Abstract
In a cohort of infants, we found that lack of the Lewis histo-blood group antigen was associated with increased susceptibility to shigellosis. Broadly inhibiting fucosylation in epithelial cells in vitro decreased invasion by Shigella flexneri. These results support a role for fucosylated glycans in susceptibility to shigellosis.
Keywords: blood-group antigens, Lewis antigens, shigellosis
Gram-negative bacteria belonging to the genus Shigella are the third leading cause of diarrhea-associated deaths in children under the age of 5 [1]. The primary treatment for shigellosis is antibiotics; however, globally, the incidence of resistance to these drugs is increasing. Additionally, despite the burden of Shigella spp. on global health, there are currently no commercially licensed vaccines. Identifying host factors that alter susceptibility to Shigella spp. is an important first step in devising novel strategies to protect against this infection.
Blood-group antigens are a polymorphic trait that is associated with altered susceptibility to numerous infectious agents, including bacteria such as Helicobacter pylori and Campylobacter jejuni, and viruses [2]. The Lewis blood-group antigens are highly expressed in the gastrointestinal tissues, making them a plausible target for mucosal pathogens such as Shigella spp. Lewis antigens are generated via the action of 2 fucosyltransferases, FUT2/Secretor (α1,2-fucosyltransferase) and FUT3/Lewis (α1, 3/4-fucosyltransferase) [2]. FUT2 modifies a precursor type 1 oligosaccharide to make the H-antigen, after which FUT3 adds a second fucose to produce the Lewis B antigen. Individuals expressing both enzymes will primarily have Lewis B antigens, while individuals negative for FUT2/Secretor will only express Lewis A antigen. If an individual is FUT3/Lewis negative they will produce neither the Lewis A nor Lewis B glycans.
In a cohort of Bangladeshi infants, we sought to determine if the Lewis histo-blood-group antigens were associated with altered susceptibility to shigellosis in the first year of life. We found significant differences in the survival probability free from diarrhea associated with Shigella spp. within the first year of life based on inferred Lewis/Secretor genotypes. Using an in vitro assay, we showed that broadly inhibiting fucosylation on epithelial cells decreases invasion by Shigella flexneri. Together, these data suggest a role for fucosylated blood-group antigens in susceptibility to shigellosis.
METHODS
Study Population
We performed a substudy analysis from the Performance of Rotavirus and Oral Polio Vaccines in Developing Countries (PROVIDE) cohort. The cohort and study design have been previously described [3]. Briefly, 700 infants born in urban Dhaka, Bangladesh, were enrolled within the first 7 days of life. During the study, diarrheal episodes were monitored and fecal samples were collected. DNA extracted from the diarrheal fecal samples were used to detect common enteric pathogens using a custom developed TaqMan array card as described previously [4]. We identified infants with a complete 1-year follow-up who were successfully phenotyped for Lewis antigens. To exclude subclinical infections, Shigella positivity was determined by attributable fraction based on pathogen quantity as previously described [5]. All families participating in the study provided consent. The PROVIDE was approved by the ethical review boards of the International Center for Diarrhoeal Disease Research, Bangladesh; the University of Virginia; and the University of Vermont. The trial was registered at ClinicalTrials.gov (NCT01375647).
Secretor Status and Lewis Antigen Phenotyping
Lewis and Secretor status were inferred from LeA and LeB antigen phenotyping of stored saliva specimens using a dot-blot assay as previously described [6].
In Vitro Shigella flexneri Infections
Shigella flexneri infections were carried out as previously described [7] with the following modifications. HT-29 cell lines stably expressing yellow fluorescent protein membrane marker were maintained in McCoy’s 5A Medium (Gibco) with 10% heat-inactivated fetal bovine serum (Invitrogen). Four days before infection, cells were added to a 96-well cell culture–treated plate (Corning). The following day, the medium was replaced with medium supplemented with 250 μM 2F-peracetyl-fucose (2F-PAF), a cell-permeable inhibitor of fucosyltransferases (2F-PAF; Millipore Sigma), or vehicle (dimethyl sulfoxide) [8]. Drug (or vehicle)-supplemented medium was replaced each day thereafter, for a total of 3 days of treatment. The effect of the drug was evaluated by flow cytometry as previously described [8] (also see Supplementary Methods). On the day of infection, the medium was replaced with fresh medium without drug. Cells were infected using a frozen stock made from exponential phase S. flexneri serotype 2a 2457T expressing cyan fluorescent protein (CFP) under a β-D thiogalactopyranoside (IPTG)–inducible promoter. Maintenance of virulence plasmid was confirmed by presence of red colonies on plates containing Congo Red. Infection was started by centrifuging the plate for 5 minutes at 1000 rpm and internalization of the bacteria was allowed to progress for 1 hour before IPTG (10 mM final concentration) and gentamicin (50 μM) were added to the plate to induce expression of CFP and kill the remaining extracellular bacteria, respectively. Intracellular infection proceeded for another 7 hours at which point cells were fixed with 4% paraformaldehyde and the plates were imaged using the ImageXpress Micro imaging system (Molecular Devices). The number of infection foci per well were manually counted, while infection foci area (size) was measured with ImageXpress imaging software [9].
Statistical Analysis
Statistical analysis was performed in R version 3.6.3 using standard downloadable packages. The Kaplan–Meier (KM) method was used to estimate the probability free from the first Shigella-attributable diarrheal episode, and KM survival plots were generated using the packages survminer, survival, and survMisc. The log-rank test was used to compare the KM survival curves between Lewis and Secretor groups. The Cox proportional hazards model was used to evaluate the risks of Shigella-attributable diarrhea among Lewis and Secretor groups. A 2-tailed Mann–Whitney U nonparametric test was used to compare foci number and area between treatment groups. Data were graphed using the package ggpubR.
RESULTS
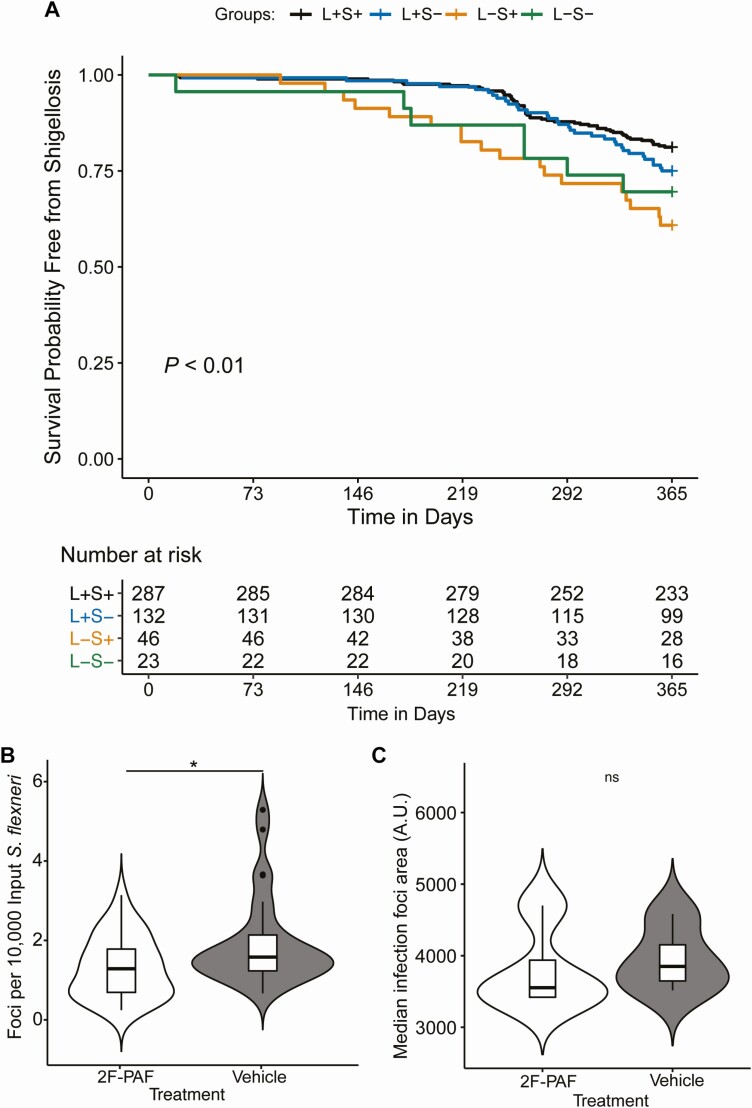
To determine if Lewis or Secretor status is associated with altered susceptibility to shigellosis, we performed a KM survival analysis. Infants who did not have a diarrheal episode attributable to Shigella spp. were censored at 1 year of age. In this analysis, we found that Lewis histo-blood-group status was significantly associated with decreased probability of shigellosis-free survival in the first year of life (P < .01), and Le−/Se+ infants were more likely to suffer such illness with a significantly increased hazard ratio of 2.4 (95% confidence interval, 1.4−4.2; P = .001) compared with Le+/Se+ infants (Figure 1A, Supplementary Figure 1).
Figure 1. .
Lewis antigens are associated with altered susceptibility to shigellosis. A, Kaplan-Meier curve showing survival probability free from Shigella spp.–attributed diarrheal episode in the first year of life based on inferred Lewis and Secretor status (P < .01, log-rank test). The numbers of children who were at risk for Shigella spp.–attributed diarrhea are listed at the bottom of the table. B, HT-29 cells were treated with 2F-PAF or vehicle control for 3 days before infection. 2F-PAF treatment decreased the number of S. flexneri infection foci; the y axis shows infections per well normalized to input inoculum. n = 32 wells per treatment group. Data are combined from 4 independent experiments. C, 2F-PAF treatment did not alter S. flexneri infection foci area (spread). n = 28 wells per treatment group. Data are combined from 3 independent experiments Significance for both B and C was determined by a 2-tailed nonparametric Mann–Whitney U test, *P < .05. Abbreviations: A.U., arbitrary units; L+, Lewis positive; L−, Lewis negative; ns, not significant; S+, Secretor positive; S–, Secretor negative; 2F-PAF, 2F-peracetyl-fucose.
To test the biological plausibility of this finding, we asked if inhibition of fucosylation in human colonic epithelial cells altered S. flexneri infection and/or dissemination. To do this, we utilized a cell-permeable fluorinated fucose derivate 2F-PAF. Following uptake and metabolism of 2F-PAF into a guanosine diphosphate (GDP)-fucose memetic, the metabolite broadly inhibits cellular fucoslytransferases. As previously established, treatment of HT-29 cells with 2F-PAF resulted in decreased levels of the fucosylated glycans Lewis A, Lewis B, as well as the Lewis B precursor H-antigen compared with vehicle-treated cells (Supplementary Figure 2A) [8]. The drug treatment did not alter the viability of cells (Supplementary Figure 2B). Infection of 2F-PAF–treated colonic epithelial cells resulted in significantly decreased numbers of infection foci per well relative to vehicle control (Figure 1B). However, median infection foci area was not significantly different between the treatments (Figure 1C). Together, these results suggest that inhibition of fucosyltransferases in colonic epithelial cells decreases S. flexneri invasion but does not impact cell-to-cell spread.
DISCUSSION
In this study, we found that Lewis-negative status was associated with increased susceptibility to shigellosis in Bangladeshi infants in their first year of life. This discovery of the role of fucosylation in susceptibility enriches our understanding of Shigella pathogenesis and may provide new avenues towards the prevention or treatment of this antimicrobial-resistant bacterium.
We demonstrated that inhibition of fucosyltransferases in colonic epithelial cells decreases S. flexneri invasion without impacting dissemination. The results of our in vitro assay were surprising at first, as one might predict that decreasing fucosylation would mimic the phenotype of the susceptible Lewis-negative infants, resulting in an increase in invasion rather than the observed decrease. However, since the inhibitor used blocks many fucosyltransferases it is possible that our data point to a complex role for other fucosylated glycans in mediating S. flexneri invasion of the colonic epithelium. Other groups have found that S. flexneri adheres to epithelial cells via glycan–glycan interaction between bacterial lipopolysaccharide and host-cell ABO blood-group antigens (which are also fucosylated) in addition to Lewis antigens [10].
A limitation of the study is that the children were infected with multiple other enteropathogens in addition to Shigella. However, the use of attributable fraction rather than solely polymerase chain reaction positivity helped to ascribe a diarrheal infection as shigellosis [5]. An additional limitation was that our detection method targeted the virulence factor ipaH, which is present in all 4 species of Shigella as well enteroinvasive Escherichiacoli. Shigella flexneri is one of the most common causes of shigellosis in the region of Bangladesh our cohort was from; thus, we used S. flexneri for our in vitro assays [11].
In addition to bacteria–epithelial cell interactions during infection, Lewis antigens impact neutrophil binding and transepithelial migration [12]. Neutrophil influx is a hallmark of shigellosis, and neutrophils are critical for control of the pathogen [13–15]. While we only tested the effect of fucosylated glycans on S. flexneri invasion and dissemination in tissue culture epithelial cells, it is possible that Lewis blood-group antigens additionally alter susceptibility to S. flexneri via their ability to facilitate recruitment of leukocytes in vivo. Together, the data we present support the concept that fucosylated glycans play a role in susceptibility to shigellosis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. L. L., with help from E. W., L. K. Y., and H. F. A., carried out the in vitro experiments. J. L. L., M. L. J., Y. L., J. A. P.-M., and J. Z. M. analyzed the clinical data. R. H. founded the birth cohort and directed all work in Bangladesh. J. L. L., B. L., H. F. A., J. A. P.-M., B. D. K., R. H., and W. A. P. formulated the study concept. U. N. curated and maintained the clinical data. J. L. L. wrote the manuscript with input from W. A. P., H. F. A., J. Z. M., and M. J. L. All authors had access to the draft manuscript.
Financial support. This study was supported by funding from the Bill and Melinda Gates Foundation and the National Institute of Allergy and Infectious Diseases (grant numbers R01AI43596 to W. A. P. and T32AI007496 to J. L. L.).
Potential conflicts of interest. J. Z. M., B. L., and U. N. report grants from the Bill and Melinda Gates Foundation during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kotloff KL. Shigella infection in children and adults: a formidable foe. Lancet Glob Health 2017; 5:e1166–7. [DOI] [PubMed] [Google Scholar]
- 2. Cooling L. Blood groups in infection and host susceptibility. Clin Microbiol Rev 2015; 28:801–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirkpatrick BD, Colgate ER, Mychaleckyj JC, et al. ; PROVIDE Study Teams . The “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. Am J Trop Med Hyg 2015; 92:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taniuchi M, Platts-Mills JA, Begum S, et al. Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants. Vaccine 2016; 34:3068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schnee AE, Haque R, Taniuchi M, et al. Identification of etiology-specific diarrhea associated with linear growth faltering in Bangladeshi infants. Am J Epidemiol 2018; 187:2210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee B, Dickson DM, deCamp AC, et al. Histo-blood group antigen phenotype determines susceptibility to genotype-specific rotavirus infections and impacts measures of rotavirus vaccine efficacy. J Infect Dis 2018; 217:1399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weddle E, Agaisse H. Spatial, temporal, and functional assessment of LC3-dependent autophagy in Shigella flexneri dissem ination. Infect Immun 2018; 86:e00134–18. doi: 10.1128/IAI.00134-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barbé L, Le Moullac-Vaidye B, Echasserieau K, et al. Histo-blood group antigen-binding specificities of human rotaviruses are associated with gastroenteritis but not with in vitro infection. Sci Rep 2018; 8:12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dragoi AM, Agaisse H. The serine/threonine kinase STK11 promotes Shigella flexneri dissemination through establishment of cell-cell contacts competent for tyrosine kinase signaling. Infect Immun 2014; 82:4447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Day CJ, Tran EN, Semchenko EA, et al. Glycan:glycan interactions: high affinity biomolecular interactions that can mediate binding of pathogenic bacteria to host cells. Proc Natl Acad Sci U S A 2015; 112:E7266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Das SK, Ahmed S, Ferdous F, et al. Changing emergence of Shigella sero-groups in Bangladesh: observation from four different diarrheal disease hospitals. PLoS One 2013;8:e62029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brazil JC, Sumagin R, Stowell SR, et al. Expression of Lewis-A glycans on polymorphonuclear leukocytes augments function by increasing transmigration. J Leukoc Biol 2017; 102:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raqib R, Mia SM, Qadri F, et al. Innate immune responses in children and adults with Shigellosis. Infect Immun 2000; 68:3620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sansonetti PJ, Arondel J, Huerre M, Harada A, Matsushima K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect Immun 1999; 67:1471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Z, Jin L, Champion G, Seydel KB, Stanley SL Jr. Shigella infection in a SCID mouse-human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect Immun 2001; 69:3240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.