ABSTRACT
Increasing problems with antibiotic resistance have directed interest toward phage therapy in the aquaculture industry. However, phage resistance evolving in target bacteria is considered a challenge. To investigate how phage resistance influences the fish pathogen Flavobacterium columnare, two wild-type bacterial isolates, FCO-F2 and FCO-F9, were exposed to phages (FCO-F2 to FCOV-F2, FCOV-F5, and FCOV-F25, and FCO-F9 to FCL-2, FCOV-F13, and FCOV-F45), and resulting phenotypic and genetic changes in bacteria were analyzed. Bacterial viability first decreased in the exposure cultures but started to increase after 1 to 2 days, along with a change in colony morphology from original rhizoid to rough, leading to 98% prevalence of the rough morphotype. Twenty-four isolates (including four isolates from no-phage treatments) were further characterized for phage resistance, antibiotic susceptibility, motility, adhesion, and biofilm formation, protease activity, whole-genome sequencing, and virulence in rainbow trout fry. The rough isolates arising in phage exposure were phage resistant with low virulence, whereas rhizoid isolates maintained phage susceptibility and high virulence. Gliding motility and protease activity were also related to the phage susceptibility. Observed mutations in phage-resistant isolates were mostly located in genes encoding the type IX secretion system, a component of the Bacteroidetes gliding motility machinery. However, not all phage-resistant isolates had mutations, indicating that phage resistance in F. columnare is a multifactorial process, including both genetic mutations and changes in gene expression. Phage resistance may not, however, be a challenge for development of phage therapy against F. columnare infections since phage resistance is associated with decreases in bacterial virulence.
IMPORTANCE Phage resistance of infectious bacteria is a common phenomenon posing challenges for the development of phage therapy. Along with a growing world population and the need for increased food production, constantly intensifying animal farming has to face increasing problems of infectious diseases. Columnaris disease, caused by Flavobacterium columnare, is a worldwide threat for salmonid fry and juvenile farming. Without antibiotic treatments, infections can lead to 100% mortality in a fish stock. Phage therapy of columnaris disease would reduce the development of antibiotic-resistant bacteria and antibiotic loads by the aquaculture industry, but phage-resistant bacterial isolates may become a risk. However, phenotypic and genetic characterization of phage-resistant F. columnare isolates in this study revealed that they are less virulent than phage-susceptible isolates and thus not a challenge for phage therapy against columnaris disease. This is valuable information for the fish farming industry globally when considering phage-based prevention and curing methods for F. columnare infections.
KEYWORDS: bacteriophage, colony morphology, Flavobacterium columnare, gliding motility, mutation, phage resistance, type IX secretion system, virulence
INTRODUCTION
Aquaculture has a central role in supporting the increasing demand for high quality protein and healthy food. However, the use of chemotherapy in disease treatment in the industry has led to increased resistance of disease-causing agents to commonly used antibiotics (1, 2). Further, in the face of climate warming, the production of protein with a smaller carbon footprint is of increasing importance. This has put pressure on the aquaculture industry to increase efficiency in food production, which also means developing more effective ways to fight infectious diseases in intensive farming, including reducing the use of antibiotics. Although vaccines against many microbial diseases are in use globally in aquaculture, there are still many diseases with no potent immunization method available (3). This applies especially to infections of fish fry, where efficiency of vaccination is poor due to lack of development of fish secondary immunity at the early life stage.
One of these diseases affecting fry is caused by the fish pathogenic bacterium Flavobacterium columnare, the infectious agent of columnaris disease. Columnaris infections cause extensive losses in farmed salmonid fry and juveniles, populations of different catfish species, and ayu (Plecoglossus altivelis) around the world in water temperatures above 18°C. The only effective treatment method is antibiotic treatment. However, infections often occur repeatedly and may cause up to 100% mortality in rainbow trout fry populations if not treated, thus causing major economic losses to the industry (4, 5). In addition, elevated water temperatures due to warmer summers in the recent years are suggested to enhance virulence development in F. columnare (5). Although antibiotic resistance in this bacterium is not yet as severe a problem as in related pathogens (e.g., Flavobacterium psychrophilum [6, 7] or Vibrio species [8, 9]), strains that have acquired resistance toward commonly used antibiotics already exist (10).
Bacteriophages (phages) are viruses that specifically infect their host bacteria without harming the surrounding microbial community (reviewed in reference 11). Among the alternatives to traditional antibiotics, phage therapy (i.e., the use of phages against bacterial infections) has demonstrated a strong potential for controlling disease outbreaks in aquaculture (12–14). Promising results have been gained also in phage therapy trials of flavobacterial infections. In a study by Castillo et al. (15), phage treatment reduced the mortality of F. psychrophilum-infected Atlantic salmon (Salmo salar) by 60% and rainbow trout (Oncorhynchus mykiss) by 67%. In studies with columnaris infections, mortality of zebra fish (Danio rerio) and rainbow trout were reduced by 100% and nearly 42%, respectively, in the presence of phages (16). In addition, precolonization of fish with phage significantly slowed down the infection and reduced the mortality of rainbow trout (17).
One of the biggest challenges for phage therapy is the imposed selection for phage resistance among phage-exposed bacteria. Bacteria have developed a variety of phage defense strategies, including surface modification and cell aggregation, inactivation of intruding phage DNA by restriction modification and CRISPR-Cas systems, proteolytic digestion of phage particles, and quorum sensing regulation of phage receptor expression (18–20). Prevalence and control of these resistance mechanisms depend specifically on the phage-bacterium interaction, on the type and function of the receptor, and the costs of engaging the different mechanisms under various environmental conditions. In many pathogenic bacteria, the cell surface molecules are functioning as virulence factors, and phage-driven changes in these structures leading to phage resistance often lead to simultaneous reduction in virulence (21). This trade-off has been detected also among several bacterial fish pathogens (e.g., in Pseudomonas plecoglossicida [22], F. psychrophilum [23], and Vibrio anguillarum [24]).
Exposing F. columnare to phages has been observed to cause a change in colony morpohotype from the ancestral rhizoid form to rough form, which is associated with loss of gliding motility and virulence (25–27). Since a change in colony morphology and loss of virulence have been observed previously by deletion of genes in the type IX secretion system involved in gliding motility of F. columnare (28), it is likely that mutations in this secretion system are also linked with phage resistance in F. columnare (29). Yet, the exact mechanisms by which phages select for colony morphology change in F. columnare, and the functional implications for the bacteria have not been previously explored.
Understanding the mechanisms and consequences of phage resistance in the target bacteria is central for development of successful phage therapy. Thus, in this study, we exposed two F. columnare isolates (FCO-F2 and FCO-F9) separately to three different phages and studied infection dynamics, bacterial viability, and colony morphology and isolated phage-resistant bacteria. Twenty-four phage-exposed and no-phage control isolates were further characterized for their phage resistance, antibiotic susceptibility, motility, adhesion, and biofilm formation on a polystyrene surface, protease (elastinase, gelatinase, and caseinase) activity, virulence on rainbow trout fry, and whole-genome sequence. Our results show that if phage resistance in F. columnare is gained via surface modification leading to morphotype change, virulence decreases. However, if the colony morphology remains rhizoid, the isolates remain highly virulent with reduced susceptibility to phage compared to the ancestral wild-type strain.
RESULTS
Isolates from phage exposures: growth, colony morphology, and phage resistance.
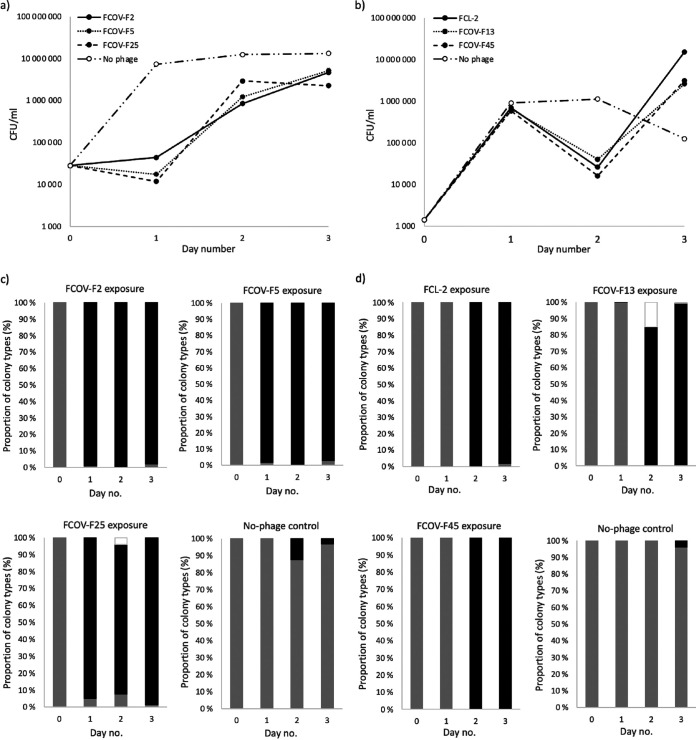
In all phage exposure cultures of FCO-F2, there was a strong initial phage control of the host population during the first day in all the phage-exposed cultures compared with control culture without phages (Fig. 1a). After this, the bacterial density started to recover. The phage-free cultures grew exponentially during the first day, after which they reached a plateau phase. Along with the population decline on day 1, bacterial colony morphotype changed from ancestral rhizoid to rough (Fig. 2). From day 1 onwards, more than 88% of the colonies formed by phage-exposed bacterial isolates were rough, the amount reaching at least 98% at the end of the experiment (Fig. 1c). In addition, in FCOV-F25 exposure, few soft colonies were observed on day 2 (Fig. 2), and in no-phage control cultures, some rough colonies appeared among the prevailing rhizoid colonies.
FIG 1.
(a, b) Bacterial growth determined from three replicate phage exposure and no-phage control cultures represented as CFU ml−1 (± standard error [SE]). (c, d) Proportion (%) of different colony types of Flavobacterium columnare isolates FCO-F2 (c) and FCO-F9 (d) during the 3-day exposure to phages FCOV-F2, FCOV-F5, FCOV-F25, FCL-2, FCOV-F13, and FCOV-F45. The dark gray bar indicates the proportion of isolates forming a rhizoid colony morphology, the black bar indicates the proportion of isolates forming a rough colony morphology, and the white bar indicates the proportion of isolates forming a soft colony morphology.
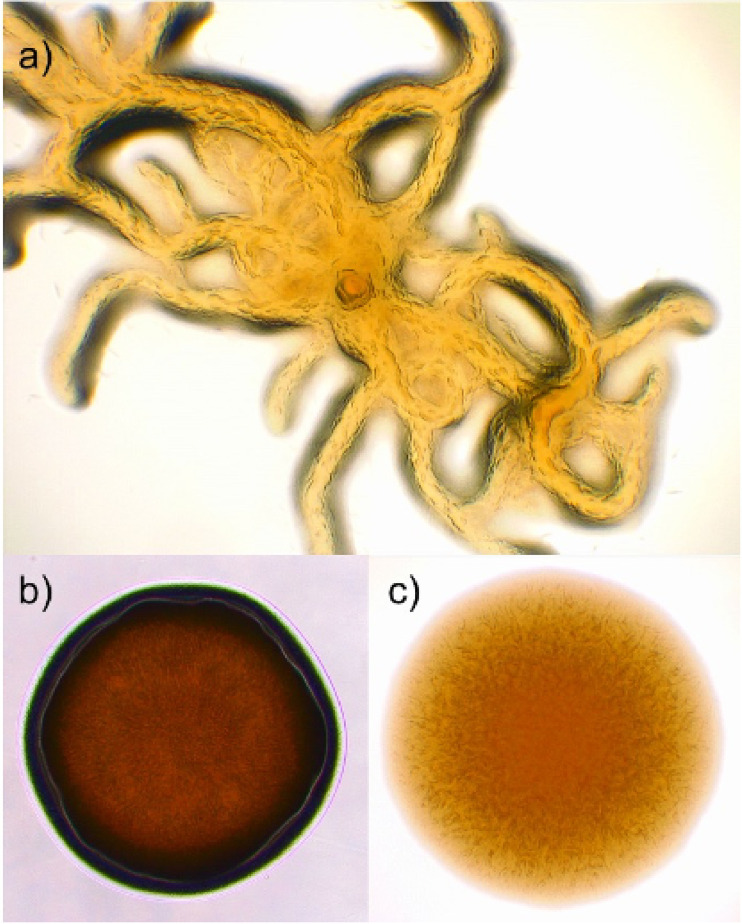
FIG 2.
Different colony morphologies formed by Flavobacterium columnare on Shieh agar plates after phage exposure: (a) rhizoid, (b) rough, and (c) soft. For the approximate size of each colony morphology, see Fig. 3.
FCO-F9 showed slightly different growth dynamics. The bacterial population size increased exponentially during the first day in all cultures (Fig. 1b) but decreased drastically on day 2 in response to phage exposure and then reached exponential growth again. The phage-free cultures reached a plateau phase on day 2, after which the amount of culturable bacteria decreased. From the day 2 population crash and onwards, more than 85% of the colonies formed by phage-exposed bacteria had rough morphology (Fig. 1d). At the end of the experiment, more than 98% of the colonies were rough. In FCOV-F13 exposure, a few rough colonies were observed already on day 1 and some soft colonies on days 2 and 3. In no-phage control cultures, some (4%) rough colonies appeared among the rhizoid colonies on day 3.
Out of 189 colonies collected from phage exposures, 20 phage-exposed and 4 no-phage control isolates were characterized further (Table 1). Of these isolates, the no-phage control isolates all formed rhizoid colonies similar to their wild-type parent phage-susceptible isolates FCO-F2 and FCO-F9. Most of the phage-exposed isolates were of rough colony morphology, but F2R58, F2R66, and F9R56 had a rhizoid morphology and F9R69 had a soft colony morphology. It should be noted, however, that rough colonies also appeared spontaneously in the no-phage control treatments (Fig. 1).
TABLE 1.
Experimental setup of phage exposure of two phage-susceptible wild-type Flavobacterium columnare isolates FCO-F2 (high-virulence, genotype C; exposed for phages FCOV-F2, FCOV-F5, and FCOV-F25) and FCO-F9 (medium-virulence, genotype G; exposed for phages FCL-2, FCOV-F13, and FCOV-F45), and colony morphologies and phage susceptibilities of the 20 phage-exposed (F2R- and F9R-) and 4 no-phage control (F2S- and F9S-) isolates obtained from the exposure cultures
| Wild-type isolate | Phage | Phage-exposed isolatea | No-phage control isolatea | Colony morphology of the isolate | Phage susceptibility of the isolateb |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| FCOV-F2 | FCOV-F5 | FCOV-F25 | FCL-2 | FCOV-F13 | FCO-F45 | |||||
| FCO-F2 | Rhizoid | + | + | + | − | − | − | |||
| FCOV-F2 | F2R58 | Rhizoid | ± | ± | ± | − | − | − | ||
| F2R60 | Rough | − | − | − | − | − | − | |||
| F2R62 | Rough | − | − | − | − | − | − | |||
| FCOV-F5 | F2R64 | Rough | − | − | − | − | − | − | ||
| F2R65 | Rough | − | − | − | − | − | − | |||
| F2R66 | Rhizoid | ± | ± | ± | − | − | − | |||
| F2R67 | Rough | − | − | − | − | − | − | |||
| F2R68 | Rough | − | − | − | − | − | − | |||
| FCOV-F25 | F2R70 | Rough | i | i | i | − | − | − | ||
| F2R72 | Rough | − | − | − | − | − | − | |||
| F2R74 | Rough | − | − | − | − | − | − | |||
| No phage | F2S4 | Rhizoid | + | + | + | − | − | − | ||
| F2S17 | Rhizoid | + | + | + | − | − | − | |||
| FCO-F9 | Rhizoid | − | − | − | + | + | + | |||
| FCL-2 | F9R56 | Rhizoid | − | − | − | ± | ± | ± | ||
| F9R58 | Rough | − | − | − | i | i | i | |||
| F9R61 | Rough | − | − | − | i | i | i | |||
| FCOV-F13 | F9R64 | Rough | − | − | − | − | − | − | ||
| F9R66 | Rough | − | − | − | i | i | i | |||
| F9R69 | Soft | − | − | − | i | i | i | |||
| FCOV-F45 | F9R72 | Rough | − | − | − | i | i | i | ||
| F9R75 | Rough | − | − | − | i | i | − | |||
| F9R78 | Rough | − | − | − | − | − | − | |||
| No phage | F9S15 | Rhizoid | − | − | − | + | + | + | ||
| F9S17 | Rhizoid | − | − | − | + | + | + | |||
Isolates are shown according to the phage to which they were exposed.
The following indicate the susceptibility of the isolates to phages used in exposures: +, susceptible; –, resistant; ±, susceptibility decreased compared to the parent wild-type isolate; i, inhibition of bacterial growth, considered phage resistance.
All the phage-exposed rough isolates were resistant to all the phages used to infect the ancestor wild-type bacteria (Table 1). In addition, in some cases, phage caused visible inhibition of bacterial growth in the double layer agar assay, but colonies were considered phage resistant because no clear plaques due to phage infection were detected. The rhizoid phage-exposed isolates turned out to be partly phage resistant, with a 5.5 × 105- to 11 × 105-fold reduction in phage susceptibility (efficiency of plating) compared to the wild-type isolates, depending on the specific phage (results not shown). Throughout this paper, these isolates with decreased phage susceptibility are grouped together with the phage-susceptible isolates.
Antibiotic susceptibility.
In general, phage-resistant isolates showed antibiotic susceptibility patterns similar to the parent wild-type isolates; however, some differences were also observed (Fig. S1 and Table S1 in the supplemental material). For example, in most cases, the inhibition zone of tetracycline (used against columnaris) increased in the phage-resistant strains compared to the ancestral type, which may indicate increased susceptibility. However, the assay was not replicated, so exact conclusions cannot be drawn.
Motility, adhesion, and biofilm formation.
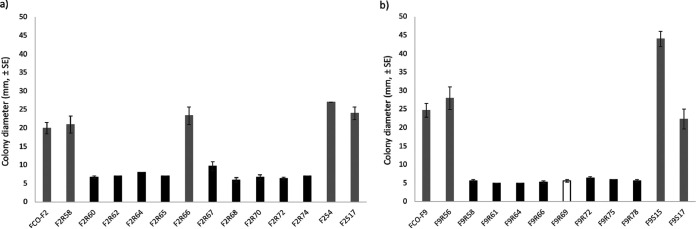
Phage-susceptible bacteria forming rhizoid colonies were significantly more motile (determined as colony spreading) than phage-resistant rough or soft morphotypes, irrespective of isolation history (F2 isolates, P < 0.001, one-way analysis of variance [ANOVA], log10 transformation; F9 isolates, P ≤ 0.004, Mann-Whitney test) (Fig. 3).
FIG 3.
Colony spreading of Flavobacterium columnare wild-type FCO-F2 (a) and FCO-F9 (b) isolates and their phage-exposed (F2R- and F9R-) and no-phage control (F2S- and F9S-) isolates expressed as colony diameter (mm, ±SE) on TYES agar. All the phage-susceptible rhizoid colony-forming isolates (dark gray bars) produced significantly more spreading than phage-resistant rough (black bar) or soft (white bar) morphology isolates (F2 isolates, P < 0.001, one-way ANOVA, log10 transformation; F9 isolates, P ≤ 0.004, Mann-Whitney test).
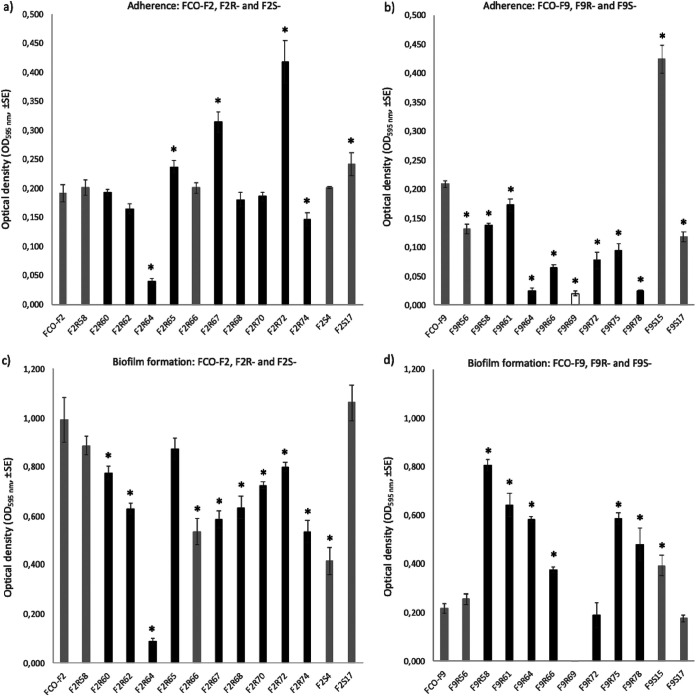
Compared to the parent wild-type FCO-F2 isolate, there was a large variability in the adhesion capacity of individual phage-resistant F2 isolates (Fig. 4a). Phage susceptibility (rhizoid versus rough colony type) or phage used in the coculture experiment did not influence bacterial adhesion capacity (P = 0.3, Mann-Whitney test, and P = 0.564, Kruskal-Wallis test, respectively).
FIG 4.
Adherence (a, b) and biofilm-forming capacity (c, d) of Flavobacterium columnare wild-type FCO-F2 (a, c) and FCO-F9 (b, d) isolates and their phage-exposed (F2R- and F9R-) and no-phage control (F2S- and F9S-) isolates on a polystyrene surface measured as optical density (OD595, ±SE). Asterisks indicate a statistically significant difference (P < 0.05) compared to the parent wild-type isolate. F9R69 did not form any biofilm and was thus excluded from the statistical analyses. Dark gray bars, phage-susceptible isolates forming a rhizoid colony morphology; black bars, phage-resistant isolates forming a rough morphology; white bar, phage-resistant isolate forming a soft morphology.
Most of the individual phage-exposed and no-phage control F2 isolates had significantly lower biofilm-forming capacity than parent wild-type FCO-F2 (P ≤ 0.017, one-way ANOVA, least significant difference [LSD] multiple comparisons, square root transformation) (Fig. 4c). Still, there was no statistical difference in biofilm formation between phage-susceptible rhizoid and phage-resistant rough morphology F2 isolates (P = 0.062, one-way ANOVA).
Again, the bacterial strain F9 behaved differently compared to F2. In contrast to the phage-resistant F2 isolates, the phage-resistant rough and soft morphology F9 isolates had significantly lower adherence than susceptible rhizoid isolates (P < 0.001, one-way ANOVA, LSD multiple comparisons, square root transformation) (Fig. 4b). In addition, isolates exposed to phages isolated in 2017, FCOV-F13 and FCOV-F45 (F9R64, F9R66, and F9R69, and F9R72, F9R75, and F9R78, respectively), had significantly lower adhesion capacity than isolates exposed to FCL-2 (F9R56, F9R58, and F9R61) isolated in 2008 (P < 0.001, Mann-Whitney test). This may indicate that phage FCL-2 uses a different phage receptor (see below).
In contrast to adhesion ability, biofilm-forming capacity of most of the individual phage-exposed and no-phage control F9 isolates was significantly higher than that in the wild-type parent isolate (P ≤ 0.004, one-way ANOVA, LSD multiple comparisons) (Fig. 4d). F9R69 with soft colony morphology did not form any biofilm and was thus excluded from the multiple comparisons. Phage-resistant rough F9 isolates had significantly higher biofilm-forming capacity than the phage-susceptible rhizoid morphotypes (P < 0.001, one-way ANOVA, square root transformation).
Protease activity: elastinase, gelatinase, and caseinase.
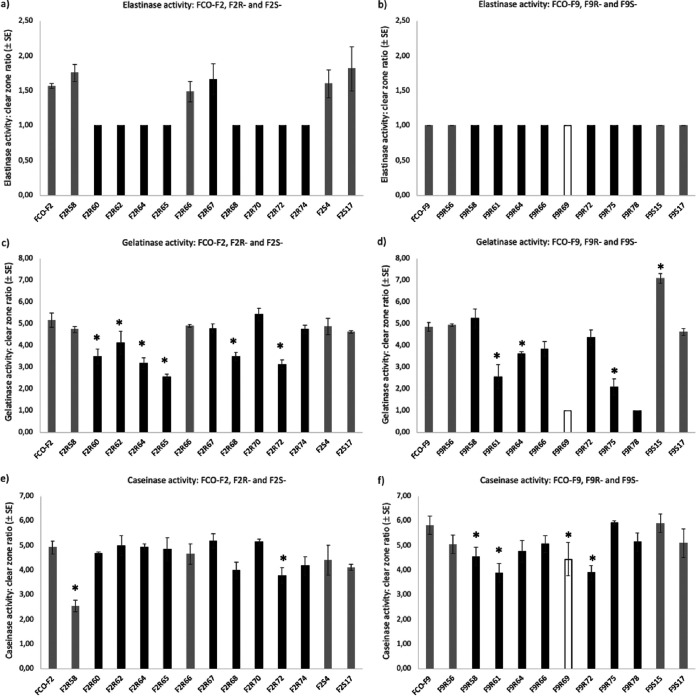
Elastinase activity was detected in the wild-type and all the phage-susceptible rhizoid FCO-F2 isolates and one resistant, rough F2 isolate (clear zone ratio of >1), whereas all remaining resistant, rough morphology isolates had completely lost the ability to degrade elastin (Fig. 5a). There were no differences in elastinase activity between the elastinase-positive isolates (P = 0.843, one-way ANOVA). Elastinase activity was not detected in any of the F9 isolates (clear zone ratio of 1) (Fig. 5b).
FIG 5.
Elastinase (a, b), gelatinase (c, d), and caseinase (e, f) activity of the Flavobacterium columnare FCO-F2 (a, c, e) and FCO-F9 (b, d, f) isolates and their phage-exposed (F2R- and F9R-) and no-phage control (F2S- and F9S-) isolates. Activity was measured as the clear zone ratio (clear zone diameter/colony diameter, ±SE) on TYES agar supplemented with elastin, gelatin, and skim milk (caseinase). The asterisk indicates a significant reduction in protease activity (P < 0.05) compared to the parent wild-type isolate. A clear zone ratio of 1 indicates no protease activity. Isolates with no activity were excluded from the statistical analyses. Dark gray bars, phage-susceptible isolates forming a rhizoid colony morphology; black bars, phage-resistant isolates forming a rough morphology; white bar, phage-resistant isolate forming a soft morphology.
There were variations in gelatinase activity between individual F2 and F9 isolates (one-way ANOVA, LSD multiple comparisons) (Fig. 5c and d). However, among both F2 and F9 isolates, gelatinase activity of phage-resistant rough morphotypes was lower than that of susceptible rhizoid morphotypes (F2 isolates, P = 0.018, one-way ANOVA, exponential transformation; F9 isolates, P < 0.001, one-way ANOVA). Two of the phage-exposed F9 isolates (F9R69 and F9R78) did not have any gelatinase activity and were thus excluded from the multiple comparisons.
Less variation in caseinase activity between individual isolates was observed (one-way ANOVA, LSD multiple comparisons) (Fig. 5e and f), and phage-susceptible rhizoid and resistant rough F2 isolates did not differ from each other (P = 0.058, one-way ANOVA). On the other hand, caseinase activity of phage-resistant rough and soft F9 isolates was lower than that of susceptible rhizoid isolates (P = 0.007, one-way ANOVA).
Virulence.
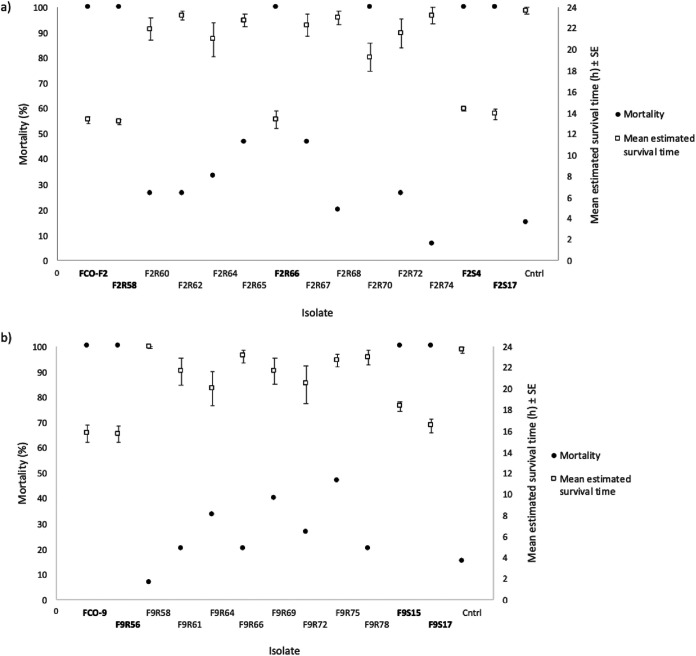
Rainbow trout fry were exposed to wild-type, phage-exposed, and no-phage control isolates, and all of them caused mortality during 24 h (Fig. 6). The phage-susceptible rhizoid morphotypes were most virulent, causing 100% mortality, whereas resistant rough and soft morphotypes were less virulent, causing 46.7% mortality at the highest (except for phage-resistant rough morphotype F2R70, which caused 100% mortality). Mortality of control fish was 15%, but no bacterial growth was observed from these fish. This mortality is most likely caused by the high water temperature used in the experiment (+25°C). However, F. columnare growth was observed from all fish exposed to bacteria. Colony morphotype of the bacterial isolates did not change during the infection.
FIG 6.
Mortality percent and estimated survival time (±SE) of rainbow trout (Oncorhynchus mykiss) during a 24-h experimental infection with wild-type Flavobacterium columnare FCO-F2 (a) and FCO-F9 (b) and their phage-exposed (F2R- and F9R-) and no-phage control (F2S- and F9S-) isolates. Phage-susceptible rhizoid colony-forming isolates are written in bold. Cntrl, control with no bacterial infection.
When comparing the data according to phage susceptibility and thus colony morphology, cumulative mortality of fish infected with phage-susceptible rhizoid morphotypes, irrespective of if they were wild-type, phage-exposed, or no-phage control isolates, was significantly higher than mortality caused by phage-resistant rough or soft morphotypes among both F2 and F9 isolates (P < 0.001, Kaplan-Meier survival analysis). Also, the estimated survival time (Kaplan-Meier survival analysis) was shortest in fish infected with susceptible rhizoid isolates (Fig. 6). In the case of F2 isolates, mortality caused by phage-resistant rough isolates was also significantly higher than mortality of control fish, but mortality caused by resistant rough and soft F9 isolates did not differ from each other or from the control fish mortality. Mortality caused by rhizoid phage-susceptible F2 isolates started to peak at 12 h postinfection (h p.i.) and in F9 at 16 h p.i. (P < 0.001, Kaplan-Meier survival analysis), but between rough phage-resistant F2 and F9 isolates, the mortality patterns were more similar, starting to increase slowly at 2 to 3 h p.i. (P = 0.217, Kaplan-Meier survival analysis). However, there were differences in cumulative mortalities caused by individual isolates in each morphology group (Data Set S1).
Whole-genome sequencing.
The aim of the genomic analysis was to identify the mutations selected by phage exposure; therefore, the spontaneously formed rough colonies were not sequenced. Genomic data of wild-type F. columnare isolates FCO-F2 and FCO-F9 are presented in Table 2.
TABLE 2.
Data on genomes of wild-type Flavobacterium columnare strains FCO-F2 and FCO-F9
| Wild-type isolate | Genetic group | Genome size (bases) | No. of ORFsa | GC % |
|---|---|---|---|---|
| FCO-F2 | C | 3,221,312 | 3,280 | 31.7 |
| FCO-F9 | G | 3,261,403 | 3,374 | 31.7 |
ORFs, open reading frames.
Genomic comparisons between F2 wild-type and phage-exposed isolates revealed a limited number of genomic changes. In 7 out of 11 isolates, single mutation leading to the formation of wrong or truncated proteins was observed in the phage-resistant mutants (Table 3). Notably, the majority of the mutations were located in genes encoding gliding motility proteins gldB (F2R67), gldN (F2R72), and sprA (F2R60, F2R64, F2R65, and F2R74), of which gldN and sprA are also parts of the type IX secretion system (29). Isolate F2R70, the only virulent rough isolate, had one nucleotide insertion in an outer membrane protein H (OmpH) family protein-coding gene. Three isolates (F2R62, F2R66, and F2R68) did not show any genomic changes relative to the wild type. In isolate F2R58 with decreased phage susceptibility, one nucleotide change in the rlmF gene (encoding rRNA large subunit methyltransferase F) did not lead to an amino acid change. No mutations were observed in the no-phage control isolates. At certain points of rRNA operons in all phage-exposed and no-phage control isolates, and also in a 736,221-bp sequence (hypothetical protein coding sequence in the wild-type FCO-F2 genome used as a reference) in phage-exposed isolates F2R66 and F2R68, there was poor coverage of reads leading to unclear sequences, which prevented detection of possible mutations in this region.
TABLE 3.
Mutations revealed by whole-genome sequencing (Illumina) in F2 phage-exposed Flavobacterium columnare isolates compared to their wild-type (wt) isolate FCO-F2
| (Phage) phage-exposed isolatea | Colony morphology | Gene/CDSb | Mutation | Location (base no.) in wt genome | Outcome |
|---|---|---|---|---|---|
| (FCOV-F2) | |||||
| F2R58 | Rhizoid | rlmF (rRNA large subunit methyltransferase F) | T → A | 21,350 | No aa change |
| F2R60 | Rough | sprA (component of type IX SS) | Ins GT | 1,314,323–1,314,324 | Change in reading frame → stop codon → two truncated proteins |
| F2R62 | Rough | ||||
| (FCOV-F5) | |||||
| F2R64 | Rough | sprA (component of type IX SS) | Ins G | 1,317,523 | Change in reading frame → stop codon → two truncated proteins |
| F2R65 | Rough | sprA (component of type IX SS) | Ins G | 1,317,524 | Change in reading frame → stop codon → two truncated proteins |
| F2R66 | Rhizoid | ||||
| F2R67 | Rough | gldB (gliding motility machinery protein) | Del T | 1,122,801 | Truncated/wrong protein |
| F2R68 | Rough | ||||
| (FCOV-F25) | |||||
| F2R70 | Rough | OmpH family outer membrane protein | Ins G | 1,275,242 | Change in reading frame → wrong protein |
| F2R72 | Rough | gldN (component of type IX SS) | Ins TCTAC | 1,013,274–1,013,278 | Change in reading frame → stop codon → two truncated proteins |
| F2R74 | Rough | sprA (component of type IX SS) | Del A | 1,313,911 | Change in reading frame → stop codon → two truncated proteins |
Isolates are shown according to the phage to which they were exposed.
→, change to; aa, amino acid; CDS, coding sequence; Del, deletion; Ins, insertion; SS, secretion system.
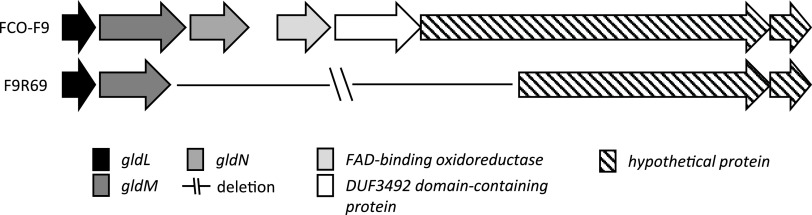
In F9 phage-exposed isolates, excluding F9R58, one or two mutations per isolate were observed (Table 4). Mutations in isolates exposed to FCOV-F45 had insertions, whereas FCOV-F13-exposed isolates had deletions or single nucleotide changes in genes encoding gliding motility proteins gldG (F9R72), gldM (F9R64, F9R69, and F9R78), and gldN (F9R69 and F9R75), leading to formation of wrong or truncated proteins. Interestingly, in the isolate F9R69 (exposed to FCOV-F13) with a soft colony type, deletion of a genomic region of 4,701 bp was observed, spanning over gliding motility genes gldM and gldN and sequences encoding flavin adenine dinucleotide (FAD)-binding oxidoreductase, DUF3492 domain-containing protein, and a hypothetical protein (Fig. 7).
TABLE 4.
Mutations revealed by whole-genome sequencing (Illumina) in F9 phage-exposed Flavobacterium columnare isolates compared to their wild-type (wt) isolate FCO-F9
| (Phage) phage-exposed isolatea | Colony morphology | Gene/CDSb | Mutation | Location (base no.) in wt genome | Outcome |
|---|---|---|---|---|---|
| (FCL-2) | |||||
| F9R56 | Rhizoid | DegT/DnrJ/EryC1/StrS family aminotransferase | C → T | 657,725 | Cys → Tyr |
| DUF255 domain-containing protein | C → T | 2,542,435 | Stop codon → truncated protein | ||
| F9R58 | Rough | ||||
| F9R61 | Rough | Cystathionine gamma-synthase | G → A | 1,720,857 | His → Tyr |
| (FCOV-F13) | |||||
| F9R64 | Rough | gldM (component of type IX SS) | Del CAA | 2,732,551 | Del Thr |
| F9R66 | Rough | Gliding motility protein | G → A | 1,849,668 | Stop codon → truncated protein |
| F9R69 | Soft | gldM (component of type IX SS) | Del 255 3′ nt | 2,732,457– | No/truncated protein |
| gldN (component of type IX SS) | Del CDS | No protein | |||
| FAD-binding oxidoreductase | Del CDS | No protein | |||
| DUF3492 domain containing protein | Del CDS | No protein | |||
| Hypothetical protein | Del 454 5′ nt | –2,737,157 | No/truncated protein | ||
| (FCOV-F45) | |||||
| F9R72 | Rough | gldG (gliding motility machinery protein) | Ins T | 3,023,647 | Change in reading frame → wrong protein |
| F9R75 | Rough | gldN (component of type IX SS) | Ins G | 2,733,099 | Start and stop codon → two truncated proteins |
| F9R78 | Rough | gldM component of type IX SS) | Ins A | 2,731,567 | Change in reading frame → stop codon → truncated protein |
Isolates are shown according to the phage to which they were exposed.
→, change to; CDS, coding sequence; Del, deletion; Ins, insertion; nt, nucleotide; SS, secretion system.
FIG 7.
Deletion of genomic region covering 4,701 bp in FCOV-F13-exposed soft colony-forming phage-resistant Flavobacterium columnare isolate F9R69.
On the contrary, no mutations in gliding motility genes were observed in the sequenced F9 isolates exposed to FCL-2; but, instead, two of these isolates had one nucleotide change in genes encoding DegT/DnrJ/EryC1/StrS family aminotransferase, DUF255-domain containing protein (F9R56), and cystathionine gamma-synthase (F9R61), leading to either one amino acid change or truncated protein. No mutations were observed in no-phage control isolates. Around 2,000,620 bp (hypothetical protein coding sequence in the B185 genome used as a reference), there was poor coverage of reads, leading to unclear sequence in both wild-type FCO-F9, phage-exposed, and no-phage control isolates, which prevented detection of possible mutations in this region.
DISCUSSION
Phage therapy is seen as an attractive option to treat and prevent bacterial diseases, but the development of phage resistance in target bacteria is considered one of the main problems related to the use of phages. Our results describe the selection for phage resistance in two different F. columnare isolates upon exposure to six specific phages. We show that phage resistance is associated with reduction in virulence and virulence-related phenotypic changes in the bacterium. Our genetic data indicate that, in most cases, phage resistance is linked to surface modifications, often related to the type IX secretion system connected to Bacteroidetes gliding motility machinery. Mutations in the genes encoding an outer membrane protein or genes related to gliding motility seem to be phage specific and likely prevent phage attachment, possibly in a phage-specific manner, selecting morphology change and loss of virulence.
In the present study, phage exposure caused significant changes in bacterial phenotypic characteristics (motility, adhesion, protein secretion, and virulence; see details below), leading to phage resistance. In most isolates, these changes could be linked to changes in gliding motility-related genes. Flavobacteria show gliding motility on surfaces (29), and mutations in any of the genes encoding gliding motility machinery proteins have been shown to lead to loss of motility (30, 31). Gliding is also connected to virulence, since part of the gliding motility machinery (GldK, GldL, GldM, GldN, PorV, SprA, SprE, SprF, and SprT) is used as a type IX secretion system found in Bacteroidetes (28, 32). Indeed, phage resistance due to loss of motility has been linked to decreased virulence in F. columnare previously (27), and F. columnare gldN mutants have been shown to exhibit both decreased proteolytic and chondroitinase activity and virulence on rainbow trout (28). Similarly, phage resistance was associated with loss of motility and mutations in genes related to cell surface properties and gliding motility in F. psychrophilum (23) and in F. johnsoniae (31, 33). It has been suggested that surface proteins secreted by the type IX secretion system in F. johnsoniae (such as SprB and RemA) may function as phage receptors (31, 34). Mutations in either gliding (gldB, gldG, gldL, gldM, gldN) or type IX secretion system (sprA, gldL, gldM, gldN) genes in F. johnsoniae will disrupt SprB/RemA secretion, resulting in phage resistance due to the lack of receptors on the host cell surface. Together, the results suggest that the type IX secretion system is a key target for infection by a wide range of phages and across the Flavobacterium genus, and that phage exposure selects for mutations linked with morphology changes and loss of motility in this bacterial group.
Exposure to a specific phage selected for different gliding motility mutations in different F. columnare isolates, as has also been seen in phage-resistant F. psychrophilum (23), indicating that several genes are involved in phage attachment and infection of F. columnare phages. Furthermore, genomic analysis of one soft colony isolate revealed a large deletion (4,701 bp) spanning over two gliding motility genes. It is possible that this region is important for adhesion and biofilm formation. However, although all rough colony-forming isolates were phage resistant, not all of these isolates (F2R62, F2R68, and F9R58) had mutations in genes encoding proteins related to gliding motility or elsewhere in their genomes. This may indicate that development of phage resistance and colony morphology change are also influenced by gene expression or epigenetic modifications, leading to variation in colony morphology, as suggested previously (35). For example, in Bordetella spp., phage resistance is regulated via phase variation in virulence-related factors, such as some adhesins, toxins, and the type III secretion system (reviewed in reference 36). Furthermore, our data indicate that phage exposure can also select for mutations encoding other outer membrane proteins versus those related to gliding motility, such as ompH in the case of F2R70. Although it had a rough colony morphotype, this isolate was virulent in rainbow trout. Interestingly, isolates exposed to FCL-2 did not have mutations in gliding motility-related genes. To uncover if FCL-2 uses different receptors for infection of F. columnare than the other phages in this study, more phage-resistant isolates should be sequenced. However, FCL-2 differs genetically from other phages infecting genetic group G bacteria (this article was submitted to an online preprint archive [37]), supporting this suggestion.
Generally, point mutations and changes in receptor expression enable a rapid and efficient response of bacterial populations to phage exposure. However, the large phenotypic costs of mutation-derived phage resistance observed in F. columnare in this study suggest that these mutations may be dynamic and likely also rapidly revert back to the susceptible form in F. columnare. Indeed, reversion of both phage-driven and spontaneously formed rough colony types back to rhizoid has been observed to happen in F. columnare subcultures, similar to the occasional appearance of spontaneous rough colonies (27) as also observed in this study. Various mechanisms to regain phage resistance have also been found in fish pathogenic F. psychrophilum (23) and V. anguillarum (24), in which a rapid reversion back to a phage-susceptible phenotype has been shown to occur. These sorts of dynamics in phage resistance have also been observed in a human symbiont Bacteroides thetaiotaomicron (38), suggesting that the phenomenon may be common among a wide variety of bacteria.
Phage-exposed F. columnare isolates F2R58, F2R66, and F9R56 did not respond to phage infection with colony morphotype change but maintained their original rhizoid colony morphotype and high virulence. These rhizoid isolates were not completely resistant to phage, although phage infection efficiency dropped markedly (up to a one-million-fold decrease on efficiency of plating), suggesting some other mechanism for reducing infection efficiency. F. columnare has two functional CRISPR systems that have been shown to adapt under phage exposure at fish farms (39). However, we did not observe additional CRISPR spacers in whole-genome sequencing. The same was observed in phage-exposed F. psychrophilum isolates in which no differences to the wild-type strain’s CRISPR composition were found (23). In our experience, CRISPR adaptation in F. columnare requires a different experimental setup with longer coculture time in low-nutrient medium, followed by enrichment in high-nutrient medium (40). Thus, the decreased phage susceptibility of rhizoid phage-exposed isolates is likely a consequence of as of yet unknown functions that need to be studied in the future.
In addition to the type IX secretion system, type I and VI secretion systems are also known to function in F. columnare (41). Possible secretion of virulence-related factors through type I and VI secretion systems in F. columnare could be one of the reasons why rough phage-resistant isolates caused some mortality in fish and explains their gelatinase and caseinase activity despite morphology change. It has also been shown recently that virulence of F. columnare increases in the mucus and with increasing mucin concentration (17). As the mucus-covered fish surface is the main infection route of F. columnare, it is probable that some F. columnare virulence factors, such as proteinase activity, are expressed differently in growth media compared to in an in vivo infection situation. This possible differential expression could also explain the mortality caused by phage-resistant rough isolates.
The ability to adhere and form biofilms has a major role in bacterial infections and in colonizing niches (42). In F. columnare, adhesion and biofilm-forming capacity may have a central role in their persistence in the farming environment (e.g., tanks and water systems) (43) but also in establishing the first steps of infection on the fish surfaces (44). Our results indicate that F. columnare strains differ in their adherence and biofilm-forming characteristics, and the effect of phage resistance on bacterial phenotype is not straightforward. This is probably associated with different mutations in different isolates and general biological variability. Whereas phage exposure had no clear effect on the adhesion capacity of the F2 isolates, phage resistance led to a decrease in biofilm-forming capacity in most of the individual phage-resistant F2 isolates. This is in agreement with the systematic reduction in biofilm-forming properties of phage-resistant F. psychrophilum relative to the wild type (23). Adhesion capacity of F9 phage-resistant isolates, on the other hand, was significantly lower than in the wild-type parent isolate, but rough phage-resistant F9 isolates had a significantly higher biofilm-forming capacity than rhizoid susceptible isolates. These results partly differ from what we have found earlier (25, 26), most likely because in the previous studies, the rough colonies were formed spontaneously without phage exposure. Indeed, morphology of spontaneously formed rough colonies and these morphotypes’ ability to move when cultured in low-nutrient media differ from rough morphotypes formed under phage exposure (27). Furthermore, the high variability in the results of biofilm and adhesion assays may reflect the biology of the bacterium, which may have natural variance in its phenotype. As the bacterium has the capacity to survive long periods in the environment outside of the host, different strategies can improve survival. Furthermore, although adhesion is a prerequisite for biofilm formation, these are different steps of the infection process. It is possible that once the surface adhesion has been established, bacterial gene expression changes to initiate biofilm formation. Nevertheless, together, our results indicate that since F. columnare phages are genetically group specific, they might be using different receptors, which, in turn, causes differences in bacterial resistance mechanisms and phenotypic characteristics between genetic groups.
Phage resistance can influence bacterial susceptibility to antibiotics (45–47). In this study, phage resistance did not affect the antibiotic susceptibility of any of the isolates studied. Lack of association between development of antibiotic resistance and bacteriophage resistance has previously been shown (e.g., in Escherichia coli [48]). Based on our results, phage resistance may not increase a risk of antibiotic resistance development in F. columnare. Thus, phage therapy given at fish farms is not likely to hamper possible concomitant use of antibiotics as therapeutic agents against columnaris infections. Indeed, it has been shown with Pseudomonas fluorescens that applying phages together with antibiotic treatments may inhibit the evolution of antibiotic resistance in pathogenic bacteria (49). However, since some changes in inhibition zone tests were detected in our study with F. columnare, more thorough analysis of antibiotic resistance would be beneficial to fully describe the association with phage resistance.
To summarize, our results show that even though F. columnare rapidly develops phage resistance under phage exposure, the emergence of phage resistance does not pose a high risk in the development of phage therapy against columnaris infections in rainbow trout. This is because, in most cases, phage resistance selects for decreases in bacterial virulence, adherence to surfaces, and protease secretion. Based on our results with experiments with two genetically different wild-type bacterial isolates, development and regulation of phage resistance in F. columnare is a multifactorial process, partly affected by the formation of mutations mainly in gliding motility- and type IX secretion system-related genes and partly by other defense mechanisms against phages, the functions of which need to be studied in the future.
MATERIALS AND METHODS
Bacterial and phage isolates used in this study.
Bacteria and phages used in this study were isolated from water samples collected from fish farms during columnaris outbreaks (37) (Table 5). The bacteria were confirmed as F. columnare by restriction fragment length polymorphism (RFLP) analysis of the 16S rRNA gene and classified into genetic groups by RFLP of the 16S to 23S internal transcribed spacer (ITS) region (37). The six phages used in the experiments have been isolated from different fish farms in Finland (37, 50). Phages belong to the Myoviridae family and have been characterized with respect to host range and genomic composition previously (37). Briefly, F. columnare phages infect their host in a genotype-specific manner; phages FCOV-F2, FCOV-F5, and FCOV-F25 infect bacterial strains belonging to genetic group C (here FCO-F2), and phages FCL-2, FCOV-F13, and FCOV-F45 infect bacteria in genetic group G (here FCO-F9).
TABLE 5.
Flavobacterium columnare isolates and phages used in this study
| Bacterium isolatea | Genetic group of the bacterium | Phage isolate | Genetic group of the phage isolation host | Farm no. | Isolation yr |
|---|---|---|---|---|---|
| FCO-F2 | C | 1 | 2017 | ||
| FCO-F9 | G | 2 | 2017 | ||
| FCOV-F2 | C | 1 | 2017 | ||
| FCOV-F5 | C | 3 | 2017 | ||
| FCOV-F25 | C | 1 | 2017 | ||
| FCL-2 | G | 2 | 2008 | ||
| FCOV-F13 | G | 1 | 2017 | ||
| FCOV-F45 | G | 2 | 2017 |
Bacteria and phages were isolated from Finnish fish farms. F. columnare isolates have previously been categorized into genetic groups by restriction fragment length polymorphism analysis of internal transcribed spacer region between 16S and 23S rRNA genes (36).
Bacterial cultures and phage lysates.
For phage exposure and virulence testing, F. columnare isolates were inoculated from cryopreserved (–80°C) stocks in modified Shieh medium (51) and grown for 48 h at 25°C with 120 rpm agitation. After this, subcultures were made in modified Shieh medium and grown for 24 h at 25°C with 120 rpm agitation. The optical density (OD) of the bacterial broth suspensions was measured spectrophotometrically at 595 nm and adjusted to 5 × 105 CFU ml−1 for phage exposures and 5 × 106 CFU ml−1 for virulence experiments (based on previously determined OD/CFU relationships). For other tests, F. columnare isolates were cultured in tryptone yeast extract salts (TYES) broth (52) and washed in TYES broth by centrifugation at 5,310 × g for 15 min at 4°C. Cultures were then spectrophotometrically adjusted to an OD of 0.6 at 520 nm (approximately 108 CFU ml−1).
Phage lysates were produced using the “double layer agar” method (53) as follows: 3 ml of melted (47°C) top agar (0.5%), including 300 μl of a 24-h subculture of the host bacterium and 100 μl of phage (10-fold dilutions in Shieh medium), was poured onto Shieh agar and grown for 48 h at 25°C. Five milliliters of Shieh medium was added on top of Shieh agar plates with confluent lysis and incubated at 7°C for 12 to 18 h with constant agitation (90 rpm). Lysates were collected, filtered (polyethersulfone [PES] membrane, pore size 0.45 µm; Nalgene), and stored at +7°C or at −80°C with 20% glycerol. For phage exposure, phage lysates were diluted with Shieh medium to 5 × 105 PFU ml−1.
Phage exposure experiments and isolation of colonies.
Two phage-susceptible wild-type F. columnare isolates, the high-virulence FCO-F2 isolate (genetic group C) and the medium-virulence FCO-F9 isolate (genetic group G) (37), were each exposed to three phages in separate experiments with individual phages. Isolate FCO-F2 was exposed to phages FCOV-F2, FCOV-F5, and FCOV-F25, and isolate FCO-F9 was exposed to phages FCL-2, FCOV-F13, and FCOV-F45, in accordance with the host range of the phages. Cultures with only bacteria served as no-phage controls. The exposures were carried out in 20 ml of autoclaved fresh water (Lake Jyväsjärvi) in triplicate cultures under constant agitation (120 rpm) at 25°C for 3 days at a multiplicity of infection (MOI) at inoculation of 1 (1 × 104 CFU and PFU ml−1). The cultures were sampled every 24 h for 3 days by making a serial 10-fold dilution of samples and spreading on Shieh agar plates. After up to 4 days of incubation at room temperature, CFU and colony morphologies were determined from the plate cultures. Two to three colonies from each triplicate culture at each sampling point were picked and pure cultured directly on Shieh agar plates three times to get rid of any phage contamination. Colonies were then checked for phage resistance by spot assay on agar plates; bacterial lawns on top agar were prepared as described above, and 10 μl of 10-fold-diluted original phage lysates (used in initial exposures) was spotted on agar. After a 48-h incubation at 25°C, bacterial plates with no observed plaques or confluent lysis were considered phage resistant. Altogether, 189 colonies from phage-exposed and no-phage control exposures were isolated from plate cultures. From this collection, 20 phage-exposed and 4 no-phage control isolates were randomly selected for further analysis (Table 1).
The phage-exposed and no-phage control isolates were named according to the latter part of the wild-type bacterial host, the letter R for phage-exposed and the letter S for no-phage control isolates plus a running number for the isolated colony. For example, F2R2 is the second selected phage-exposed colony of the F. columnare wild-type isolate FCO-F2. Correspondingly, the second F. columnare isolate from no-phage control cultures was marked as F2S2. For simplicity, wild-type FCO-F2 and all its subsequent isolates from the phage and control exposures are commonly called F2 isolates in this paper. Correspondingly, wild-type FCO-F9 and its subsequent isolates are called F9 isolates.
Antibiotic sensitivity.
Changes in susceptibility of phage-exposed F. columnare isolates toward antibiotics was tested using the Kirby-Bauer disc diffusion method (54) on diluted Mueller-Hinton (55) agar medium supplemented with 5% wt/vol fetal calf serum. A 40-µl volume of each isolate suspension (109 CFU ml−1) was added to 5 ml of phosphate-buffered saline and poured onto the Mueller-Hinton agar plates. After removing excess bacterial suspension by pipetting, the antibiotic discs (oxolinic acid [2 µg], florfenicol [30 µg], sulfamethoxasol/trimethoprim [25 µg], and tetracycline [30 µg]) were placed on the plates. The plates were then incubated for 3 days at 25°C. After incubation, the inhibition zone around the antibiotic discs was measured. The susceptibility tests did not include replicates. The susceptibility patterns of the selected phage-exposed and no-phage control F. columnare isolates to the antibiotics were compared to that of the parent wild-type isolates.
Motility/colony spreading.
The effect of phage exposure on bacterial motility was tested by comparing the colony-spreading ability of phage-exposed and no-phage control isolates with that of their parent wild-type isolates. After spotting 5 μl of bacterial suspension (109 CFU ml−1) on TYES agar (0.5% agar) plates supplemented with 0.1% baker’s yeast and incubating for 3 days at 25°C, the colony diameter of each isolate was measured. Each isolate was tested in three replicates.
Adhesion and biofilm formation.
Changes in adherence or biofilm formation capacities between wild-type, phage-exposed, and no-phage control F. columnare isolates were studied in flat-bottomed 96-well microtiter plates (Nunclon Delta surface, Nunc) (56). F. columnare cells grown on TYES agar were suspended in autoclaved fresh water (Lake Littoistenjärvi) to a concentration of 108 CFU ml−1 (OD520 = 0.6). For testing of bacterial adherence, a 100-μl volume of the prepared bacterial suspensions was added in triplicate into wells of replicate microtiter plates and incubated statically for 1 h at 25°C. For testing of biofilm formation, a 100-μl volume of TYES broth was added to wells containing 100 μl of the prepared bacterial suspensions and allowed to incubate for 3 days. Autoclaved fresh water was used as a negative control. After incubation, the contents were discarded, and the wells were washed three times with sterile 0.5% NaCl to remove nonadherent cells and air dried. The wells were then stained with 0.1% crystal violet solution for 45 min and washed three times by submersion in a container of tap water and air dried. The crystal violet was solubilized with 96% ethanol for 15 min before measuring the absorbance (1 s) at 595 nm (Victor2, Wallac).
Protease activity.
Changes in protease activity were examined by spotting 1 μl of bacterial TYES broth suspension (108 CFU ml−1) of the wild-type isolates and each phage-exposed and no-phage control isolate on TYES agar (1.5% agar) supplemented with elastin (0.1%, wt/vol), gelatin (3%), and skim milk (5%) (caseinase production). The proteolytic activity of each isolate was observed by the presence of a clear zone surrounding the colony after incubation and assessed by measuring the clear zone ratio (diameter of clear zone/diameter of the colony) of three replicate samples. In the absence of a clearing zone outside the colony, the clear zone ratio was defined as 1. The measurements were made after 5 (caseinase and gelatinase) or 10 (elastinase) days of incubation at 25°C.
Virulence.
Virulence of phage-exposed and no-phage control F. columnare isolates was tested on 1.94-g (average weight) rainbow trout fry and compared to the virulence of wild-type isolates. Fifteen fish per treatment, 20 in the control treatment with no bacteria, were exposed individually in 500 ml of bore hole water (25°C) to cells of single bacterial isolates by constant immersion (5.0 × 103 CFU ml−1). Survival of the fish was monitored hourly for 24 h. Morbid fish that did not respond to stimuli were considered dead, removed from the experiment, and euthanized by decapitation. At the end of the experiment, the fish that survived infection were euthanized using 0.008% benzocaine. Bacterial cultivations from gills of all the dead fish were made on Shieh agar supplemented with tobramycin (57) to confirm the presence/absence of the bacterium. Cumulative percent mortality and estimated survival time (Kaplan-Meier survival analysis) based on observed average survival time of fish after exposure to each isolate were used as measures of virulence, with more virulent isolates having a shorter estimated survival time.
Fish experiments were conducted according to the Finnish Act of Use of Animals for Experimental Purposes under permission ESAVI/8187/2018 granted for Lotta-Riina Sundberg by the National Animal Experiment Board at the Regional State Administrative Agency for Southern Finland.
Whole-genome sequencing.
Genomes of the wild-type FCO-F2 and FCO-F9 F. columnare and selected 20 phage-exposed and four no-phage control isolates (Table 1) were sequenced using an Illumina HiSeq platform (Institute of Molecular Medicine Finland). The Illumina data reads of FCO-F9 and its phage-exposed and no-phage control isolates were mapped to a reference genome of F. columnare isolate B185 (58) using Geneious software version 11.1.5 (Biomatters Ltd.). The genome of the wild-type FCO-F2 isolate was sequenced using PacBio (BGI, China). PacBio data of FCO-F2 were assembled using >8-kbp reads with Flye (version 2.7, four iterations) and >6-kbp reads with Canu (version 1.9). These multicontig assemblies were then combined using Quickmerge (version 0.3) to produce one 3,221,312-bp contig. This contig was polished with Illumina HiSeq reads using Pilon (version 1.23), with preprocessing done using Trimmomatic (version 0.39), bowtie2 (version 2.3.5.1), and Samtools (version 1.9). The quality of the polished contig was quantified using Busco (version 4.0.2), which reported 100% completeness of the genome against the bacteria_odb10 reference set. The genome was annotated using the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) (59, 60) and used as reference genome for mapping of F2 phage-exposed and no-phage control isolates using Geneious software version 11.1.5 (Biomatters Ltd.). The mutations were considered true when the number of reads with nucleotide changes/mutations was bigger than the number of reads with a wild-type sequence.
Statistical analyses.
IBM SPSS statistics version 24 was used for statistical analysis of the data. A one-way analysis of variance (ANOVA) was used to compare means from phenotypic analyses between experimental groups (phage-exposed isolates and no-phage control isolates) and parent wild-type isolates. If needed, log10 exponential or square root transformations were made for the data to fulfill the homogeneity of variances assumption. If the homogeneity of variances could not be met by transformations, the data were analyzed using nonparametric Kruskal-Wallis and Mann-Whitney tests. In cases of elastinase and casienase activity and biofilm formation, the isolates with no activity/biofilm-forming capacity were excluded from the ANOVA LSD multiple comparison analyses. Kaplan-Meier survival analysis was used for the analysis of virulence data.
Data availability.
The whole-genome sequences of all isolates were submitted to GenBank under accession numbers presented in Table 6.
TABLE 6.
Accession numbers of whole-genome sequences of wild-type Flavobacterium columnare isolates FCO-F2 and FCO-F9 and their phage-exposed (F2R- and F9R-) and no-phage control (F2S- and F9S-) isolates submitted to GenBank
| Isolate | Accession no. |
|---|---|
| FCO-F2 | CP051861 |
| F2R58 | CP054506 |
| F2R60 | CP054505 |
| F2R62 | CP054504 |
| F2R64 | CP054503 |
| F2R65 | CP054502 |
| F2R66 | CP054501 |
| F2R67 | CP054500 |
| F2R68 | CP054499 |
| F2R70 | CP054498 |
| F2R72 | CP054497 |
| F2R74 | CP054496 |
| F2S4 | CP054495 |
| F2S17 | CP054494 |
| FCO-F9 | CP054518 |
| F9R56 | CP054517 |
| F9R58 | CP054516 |
| F9R61 | CP054515 |
| F9R64 | CP054514 |
| F9R66 | CP054513 |
| F9R69 | CP054512 |
| F9R72 | CP054511 |
| F9R75 | CP054510 |
| F9R78 | CP054509 |
| F9S15 | CP054508 |
| F9S17 | CP054507 |
ACKNOWLEDGMENTS
We acknowledge funding from the Academy of Finland (grant number 314939) and the Jane and Aatos Erkko Foundation. This work resulted from the BONUS FLAVOPHAGE project supported by BONUS (Art 185), funded jointly by the EU and Academy of Finland.
Footnotes
Supplemental material is available online only.
Contributor Information
Lotta-Riina Sundberg, Email: lotta-riina.sundberg@jyu.fi.
Karyn N. Johnson, University of Queensland
REFERENCES
- 1.Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A, Buschmann AH. 2013. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ Microbiol 15:1917–1942. 10.1111/1462-2920.12134. [DOI] [PubMed] [Google Scholar]
- 2.Watts JEM, Schreier HJ, Lanska L, Hale MS. 2017. The rising tide of antimicrobial resistance in aquaculture: sources, sinks and solutions. Mar Drugs 15:158. 10.3390/md15060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gudding R, Lillehaug A, Evensen Ø (ed). 2014. Fish vaccination. Wiley Blackwell, Fairford, UK. [Google Scholar]
- 4.Suomalainen LR, Tiirola MA, Valtonen ET. 2005. Effect of Pseudomonas sp. MT5 baths on Flavobacterium columnare infection of rainbow trout and on microbial diversity on fish skin and gills. Dis Aquat Organ 63:61–68. 10.3354/dao063061. [DOI] [PubMed] [Google Scholar]
- 5.Pulkkinen K, Suomalainen L-R, Read AF, Ebert D, Rintamäki P, Valtonen ET. 2010. Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proc Biol Sci 277:593–600. 10.1098/rspb.2009.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt AS, Bruun MS, Dalsgaard I, Pedersen K, Larsen JL. 2000. Occurrence of antimicrobial resistance in fish-pathogenic and environmental bacteria associated with four Danish rainbow trout farms. Appl Environ Microbiol 66:4908–4915. 10.1128/AEM.66.11.4908-4915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesami S, Parkman J, MacInnes JI, Gray JT, Gyles CL, Lumsden JS. 2010. Antimicrobial susceptibility of Flavobacterium psychrophilum isolates from Ontario. J Aquat Anim Health 22:39–49. 10.1577/H09-008.1. [DOI] [PubMed] [Google Scholar]
- 8.Molina-Aja A, García-Gasca A, Abreu-Grobois A, Bolán-Mejía C, Roque A, Gomez-Gil B. 2002. Plasmid profiling and antibiotic resistance of Vibrio strains isolated from cultured penaeid shrimp. FEMS Microbiol Lett 213:7–12. 10.1111/j.1574-6968.2002.tb11278.x. [DOI] [PubMed] [Google Scholar]
- 9.Mohamad N, Amal MNA, Saad MZ, Yasin ISM, Zulkiply NA, Mustafa M, Nasruddin NS. 2019. Virulence-associated genes and antibiotic resistance patterns of Vibrio spp. isolated from cultured marine fishes in Malaysia. BMC Vet Res 15:176. 10.1186/s12917-019-1907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Declercq AM, Boyen F, Van den Broeck W, Bossier P, Karsi A, Haesebrouck F, Decostere A. 2013. Antibiotic susceptibility pattern of Flavobacterium columnare isolates collected worldwide from 17 fish species. J Fish Dis 36:45–55. 10.1111/j.1365-2761.2012.01410.x. [DOI] [PubMed] [Google Scholar]
- 11.Loc-Carrillo C, Abedon ST. 2011. Pros and cons of phage therapy. Bacteriophage 1:111–114. 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakai T, Sugimoto R, Park KH, Matsuoka S, Mori K, Nishioka T, Maruyama K. 1999. Protective effects of bacteriophage on experimental Lactococcus garviae infection in yellowtail. Dis Aquat Organ 37:33–41. 10.3354/dao037033. [DOI] [PubMed] [Google Scholar]
- 13.Park SC, Nakai T. 2003. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis Aquat Organ 53:33–39. 10.3354/dao053033. [DOI] [PubMed] [Google Scholar]
- 14.Higuera G, Bastías R, Tsertsvadze G, Romero J, Espejo RT. 2013. Recently discovered Vibrio anguillarum phages can protect against experimentally induced vibriosis in Atlantic salmon, Salmo salar. Aquaculture 392:128–130. 10.1016/j.aquaculture.2013.02.013. [DOI] [Google Scholar]
- 15.Castillo D, Higuera G, Villa M, Middelboe M, Dalsgaard I, Madsen L, Espejo RT. 2012. Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold-water disease in salmonids. J Fish Dis 35:193–201. 10.1111/j.1365-2761.2011.01336.x. [DOI] [PubMed] [Google Scholar]
- 16.Laanto E, Bamford JKH, Ravantti J, Sundberg L-R. 2015. The use of phage FCL-2 as an alternative to chemotherapy against columnaris disease in aquaculture. Front Microbiol 6:829. 10.3389/fmicb.2015.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida GMF, Laanto E, Ashrafi R, Sundberg LR. 2019. Bacteriophage adherence to mucus mediates preventive protection against pathogenic bacteria. mBio 10:e01984-19. 10.1128/mBio.01984-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan D, Svenningsen SL, Middelboe M. 2015. Quorum sensing determines the choice of anti-phage defense strategy in Vibrio anguillarum. mBio 6:e00627-15. 10.1128/mBio.00627-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azam AH, Tanji Y. 2019. Bacteriophage-host arm race: an update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl Microbiol Biotechnol 103:2121–2131. 10.1007/s00253-019-09629-x. [DOI] [PubMed] [Google Scholar]
- 20.Cohen D, Melamed S, Millman A, Shulman G, Oppenheimer-Shaanan Y, Kacen A, Doron S, Amitai G, Sorek R. 2019. Cyclic GMP–AMP signalling protects bacteria against viral infection. Nature 574:691–695. 10.1038/s41586-019-1605-5. [DOI] [PubMed] [Google Scholar]
- 21.Léon M, Bastias R. 2015. Virulence reduction in bacteriophage resistant bacteria. Front Microbiol 6:343. 10.3389/fmicb.2015.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SC, Shimamura I, Fukunaga M, Mori KI, Nakai T. 2000. Isolation of bacteriophages specific for a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl Environ Microbiol 66:1416–1422. 10.1128/AEM.66.4.1416-1422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castillo D, Christiansen RH, Dalsgaard I, Madsen L, Middelboe M. 2015. Bacteriophage resistance mechanisms in the fish pathogen Flavobacterium psychrophilum: linking genomic mutations to changes in bacterial virulence factors. Appl Environ Microbiol 81:1157–1167. 10.1128/AEM.03699-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo D, Rørbo N, Jørgensen J, Lange J, Tan D, Kalatzis PG, Lo Svenningsen S, Middelboe M. 2019. Phage defence mechanisms and their genomic and phenotypic implications in the fish pathogen Vibrio Anguillarum. FEMS Microbiol Ecol 95:fiz004. 10.1093/femsec/fiz004. [DOI] [PubMed] [Google Scholar]
- 25.Kunttu HMT, Suomalainen LR, Jokinen EI, Valtonen ET. 2009. Flavobacterium columnare colony types: connection to adhesion and virulence? Microb Pathog 46:21–27. 10.1016/j.micpath.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Kunttu HMT, Jokinen EI, Sundberg L-R, Valtonen ET. 2011. Virulent and nonvirulent Flavobacterium columnare colony morphologies: characterization of chondroitin AC lyase activity and adhesion to polystyrene. J Appl Microbiol 111:1319–1326. 10.1111/j.1365-2672.2011.05149.x. [DOI] [PubMed] [Google Scholar]
- 27.Laanto E, Bamford JKH, Laakso J, Sundberg LR. 2012. Phage driven loss of virulence in a fish pathogenic bacterium. PLoS One 7:e53157. 10.1371/journal.pone.0053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li N, Zhu Y, LaFrentz BR, Evenhuis JP, Hunnicut DW, Conrad RA, Barbier P, Gullstrand GW, Roets JE, Powers JL, Kulkarni SS, Erbes DH, Garcia JC, Nie P, McBride MJ. 2017. The type IX secretion system is required for virulence of the fish pathogen Flavobacterium columnare. Appl Environ Microbiol 83:e01769-17. 10.1128/AEM.01769-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBride MJ, Nakane D. 2015. Flavobacterium gliding motility and the type IX secretion system. Curr Opin Microbiol 28:72–77. 10.1016/j.mib.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Braun TF, Khubbar MK, Saffarini DA, McBride MJ. 2005. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J Bacteriol 187:6943–6952. 10.1128/JB.187.20.6943-6952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrivastava A, Johnston JJ, van Baaren JM, McBride MJ. 2013. Flavobacterium johnsoniae GldK, GldL, GldM, and SprA are required for secretion of the cell surface gliding motility adhesins SprB and RemA. J Bacteriol 195:3201–3212. 10.1128/JB.00333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston JJ, Shrivastava A, McBride MJ. 2017. Untangling Flavobacterium johnsoniae gliding motility and protein secretion. J Bacteriol 200:e00362-17. 10.1128/JB.00362-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunnicutt DW, Kempf MJ, McBride MJ. 2002. Mutations in Flavobacterium johnsoniae gldF and gldG disrupt gliding motility and interfere with membrane localization of GldA. J Bacteriol 184:2370–2378. 10.1128/JB.184.9.2370-2378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrivastava A, Rhodes RG, Pochiraju S, Nakane D, McBride MJ. 2012. Flavobacterium johnsoniae RemA is a mobile cell surface lectin involved in gliding. J Bacteriol 194:3678–3688. 10.1128/JB.00588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penttinen R, Hoikkala V, Sundberg LR. 2018. Gliding motility and expression of motility related genes in spreading and non-spreading colonies of Flavobacterium columnare. Front Microbiol 9:525. 10.3389/fmicb.2018.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labrie S, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327. 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- 37.Runtuvuori-Salmela A, Kunttu HMT, Laanto E, Almeida GMF, Mäkelä K, Middelboe M, Sundberg LR. 2020. Prevalence of genetically similar Flavobacterium columnare phages across aquaculture environments reveals a strong potential for pathogen control. bioRxiv https://biorxiv.org/cgi/content/short/2020.09.23.309583v1. [DOI] [PMC free article] [PubMed]
- 38.Porter NT, Hryckowian AJ, Merrill BD, Fuentes JJ, Gardner JO, Glowacki RWP, Singh S, Crawford RD, Snitkin ES, Sonnenburg JL, Martens EC. 2020. Phase-variable capsular polysaccharides modify bacteriophage susceptibility in Bacteroides thetaiotaomicron. Nat Microbiol 5:1170–1181. 10.1038/s41564-020-0746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laanto E, Hoikkala V, Ravantti J, Sundberg LR. 2017. Long-term coevolution of host-parasite interaction in the natural environment. Nat Commun 8:111. 10.1038/s41467-017-00158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoikkala V, Ravantti J, Díez-Villaseñor C, Tiirola M, Conrad RA, McBride MJ, Moineau S, Sundberg L-R. 2021. Cooperation between CRISPR-Cas types enables adaptation in an RNA-targeting system. mBio 12:e03338-20. 10.1128/mBio.03338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumru S, Tekedar HC, Gulsoy N, Waldbieser GC, Lawrence ML, Karsi A. 2017. Comparative analysis of the Flavobacterium columnare genomovar I and II genomes. Front Microbiol 8:1375. 10.3389/fmicb.2017.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat Rev 14:563–575. 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 43.Cai W, De La Fuente L, Arias CR. 2013. Biofilm formation by the fish pathogen Flavobacterium columnare: development and parameters affecting surface attachment. Appl Environ Microbiol 79:5633–5642. 10.1128/AEM.01192-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Decostere A, Haesebrouck F, Turnbull JF, Charlier G. 1999. Influence of water quality and temperature on adhesion of high and low virulence Flavobacterium columnare strains to isolated gill arches. J Fish Dis 22:1–11. 10.1046/j.1365-2761.1999.00132.x. [DOI] [Google Scholar]
- 45.Cairns J, Becks L, Jalasvuori M, Hiltunen T. 2017. Sublethal streptomycin concentration and lytic bacteriophage together promote resistance evolution. Philos Trans R Soc Lond B Biol Sci 372:20160040. 10.1098/rstb.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moulton-Brown CE, Friman VP. 2018. Rapid evolution of generalized mechanisms can constrain the efficacy of phage-antibiotic treatments. Evol Appl 11:1630–1641. 10.1111/eva.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burmeister AR, Fortier A, Roush C, Lessing AJ, Bender RG, Barahman R, Grant R, Chan BK, Turner PE. 2020. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc Natl Acad Sci U S A 117:11207–11216. 10.1073/pnas.1919888117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allen RC, Pfrunder-Cardozo KR, Meinel D, Egli A, Hall AR. 2017. Associations among antibiotic and phage resistance phenotypes in natural and clinical Escherichia coli isolates. mBio 8:e01341-17. 10.1128/mBio.01341-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang QG, Buckling A. 2012. Phages limit the evolution of bacterial antibiotic resistance in experimental microcosms. Evol Appl 5:575–582. 10.1111/j.1752-4571.2011.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laanto E, Sudberg LR, Bamford JKH. 2011. Phage specificity of the freshwater fish pathogen Flavobacterium columnare. Appl Environ Microbiol 77:7868–7872. 10.1128/AEM.05574-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song YL, Fryer JL, Rohovec JS. 1988. Comparison of six media for the cultivation of Flexibacter columnaris. Fish Pathol 23:91–94. 10.3147/jsfp.23.91. [DOI] [Google Scholar]
- 52.Holt RA, Rohovec JS, Fryer JL. 1993. Bacterial cold-water disease, p 3–23. In Inglis V, Roberts RJ, Bromage NR (ed), Bacterial diseases of fish. Blackwell Scientific Publication, Oxford, UK. [Google Scholar]
- 53.Adams MH. 1959. Bacteriophages. Interscience Publishers, Inc., New York, NY. [Google Scholar]
- 54.Bauer AW, Kirby WMM, Sherris JC, Turck M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496. 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 55.Clinical and Laboratory Standards Institute. 2006. Methods for antimicrobial disk susceptibility testing of bacteria isolated from aquatic animals; approved guideline. CLSI document M42-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 56.Högfors-Rönnholm E, Norrgård J, Wiklund T. 2015. Adhesion of smooth and rough phenotypes of Flavobacterium psychrophilum to polystyrene surfaces. J Fish Dis 38:429–437. 10.1111/jfd.12250. [DOI] [PubMed] [Google Scholar]
- 57.Decostere A, Haeseborouck F, Devriese LA. 1997. Shieh medium supplemented with tobramycin for selective isolation of Flavobacterium columnare (Flexibacter columnaris) from diseased fish. J Clin Microbiol 35:322–324. 10.1128/JCM.35.1.322-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ravantti JJ, Laanto E, Papponen P, Sundberg LR. 2019. Complete genome sequence of fish pathogen Flavobacterium columnare strain B185, originating from Finland. Microbiol Resour Announc 8:e01285-19. 10.1128/MRA.01285-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. 2016. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44:6614–6624. 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haft DH, DiCuccio M, Badretdin A, Brover V, Chetvernin V, O'Neill K, Li W, Chitsaz F, Derbyshire MK, Gonzales NR, Gwadz M, Lu F, Marchler GH, Song JS, Thanki N, Yamashita RA, Zheng C, Thibaud-Nissen F, Geer LY, Marchler-Bauer A, Pruitt KD. 2018. RefSeq: an update on prokaryotic genome annotation and curation. Nucleic Acids Res 46:D851–D860. 10.1093/nar/gkx1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplemental text, Figure S1, Table S1, legend to Data Set S1. Download AEM.00812-21-s0001.pdf, PDF file, 0.2 MB (234.3KB, pdf)
Data Set S1. Download AEM.00812-21-s0002.xlsx, XLSX file, 0.03 MB (29.4KB, xlsx)
Data Availability Statement
The whole-genome sequences of all isolates were submitted to GenBank under accession numbers presented in Table 6.
TABLE 6.
Accession numbers of whole-genome sequences of wild-type Flavobacterium columnare isolates FCO-F2 and FCO-F9 and their phage-exposed (F2R- and F9R-) and no-phage control (F2S- and F9S-) isolates submitted to GenBank
| Isolate | Accession no. |
|---|---|
| FCO-F2 | CP051861 |
| F2R58 | CP054506 |
| F2R60 | CP054505 |
| F2R62 | CP054504 |
| F2R64 | CP054503 |
| F2R65 | CP054502 |
| F2R66 | CP054501 |
| F2R67 | CP054500 |
| F2R68 | CP054499 |
| F2R70 | CP054498 |
| F2R72 | CP054497 |
| F2R74 | CP054496 |
| F2S4 | CP054495 |
| F2S17 | CP054494 |
| FCO-F9 | CP054518 |
| F9R56 | CP054517 |
| F9R58 | CP054516 |
| F9R61 | CP054515 |
| F9R64 | CP054514 |
| F9R66 | CP054513 |
| F9R69 | CP054512 |
| F9R72 | CP054511 |
| F9R75 | CP054510 |
| F9R78 | CP054509 |
| F9S15 | CP054508 |
| F9S17 | CP054507 |