Abstract
Chronic abdominal pain is a common gastrointestinal (GI) symptom that characterizes many functional GI disorders/disorders of gut-brain interaction, including irritable bowel syndrome, functional dyspepsia, and centrally mediated abdominal pain syndrome. The symptoms of abdominal pain in these highly prevalent disorders are often treated with antispasmodic agents. Antispasmodic treatment includes a broad range of therapeutic classes with different mechanisms of action, including anticholinergic/antimuscarinic agents (inhibition of GI smooth muscle contraction), calcium channel inhibitors (inhibition of calcium transport into GI smooth muscle), and direct smooth muscle relaxants (inhibition of sodium and calcium transport). The aim of this review article was to examine the efficacy and safety of antispasmodics available in North America (e.g., alverine, dicyclomine, hyoscine, hyoscyamine, mebeverine, otilonium, pinaverium, and trimebutine) for the treatment of chronic abdominal pain in patients with common disorders of gut-brain interaction. For the agents examined, comparisons of studies are limited by inconsistencies in treatment dosing and duration, patient profiles, and diagnostic criteria employed. Furthermore, variability in study end points limits comparisons. Risk of selection, performance, detection, attrition, and reporting bias also differed among studies, and in many cases, risks were considered “unclear.” The antispasmodics evaluated in this review, which differ in geographic availability, were found to vary dramatically in efficacy and safety. Given these caveats, each agent should be considered on an individual basis, rather than prescribed based on information across the broad class of agents.
INTRODUCTION
Abdominal pain is the most common gastrointestinal (GI) symptom prompting an office-based outpatient or emergency department visit in the United States (1). Functional GI disorders, now more formally described as disorders of gut-brain interaction (DGBI), such as irritable bowel syndrome (IBS), functional dyspepsia (FD), and centrally mediated abdominal pain syndrome (CAPS), are the underlying cause of abdominal pain in many patients (2). IBS is a chronic disorder characterized by recurring abdominal pain associated with disordered bowel habits (3). According to Rome IV criteria, the diagnosis of IBS requires patients to have abdominal pain ≥1 day per week in the previous 3 months (3). FD is also a pain-predominant disorder (4). Rome IV diagnostic criteria for FD require patients to present with bothersome epigastric pain (≥1 day per week), epigastric burning (≥1 day per week), postprandial fullness (≥3 days per week), or early satiation (≥3 days per week) during the previous 3 months (5). Centrally mediated abdominal pain syndrome is characterized by persistent abdominal pain that interferes with daily activities; it is not associated with altered bowel habits (6,7).
Functional GI disorders are highly prevalent, resulting in impaired health-related quality of life and increased healthcare utilization (8,9). The prevalence of IBS varies based on the criteria used and the populations studied (10). In Canada and the United States, the prevalence of IBS based on Rome IV criteria has been estimated at 4.7% and 4.8%, respectively; the prevalence of IBS based on Rome III criteria was estimated at 9.7% and 8.8% in the same countries and ranged between 6.5% and 8.7% in Mexico (10,11). The prevalence of FD similarly varies depending on the criteria used to define it (12). In the United States, the prevalence of FD has been estimated at 12% based on Rome IV criteria (9,13). Data for the prevalence of CAPS are currently lacking.
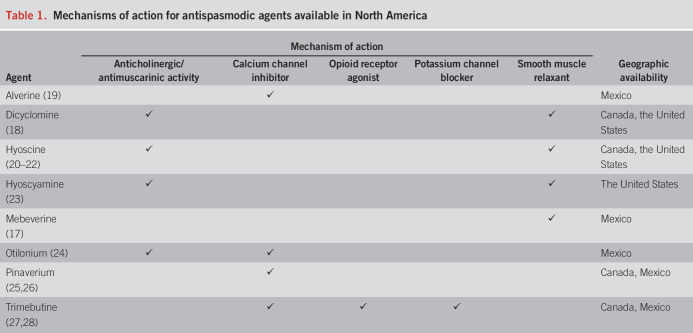
Alterations in GI motility and visceral sensation play a role in the development of abdominal pain in many patients; antispasmodics function as smooth muscle relaxants or antagonists to block excitatory neuromuscular neurotransmission (14,15). Antispasmodics are considered a mainstay treatment option for patients with IBS (Table 1; Figure 1) (16–28); indeed, online survey data indicated that 30% of 1,094 patients with IBS with diarrhea (IBS-D) previously used antispasmodics (29). However, antispasmodic therapies differ in their mechanism(s) of action, with the major classes categorized as anticholinergic/antimuscarinic agents, calcium channel inhibitors, and direct smooth muscle relaxants (30). Anticholinergic/antimuscarinic agents inhibit GI smooth muscle contraction, in part, by blocking calcium transport through calcium channels (31); furthermore, these agents decrease colonic motility (32). Calcium channel inhibitors prevent the influx of calcium into GI smooth muscle, thus inhibiting smooth muscle contraction (33). Direct smooth muscle relaxants affect GI smooth muscle by inhibiting sodium influx through sodium channels and preventing subsequent influx of calcium, all of which leads to inhibition of duodenal and colonic contraction (17,34–36).
Table 1.
Mechanisms of action for antispasmodic agents available in North America
Figure 1.
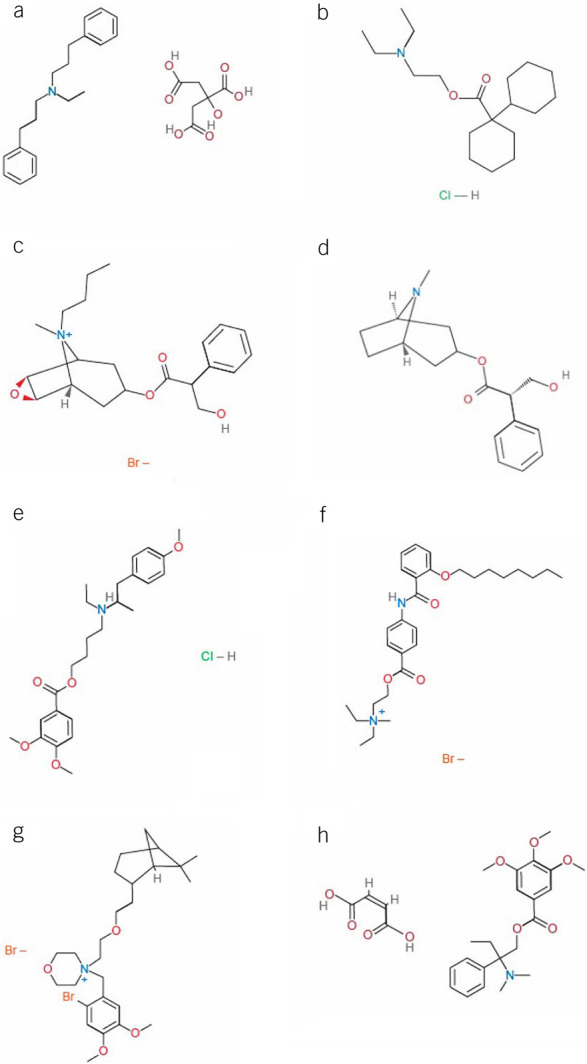
Chemical structure for antispasmodic agents available in North America. (a) alverine, (b) dicyclomine, (c) hyoscine, (d) hyoscyamine, (e) mebeverine, (f) otilonium, (g) pinaverium, (h) trimebutine. Chemical structures reprinted from PubChem, https://pubchem.ncbi.nlm.nih.gov
A 2014 American Gastroenterological Association guideline noted that antispasmodics could be used to treat IBS symptoms; a new guideline is currently under development (37). The American Gastroenterological Association provided a conditional recommendation for antispasmodics based on the low certainty of evidence (e.g., methodologic limitations and publication bias) (37). In addition, data were based on continuous, rather than as-needed, use, and not all antispasmodics evaluated are currently available in the United States (37). The 2018 American College of Gastroenterology (ACG) monograph suggested that certain antispasmodic drugs (i.e., dicyclomine, hyoscine, cimetropium, drotaverine, otilonium, and pinaverium) may improve IBS symptoms, although this was a weak recommendation based on the very low quality of evidence (38). Importantly, data are limited for the antispasmodics currently available in the United States. Recently published ACG guidelines (2021) for the treatment of IBS, which used a GRADE approach, do not recommend the use of smooth muscle antispasmodics currently available in the United States for the treatment of IBS (39). Although antispasmodics are frequently prescribed for the treatment of FD, a 2017 joint ACG/Canadian Association of Gastroenterology dyspepsia guideline does not recommend their use for this condition (40,41). There are currently no formal guidelines or recommendations regarding the use of antispasmodics for treating CAPS.
Given the discrepancies in recent recommendations, the aim of this review was to examine the efficacy and safety of individual antispasmodics available in North America (i.e., alverine, dicyclomine, hyoscine, hyoscyamine, mebeverine, otilonium, pinaverium, and trimebutine; Table 1; Figure 1) for the treatment of chronic abdominal pain in patients with these pain-predominant disorders.
METHODS
PubMed and Embase were searched electronically for full-length articles available through December 2020 (start date, 1963 [PubMed] or 1947 [Embase] to allow complete database review) that reported the results of randomized, placebo-controlled, parallel, or crossover studies of antispasmodics conducted in adults with abdominal pain because of IBS, dyspepsia/FD, and CAPS. Antispasmodics currently available in North America (United States, Canada, and Mexico) were included in this search.
Search terms were “abdominal pain,” “irritable bowel syndrome,” “dyspepsia,” “centrally mediated abdominal pain syndrome,” “antispasmodic,” “parasympatholytic,” “alverine,” “dicyclomine,” “hyoscine,” “hyoscyamine,” “mebeverine,” “otilonium,” “pinaverium,” and “trimebutine.” Reference lists from relevant review articles and the Cochrane Central Register for Controlled Trials were searched for additional references. Relevant articles published in languages other than English were translated using Google Translate. Articles eligible for inclusion examined improvement in chronic abdominal pain as an efficacy outcome in functional GI disorders in adults. Studies evaluating peppermint oil formulations were excluded from this review, as peppermint oil is considered a unique treatment class for these disorders. Trials of <10 days' treatment duration were also excluded.
The Cochrane Collaboration's “Risk of Bias” tool was used to assess the risk of bias in articles included in the review (42). Briefly, risk of bias was rated as “low,” “high,” or “unclear” for random allocation sequence generation and concealment; blinding of patients, personnel, and outcome assessments; adequately addressing incomplete outcome data; and selective outcome reporting (42). Each author independently evaluated risk of bias, with authors reaching consensus on any disagreements in ratings.
RESULTS
The PubMed and Embase database searches identified 492 publications (Figure 2). Eleven additional references were identified from reference lists in relevant review articles and the Cochrane Central Register for Controlled Trials. A total of 26 studies, including 23 IBS and 1 FD, were included. In addition, 2 studies of recurrent abdominal pain with cramping (APC) met criteria for inclusion. No studies evaluating antispasmodics in patients with CAPS were identified.
Figure 2.
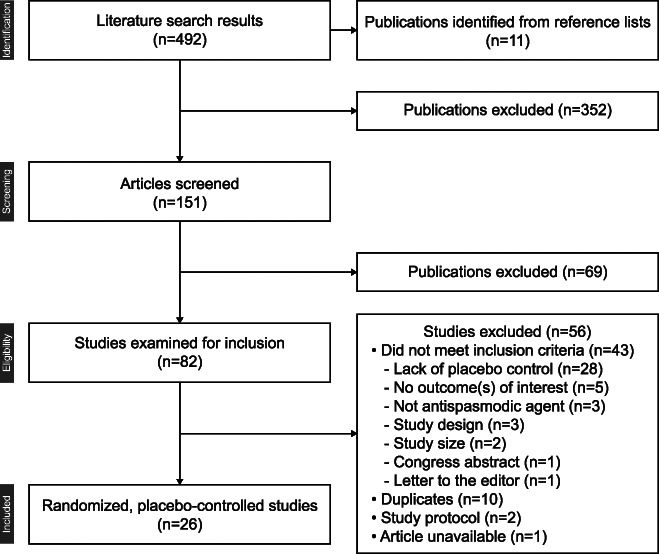
Summary of literature search.
Antispasmodics for IBS
Anticholinergic/antimuscarinic antispasmodics.
Dicyclomine.
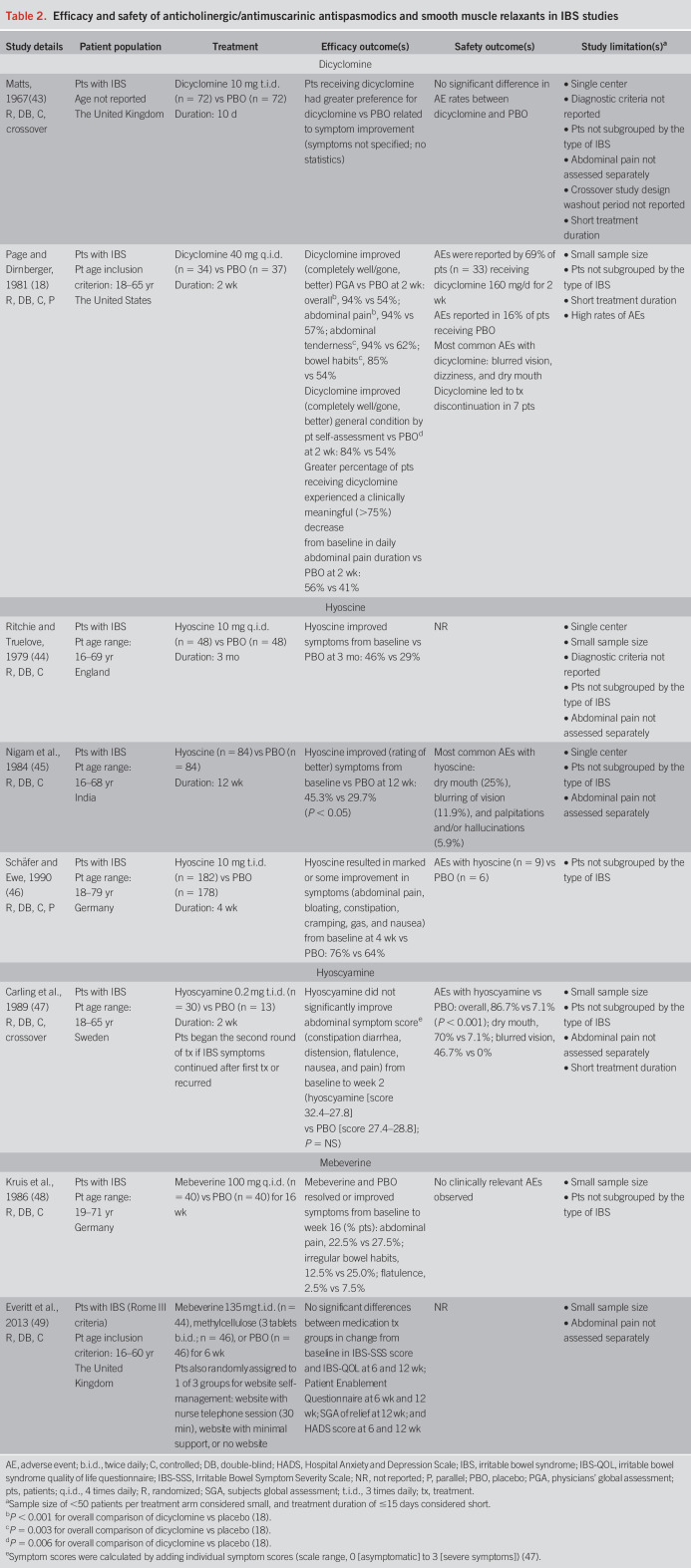
In 2 randomized, placebo-controlled studies, dicyclomine improved symptoms of IBS relative to placebo (Table 2) (18,43–49). One study reported no difference in adverse event (AE) rates with dicyclomine vs placebo (43), whereas the other reported that AEs occurred in a greater percentage of patients (69%) receiving dicyclomine 160 mg/d continuously for 2 weeks vs patients receiving placebo (16%; Table 2) (18). Although efficacy data were generally favorable, these studies used different doses of dicyclomine and had a short treatment duration (10 days–2 weeks) (18,43). Furthermore, 1 study had a high risk of allocation bias (see Supplementary Table, Supplementary Digital Content, http://links.lww.com/AJG/B987) because of AEs (15,18,43–66).
Table 2.
Efficacy and safety of anticholinergic/antimuscarinic antispasmodics and smooth muscle relaxants in IBS studies
Hyoscine.
Hyoscine, also known as scopolamine, is an anticholinergic/antimuscarinic agent and smooth muscle relaxant (20). In 3 studies, hyoscine taken for a duration of 4 weeks to 3 months was more efficacious than placebo at improving IBS symptoms (Table 2) (44–46). Only 1 study adequately reported AEs (45). Although all 3 studies reported favorable efficacy, they differed in treatment duration and definitions of IBS, and 2 studies lacked separate assessments of abdominal pain (44–46). However, the risk of bias was mostly low (44,45).
Hyoscyamine.
Hyoscyamine, an L-isomer of atropine racemate, is, like hyoscine, an anticholinergic/antimuscarinic agent and smooth muscle relaxant (23). One small crossover study (N = 40) reported that hyoscyamine 0.2 mg 3 times daily (t.i.d.) for a 2-week period (dose increased if IBS symptoms persisted) improved IBS symptoms (including pain) from baseline numerically, but not significantly, compared with placebo (P = NS; Table 2) (47). Study limitations included short treatment duration and lack of analysis by IBS subtype or abdominal pain alone. According to the authors, patients might also have been aware of treatment assignment, given the nature of the AEs reported (47).
Direct smooth muscle relaxant.
Mebeverine.
The efficacy of mebeverine was examined in 2 randomized, placebo-controlled trials (Table 2) (48,49). In 1 study, 16 weeks of treatment with mebeverine 100 mg 4 times daily was less effective for patients with IBS than placebo for improving symptoms of abdominal pain and flatulence, and irregular bowel habits. No clinically relevant AEs occurred in either treatment group (48). In a second study, a 6-week treatment with mebeverine 135 mg t.i.d. in conjunction with or without use of a self-management website had no greater efficacy than placebo for improving IBS symptoms; AEs were not reported in this study (49). Limitations included small sample sizes and lack of data for IBS subtypes (48,49). Risk of bias was mostly unclear for 1 study (48), whereas another indicated a potential placebo effect on efficacy results (49).
Calcium channel inhibitors.
Alverine.
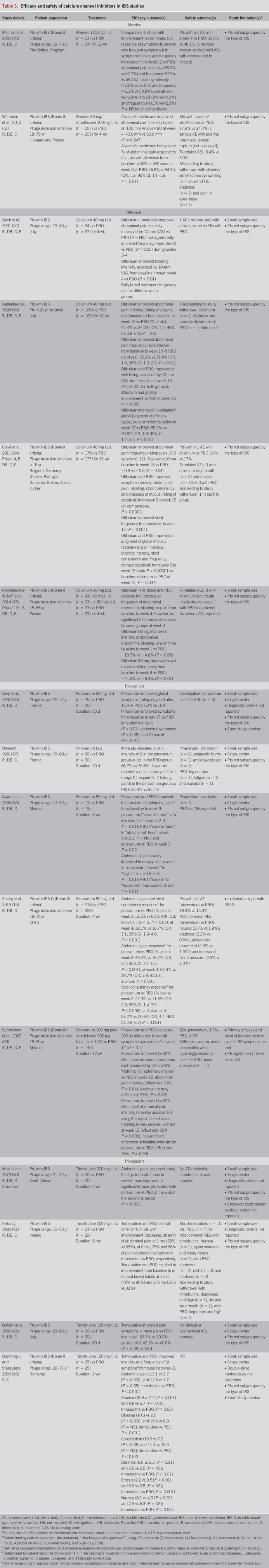
Efficacy and safety were examined for alverine, a calcium channel blocker (19), in 2 randomized, placebo-controlled studies (Table 3) (50,51). A comparable percentage of patients receiving alverine 120 mg t.i.d. or placebo for 12 weeks experienced improvements from baseline in the intensity and frequency of abdominal pain, bloating, and overall well-being at week 12; differences between groups did not achieve statistical significance (50). A lower percentage of patients receiving alverine reported ≥1 AE, compared with placebo (50). In a second study, alverine 60 mg/simethicone 300 mg t.i.d. was significantly more efficacious than placebo at improving abdominal pain in patients with IBS (P = 0.047) (51). The safety profile of alverine/simethicone was generally comparable with that of placebo (51); however, this study potentially excluded patients with more severe symptoms (51).
Table 3.
Efficacy and safety of calcium channel inhibitors in IBS studies
Otilonium.
The efficacy and safety of otilonium were examined in 4 randomized, controlled studies (Table 3) (52–55). In 3 studies, otilonium 40 mg t.i.d. decreased abdominal pain frequency compared with placebo during weeks 3–4 (52) and at week 15 (53,54). Otilonium was associated with mild nausea in 1 study, whereas no AEs were reported with placebo (52). In another study, prostate disturbance and dizziness were reported with otilonium, and skin rash with placebo; these AEs led to study withdrawal (53). In a dose-ranging study, otilonium 20, 40, and 80 mg t.i.d. decreased the intensity and frequency of abdominal pain and bloating from baseline to 4 weeks; however, no differences between otilonium and placebo were observed after treatment (55). Treatment-related AEs with otilonium were generally comparable with placebo (55). Few details regarding treatment allocation, blinding, and participant attrition were provided for 2 of the studies (52,53); thus, the risks of bias were mostly unclear. One study was at high risk of bias for selective outcome reporting because of a lack of economic data (a prespecified outcome) (54).
Pinaverium.
Pinaverium efficacy and safety were reported in 5 randomized placebo-controlled IBS studies (Table 3) (15,56–59). Three small, single-center studies published in 1995 or earlier reported that pinaverium 50 mg t.i.d. improved abdominal pain in patients with IBS (56–58). The safety profile of pinaverium in these small studies was generally comparable with that of placebo (56–58).
Two larger, multicenter, double-blind, placebo-controlled studies evaluated the efficacy and safety of pinaverium in patients with IBS diagnosed per Rome III criteria (15,59). Zheng reported that patients with IBS-D receiving pinaverium 50 mg t.i.d. experienced significant improvements in composite abdominal pain and stool consistency response versus placebo at weeks 2 and 4 (P < 0.05 and P < 0.001, respectively) (15). The most common AEs reported were nausea, dizziness, abdominal discomfort, and hypertension (15). Schmulson reported that the combination of pinaverium 100 mg plus simethicone 300 mg twice daily compared with placebo significantly improved the intensity of abdominal pain (P = 0.04) and bloating (P = 0.02); the individual contribution of each agent cannot be determined (59). The safety profile of pinaverium/simethicone was generally comparable with that of placebo.
Analysis of risk of bias in the 5 pinaverium studies was mostly unclear (15,56–59).
Trimebutine.
Across 4 small studies of trimebutine 100 or 200 mg t.i.d. administered for 2 weeks to 6 months, improvement in abdominal pain was inconsistently observed (Table 3) (60–63). Of these 4 studies, 1 evaluating trimebutine 200 mg t.i.d. did not show improvement in abdominal pain versus placebo (61). Nausea, shaky hands, and upset stomach, the most common AEs experienced with trimebutine, were not reported by any patients receiving placebo (61). The other 3 studies (100 and 200 mg t.i.d.) reported improvement in abdominal pain versus placebo (60,62,63). Safety data were not consistently presented in the 4 studies, and the risk of bias was mostly unclear (60–63).
Antispasmodics for abdominal pain in other functional GI disorders
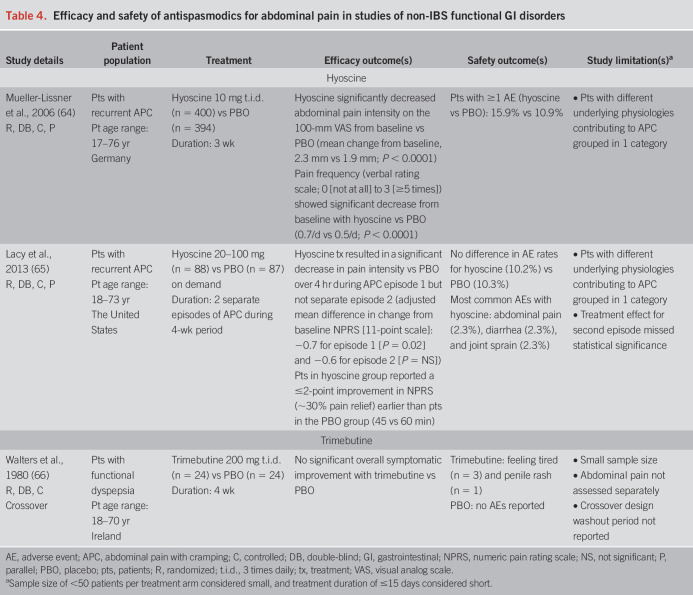
Three non-IBS functional GI disorder studies were included in this review (Table 4) (64–66).
Table 4.
Efficacy and safety of antispasmodics for abdominal pain in studies of non-IBS functional GI disorders
Hyoscine for recurrent abdominal pain.
Two multicenter studies assessed the efficacy and safety of hyoscine for the treatment of recurrent APC not linked to altered bowel habits (64,65). Mueller-Lissner et al. reported a significant decrease from baseline in abdominal pain intensity with hyoscine 10 mg t.i.d. compared with placebo (P < 0.0001) after 3 weeks of treatment; in addition, abdominal pain frequency was significantly reduced with hyoscine compared with placebo (P < 0.0001) (64). Lacy et al. reported that, during a 4-week period of study, on-demand hyoscine 20–100 mg treatment over 4 hours decreased abdominal pain intensity versus placebo during the first APC episode (P = 0.02), but not during a second, separate APC episode (65). Hyoscine was well tolerated in both studies (64,65).
Trimebutine for patients with FD.
A small crossover study with trimebutine 200 mg t.i.d. in patients with FD reported no significant improvement in overall dyspeptic symptoms (including abdominal pain) compared with placebo after 4 weeks of treatment (66). Tiredness and transient penile rash were AEs reported during trimebutine treatment, whereas no AEs were reported during placebo treatment (66).
DISCUSSION
Dicyclomine, hyoscine, and hyoscyamine are anticholinergic/antimuscarinic agents available in the United States. Although placebo-controlled efficacy and safety data related to the use of these antispasmodics in patients with IBS seem favorable, the studies of dicyclomine (18,43) and hyoscine (44–47) identified in this review were published in 1990 or earlier and used different doses, treatment durations, and outcome assessments. Furthermore, in these relatively small studies, patients with IBS were not subgrouped by IBS subtype, and definitions of IBS were inconsistent. Consequently, comparisons that can be made across studies are limited. Risk of bias was variable among studies (e.g., AEs with dicyclomine and hyoscyamine could have revealed treatment allocation) (18,47).
Two randomized, placebo-controlled studies demonstrated that the direct smooth muscle relaxant mebeverine did not improve IBS symptoms compared with placebo (48,49). However, these trials were limited by small sample sizes (48,49). Furthermore, the risk of bias was unclear in 1 of the 2 studies (48).
Calcium channel inhibitors for the treatment of chronic abdominal pain are currently available in Canada and/or Mexico, but not the United States. The efficacy of alverine was variable in 2 randomized, controlled studies, with 1 study achieving a statistically significant improvement in abdominal pain compared with placebo (50,51). Both studies had a risk of bias related to patient selection (50,51). Otilonium was evaluated in 4 clinical studies that varied in dosing and treatment duration (52–55) and also treatment allocation, blinding, and patient attrition (52,53). The high placebo response observed in 1 study was potentially because of patient selection and/or the patient-provider relationship (54). Pinaverium was examined in 5 randomized, placebo-controlled studies that differed in treatment duration, dosing, and outcomes; furthermore, 1 study included only patients with IBS-D (15,56–59). Studies generally had an unclear risk of bias (15,56–59). Trimebutine was examined in 4 clinical trials of patients with IBS with inconsistent results: in 2 studies, trimebutine 100 mg t.i.d. improved multiple IBS symptoms, a finding that differed significantly from placebo; however, 2 studies that examined trimebutine treatment at a higher dose showed the drug was no more efficacious than placebo for improving abdominal pain or bowel habits (60–63). Limitations included the absence of patient populations from multiple centers, which potentially limited the generalizability of results, and small, underpowered studies. Risk of bias in studies of trimebutine was unclear.
The definition of IBS has changed over time, and studies of antispasmodics are inconsistent in this regard. For example, Rome IV criteria no longer include abdominal discomfort as a hallmark symptom because of its ambiguous nature and a lack of the term in some languages; in addition, duration of symptom frequency increased from ≥3 d/mo with Rome III criteria to ≥1 d/wk with Rome IV (67). Furthermore, since the publication of most of these antispasmodic studies, the US Food and Drug Administration (FDA) has provided guidance for defining treatment response in clinical trials of IBS. Importantly, of all the antispasmodic trials reviewed herein, only one (15) is consistent with the current US FDA guidance (68).
Studies supporting the use of specific antispasmodics for non-IBS DGBI are limited. Hyoscine was examined in 2 studies of patients with recurrent APC (64,65), and trimebutine in 1 small study of patients with FD (66). Hyoscine improved abdominal pain frequency and intensity versus placebo in patients with recurrent APC, with a fixed dosing schedule or on-demand use; however, patients with different underlying physiologies contributing to APC were grouped in 1 broad category in these studies (64,65). Trimebutine did not show overall symptomatic improvement versus placebo in patients with FD (66).
In summary, data supporting the use of antispasmodics for the treatment of chronic abdominal pain in patients with DGBI, including IBS and FD, are limited. Limited sample size, short duration of therapy, heterogeneity in outcomes, and concerns over potential bias with study design make it difficult to recommend these agents for clinical use, especially when compared with the data sets available from large, randomized, controlled trials that characterize the current US FDA-approved IBS medications. This highlights the need to use other therapies to treat chronic abdominal pain (e.g., neuromodulators and cognitive behavioral therapy) and to develop agents to treat this debilitating symptom.
CONFLICTS OF INTEREST
Guarantor of the article: Darren M. Brenner, MD, FACG.
Specific author contributions: Both authors contributed equally to the design, research, writing, and editing of this review.
Financial support: Technical editorial and medical writing assistance was provided, under direction of the authors, by Mary Beth Moncrief, PhD, and Sophie Bolick, PhD, Synchrony Medical Communications, LLC, West Chester, PA. Funding for this assistance was provided by Salix Pharmaceuticals, Bridgewater, NJ.
Potential competing interests: D.M.B. reports serving as a consultant, advisor, and/or speaker for Alnylam, Alphasigma, Arena, Salix Pharmaceuticals, Allergan (Abbvie), Ironwood, and Takeda Pharmaceuticals. D.M.B. was also supported in research by an unrestricted grant from the Irene D. Pritzker Foundation. B.E.L. reports serving as an advisory board member for Ironwood Pharmaceuticals, Salix Pharmaceuticals, Arena Pharmaceuticals, Alphasigma, and Allakos; he also has provided consulting services to Viver and Urovant Sciences.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/B987
REFERENCES
- 1.Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2018. Gastroenterology 2019;156:254–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drossman DA. Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology 2016;150:1262–79. [DOI] [PubMed] [Google Scholar]
- 3.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393–407. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Mahadeva S, Carbone MF, et al. Functional dyspepsia. Lancet 2020;396:1689–702. [DOI] [PubMed] [Google Scholar]
- 5.Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology 2016;150:1380–92. [DOI] [PubMed] [Google Scholar]
- 6.Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil 2017;23:151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keefer L, Drossman DA, Guthrie E, et al. Centrally mediated disorders of gastrointestinal pain. Gastroenterology 2016;150:1408–19. [DOI] [PubMed] [Google Scholar]
- 8.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation Global Study. Gastroenterology 2021;160:99–114.e3. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB, Talley NJ. Systematic review: Health-related quality of life in functional dyspepsia. Aliment Pharmacol Ther 2003;18:387–93. [DOI] [PubMed] [Google Scholar]
- 10.Palsson OS, Whitehead W, Törnblom H, et al. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020;158:1262–73. [DOI] [PubMed] [Google Scholar]
- 11.Amieva-Balmori M, Meixueiro-Daza A, Cantón P, et al. Gastroesophageal reflux disease in Mexico. National study using the Rome III and PAGI-SYM questionnaires [Spanish-language article]. Rev Gastroenterol Mex 2014;79:22–3.24629570 [Google Scholar]
- 12.Ford AC, Marwaha A, Sood R, et al. Global prevalence of, and risk factors for, uninvestigated dyspepsia: A meta-analysis. Gut 2015;64:1049–57. [DOI] [PubMed] [Google Scholar]
- 13.Aziz I, Palsson OS, Törnblom H, et al. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: A cross-sectional population-based study. Lancet Gastroenterol Hepatol 2018;3:252–62. [DOI] [PubMed] [Google Scholar]
- 14.Ford AC, Sperber AD, Corsetti M, et al. Irritable bowel syndrome. Lancet 2020;396:1675–88. [DOI] [PubMed] [Google Scholar]
- 15.Zheng L, Lai Y, Lu W, et al. Pinaverium reduces symptoms of irritable bowel syndrome in a multicenter, randomized, controlled trial. Clin Gastroenterol Hepatol 2015;13:1285–92. [DOI] [PubMed] [Google Scholar]
- 16.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA 2015;313:949–58. [DOI] [PubMed] [Google Scholar]
- 17.Lindner A, Selzer H, Claassen V, et al. Pharmacological properties of mebeverine, a smooth-muscle relaxant. Arch Int Pharmacodyn Ther 1963;145:378–95. [PubMed] [Google Scholar]
- 18.Page JG, Dirnberger GM. Treatment of the irritable bowel syndrome with bentyl (dicyclomine hydrochloride). J Clin Gastroenterol 1981;3:153–6. [DOI] [PubMed] [Google Scholar]
- 19.Hayase M, Hashitani H, Suzuki H, et al. Evolving mechanisms of action of alverine citrate on phasic smooth muscles. Br J Pharmacol 2007;152:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuels LA. Pharmacotherapy update: Hyoscine butylbromide in the treatment of abdominal spasms. Clin Med Therapeutics 2009;1:647–55. [Google Scholar]
- 21.Ritchie JA, Truelove SC. Comparison of various treatments for irritable bowel syndrome. Br Med J 1980;281:1317–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shutt LE, Bowes JB. Atropine and hyoscine. Anaesthesia 1979;34:476–90. [DOI] [PubMed] [Google Scholar]
- 23.Mirakhur RK. Anticholinergic drugs. Br J Anaesth 1979;51:671–9. [DOI] [PubMed] [Google Scholar]
- 24.Evangelista S, Traini C, Vannucchi MG. Otilonium bromide: A drug with a complex mechanism of action. Curr Pharm Des 2018;24:1772–9. [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner A, Drack E, Halter F, et al. Effects of pinaverium bromide and verapamil on the motility of the rat isolated colon. Br J Pharmacol 1985;86:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fioramonti J, Frexinos J, Staumont G, et al. Inhibition of the colonic motor response to eating by pinaverium bromide in irritable bowel syndrome patients. Fundam Clin Pharmacol 1988;2:19–27. [DOI] [PubMed] [Google Scholar]
- 27.Tan W, Zhang H, Luo HS, et al. Effects of trimebutine maleate on colonic motility through Ca2+-activated K+ channels and L-type Ca2+ channels. Arch Pharm Res 2011;34:979–85. [DOI] [PubMed] [Google Scholar]
- 28.Fioramonti J, Bueno L. Centrally acting agents and visceral sensitivity. Gut 2002;51:i91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayuk GS, Wolf R, Chang L. Comparison of symptoms, healthcare utilization, and treatment in diagnosed and undiagnosed individuals with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 2017;112:892–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boeckxstaens G, Clavé P, Corazziari ES, et al. Irritable bowel syndrome: Focus on otilonium bromide. Expert Rev Gastroenterol Hepatol 2014;8:131–7. [DOI] [PubMed] [Google Scholar]
- 31.Tobin G, Giglio D, Lundgren O. Muscarinic receptor subtypes in the alimentary tract. J Physiol Pharmacol 2009;60:3–21. [PubMed] [Google Scholar]
- 32.Centonze V, Imbimbo BP, Campanozzi F, et al. Oral cimetropium bromide, a new antimuscarinic drug, for long-term treatment of irritable bowel syndrome. Am J Gastroenterol 1988;83:1262–6. [PubMed] [Google Scholar]
- 33.Evangelista S. Quaternary ammonium derivatives as spasmolytics for irritable bowel syndrome. Curr Pharm Des 2004;10:3561–8. [DOI] [PubMed] [Google Scholar]
- 34.Subissi A, Brunori P, Bachi M. Effects of spasmolytics on K+-induced contraction of rat intestine in vivo. Eur J Pharmacol 1983;96:295–301. [DOI] [PubMed] [Google Scholar]
- 35.Den Hertog A, Van den Akker J. The action of mebeverine and metabolites on mammalian non-myelinated nerve fibres. Eur J Pharmacol 1987;139:353–5. [DOI] [PubMed] [Google Scholar]
- 36.Den Hertog A, Van den Akker J. Modification of alpha 1-receptor-operated channels by mebeverine in smooth muscle cells of Guinea-pig taenia caeci. Eur J Pharmacol 1987;138:367–74. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg DS, Smalley W, Heidelbaugh JJ, et al. American Gastroenterological Association Institute Guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology 2014;147:1146–8. [DOI] [PubMed] [Google Scholar]
- 38.Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol 2018;113:1–18. [DOI] [PubMed] [Google Scholar]
- 39.Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: Management of irritable bowel syndrome. Am J Gastroenterol 2021;116:17–44. [DOI] [PubMed] [Google Scholar]
- 40.Brun R, Kuo B. Functional dyspepsia. Therap Adv Gastroenterol 2010;3:145–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moayyedi P, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: Management of dyspepsia. Am J Gastroenterol 2017;112:988–1013. [DOI] [PubMed] [Google Scholar]
- 42.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell: Chichester, England, 2008. [Google Scholar]
- 43.Matts SGF. An assessment of dicyclomine hydrochloride (‘Merbentyl’) in the irritable colon syndrome. Br J Clin Pract 1967;21:549–51. [PubMed] [Google Scholar]
- 44.Ritchie JA, Truelove SC. Treatment of irritable bowel syndrome with lorazepam, hyoscine butylbromide, and ispaghula husk. Br Med J 1979;1:376–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nigam P, Kapoor KK, Rastog CK, et al. Different therapeutic regimens in irritable bowel syndrome. J Assoc Physicians India 1984;32:1041–4. [PubMed] [Google Scholar]
- 46.Schäfer E, Ewe K. The treatment of irritable colon. Efficacy and tolerance of Buscopan Plus, Buscopan, paracetamol and placebo in ambulatory patients with irritable colon [in German]. Fortschr Med 1990;108:488–92. [PubMed] [Google Scholar]
- 47.Carling L, Svedberg L-E, Hulten S. Short term treatment of the irritable bowel syndrome: A placebo-controlled trial of peppermint oil against hyoscyamine. Opuscula Medica 1989(34):55–7. [Google Scholar]
- 48.Kruis W, Weinzierl M, Schüssler P, et al. Comparison of the therapeutic effect of wheat bran, mebeverine and placebo in patients with the irritable bowel syndrome. Digestion 1986;34:196–201. [DOI] [PubMed] [Google Scholar]
- 49.Everitt H, Moss-Morris R, Sibelli A, et al. Management of irritable bowel syndrome in primary care: The results of an exploratory randomised controlled trial of mebeverine, methylcellulose, placebo and a self-management website. BMC Gastroenterol 2013;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell SA, Mee AS, Smith GD, et al. Alverine citrate fails to relieve the symptoms of irritable bowel syndrome: Results of a double-blind, randomized, placebo-controlled trial. Aliment Pharmacol Ther 2002;16:1187–95. [DOI] [PubMed] [Google Scholar]
- 51.Wittmann T, Paradowski L, Ducrotté P, et al. Clinical trial: The efficacy of alverine citrate/simeticone combination on abdominal pain/discomfort in irritable bowel syndrome—A randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther 2010;31:615–24. [DOI] [PubMed] [Google Scholar]
- 52.Baldi F, Longanesi A, Blasi A, et al. Clinical and functional evaluation of the efficacy of otilonium bromide: A multicenter study in Italy. Ital J Gastroenterol 1991;23:60–3. [PubMed] [Google Scholar]
- 53.Battaglia G, Morselli-Labate AM, Camarri E, et al. Otilonium bromide in irritable bowel syndrome: A double-blind, placebo-controlled, 15-week study. Aliment Pharmacol Ther 1998;12:1003–10. [DOI] [PubMed] [Google Scholar]
- 54.Clavé P, Acalovschi M, Triantafillidis JK, et al. Randomised clinical trial: Otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2011;34:432–42. [DOI] [PubMed] [Google Scholar]
- 55.Chmielewska-Wilkón D, Reggiardo G, Egan CG. Otilonium bromide in irritable bowel syndrome: A dose-ranging randomized double-blind placebo-controlled trial. World J Gastroenterol 2014;20:12283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy C, Charbonnier A, Cachin M. Pinaverium bromide and functional colonic disease (double-blind study) [Article in French]. Sem Hop Ther 1977;53:372–4. [PubMed] [Google Scholar]
- 57.Delmont J. The value of adding an antispasmodic musculotropic agent in the treatment of painful constipation in functional colopathies with bran. Double-blind study [French]. Med Chir Dig 1981;10:365–70. [PubMed] [Google Scholar]
- 58.Awad R, Dibildox M, Ortiz F. Irritable bowel syndrome treatment using pinaverium bromide as a calcium channel blocker. A randomized double-blind placebo-controlled trial. Acta Gastroenterol Latinoam 1995;25:137–44. [PubMed] [Google Scholar]
- 59.Schmulson MJ, Chiu-Ugalde J, Sáez-Ríos A, et al. Efficacy of the combination of pinaverium bromide 100 mg plus simethicone 300 mg in abdominal pain and bloating in irritable bowel syndrome: A randomized, placebo-controlled trial. J Clin Gastroenterol 2020;54:e30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moshal MG, Herron M. A clinical trial of trimebutine (Mebutin) in spastic colon. J Int Med Res 1979;7:231–44. [DOI] [PubMed] [Google Scholar]
- 61.Fielding JF. Double blind trial of trimebutine in the irritable bowel syndrome. Ir Med J 1980;73:377–9. [PubMed] [Google Scholar]
- 62.Ghidini O, Saponati G, Intrieri L. Single drug treatment for irritable colon: Rociverine versus trimebutine maleate. Curr Ther Res 1986;39:541–8. [Google Scholar]
- 63.Dumitraşcu DL, Stănculete M. The effect of trimebutine on the psychosocial adjustment to illness in the irritable bowel syndrome. Rom J Intern Med 2006;44:273–80. [PubMed] [Google Scholar]
- 64.Mueller-Lissner S, Tytgat GN, Paulo LG, et al. Placebo- and paracetamol-controlled study on the efficacy and tolerability of hyoscine butylbromide in the treatment of patients with recurrent crampy abdominal pain. Aliment Pharmacol Ther 2006;23:1741–8. [DOI] [PubMed] [Google Scholar]
- 65.Lacy BE, Wang F, Bhowal S, et al. On-demand hyoscine butylbromide for the treatment of self-reported functional cramping abdominal pain. Scand J Gastroenterol 2013;48:926–35. [DOI] [PubMed] [Google Scholar]
- 66.Walters JM, Crean P, McCarthy CF. Trimebutine, a new antispasmodic in the treatment of dyspepsia. Ir Med J 1980;73:380–1. [PubMed] [Google Scholar]
- 67.Aziz I, Törnblom H, Palsson OS, et al. How the change in IBS criteria from Rome III to Rome IV impacts on clinical characteristics and key pathophysiological factors. Am J Gastroenterol 2018;113:1017–25. [DOI] [PubMed] [Google Scholar]
- 68.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for industry: Irritable bowel syndrome—Clinical evaluation of drugs for treatment. May 2012. (http://www.fda.gov/downloads/Drugs/Guidances/UCM205269.pdf). Accessed April 5, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.