ABSTRACT
Background: This study examined the frequency of inherited retinal diseases (IRDs) as the reason for blindness registrations over the last two decades and the demographic and clinical phenotypes of inherited retinal disease (IRD)-related registrations.
Materials and methods: Retrospective, observational study of individuals registered with a state-wide blind and vision-impaired registry. Low-vision or blindness-only (≤20/200 or ≤20°) certificates issued to children (0-15 years), working-age (16-64 years) and older-age (65 and older) adults were assessed. Sex and age distributions were examined for the top 20 reasons for certification. Demographic and clinical features of specific phenotypes of IRDs listed in the registry were examined.
Results: Amongst 11824 low-vision certificates issued between July 1995 and January 2017, 679 (5.7%) listed an IRD as the reason for registration. In individuals with blindness-only certification (N=4919), IRDs was the second most common diagnosis (8.3%), overtaking glaucoma (8.1%) and diabetic retinopathy (5.4%). IRD was the second most common reason for low-vision certification amongst children (11.6%) and the most common reason amongst working-age population (23.3%). The mean±SD age for IRD-related blindness-only certification was 46±20 years. The top three phenotypes of IRD-related low-vision certification were non-syndromic retinitis pigmentosa (54%), Stargardt disease (12%) and macular dystrophy (8%).
Conclusion: Our findings of IRDs as a common cause of blindness in all ages justify continued funding for providing low-vision services and developing treatments for these conditions.
KEYWORDS: Epidemiology, retinal Dystrophy, blind Registry, retinitis Pigmentosa, stargardt Disease
Background
Visual impairment and blindness can significantly impact on an individual’s opportunity for education, employment, recreational experiences, health and life expectancies. Australian data have revealed a strong association between blindness in the working–age population and the frequency and length of hospital admissions as well as a significantly higher mortality rate(1). Inherited retinal diseases (IRDs) account for 20-25% of blindness in the working-age population (1,2). Retinitis pigmentosa (RP), the most common form of IRD, has also been associated with a 6.6-fold higher rate of suicide in males in Korea as compared to the same age group without RP. Given the emergence of IRD gene therapies, it is vital to investigate IRD–related vision impairment and blindness in the community(3). Thus, there is a need for epidemiological data to inform and support research and development into novel treatments that may have the potential to reduce mortality through prevention of vision loss.
To date there remains a paucity of studies on the epidemiology of blindness attributed due to IRDs. Despite several population studies that examined the prevalence of IRDs (4–6), there are only a few that reported the incidence of IRDs or IRD–related blindness on a population scale (7–11). A significant proportion of childhood blindness, across different countries, has been attributed to IRD (9,10,12–15). This is consistent with data from the UK in which 13% of all childhood blindness certificates were attributed to IRD(16). Over the past decade, Rahman et al (17) reported trends in vision-impairment certifications and also found IRD to be the leading cause of sight loss in the working-age population. Similarly, in working–age adults (16–64 years), Liew et al (2). found the main cause of blindness was due to IRDs, accounting for 20.2%. However, all IRDs were grouped as ‘hereditary retinal disorders’ and no further analysis on the spectrum of diseases registered within this heterogeneous category was provided. In Australia, blindness registration improves access to early financial, educational and social supports. The Association for the Blind of Western Australia (ABWA), known as VisAbility from 2014, maintains the only Australian state–wide register of blind individuals. Despite the ascertainment limitations of a registry (18–20) the diagnostic accuracy of this registry was shown to have high positive predictive value and sensitivity(21). This resource provides a unique opportunity to examine IRD-related low vision on a population level.
We examined the low-vision and blindness-only registrations from 1996 to 2016 in order to provide a unique perspective of the causes of blindness in children, working-age and older-age adults over the last two decades, and the demographic and clinical phenotypes of IRD-related blindness.
Methods
This was a retrospective, observational study of low-vision and blindness-only registrations in Western Australia(WA) from 1996 to 2016. Anonymised electronic records were obtained from the ABWA/VisAbility Blind and Vision Impaired Registry, a not-for-profit state-based organisation founded in 1977 that provides rehabilitation services for blind and visually impaired residents.
In WA, ophthalmologists and optometrists have no formal obligation to ensure patients satisfying the criteria below are referred to or informed about the ABWA registry nor is there any financial incentive for the referrer to send a patient to the registry. Registration requires an ophthalmologist or an optometrist to provide the following data: sex, age, best-corrected VA (BCVA) in each eye, visual field span in each eye, and an ocular diagnosis of the cause of visual impairment in each eye. Multiple diagnoses are allowed, with the most relevant to vision loss identified .
In the registry, BCVA in Snellen fraction was converted to logarithm of minimum angle of resolution (logMAR) units. Blindness was defined as logMAR ≥1.0 (20/200 or worse) in the better-seeing eye or visual field constriction ≤ 20° diameter with both eyes. In Australia, legal blindness is defined by logMAR>1.0 (less than 20/200). Individuals with logMAR = 1.0 were assigned as “borderline” blindness. Those who did not meet these criteria were assigned a vision-impaired status. Some records did not have any visual function data and these were assigned as vision unknown. These certificates were generally associated with registration in pre-verbal children or elderly individuals with dementia. All certificate entries in the electronic database were examined individually for a primary diagnosis by FKC to ensure a uniform system was used for diagnostic labelling. Duplicates were removed except for a few cases where an initial registration was assigned vision–impaired and later upgraded to blindness. For children (age 0–15 years), the diagnostic categories were assigned according to anatomical sites as recommended by Rahi et al (10). For adults, the diagnoses were grouped into locations: brain, optic nerve, retina/choroid, lens, cornea and other (location not specified). Three different registration forms were used by the ABWA registry throughout the study period (Supplementary Material S1).
Analyses were performed on all low-vision certificates (combined legal blindness, borderline blindness, vision-impaired and vision unknown) and repeated for the blindness-only (legal and borderline blindness) certificates. The proportions of low-vision and blindness–only certifications attributed to major diagnostic categories in childhood (0–15 years), working–age (16–64 years) and older–age (65 and older) adult populations were determined. These were separated into four time frames for examining the trends over the study period: 1995–2000, 2001–2005, 2006–2010 and 2011-2016. Sex and age distributions were examined for the top 20 causes of blindness. The age distribution for five common diagnostic categories (age-related macular degeneration [AMD], glaucoma, IRDs, diabetic retinopathy [DR] and optic atrophy(OA)) was analysed by plotting a frequency distribution (density function) curve against each decade of life. The demographic features and BCVA in the better-seeing eye were further examined for each type of IRD.
This study was exempted from human research ethics review by the Human Ethics Office of Research Enterprise at The University of Western Australia (2021/ET000001).
Results
Study population
Amongst the 11824 low-vision registration certificates issued to 11037 individuals between July 1995 and January 2017, 679 or 5.7% (644 or 5.8% of the individuals) had an IRD listed as the cause of vision loss. Of all IRD-related certifications (N = 679), 60% met the blindness definition whilst 37% had vision that did not meet this criterion (Table 1). Of those issued with a blindness certificate, IRD (8.3%, 406/4919) was the second most common diagnosis after AMD, (54.6%, 2684/4919, Table 2). The frequency of IRD as a reason for certification was then compared to other causes in children (age 15 years and under), working–age (age 16–64 years) and older-age adults (age 65 years or older).
Table 1.
Distribution of certificates for inherited retinal diseases–related vision loss according to the level of vision loss and year of registration
| Certificate Year | Legal Blindness | Borderline Blindness | Vision Impaireda | Vision Unknowna | All cases (% of total) |
|---|---|---|---|---|---|
| Epoch 1: 1995–2000 | |||||
| 1995 | 9 | 2 | 3 | 0 | 14 |
| 1996 | 6 | 5 | 1 | 0 | 12 |
| 1997 | 15 | 0 | 0 | 0 | 15 |
| 1998 | 10 | 2 | 1 | 0 | 13 |
| 1999 | 6 | 1 | 0 | 0 | 7 |
| 2000 | 5 | 1 | 1 | 0 | 7 |
| Total | 51 | 11 | 6 | 0 | 68 (10.0%) |
| Epoch 2: 2001–2005 | |||||
| 2001 | 14 | 1 | 0 | 0 | 15 |
| 2002 | 5 | 0 | 1 | 0 | 6 |
| 2003 | 25 | 1 | 10 | 0 | 36 |
| 2004 | 21 | 1 | 19 | 1 | 42 |
| 2005 | 18 | 2 | 15 | 2 | 37 |
| Total | 83 | 5 | 45 | 3 | 136 (20.0%) |
| Epoch 3: 2006–2010 | |||||
| 2006 | 20 | 4 | 23 | 1 | 48 |
| 2007 | 21 | 4 | 28 | 1 | 54 |
| 2008 | 16 | 4 | 14 | 0 | 34 |
| 2009 | 22 | 2 | 26 | 1 | 51 |
| 2010 | 15 | 7 | 24 | 2 | 48 |
| Total | 94 | 21 | 115 | 5 | 235 (34.6%) |
| Epoch 4: 2011-2016 | |||||
| 2011 | 13 | 6 | 19 | 2 | 40 |
| 2012 | 28 | 7 | 18 | 5 | 58 |
| 2013 | 20 | 5 | 16 | 4 | 45 |
| 2014 | 25 | 4 | 11 | 4 | 44 |
| 2015 | 20 | 1 | 10 | 1 | 32 |
| 2016 | 11 | 1 | 7 | 1 | 20 |
| Total | 117 | 24 | 81 | 17 | 239 (35.2%) |
| No date | 0 | 0 | 1 | 0 | 1 (0.15%) |
| Grand Total (% of total) |
345 (50.8%) | 61 (9.0%) | 248 (36.5%) | 25 (3.7%) | 679b |
aThere were a total of 33 cases with vision impairment and 2 cases with vision unknown which were later registered as borderline blindness or legal blindness; bThis total included the 35 cases that were vision impaired as well as those where vision was unknown on the first certificate, but subsequent registration showed borderline blindness or legal blindness.
Table 2.
Frequency and demographic features for the top 20 most common reasons for blindness certification
| Diagnosis | Number of cases (% of totalb) | Female (% of total) | Age at certification Mean±SD (years) | Childhooda (%) | Working–agea (%) | Older adultsa (%) |
|---|---|---|---|---|---|---|
| AMD | 2684 (54.6%) | 64.7% | 84 ± 7 | 0.0% | 1.3% | 98.7% |
| IRD | 406 (8.3%) | 50.5% | 47 ± 20 | 7.9% | 73.8% | 18.3% |
| Glaucoma | 398 (8.1%) | 49.7% | 79 ± 13 | 0.0% | 13.9% | 86.1% |
| Diabetic Retinopathy | 264 (5.4%) | 53.0% | 65 ± 14 | 0.0% | 41.4% | 58.6% |
| Optic Atrophy | 237 (4.8%) | 47.9% | 55 ± 23 | 8.0% | 54.0% | 38.0% |
| Retina Vascular Disease | 153 (3.1%) | 55.6% | 79 ± 11 | 0.0% | 10.5% | 89.5% |
| Cerebral Defect | 88 (1.8%) | 53.4% | 56 ± 27 | 14.9% | 37.9% | 47.1% |
| Retina Surgical | 85 (1.7%) | 38.8% | 64 ± 22 | 3.6% | 38.1% | 58.3% |
| Retina Congenital | 73 (1.5%) | 49.3% | 17 ± 20 | 63.0% | 31.5% | 5.5% |
| Corneal Scar | 41 (0.8%) | 46.3% | 66 ± 20 | 2.4% | 34.1% | 63.4% |
| Uveitis | 38 (0.8%) | 50.0% | 59 ± 21 | 2.8% | 61.1% | 36.1% |
| Cataract Adult | 35 (0.7%) | 60.0% | 80 ± 9 | 0.0% | 5.7% | 94.3% |
| Myopic Macular Disease | 34 (0.7%) | 52.9% | 67 ± 15 | 0.0% | 41.2% | 58.8% |
| Amblyopia | 27 (0.5%) | 48.1% | 38 ± 30 | 25.9% | 55.6% | 18.5% |
| Corneal Dystrophy | 21 (0.4%) | 76.2% | 61 ± 22 | 4.8% | 42.9% | 52.4% |
| Optic Nerve Tumour | 21 (0.4%) | 61.9% | 39 ± 23 | 23.8% | 57.1% | 19.0% |
| Optic Nerve Congenital | 21 (0.4%) | 57.1% | 19 ± 22 | 61.9% | 33.3% | 4.8% |
| Cataract Congenital | 13 (0.3%) | 30.8% | 26 ± 17 | 30.8% | 69.2% | 0.0% |
| HON | 10 (0.2%) | 30.0% | 36 ± 22 | 0.0% | 80.0% | 20.0% |
| Retinal Trauma | 8 (0.2%) | 37.5% | 56 ± 25 | 0.0% | 75.0% | 25.0% |
aPercentage total for each diagnosis; childhood included those aged between 0–15 years, working age was 16-64 years and older adults were those 65 years and over.
bThe total number of blindness certificates issued were 4919.
AMD; age-related macular degeneration, IRD; inherited retinal disease, HONs; hereditary optic neuropathy.
Frequency of low-vision or blindness due to IRD
Amongst children, IRD was the second most common reason for low-vision certification (78/670, 11.6%) after cerebral visual impairment (CVI, 90/670, 13.4%, Supplementary Material S2) and this remained unchanged over the four time frames (Figure 1). Amongst those with blindness-only certification (N = 174), the three most common retinal disease-related causes (N = 68) were IRD (32/68, 47%), albinism (21/68, 31%) and retinopathy of prematurity (6/68, 9%, Supplementary Material S3).
Figure 1.

Graphs showing the proportion of all low-vision (first column) or blindness-only (second column) certificates issued for cerebral vision impairment (CVI), inherited retinal disease (IRD), albinism (Alb), optic atrophy (OA), age–related macular degeneration (AMD), optic hypoplasia (OH), diabetic retinopathy (DR), retinal vascular disease (RVD), glaucomatous optic neuropathy (GON), central nervous system diseases (CNS) over the four time frames 1995–2000 (blue), 2001–2005 (red), 2006–2010 (green) and 2011-2016 (purple) for those with childhood (A, B), working-age adult (C, D) or older-age adult (E, F) age of registration
In the working-age population, IRD was the most common reason for low-vision certification (418/1793, 23.3%) followed by DR (11.3%) and OA (10.7%, Supplementary Material S4). Amongst those with blindness-only certification (N = 943, Supplementary Material S5), IRD accounted for 31.6% of registrations followed by OA (13.6%) and DR (11.6%). There was no significant change in the proportions of IRD, OA and DR as the cause of low-vision or blindness over the four time frames (Figure 1).
In older-age adults, IRD was the equal fifth and sixth most common reason for low-vision certification along with surgical retinal diseases (both at 144/8499, 1.7%) after AMD (70.2%), glaucoma (6.9%) and, in equal third and fourth, DR (3.4%) and retinal vascular diseases (3.4%, Supplementary Material S6). Amongst those with blindness-only certification (N = 3772), IRD was the sixth most common (2.0%) reason for registration after AMD (69.9%), glaucoma (9.1%), DR (4.1%), retinal vascular diseases (3.6%) and OA (2.4%, Supplementary Material S7).
Demographic characteristics of IRD-related vision loss
IRD ranked 14th amongst the top 20 most commonly listed causes for age at low-vision certification with a mean±SD of 46 ± 23 years (Figure 2). Similarly, IRD also ranked 14th in age amongst the top 20 most commonly listed causes of blindness-only certification (Figure 2, Table 2). Females accounted for 50.5% of registrations and their mean±SD age was 47 ± 20 years at the time of certification. Most certificates (74%) were issued during the working-age, with some in the older-adult cohort (18%) and the remaining in childhood (8%).
Figure 2.
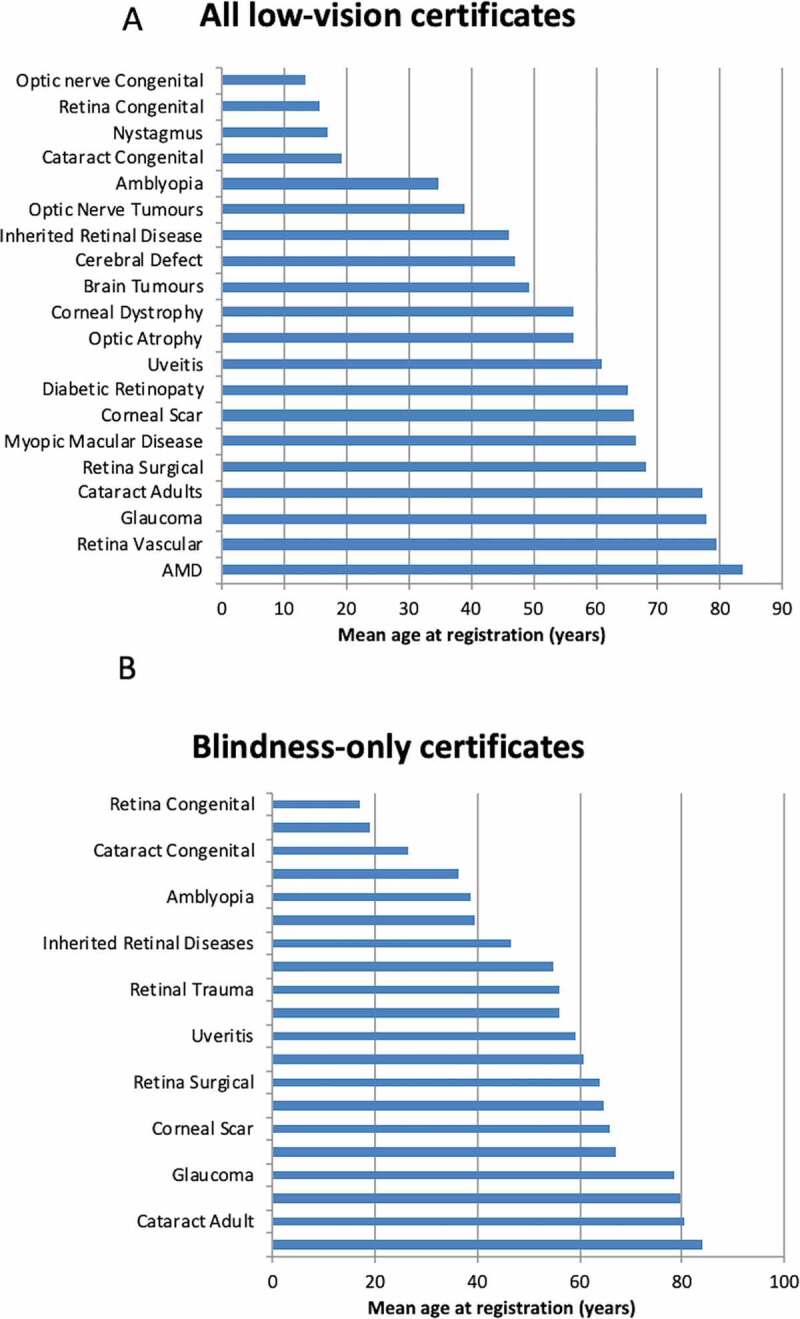
Ranking for the mean age at registration for all low-vision certificates (A) and blindness-only certificates (B) for the top 20 ophthalmic diagnoses. AMD; age-related macular degeneration
The age distributions for AMD and glaucoma were similar, peaking in the 9th decade. DR peaked during the 7th and 8th decade. In contrast, the age distribution for OA and IRDs was flatter, with a broader peak spanning the 7th to 9th decades and 4th to 6th decades, respectively (see density function plots in Supplementary Material S8). There was a trend for increasing age at registration for AMD, glaucoma and IRD (Supplementary Material S9). However, the age at registration declined for DR and OA during the four time frames (Supplementary Material S9).
Spectrum of IRDs causing vision loss
Amongst all IRD-related low-vision certificates (N = 640), 96% listed a specific retinal or syndromic phenotype. The three most common phenotypes were RP (N = 374, 58%), Stargardt disease (N = 77, 12%) and macular dystrophy (N = 53, 8%, Table 3) within the low-vision certified cohort. In the blindness-only certificate cohort (N = 404), RP made up the majority (69%), followed by Stargardt disease (12%) and macular dystrophy (4.2%, Figure 3, Table 3). Inherited macular dystrophies (unspecified macular dystrophy, vitelliform dystrophy, Stargardt disease and angioid streaks) were the predominant cause in those with vision-impaired or vision-unknown certificates (Figure 3).
Table 3.
Demographic and clinical features for inherited retinal diseases related certification
| Diagnosis | Number of cases (% of total) |
Female (% of total) |
Age at certification Mean±SD (years) |
Childhood a (%) |
Working-agea (%) |
Older–agea (%) |
VA in better eye Mean±SD (logMAR) |
|---|---|---|---|---|---|---|---|
| All low vision certificates (N = 640) | |||||||
| RP | 349 (54.2%) | 49.3% | 46 ± 20 | 8.9% | 72.5% | 18.6% | 0.67 ± 0.66 |
| Stargardt disease | 77 (12.0%) | 59.5% | 37 ± 20 | 15.6% | 72.7% | 11.7% | 1.03 ± 0.49 |
| Macular dystrophy | 53 (8.2%) | 48.1% | 55 ± 24 | 0.0% | 62.3% | 37.7% | 0.73 ± 0.50 |
| Vitelliform dystrophy | 40 (6.2%) | 52.5% | 71 ± 23 | 5.0% | 22.5% | 72.5% | 0.50 ± 0.24 |
| Angioid streak/PXE | 25 (3.9%) | 56.0% | 59 ± 13 | 0.0% | 72.0% | 28.0% | 1.15 ± 0.63 |
| Syndromic RP | 25 (3.9%) | 56.0% | 38 ± 18 | 20.0% | 76.0% | 4.0% | 0.68 ± 0.71 |
| LCA | 20 (3.1%) | 45.0% | 16 ± 20 | 65.0% | 30.0% | 5.0% | 1.60 ± 0.84 |
| CDS/COD/CORD | 14 (2.2%) | 21.4% | 34 ± 20 | 21.4% | 64.3% | 14.3% | 1.16 ± 0.52 |
| Otherb | 37 (5.7%) | 37.8% | 41 ± 29 | 32.4% | 40.5% | 27.0% | 0.85 ± 0.62 |
| Blindness certificates (N = 404) | |||||||
| RP | 257 (63.6%) | 49.6% | 48 ± 18 | 4.7% | 76.3% | 19.1% | 0.77 ± 0.72 |
| Stargardt disease | 49 (12.1%) | 66.0% | 40 ± 21 | 10.2% | 75.5% | 14.3% | 1.29 ± 0.40 |
| Syndromic RP | 22 (5.4%) | 63.6% | 39 ± 18 | 18.2% | 77.3% | 4.5% | 0.67 ± 0.76 |
| Macular dystrophy | 18 (4.5%) | 33.3% | 57 ± 22 | 0.0% | 66.7% | 33.3% | 1.25 ± 0.46 |
| Angioid streak/PXE | 17 (4.2%) | 52.9% | 60 ± 12 | 0.0% | 70.6% | 29.4% | 1.47 ± 0.49 |
| LCA | 12 (3.0%) | 33.3% | 24 ± 23 | 50.0% | 41.7% | 8.3% | 1.91 ± 0.74 |
| CDS/COD/CORD | 9 (2.2%) | 22.2% | 33 ± 18 | 22.2% | 66.7% | 11.1% | 1.41 ± 0.47 |
| Vitelliform dystrophy | 4 (1.0%) | 50.0% | 47 ± 37 | 25.0% | 50.0% | 25.0% | 1.00 ± 0.00 |
| Otherb | 16 (4.0%) | 43.8% | 48 ± 22 | 12.5% | 68.8% | 18.8% | 1.28 ± 0.65 |
aPercentage of the total for each diagnosis, childhood is 0-15 years, working–age is 16-64 years and older-age is 65 years and over.
bOther includes retinal dystrophy not otherwise specified, gyrate atrophy, Bietti crystalline retinopathy and retinoschisis.
RP; retinitis pigmentosa; PXE = pseudoxanthoma elasticum, LCA; Leber congenital amaurosis, CDS; cone dysfunction syndrome, COD; cone dystrophy, CORD; cone-rod dystrophy, VA; visual acuity, SD; standard deviation, logMAR; logarithm of minimum angle of resolution, VA; visual acuity, PXE; pseudoxanthoma elasticum.
Figure 3.
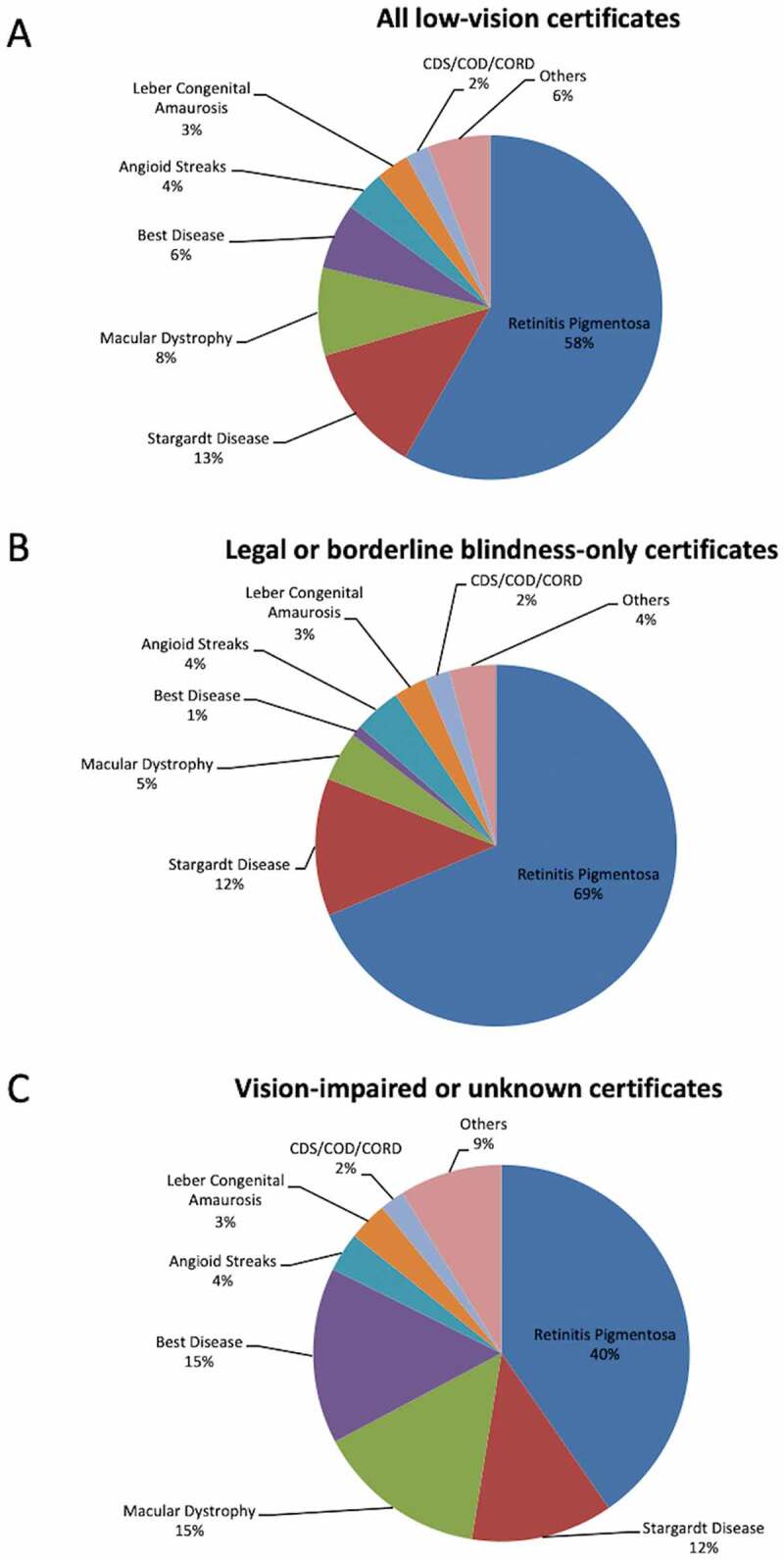
Pie charts showing the proportion of all low-vision certificates (A), legal (<20/200, ≤20°) or borderline (at 20/200, >20°) blindness-only certificates (B) and vision-impaired or unknown certificates (C) attributed to different types of inherited retinal diseases. CDS; cone dysfunction syndrome, COD; cone dystrophy, CORD; cone rod dystrophy
Age at low-vision registration was oldest in vitelliform dystrophy (71 ± 23 years) and youngest in Leber congenital amaurosis (LCA, 16 ± 20 years). Conversely, BCVA (logMAR) was worst in those with LCA (1.60 ± 0.84) and best in vitelliform dystrophy (0.50 ± 0.24). Older individuals with vitelliform dystrophy accounted for the better visual acuities. There were insufficient clinical data in the database to distinguish between adult-onset (foveomacular vitelliform dystrophy) and early-onset vitelliform dystrophy (the classic juvenile Best disease, Supplementary Material S10). The peak age for RP-associated low-vision and blindness-only registration was during the 5th and 6th decade respectively. There was a bimodal peak age for Stargardt disease associated with low-vision registration during the 2nd and 5th decade (Supplementary Material S10). Stargardt disease had a female preponderance (60% female) whilst cone diseases (achromatopsia, cone dystrophy and cone-rod dystrophy) had a male preponderance (79% male, Table 3).
Discussion
This is the first detailed examination of IRD-related blindness in a population registry of individuals with low vision. IRDs ranked second after AMD, above glaucoma and DR, as the reason for blindness certification. The early age of registration for IRD-related blindness and the heterogeneity of clinical phenotypes in this cohort herein highlight the challenges facing low-vision service providers and researchers developing treatment to prevent blindness due to IRDs.
Our study found IRDs (8.3%, 406/4919) were the second most common cause of legal or borderline blindness after AMD for all ages. During 2009, the Epidemiology of Blinding Eye Disease Study (22) surveyed 122 legally blind patients aged 2 to 96 years and found AMD was the most common cause of blindness – 45%– compared to retinal dystrophies at 8%. Interestingly, at that time only one-third of patients had not been in contact with ABWA, the same registry from which our data were extracted. Yong et al (11) estimated whole-population-based incidence rates of all blindness registrations in WA between 1984 and 2002 and found an overall decline in the incidence of registered blindness, with AMD (54%), glaucoma (11%) and optic neuropathies (5%) the most common causes of blindness. Our study expands on the work of Yong et al (11) by including vision impairment in addition to blindness registrations over a more recent period up to 2017. In children with legal or borderline blindness, IRD was the second most common cause after CVI and the most common cause amongst retinal disease–related blindness. This is similar to data from the UK where IRD was ranked second after CVI as a cause of visual impairment (17% vs 22%) or blindness (16% vs 31%) in 2009-2010(8). Our data showed a disproportionate number with “nystagmus” as a diagnosis, which may explain the unusually low percentages of blindness attributed to CVI. In a retrospective matched cohort study of 419 blind individuals aged between 18 and 65 years from the WA state blind register, Crewe et al (1) found almost 30% of blindness to be attributed to IRD. Similarly, Rahman et al (17) examined trends in certificates of visual impairment over 2017/18 and found IRD to be the leading cause of sight loss in the working–age population in Wales and England. However, these data were only representative of the hospital population and not the whole population. Liew et al (2) used a national database of blindness certificates to compare the causes of blindness in England and Wales between 1999/20 and 2009/10. They found IRD to be the main cause of blindness, accounting for 20.2% in those aged between 16 and 64 years of age as compared to DR (14.4%), OA (14.1%) and glaucoma (5.9%). We also observed a similar ranking with IRD the most common (31.6%), followed by OA (13.6%), DR (11.6%) and glaucoma (5.6%). However, unlike Liew et al.’s report, we did not find a declining trend in the proportion attributed to DR (9.2% in 1995-2000 vs 11.7% in 2011–2016) or an increasing trend in the proportion attributed to IRDs (30.1% in 1995–2000 vs 30.8% in 2011–2016)(2). It is important to note that even within the older population, IRD was the fifth or sixth most common cause of low vision or blindness as some IRDs may have a late onset(23).
Our study is unique as we examined the age and sex distribution for the most common causes of blindness across all age groups. AMD and glaucoma had a similar age distribution, but AMD showed a greater proportion of females (65% vs 50%). DR tended to occur in a younger cohort, peaking in the 7th decade with only a slightly female predominance (53%). The declining trend in the age at registration for DR–associated blindness (68.7 years in 1995-2000 vs 61.9 years in 2011–2016) was concerning and this may be related to an earlier onset of diabetes in the general population(24). More recently, blindness from IRD was registered at an older age (43.8 years in 1995–2000 vs 55.1 years in 2011–2016) with an equal sex distribution. This increase in the mean age may be due to older IRD-affected individuals seeking blindness registration in order to access improved low–vision aid technology. Crewe et al (1) found the median age at the index date of blindness for IRD was 47 years (1999–2010), similar to the mean age in our study. However, they only included those between 18-65 years. We found one-quarter of IRD-related blindness registered outside the working–age group (8% in childhood and 18% in 65 or older). Yong et al (11) also reported the mean age at registration of bilateral blindness in WA using the ABWA data. However, only one type of IRD labelled ‘pigmentary retinopathy’ (RP) was reported, with a wide variation in the mean age (28–70 years) and no statistically significant trend observed from 1984 to 2002. As IRDs incorporates a heterogeneous group with a diverse range of onset, we sought to investigate the demographic distribution in each type of IRD listed in the ABWA register.
The most common form of IRD listed in our vision-impaired and blind register was RP, accounting for 54% and 64%, respectively, comparable to the 61% reported in the Australian national IRD register (25) and the 54% reported in the Norwegian national IRD register(26). Therefore, previous reports that only considered RP or pigmentary retinopathy would have missed up to half of all IRD cases(11). A nation–wide Korean population prevalence study of RP in 2011–2014 found a mean age at diagnosis of 45 years (age range 0-95 years), only 2 years earlier than the mean age of IRD-related blindness registration recorded in our study(6). This age of diagnosis appears markedly delayed when compared to the national registry study by Holtan et al (26) of IRDs in Norway in which 50% of RP patients reported an onset of symptoms before the age of 20 and the mean age of diagnosis was 24.4 years (ranging from 0 to 83 years)(26). Although our study did not include age at symptom onset, we suspect that by the time of diagnosis, most patients have already experienced significant visual field loss to ≤20° and thus may already be eligible for blindness registration.
The second most common IRD diagnosis was Stargardt disease, contributing to 12% of IRD–related vision loss or blindness, which is higher than that reported in the Australian and Nowegian registries: 9–10% and 6-7% respectively (25,26). Interestingly, there was a female predominance, which has also been recently reported in those with mild or hypomorphic mutations(27). Although 76% of registrations related to Stargardt disease were in the working-age group, we found 10% were registered up to the age of 15 and 14% were registered at 65years or older, with a bimodal peak. This is consistent with the extreme variability in phenotype ranging from severe childhood-onset to late-onset disease, which has a median age of onset in the mid-50 with up to 11% of these individuals eligible for blindness registration (23,28). Another retrospective cross-sectional study of 361 patients with Stargardt disease found 59% met the criteria for blindness registration and the median time to develop this degree of visual impairment varied from 22 to 29 years depending on the BCVA at the patient’s initial visit(29). In contrast, a retrospective cross-sectional study of Stargardt disease by Kim et al (30) showed that only 5.9% of their patients met the requirements for blindness registration. We also found unspecified macular dystrophy, vitelliform dystrophy and angioid streaks were significant reasons for IRD-related low-vision certification, accounting for 8.2%, 6.2% and 3.9%, respectively. However, vitelliform dystrophy only accounted for 1% of IRD-related blindness, consistent with the mild visual impairment particularly in the adult-onset subtype. To our knowledge the contribution of various inherited macular dystrophies to IRD-related blindness has not been previously reported. However, the Norwegian registry did report lower frequencies of 4% and 3.6% of the 866 cases that were attributed to Best vitelliform dystrophy and macular dystrophy, respectively(26). In contrast, LCA was noted in 3.0% of those with an IRD-related blindness registration, similar to a previous finding of a 5% contribution of LCA to IRDs (26,31). Chao et al (32) found most patients with RPE65-related LCA were legally blind by the age of 20 and by the fourth decade all patients were legally blind. Our data on the contribution of various forms of IRD to low-vision and blindness-only registrations may help estimate the impact of emerging gene-based therapies(33).
Strengths of our study include the use of data from the only Australian state-wide blindness registry spanning two decades. However, it was limited by its retrospective design, three different registration forms used during the study period and the absence of a state-wide compulsory registration program for individuals with visual impairment. Consequently, underreporting of blindness would have been a confounding factor, as demonstrated by a previous capture–recapture validation study where only 39 and 55% of legally blind adults and children, respectively, were on the ABWA register in 2008-2009(20). It has yet to be determined if the distribution of diagnoses in those who were not registered were similar to those who were registered. Furthermore, clinician referral patterns and patient preferences to participate in the registry may have changed during the study period where a clinicians’ delay or inability to diagnose and report IRD related blindness may have led to an underestimation of the frequency in the earlier period. Also, childhood blinding eye diseases may have been missed in the legally or borderline blind cohort due to lack of documentation of the exact visual acuity level. To compensate for this data artefact, we examined all low-vision certificates including those without a visual acuity level. Furthermore, a perceived possibility of vision improvement in the childhood group may have led to delayed certification. In our study, some patients had multiple diagnoses listed as the cause of their vision loss and the main contributor to blindness had to be chosen, thus underestimating the contribution of individual causes. As our patients received financial benefits once registered as legally blind, it is likely a larger proportion of blindness in the working-age group was registered by the ABWA. Conversely, the older-age blind individuals may be underrepresented as they did not receive additional financial benefit for being on the blind registry since they were already receiving government aged-care support.
In this study, IRDs were the second most common cause of low-vision and blindness-only certification across all ages, overtaking glaucoma and DR. In working-age adults, IRDs were the most common reason for low-vision and blindness-only certification. Given the recent progress in IRD clinical trials and the recent Therapeutic Goods Administration (TGA) approval of Luxturna™, it is necessary for state and federal governments to be aware of the proportion of low-vision and blindness-only registrations in children, working-age and older-age adults attributed to IRD. Our findings support continued funding allocation for providing low-vision services and developing treatments for IRDs.
Supplementary Material
Acknowledgments
The authors would like to thank Ms Julie Crewe and Mr James Semmens for their input into study design at the outset of the study, and Drs Wilson Heriot, Alex P. Hunyor and Jennifer Arnold for their assistance with finding the old MBS codes for photodynamic therapy.
Funding Statement
This research was supported by the Australian National Health and Medical Research Council under GNT116360 (FKC), GNT1188694 (FKC), GNT1054712 (FKC) and MRF1142962 (FKC), McCusker Charitable Foundation (FKC) and the Miocevich Retina Fellowship (RCHJ).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Crewe JM, Morlet N, Morgan WH, Spilsbury K, Mukhtar AS, Clark A, Semmens JB.. Mortality and hospital morbidity of working-age blind. Br J Ophthalmol. 2013;97(12):1579–85. doi: 10.1136/bjophthalmol-2013-303993. [DOI] [PubMed] [Google Scholar]
- 2.Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open. 2014;4:e004015. doi: 10.1136/bmjopen-2013-004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maguire AM, Bennett J, Aleman EM, Leroy BP, Aleman TS. Clinical perspective: treating RPE65-associated retinal dystrophy. Mol Ther. 2020. [online ahead of print]. doi: 10.1016/j.ymthe.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertelsen M, Jensen H, Larsen M, Lorenz B, Preising MN, Rosenberg T. Prevalence and diagnostic spectrum of generalized retinal dystrophy in danish children. Ophthalmic Epi. 2013;20:164–69. [DOI] [PubMed] [Google Scholar]
- 5.Bertelsen M, Jensen H, Bregbhoj JF, Rosenberg T. Prevalence of generalized retinal dystrophy in Denmark. Ophthalmic Epidemiol. 2014;21:217–23. [DOI] [PubMed] [Google Scholar]
- 6.Na K-H, Kim HJ, Kim KH, Han S, Kim P, Hann HJ, Ahn HS. Prevalence, age at diagnosis, mortality, and cause of death in retinitis pigmentosa in Korea-A nationwide population-based study. Am J Ophthalmol. 2017;176:157–65. [DOI] [PubMed] [Google Scholar]
- 7.Hamblion EL, Moore AT, Rahi JS. Incidence and patterns of detection and management of childhood-onset hereditary retinal disorders in the UK. Br J Ophthalmol. 2012;96:360e365. [DOI] [PubMed] [Google Scholar]
- 8.Mitry D, Bunce C, Wormald R, Leamon S, Simkiss P, Cumberland P, Rahi J, Bowman R. Causes of certifications for severe sight impairment (blind) and sight impairment (partial sight) in children in England and Wales. Br J Ophthalmol. 2013;97:1431–36. [DOI] [PubMed] [Google Scholar]
- 9.Durnian JM, Cheeseman R, Kumar A, Raja V, Newman W, Chandna A. Childhood sight impairment: a 10-year picture. Eye (Lond). 2010;24(1):112–17. doi: 10.1038/eye.2009.32. [DOI] [PubMed] [Google Scholar]
- 10.Rahi JS, Cable N. Severe visual impairment and blindness in children in the UK. Lancet. 2003;362(9393):1359–65. doi: 10.1016/S0140-6736(03)14631-4. [DOI] [PubMed] [Google Scholar]
- 11.Yong VK, Morgan WH, Cooper RL, Shaw M, Bremner AP, Bulsara M, Yu D-Y, et al. Trends in registered blindness and its causes over 19 years in Western Australia. Ophthalmic Epidemiol. 2006;13(1):35–42. doi: 10.1080/09286580500473779. [DOI] [PubMed] [Google Scholar]
- 12.Gao Z, Muecke J, Edussuriya K, Dayawansa R, Hammerton M, Kong A, Sennanayake S, Senaratne T, Marasinghe N, Selva D, et al. A survey of severe visual impairment and blindness in children attending thirteen schools for the blind in Sri Lanka. Ophthalmic Epidemiol. 2011;18(1):36–43. doi: 10.3109/09286586.2010.545504. [DOI] [PubMed] [Google Scholar]
- 13.Haddad MA, Sei M, Sampaio MW, Kara-Jose N. Causes of visual impairment in children: a study of 3210 cases. J Pediatr Ophthalmol Strabismus. 2007;44(4):232–40. doi: 10.3928/01913913-20070701-04. [DOI] [PubMed] [Google Scholar]
- 14.Augestad LB, Klingenberg O, Fosse P. Braille use among Norwegian children from 1967 to 2007: trends in the underlying causes. Acta Ophthalmol. 2012;90(5):428–34. doi: 10.1111/j.1755-3768.2010.02100.x. [DOI] [PubMed] [Google Scholar]
- 15.Limburg H, Gilbert C, Hon DN, Dung NC, Hoang TH. Prevalence and causes of blindness in children in Vietnam. Ophthalmology. 2012;119(2):355–61. doi: 10.1016/j.ophtha.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England & Wales. BMC Public Health. 2006;6:58. doi: 10.1186/1471-2458-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman F, Zekite A, Bunce C, Jayaram H, Flanagan D, et al. Recent trends in vision impairment certifications in England and Wales. Eye. 2020;34:1271–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, Vesti Nielsen N. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults. Ophthalmology. 2004;111:53–61. [DOI] [PubMed] [Google Scholar]
- 19.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 20.Crewe JM, Morgan WH, Morlet N, Clark A, Lam G, Parsons R, Mukhtar A, Ng J, Crowley M, Semmens J, et al. Prevalence of blindness in Western Australia: a population study using capture and recapture techniques. Br J Ophthalmol. 2012;96(4):478–81. doi: 10.1136/bjophthalmol-2011-300908. [DOI] [PubMed] [Google Scholar]
- 21.Crewe JM, Morgan WH, Morlet N, Spilsbury K, Mukhtar A, Clark A, Ng JQ, Crowley M, Semmens JB. Assessing the diagnostic validity of a blind register. Clin Exp Ophthalmol. 2011;39(6):494–500. doi: 10.1111/j.1442-9071.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 22.Crewe JM, Morgan WH, Morlet N, Crowley M, Semmens JB. Utilization of services by legally blind patients in Western Australia. Clin Exp Ophthalmol. 2010;736–37. doi: 10.1111/j.1442-9071.2010.02310.x. [DOI] [PubMed] [Google Scholar]
- 23.Runhart EH, Valkenburg D, Cornelis SS, Khan M, Sangermano R, Albert S, Bax NM, Astuti GD, Gilissen C, Pott JW, et al. Late-onset Stargardt disease due to mild, deep-intronic ABCA4 alleles. Retina. 2019;60:4249–56. [DOI] [PubMed] [Google Scholar]
- 24.Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F, Silink M. Type 2 diabetes in the young: the evolving epidemic: the international diabetes federation consensus workshop. Diabetes Care. 2004;27(7):1798–811. doi: 10.2337/diacare.27.7.1798. [DOI] [PubMed] [Google Scholar]
- 25.De Roach JN, McLaren TL, Paterson RL, O’Brien EC, Hoffmann L, Mackey DA, Hewitt AW, Lamey TM. Establishment and evolution of the Australian inherited retinal disease register and DNA bank. Clin Exp Ophthalmol. 2013;41(5):476–83. doi: 10.1111/ceo.12020. [DOI] [PubMed] [Google Scholar]
- 26.Holtan JP, Selmer KK, Heimdal KR, Bragadottir R. Inherited retinal disease in Norway– a characterization of current clinical and genetic knowledge. Acta Ophthalmol. 2019;98:286–95. doi: 10.1111/aos.14218. [DOI] [PubMed] [Google Scholar]
- 27.Runhart EH, Khan M, Cornelis SS, Roosing S, Del Pozo-Valero M, Lamey TM, Liskova P, Roberts L, Stöhr H, Klaver CCW, et al. Association of sex with frequent and mild ABCA4 alleles in stargardt disease. JAMA Ophthalmol. 2020;138:1035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collison FT, Fishman GA. Visual acuity in patients with stargardt disease after age 40. Retina. 2018;38:2387–94. doi: 10.1097/IAE.0000000000001903. [DOI] [PubMed] [Google Scholar]
- 29.Rotenstreich Y, Fishman GA, Anderson RJ. Visual acuity loss and clinical observations in a large series of patients with Stargardt disease. Ophthalmology. 2003;110:1151–58. doi: 10.1016/S0161-6420(03)00333-6. [DOI] [PubMed] [Google Scholar]
- 30.Kim LS, Fishman GA. Comparison of visual acuity loss in patients with different stages of Stargardt’s disease. Ophthalmology. 2006;113:1748–51. doi: 10.1016/j.ophtha.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Schappert-Kimmijser J, Henkes HE, Den Bosch JV. Amaurosis congenita (Leber). AMA Arch Ophthalmol. 1959;61:211–18. doi: 10.1001/archopht.1959.00940090213003. [DOI] [PubMed] [Google Scholar]
- 32.Chao DL, Burr A, Pennesi M. 2019. RPE65-related Leber congenital amaurosis early-onset severe retinal dystrophy. GeneReviews® [Internet] , Editors. Seattle: University of Washington, Seattle. p. 1993–2021. [PubMed] [Google Scholar]
- 33.Loyd A, Piglowska N, Ciulla T, Pitluck S, Johnson S, Buessing M, O’Connell T. Estimation of impact of RPE65-mediated inherited retinal disease on quality of life and the potential benefits of gene therapy. B J Ophthamol. 2019;103:1610–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


