labour. Accessed June 30, 2020. [Google Scholar]
- 236.Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita AT. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. Am J Obstet Gynecol 2013;209:51.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol 2017;130:527–38. [DOI] [PubMed] [Google Scholar]
- 238.Haas DM, Morgan S, Contreras K, Enders S. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev 2018;7:CD007892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Roeckner JT, Sanchez-Ramos L, Mitta M, Kovacs A, Kaunitz AM. Povidoneiodine 1% is the most effective vaginal antiseptic for preventing post-cesarean endometritis: a systematic review and network meta-analysis. Am J Obstet Gynecol 2019;221:261.e1–261.e20. [DOI] [PubMed] [Google Scholar]
- 240.Kanninen TT, Lakhi N. Is povidone-iodine 1% the most effective vaginal antiseptic? Am J Obstet Gynecol 2020;222:284. [DOI] [PubMed] [Google Scholar]
- 241.Roeckner JT, Sanchez-Ramos L. Reply. Am J Obstet Gynecol 2020;222:285. [DOI] [PubMed] [Google Scholar]
- 242.Duffy CR, Garcia-So J, Ajemian B, Rubinstein MR, Gyamfi-Bannerman C, Han YW. A randomized trial of the bactericidal effects of chlorhexidine vs. povidone iodine vaginal preparation. Am J Obstet Gynecol 2020;222(Suppl 1):S41. [DOI] [PubMed] [Google Scholar]
- 243.Osborne NG, Wright RC. Effect of preoperative scrub on the bacterial flora of the endocervix and vagina. Obstet Gynecol 1977;50:148–51. [PubMed] [Google Scholar]
- 244.Monif GR, Thompson JL, Stephens HD, Baer H. Quantitative and qualitative effects of povidone-iodine liquid and gel on the aerobic and anaerobic flora of the female genital tract. Am J Obstet Gynecol 1980;137:432–8. [DOI] [PubMed] [Google Scholar]
- 245.Vorherr H, Vorherr UF, Mehta P, Ulrich JA, Messer RH. Antimicrobial effect of chlorhexidine and povidone-iodine on vaginal bacteria. J Infect 1984;8:195–9. [DOI] [PubMed] [Google Scholar]
- 246.Wang X, Svedin P, Nie C, et al. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann Neurol 2007;61:263–71. [DOI] [PubMed] [Google Scholar]
- 247.Beloosesky R, Ginsberg Y, Khatib N, et al. Prophylactic maternal N-acetylcysteine in rats prevents maternal inflammation-induced offspring cerebral injury shown on magnetic resonance imaging. Am J Obstet Gynecol 2013;208:213.e1–6. [DOI] [PubMed] [Google Scholar]
- 248.Sharabi H, Khatib N, Ginsberg Y, et al. Therapeutic N-Acetyl-Cysteine (Nac) following initiation of maternal inflammation attenuates long-term offspring cerebral injury, as evident in magnetic resonance imaging (MRI). Neuroscience 2019;403:118–24. [DOI] [PubMed] [Google Scholar]
- 249.Kannan S, Dai H, Navath RS, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med 2012;4:130ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wiest DB, Chang E, Fanning D, Garner S, Cox T, Jenkins DD. Antenatal pharmacokinetics and placental transfer of N-acetylcysteine in chorioamnionitis for fetal neuroprotection. J Pediatr 2014;165:672–7.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Jenkins DD, Wiest DB, Mulvihill DM, et al. Fetal and neonatal effects of N-Acetylcysteine when used for neuroprotection in maternal chorioamnionitis. J Pediatr 2016;168:67–76.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Buhimschi CS, Bahtiyar MO, Abdelghany O, et al. Randomized controlled trial of n-acetylcysteine to prevent adverse neonatal outcome in pregnancies with intra-amniotic infection/inflammation. Am J Obstet Gynecol 2019;220(Suppl):S9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


