Abstract
In this study, we described the synthesis of 1,4- and 1,5-disubstituted-1,2,3-triazolo-nucleosides from various alkynes with 1′-azido-2′,3′,5′-tri-O-acetylribose using either copper-catalyzed azide-alkyne cycloaddition (CuAAC) or ruthenium-catalyzed azide-alkyne cycloaddition (RuAAC), respectively. Optimized RuAAC conditions were realized with the commercially available [Cp*RuCl(PPh3)2] under microwave heating, which allows a significant acceleration of the reaction times (from 6 h to 5 min). This reaction can work under water-containing system. RuAAC and CuAAC are useful tools for the synthesis of 1,2,3-triazolyl-nucleosides small libraries.
1. Introduction
In recent years, many nucleoside analogues have been successfully developed into DNA virus and retrovirus therapeutic agents,1 against different human immunodeficiency virus (HIV) strains and viruses with related-polymerases such as hepatitis B (HBV) or C (HCV). In the case of HCV, the template and substrate specificity of HCV RNA-dependent RNA polymerase differs from the host DNA-dependent polymerase, increasing thus the probability of developing potent HCV-specific nucleoside chain terminator. Figure 1 shows some examples of anti-HCV nucleosides and some triazolo derivatives (Fig. 1). The first nucleoside to show a therapeutic effect in HCV infection was ribavirin2 (1) whose mechanism of action is a subject of debate.
Figure 1.
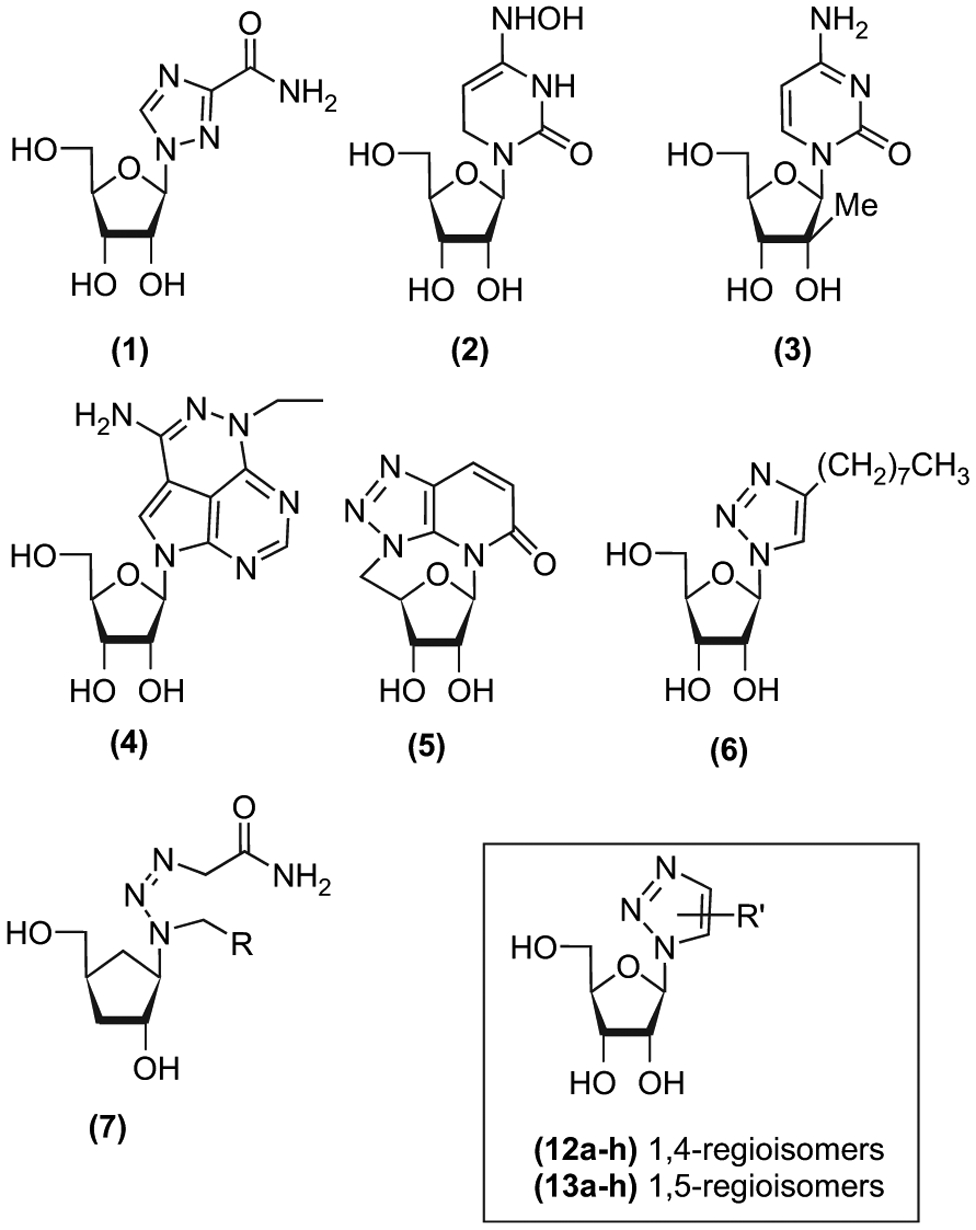
Some anti-HCV triazolo-nucleosides and target compounds (12 and 13).
Stuyver et al.3 reported the activity of a N4-hydroxycytidine (NHC) analogue (2), meanwhile several reports4 describe the anti-HCV replicon activity of nucleosides modified at the 2′-position (3). Another approach to discover a potent anti-HCV compounds was reported by Smith et al.5 in which analogues of triciribine (4), a cyclic sangivamycin analog with anti-cancer and anti-viral activity was reported. The synthesis of various triazolo compounds has been reported by Schinazi et al. (for 5),6 Benhida et al. (for 6),7 and by our team (for 7).8 As part of our drug discovery program, we report herein the synthesis of new 1,4-disubstituted-1,2,3-triazolonucleosides through well established Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC). We have then investigated the Ru(II)-catalyzed azide-alkyne cycloaddition (RuAAC) under microwave conditions for the synthesis of 1,5-regiomers. All obtained compounds were evaluated for their anti-HCV activity in vitro.
2. Results and discussion
The Huisgen 1,3-dipolar cycloaddition of alkynes and azides (AAC)9 to give substituted-1,2,3-triazoles has emerged as a powerful linking reaction in both uncatalyzed10 and copper (I)-catalyzed leading to the sole 1,4-regioisomers.11 The copper-catalyzed version of the reaction (CuAAC) has proven to be popular in many conditions, ranging from drug discovery to surface science, where rapid and reliable bond formation is required. Such 1,4 selectivity has been already reported in a very similar system under microwave conditions by Benhida et al.7 Nevertheless, we have synthesized new compounds and evaluated them against HCV. Starting with the protected β-azido-ribose (8),12 the synthesis of various protected 1,4-disubstituted-1,2,3-triazolyl-nucleosides (Scheme 1) was performed regioselectively with Cu(0)/CuSO4 as catalyst precursor. The desired new compounds 9a–h were obtained in yields ranging from 83 to 93% (Table 1, entries 1–7) except for ethoxy-ethyne (Table 1, entry 8). This system has the advantage of being simple and the products can be obtained from the reaction mixture by simple extraction.
Scheme 1.
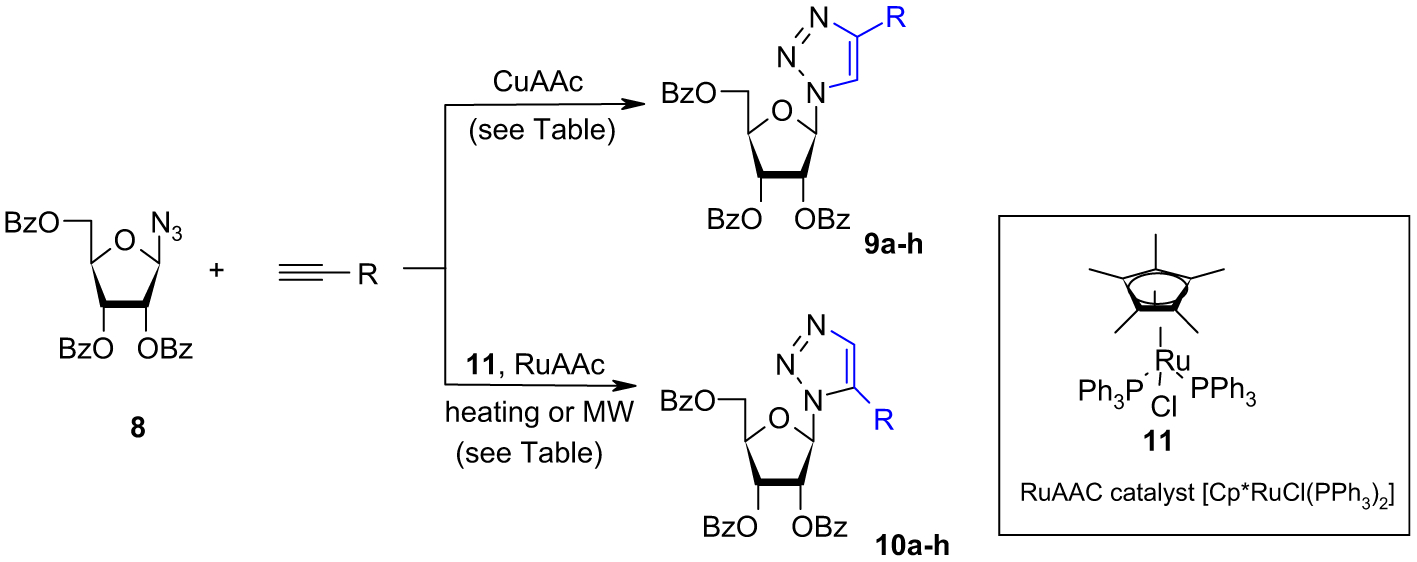
Table 1.
| Entry | Alkyne | R | 1,4-Regioisomers (CuAAC) (%) | 1,5-Regioisomers (RuAAC)b | Ratio 1,4/1,5 | |
|---|---|---|---|---|---|---|
| Δ | Δ | MW yield % (Conv. %) | ||||
| 1 | 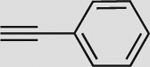 |
a | 86 | 79 | 88 (93) | 4:96 |
| 2 | 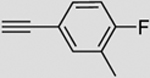 |
b | 88 | 83 | 92 (100) | 3:93 |
| 3 | 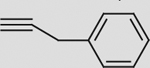 |
c | 93 | 71 | 92 (100) | 4:96 |
| 4 | 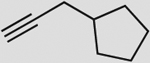 |
d | 87 | 79 | 87 (100) | 5:95 |
| 5 | e | 89 | 82 | 91 (96) | 4:96 | |
| 6 | f | 83 | 68 | 87 (100) | 5:95 | |
| 7 | 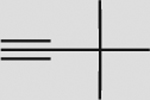 |
g | 92 | 77 | 95 (100) | 5:95 |
| 8 | h | 63 | 54 | 93 (100) | 3:97 | |
Conditions: azide 1 (1 equiv), alkyne (1.1 equiv), Cu powder (4 equiv), CuSO4·5H2O (0.2 equiv), tBuOH/H2O.
Conditions: azide 1 (1 equiv, 0.1 M in THF), alkyne (1.5 equiv), Cp*RuCl(PPh3)2 (0.05 equiv), THF, thermal heating (Δ): 6 h, T=50 °C or microwave conditions (MW): 5 min, T=100 °C.
We then turn our attention to the ruthenium-catalyzed version of the reaction (RuAAC), which is less extensively described and led mainly to 1,5-regioisomers.13 Generally, the common approaches to 1,5-regioisomers were based on Grignard reagents,14 or 1-trimethylsilylalkynes;15 nevertheless, these approaches suffer from some limitations including the number of steps and the chemical behavior of some functional groups toward those conditions. Thus, under classical heating, the azido-ribose (8) was reacted with various alkynes and 5 mol % Cp*RuCl(PPh3)2 catalyst16 (11) in THF at 50 °C. After 6 h, the desired triazolo compounds 10a–h were obtained in moderate to good yield (54–83%), after purification by column chromatography. Except for the ethyl ethynyl ether derivative (54%), the yields of all other alynes are almost identical (from 71 to 83%) for the RuAAc reaction showing that the influence of the steric hindrance of alkyne is low.
During the RuAAC, 3 to 7% of the 1,4-regioisomers, separable by column chromatography on silica gel, were obtained. Their structures were unambiguously confirmed by NMR (e.g., δH1=6.44 ppm (for 1,4-isomer) and δH1=6.15 ppm (for 1,5-isomer)) and TLC comparison with those obtained under CuAAC conditions. It should be noted that some catalyst deactivation has been encountered during long heating times. Thus, to circumvent this limitation, we decided to work under microwave activation17 (Table 1). Microwave heating is known as powerful tool to promote a variety of chemical reactions.18 While yields and purities of the coupling products were comparable to heating conditions, microwave heating allows a significant acceleration of the reaction from 6 h to 5 min (Table 1).
Working in THF at 100 °C, we first investigated the optimal conditions for the cycloaddition of azido-ribose 8 and hexyne with different concentration of catalyst 11 (Table 2). A 95% conversion was obtained after only 3 min of reaction with 5% catalyst. The total conversion of starting azido-ribose was obtained after 10 min with 3.5% catalyst loading and in 5 min for 5% catalyst loading. For lower catalyst loading, conversion was not complete, even after 10 min reaction.
Table 2.
Optimization of RuAAC under microwave conditions for 11e
| Catalyst loading (mol %) | Irradiation time | ||
|---|---|---|---|
| 3 min | 5 min | 10 min | |
| 1 | — | — | 36% |
| 2 | — | — | 57% |
| 3.5 | — | 84% | 100% |
| 5 | 94% | 100% | — |
Thus, 5 mol % catalyst and 1.5 equiv of alkyne in THF at 100 °C for 5 min under microwave irradiation allowed the isolation of the desired compound 10e. It is interesting to note that anhydrous conditions are not necessary (e.g., undistilled THF, or THF-containing 2 equiv of water can be used to run this RuAAC). With this optimized conditions in hand, we probed the scope of the reaction on protected azido-ribose (8) with various alkynes. The desired 1,5-disubstituted-1,2,3-triazolyl-nucleosides 10a–h were obtained in good yields (Table 1). In most examples (Table 1, entries 1–7), microwave irradiation had evident beneficial effects in terms of reaction time and yield. For example, using classical heating, sluggish reactions with ethyl ethynyl ether were observed (54% after 6 h), meanwhile under microwave irradiation, complete conversion was achieved with [Cp*RuCl(PPh3)2] after short reaction time (5 min). The deacylation of 1,4-regioisomers (9a–h) and 1,5-regioisomers (10a–h) was carried out using a 7 N solution of ammonia in methanol at 0 °C over 12 h and led quantitatively to the final 1,4-disubstituted-1,2,3-triazolo-nucleosides (12a–h) and 1,5-disubstituted-1,2,3-triazolo-nucleosides (13a–h), respectively (Scheme 2).
Scheme 2.
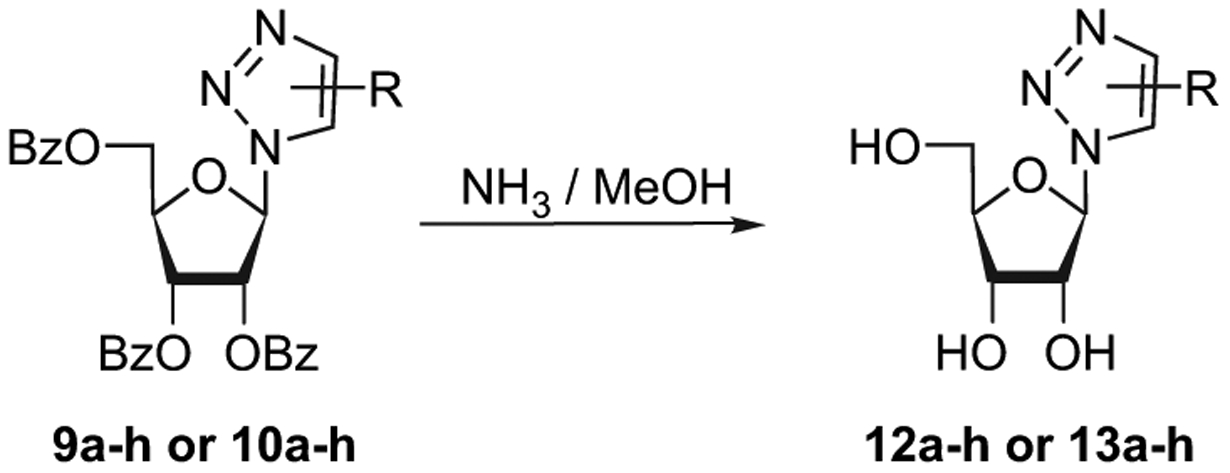
Cleavage of benzoyl protective group.
Finally, the scope of this reaction with respect to unprotected azido-ribose (14) was next investigated on the hexyne and after 5 min of irradiation with 5 mol % RuAAC catalyst, the desired product (13e) was obtained in 95% yield (Scheme 3). This result confirms the robustness of the RuACC reaction conditions.
Scheme 3.
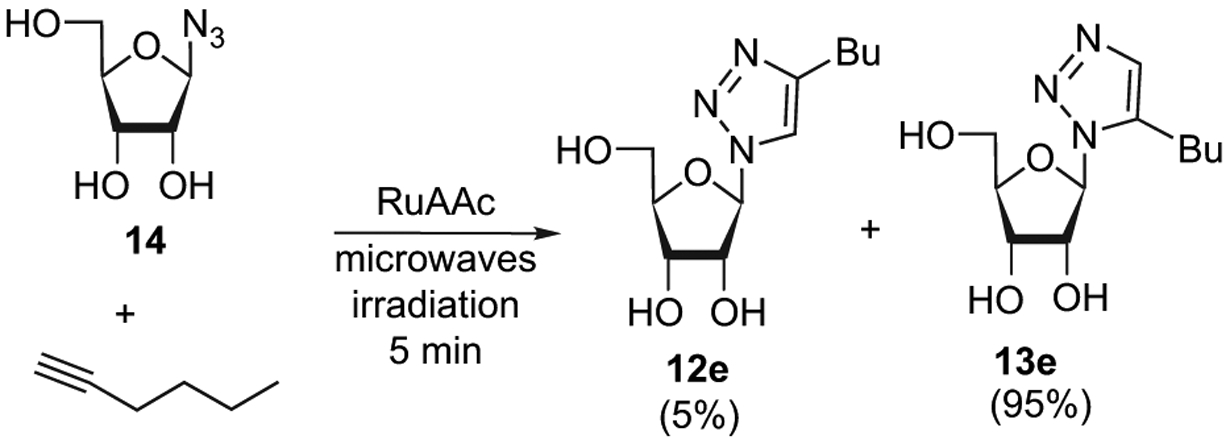
RuAAC on deprotected azido-ribose under thermal heating.
3. Biological results
The biological activity and toxicity of the synthesized triazoles against HCV were investigated in a replicon system in Huh-7 cells, and these compounds did not exhibit any marked activity or toxicity. The anti-viral19 and cytotoxicity20 assays were done as previously described.
4. Conclusion
In summary, we have used either the Cu(0)/CuSO4 catalytic system for CuAAC or the Cp*RuCl(PPh3)2 (11) under microwave conditions for RuAAC to reach hitherto unknown 1,4- and 1,5-di-substituted-1,2,3-triazolyl-nucleosides. The cooperative effect of the catalyst 11 and the microwave activation afforded the desired compounds in a few minutes in high yields. This approach allows an easy access to a small library of 1,5-disubstituted-triazolo derivatives under RuAAC and 1,4-regioisomers under CuAAC.
5. Experimental
5.1. General
Commercially available chemicals were of reagent grade and used as received. THF was distilled from sodium/benzophenone ketyl; CH2Cl2 from CaH2 immediately prior use and benzene over Na. The microwave was a Biotage AB Initiator EXP EU with a maximum power of 300 W. The vials used in the microwave were Emrys™ process vials 0.5–2 mL. The reactions were monitored by thin layer chromatography (TLC) analysis using silica gel plates (Kieselgel 60F254, E. Merck). Column chromatography was performed on Silica Gel 60M (0.040–0.063 mm, E. Merck). Melting points are uncorrected and were measured on a Kofler apparatus. The 1H and 13C NMR spectra were recorded on a Bruker AVANCE DPX 250 and Varian InovaUnity 400 spectrometer (1H: 250 MHz, 13C: 100 MHz) in (d4) methanol and CDCl3, shift values in parts per million relative to SiMe4 as internal reference, the 31P spectra were reported using aqueous phosphoric acid as external reference (31P: 161.97 MHz) in CD3OD, unless otherwise stated; J in hertz. Evidence of purity has been done from a proton-decoupled 13C NMR spectrum with a signal-to-noise ratio sufficient to permit seeing peak with 5% of the intensity of the strongest peak.
5.2. General procedure under CuAAC condition
To a solution of selected alkyne (1.1 mmol) and azido-ribose (8) (1 mmol) in H2O/tBuOH (1 mL) were added Cu powder (4 mmol) and CuSO4 (0.2 mmol). The resulting suspension was stirred overnight at room temperature, then the mixture was extracted twice with ethyl acetate (50 mL), and dried over MgSO4. The solvents were removed under reduced pressure and the obtained residue was purified on silica gel (petroleum ether/ethyl acetate, 8:2, v/v) to give the desired compound.
5.2.1. 2′,3′,5′-Tri-O-benzoyl-1′-[4-phenyl-[1,2,3]triazol-1yl]ribofuranose (9a)
Prepared from compound 8 with the typical procedure described before to give 9a (86%) as an oil. IR: 1717, 1451, 1316, 1124, 1093,1040, 703 cm−1; 1H NMR (CDCl3): δ 8.06 (d, J=7.2 Hz, 2H, HAr), 8.00–7.95 (m, 5H, HAr and H5), 7.65 (d, J=7.5 Hz, 2H, HAr), 7.58–7.50 (m, 3H, HAr), 7.41–7.30 (m, 9H, HAr), 6.53 (d, J=3.8 Hz, 1H, H1′), 6.30 (dd, J=5.3, 3.8 Hz, 1H, H2′), 6.18 (t, J=5.3 Hz, 1H, H3′), 4.86–4.92 (m, 2H, H4′ and H5′), 4.62 (dd, J=12.0, 3.6 Hz, 1H, H5′); 13C NMR (CDCl3): δ 166.0 (C(O)), 165.1 (C(O)), 165.0 (C(O)), 148.2 (C4), 133.8 (CAr), 133.6 (CAr), 133.3 (CAr), 129.8 (2C, CAr), 129.7 (CAr), 129.6 (CAr), 129.2 (CAr), 128.6 (2C, CAr), 128.5 (2C, CAr), 128.4 (CAr), 128.4 (CAr), 128.3 (CAr), 125.7 (CAr), 118.4 (C5), 90.3 (C1′), 81.1 (C4′), 75.2 (C2′), 71.5 (C3′), 63.5 (C5′); CAS: 26295-47-6; MS (ESI): m/z [M+Na]+ calcd for C34H27N3NaO7: 612.6, found: 612.5.
5.2.2. 2′,3′,5′-Tri-O-benzoyl-1′-[4–4-fluoro-3-methylphenyl-[1,2,3]triazol-1-yl]ribofuranose (9b)
Prepared from compound 8 with the typical procedure described before to give 9b (88%) as a slight yellow oil. IR: 1720, 1602, 1493, 1451, 1259, 1091, 1069, 1024, 799, 705 cm−1; 1H NMR (CDCl3): δ 8.07 (d, J=8.3 Hz, 2H, HAr), 8.00 (d, J=8.3 Hz, 2H, HAr), 7.97 (d, J=8.3 Hz, 2H, HAr), 7.89 (s, 1H, H5), 7.61–7.50 (m, 4H, HAr), 7.44–7.37 (m, 7H, HAr), 6.98 (t, J=9.0 Hz, 1H, HAr), 6.53 (d, J=3.9 Hz, 1H, H1′), 6.28 (dd, J=5.2, 3.9 Hz, 1H, H2′), 6.15 (t, J=5.2 Hz, 1H, H3′), 4.93–4.87 (m, 2H, H4′ and H5′), 4.62 (dd, J=13.2, 4.8 Hz, 1H, H5′), 2.28 (d, J=1.7 Hz, 3H, CH3); 13C NMR (CDCl3): δ 166.1 (C(O)), 165.2 (C(O)), 165.1 (C(O)), 147.7 (C4), 133.9 (CAr), 133.8 (CAr), 133.5 (CAr), 129.9 (CAr), 129.8 (CAr), 129.7 (CAr), 129.2 (CAr), 129.0 (CAr), 128.9 (CAr), 128.7 (CAr), 128.6 (3C, CAr), 128.5 (CAr), 125.8 (2C, CAr), 125.4 (CAr), 125.2 (CAr), 124.9 (CAr), 124.8 (CAr), 118.0 (C5), 115.5 (CAr), 115.2 (CAr), 90.4 (C1′), 81.3 (C4′), 75.3 (C2′), 71.6 (C3′), 63.6 (C5′), 14.6, 14.5 (CH3); MS (ESI): m/z [M+Na]+ calcd for C35H28FN3NaO7: 644.6, found: 644.5.
5.2.3. 2′,3′,5′-Tri-O-benzoyl-1′-[4-benzyl-[1,2,3]triazol-1-yl]ribofuranose (9c)
Prepared from compound 8 with the typical procedure described before to give 9c (93%) as white solid. Mp: 158 °C (CHCl3); IR: 1727, 1708, 1601, 1450, 1270, 1123, 1095, 1068, 710 cm−1; 1H NMR (CDCl3): δ 8.09–7.96 (m, 6H, HAr), 7.64–7.56 (m, 3H, HAr), 7.50–7.38 (m, 7H, HAr and H5), 7.33–7.21 (m, 5H, HAr), 6.43 (d, J=3.1 Hz, H1′), 6.28–6.25 (m, 1H, H2′), 6.19 (t, J=5.5 Hz, 1H, H3′), 4.95–4.88 (m, 1H, H4′), 4.83 (dd, J=12.2, 3.3 Hz, 1H, H5′), 4.63 (dd, J=12.2, 4.5 Hz, 1H, H5′), 4.02–3.88 (m, 1H, CH2Ph); 13C NMR (CDCl3): δ 166.0 (C(O)), 165.6 (C(O)), 165.0 (C(O)), 138.4 (C4), 133.8 (CAr), 133.6 (CAr), 133.3 (CAr), 129.8 (CAr), 129.7 (2C, CAr), 129.2 (CAr), 128.7 (CAr), 128.6 (CAr), 128.5 (CAr), 128.4 (CAr), 126.5 (C5), 90.1 (C1′), 81.0 (C4′), 75.2 (C2′), 71.6 (C3′), 63.7 (C5′), 32.1 (CH2Ph); MS (ESI): m/z [M+Na]+ calcd for C35H29N3NaO7: 626.6, found: 626.5.
5.2.4. 2′,3′,5′-Tri-O-benzoyl-1′-[4-methylcyclopentyl-[1,2,3]triazol-1-yl]ribofuranose (9d)
Prepared from compound 8 with the typical procedure described before to give 9d (87%) as white solid. Mp: 121 °C (CHCl3); IR: 1716, 1601, 1451, 1261, 1093, 1068, 1023, 803, 706 cm−1; 1H NMR (CDCl3): δ 8.05 (d, J=8.2 Hz, 2H, HAr), 7.97 (d, J=8.2 Hz, 2H, HAr), 7.94 (d, J=8.2 Hz, 2H, HAr), 7.57–7.49 (m, 4H, HAr and H5), 7.44–7.31 (m, 6H, HAr), 6.46 (d, J=3.6 Hz, 1H, H1′), 6.23 (dd, J=5.2, 3.6 Hz, 1H, H2′), 6.15 (t, J=5.4 Hz, 1H, H3′), 4.89–4.79 (m, 2H, H4′ and H5′), 4.59 (dd, J=12.1, 4.2 Hz, 1H, H5′), 2.63 (d, J=6.9 Hz, 2H, CH2–CHCH2CH2), 2.11–2.01 (m, 1H, CH2–CHCH2CH2), 1.73–1.62 (m, 2H, CH2–CHCH2CH2), 1.60–1.52 (m, 2H, CH2–CHCH2CH2), 1.50–1.42 (m, 2H, CH2–CHCH2CH2), 1.17–1.07 (m, 2H, CH2–CHCH2CH2); 13C NMR (CDCl3): δ 166.1 (C(O)), 165.2 (C(O)), 165.1 (C(O)), 148.6 (C4), 133.8 (CAr), 133.7 (CAr), 133.4 (CAr), 130.1 (CAr), 129.9 (CAr), 129.8 (2C, CAr), 129.3 (CAr), 128.7 (CAr), 128.6 (2C, CAr), 128.5 (CAr), 128.3 (CAr), 112.0 (C5), 90.2 (C1′), 81.0 (C4′), 75.2 (C2′), 71.7 (C3′), 63.8 (C5′), 39.7 (CH2–CHCH2CH2), 32.5 (2C, CH2–CHCH2CH2), 31.6 (CH2–CHCH2CH2), 25.1 (CH2–CHCH2CH2); MS (ESI): m/z [M+Na]+ calcd for C34H33N3NaO7: 618.7, found: 618.5.
5.2.5. 2′,3′,5′-Tri-O-benzoyl-1′-[4-butyl-[1,2,3]triazol-1-yl]ribofuranose (9e)
Prepared from compound 8 with the typical procedure described before to give 9e (89%) as white solid. Mp: 98 °C (CHCl3); IR: 1726, 1601, 1451, 1254, 1124, 1090, 1068, 1045, 706 cm−1; 1H NMR (CDCl3): δ 8.05 (d, J=8.5 Hz, 2H, HAr), 7.99 (d, J=8.5 Hz, 2H, HAr), 7.95 (d, J=8.5 Hz, 2H, HAr), 7.58–7.52 (m, 3H, HAr), 7.49 (s, 1H, H5), 7.46–7.34 (m, 6H, HAr), 6.45 (d, J=3.6 Hz, 1H, H1′), 6.23 (dd, J=5.3, 3.6 Hz, 1H, H2′), 6.15 (t, J=5.3 Hz, 1H, H3′), 4.90–4.80 (m, 2H, H4′ and H5′), 4.60 (dd, J=12.1, 4.2 Hz, 1H, H5′), 2.64 (td, J=7.6, 2.3 Hz, 2H, CH2CH2CH2CH3), 1.59–1.50 (m, 2H, CH2CH2CH2CH3), 1.39–1.28 (m, 2H, CH2CH2CH2CH3), 0.89 (t, J=7.3 Hz, 3H, CH2CH2CH2CH3); 13C NMR (CDCl3): δ 166.0 (C(O)), 165.0 (C(O)), 164.9 (C(O)), 148.9 (C4), 133.7 (CAr), 133.6 (CAr), 133.3 (CAr), 129.7 (2C, CAr), 129.6 (CAr), 129.2 (CAr), 128.6 (CAr), 128.5 (CAr), 128.4 (2C, CAr), 119.5 (C5), 90.0 (C1′), 80.9 (C4′), 75.1 (C2′), 71.6 (C3′), 63.6 (C5′), 31.0 (CH2CH2CH2CH3), 25.1 (CH2CH2CH2CH3), 22.2 (CH2CH2CH2CH3), 13.7 (CH2CH2CH2CH3); MS (ESI): m/z [M+Na]+ calcd for C32H31N3NaO7: 592.6, found: 592.5.
5.2.6. 2′,3′,5′-Tri-O-benzoyl-1′-[4-(3-chloropropyl)-[1,2,3]triazol-1-yl]ribofuranose (9f)
Prepared from compound 8 with the typical procedure described before to give 9f (83%) as white solid. Mp: 132 °C (CHCl3); IR: 1716, 1601, 1451, 1264, 1124, 1099, 1070, 1047, 702 cm−1; 1H NMR (CDCl3): δ 8.03 (d, J=8.5 Hz, 2H, HAr), 7.98 (d, J=8.4 Hz, 2H, HAr), 7.94 (d, J=8.4 Hz, 2H, HAr), 7.58–7.52 (m, 4H, HAr and H5), 7.45–7.33 (m, 6H, HAr), 6.44 (d, J=3.5 Hz, 1H, H1′), 6.24 (dd, J=5.4, 3.5 Hz, 1H, H2′), 6.14 (t, J=5.4 Hz, 1H, H3′), 4.90–4.79 (m, 2H, H4′ and H5′), 4.58 (dd, J=12.2, 4.3 Hz, 1H, H5′), 3.51 (t, J=6.4 Hz, 2H, CH2CH2CH2Cl), 2.79 (t, J=6.4 Hz, 2H, CH2CH2CH2Cl), 2.07–2.00 (m, 2H, CH2CH2CH2Cl); 13C NMR (CDCl3): δ 165.9 (C(O)), 165.0 (C(O)), 164.9 (C(O)), 146.7 (C4), 133.7 (CAr), 133.6 (CAr), 133.3 (CAr), 129.7 (CAr), 129.6 (2C, CAr), 129.2 (CAr), 128.5 (2C, CAr), 128.4 (2C, CAr), 128.3 (CAr), 120.1 (C5), 90.0 (C1′), 80.9 (C4′), 75.1 (C2′), 71.5 (C3′), 63.5 (C5′), 44.0 (CH2CH2CH2Cl), 31.4 (CH2CH2CH2Cl), 22.5 (CH2CH2CH2Cl); MS (ESI): m/z [M+Na]+ calcd for C31H28ClN3NaO7: 612.6, found: 612.5.
5.2.7. 2′,3′,5′-Tri-O-benzoyl-1′-[4-terbutyl-[1,2,3]triazol-1-yl]ribofuranose (9g)
Prepared from compound 8 with the typical procedure described before to give 9g (92%) as white solid. Mp: 129 °C (CHCl3); IR: 1718, 1600, 1450, 1265, 1094, 1069, 1037, 706 cm−1; 1H NMR (CDCl3): δ 8.07 (d, J=8.0 Hz, 2H, HAr), 7.99 (d, J=8.3 Hz, 2H, HAr), 7.94 (d, J=8.3 Hz, 2H, HAr), 7.59–7.51 (m, 3H, HAr), 7.46–7.33 (m, 7H, HAr and H5), 6.46 (d, J=3.1 Hz, 1H, H1′), 6.20–6.14 (m, 2H, H2′ and H3′), 4.90–4.80 (m, 2H, H4′ and H5′), 4.61 (dd, J=11.9, 4.1 Hz, 1H, H5′), 1.27 (s, 9H, C(CH3)3); 13C NMR (CDCl3): δ 166.1 (2C, C(O)), 165.1 (2C, C(O)), 158.2 (C4), 133.8 (CAr), 133.7 (CAr), 133.4 (CAr), 129.8 (CAr), 129.7 (2C, CAr), 129.3 (CAr), 128.6 (2C, CAr), 128.5 (3C, CAr), 117.4 (C5), 90.1 (C1′), 81.0 (C4′), 75.2 (C2′), 71.8 (C3′), 63.8 (C5′), 30.7 (CHCH3), 30.1 (CHCH3); MS (ESI): m/z [M+Na]+ calcd for C32H31N3NaO7: 592.6, found: 592.0.
5.2.8. 2′,3′,5′-Tri-O-benzoyl-1′-[4-O-ethoxy-[1,2,3]triazol-1-yl]ribofuranose (9h)
Prepared from compound 8 with the typical procedure described before to give 9h (63%) as yellow solid. Mp: 117 °C (CHCl3); IR: 1715, 1568, 1451, 1282, 1265, 1127, 1092, 1024, 701 cm−1; 1H NMR (CDCl3): δ 8.06 (d, J=8.4 Hz, 2H, HAr), 7.98 (d, J=8.4 Hz, 2H, HAr), 7.95 (d, J=8.4 Hz, 2H, HAr), 7.60–7.53 (m, 3H, HAr), 7.48–7.34 (m, 6H, HAr), 7.16 (s, 1H, H5), 6.36 (d, J=3.8 Hz, 1H, H1′), 6.22 (dd, J=5.3, 3.8 Hz, 1H, H2′), 6.10 (t, J=5.3 Hz, 1H, H3′), 4.88–4.79 (m, 2H, H4′ and H5′), 4.69 (dd, J=12.1, 4.2 Hz, 1H, H5′), 4.12 (q, J=7.1 Hz, 2H, OCH2CH3), 1.35 (t, J=7.1 Hz, 3H, OCH2CH3); 13C NMR (CDCl3): δ 166.0 (C(O)), 165.1 (C(O)), 165.0 (C(O)), 161.2 (C4), 133.8 (CAr), 133.7 (CAr), 133.4 (CAr), 129.8 (CAr), 129.7 (CAr), 128.6 (2C, CAr), 128.5 (2C, CAr), 104.5 (C5), 90.7 (C1′), 81.1 (C4′), 74.9 (C2′), 71.6 (C3′), 66.3 (OCH2CH3), 63.7 (C5′), 14.7 (OCH2CH3); MS (ESI): m/z [M+Na]+ calcd for C30H27N3NaO8: 580.6, found: 580.5.
5.3. General procedure for the RuAAC reaction
In a microwave sealed reactor, a mixture of azido-ribose (8) (1 mmol), the selected alkyne (1.5 mmol), and Cp*RuCl(PPh3)2 (0.05 mmol) in THF (10 mL) was irradiated for 5 min at 100 °C (200 W, normal mode). The mixture was then evaporated under reduced pressure and the residue purified on silica gel (petroleum ether/ethyl acetate; 7:3) to give the desired product.
5.3.1. 2′,3′,5′-Tri-O-benzoyl-1′-[5-phenyl-[1,2,3]triazol-1-yl]ribofuranose (10a)
Prepared from compound 8 with the typical procedure described before to give 10a (see Table 1) as a slightly brown oil. IR: 1721, 1601, 1451, 1261, 1090, 1069, 704 cm−1; 1H NMR (CDCl3): δ 8.05–7.90 (m, 6H, HAr), 7.74 (s, 1H, H4), 7.59–7.47 (m, 8H, HAr), 7.43–7.30 (m, 6H, HAr), 6.54–6.47 (m, 2H, H2′ and H3′), 6.15 (d, J=1.6 Hz, 1H, H1′), 4.91 (m, 1H, H4′), 4.78 (dd, J=12.12, 3.96 Hz, 1H, H5′), 4.61 (dd, J=12.12, 5.13 Hz, 1H, H5′); 13C NMR (CDCl3): δ 166.2 (C(O)), 165.0 (2C, C(O)), 139.1 (C5), 133.7 (CAr), 133.5 (CAr), 133.1 (CAr), 133.0 (C5), 129.9 (CAr), 129.8 (2C, CAr), 129.4 (CAr), 129.2 (2C, CAr), 128.8 (CAr), 128.7 (CAr), 128.5 (CAr), 128.4 (CAr), 128.3 (CAr), 126.0 (C4), 88.2 (C1′), 80.9 (C4′), 75.6 (C2′), 72.2 (C3′), 63.7 (C5′); MS (ESI): m/z [M+Na]+ calcd for C34H27N3NaO7: 612.6, found: 612.5.
5.3.2. 2′,3′,5′-Tri-O-benzoyl-1′-[5–4-fluoro-3-methylphenyl-[1,2,3]triazol-1-yl]ribofuranose (10b)
Prepared from compound 8 with the typical procedure described before to give 10b (see Table 1) as a slight brown oil. IR: 1720, 1602, 1492, 1451, 1253, 1084, 705 cm−1; 1H NMR (CDCl3): δ 8.05–7.91 (m, 6H, HAr), 7.70 (s, 1H, H4), 7.60–7.48 (m, 3H, HAr), 7.44–7.30 (m, 9H, HAr), 7.16–7.08 (t, J=8.0 Hz, 1H, HAr), 6.51–6.45 (m, 2H, H2′ and H3′), 6.10 (d, J=1.2 Hz, 1H, H1′), 4.93–4.89 (m, 1H, H4′), 4.78 (dd, J=12.1, 3.9 Hz, 1H, H5′), 4.61 (dd, J=12.1, 5.2 Hz, 1H, H5′), 2.32 (d, J=1.8 Hz, 3H, CH3); 13C NMR (CDCl3): δ 166.2 (C(O)), 165.1 (C(O)), 165.0 (CAr), 163.5 (CAr), 161.0 (CAr), 138.4 (CAr), 133.8 (CAr), 133.5 (CAr), 133.2 (CAr), 132.9 (CAr), 132.6 (2C, CAr), 129.8 (3C, CAr), 129.4 (CAr), 128.7 (2C, CAr), 128.5 (CAr), 128.4 (CAr), 128.3 (CAr), 126.3 (C4), 126.1 (C4), 121.7 (CAr), 121.6 (CAr), 116.0 (CAr), 115.8 (CAr), 88.1 (C1′), 80.8 (C4′), 75.7 (C2′), 72.1 (C3′), 63.7 (C5′), 14.5 (2C, CH3); MS (ESI): m/z [M+Na]+ calcd for C35H28FN3NaO7: 644.6, found: 644.5.
5.3.3. 2′,3′,5′-Tri-O-benzoyl-1′-[5-methylbenzyl-[1,2,3]triazol-1-yl]ribofuranose (10c)
Prepared from compound 8 with the typical procedure described before to give 10c (see Table 1) as a slight brown oil. IR: 1720, 1577, 1451, 1261, 1091, 705 cm−1; 1H NMR (CDCl3): δ 7.93–7.80 (m, 6H, HAr), 7.51–7.40 (m, 3H, HAr), 7.34–7.22 (m, 7H, HAr and H4), 7.21–7.09 (m, 3H, HAr), 7.09–7.04 (m, 2H, HAr), 6.35 (dd, J=5.2, 2.1 Hz, 1H, H2′), 6.21 (dd, J=7.0, 5.2 Hz, 1H, H3′), 6.07 (d, J=2.1 Hz, 1H, H1′), 4.80–4.74 (m, 1H, H4′), 4.65 (dd, J=12.1, 3.8 Hz, 1H, H5′), 4.46 (dd, J=12.1, 5.1 Hz, 1H, H5′), 4.03 (q, J=16.6 Hz, 2H, CH2Ph); 13C NMR (CDCl3): δ 166.1 (C(O)), 165.0 (2C, C(O)), 137.0, 135.6 (CAr and C5), 133.8 (CAr), 133.7 (CAr), 133.5 (CAr), 133.1 (CAr), 129.7 (2C, CAr), 129.3 (CAr), 128.9, 128.7, 128.6, 128.5, 128.4 (2C), 128.3 (CAr and C4), 127.2 (CAr), 88.0 (C1′), 80.8 (C4′), 75.1 (C2′), 71.8 (C3′), 63.6 (C5′), 29.0 (CH2Ph); MS (ESI): m/z [M+Na]+ calcd for C35H29N3NaO7: 626.6, found: 626.5.
5.3.4. 2′,3′,5′-Tri-O-benzoyl-1′-[5-methylcyclopentyl-[1,2,3]triazol-1-yl]ribofuranose (10d)
Prepared from compound 8 with the typical procedure described before to give 10d (see Table 1) as a slight brown oil. IR: 1721, 1601, 1580, 1451, 1262, 1091, 706 cm−1; 1H NMR (CDCl3): δ 8.06–7.92 (m, 6H, HAr), 7.62–7.49 (m, 3H, HAr), 7.47 (s, 1H, H4), 7.46–7.32 (m, 6H, HAr), 6.48 (dd, J=5.2, 1.8 Hz, 1H, H2′), 6.36 (dd, J=7.1, 5.2 Hz, 1H, H3′), 6.20 (d, J=1.8Hz, 1H, H1′), 4.93–4.87 (m, 1H, H4′), 4.73 (dd, J=12.1, 3.9 Hz, 1H, H5′), 4.53 (dd, J=12.1, 5.3 Hz, 1H, H5′), 2.73 (d, J=7.4 Hz, 2H, CH2–CHCH2CH2), 2.25–2.12 (m, 1H, CH2–CHCH2CH2), 1.85–1.73 (m, 2H, CH2–CHCH2CH2), 1.68–1.47 (m, 4H, CH2–CHCH2CH2), 1.24–1.12 (m, 2H, CH2–CHCH2CH2); 13C NMR (CDCl3): δ 166.1 (C(O)), 165.1 (C(O)), 165.0 (C(O)), 138.0 (C5), 133.7 (CAr), 133.5 (CAr), 133.1 (CAr), 132.8 (C4), 129.8 (CAr), 129.7 (CAr), 129.3 (CAr), 128.8 (CAr), 128.7 (CAr), 128.5 (CAr), 128.4 (CAr), 128.3 (CAr), 87.8 (C1′), 80.8 (C4′), 75.4 (C2′), 72.1 (C3′), 63.8 (C5′), 38.7 (CH2–CHCH2CH2), 32.5 (2C, CH2–CHCH2CH2), 28.9 (CH2–CHCH2CH2), 25.0 (CH2–CHCH2CH2); MS (ESI): m/z [M+Na]+ calcd for C34H33N3NaO7: 618.7, found: 618.5.
5.3.5. 2′,3′,5′-Tri-O-benzoyl-1′-[5-butyl-[1,2,3]triazol-1-yl]ribofuranose (10e)
Prepared from compound 8 with the typical procedure described before to give 10e (see Table 1) as a brown oil. IR: 1721, 1602, 1451, 1261, 1092, 706 cm−1; 1H NMR (CDCl3): δ 8.05–7.93 (m, 6H, HAr), 7.62–7.50 (m, 3H, HAr), 7.46 (s, 1H, H4), 7.45–7.33 (m, 6H, HAr), 6.46 (dd, J=5.2, 1.8 Hz, 1H, H2′), 6.35 (dd, J=7.2, 5.2 Hz, 1H, H3′), 6.19 (d, J=1.8 Hz, 1H, H1′), 4.92–4.88 (m, 1H, H4′), 4.73 (dd, J=12.1, 3.9 Hz, 1H, H5′), 4.54 (dd, J=12.1, 5.5 Hz, 1H, H5′), 2.73 (t, J= 7.8 Hz, 2H, CH2CH2CH2CH3), 1.71–1.62 (m, 2H, CH2CH2CH2CH3), 1.44–1.33 (m, 2H, CH2CH2CH2CH3), 0.96–0.89 (t, J=7.4 Hz, 3H, CH2CH2CH2CH3); 13C NMR (CDCl3): δ 166.1 (C(O)), 165.2 (C(O)), 165.0 (C(O)), 138.4 (C5), 133.8 (CAr), 133.5 (CAr), 133.2 (CAr), 132.4 (C4), 129.8 (2C, CAr), 129.4 (CAr), 128.8 (2C, CAr), 128.6 (CAr), 128.4 (CAr), 128.3 (CAr), 87.9 (C1′), 80.8 (C4′), 75.4 (C2′), 72.0 (C3′), 63.8 (C5′), 30.2 (CH2CH2CH2CH3), 22.6 (CH2CH2CH2CH3), 22.2 (CH2CH2CH2CH3), 13.6 (CH2CH2CH2CH3); MS (ESI): m/z [M+Na]+ calcd for C32H31N3NaO7: 592.6, found: 592.5.
5.3.6. 2′,3′,5′-Tri-O-benzoyl-1′-[5-(3-chloropropyl)-[1,2,3]triazol-1-yl]ribofuranose (10f)
Prepared from compound 8 with the typical procedure described before to give 10f (see Table 1) as a brown oil. IR: 1719, 1602, 1451, 1260, 1092, 706 cm−1; 1H NMR (CDCl3): δ 8.05–7.94 (m, 6H, HAr), 7.61–7.50 (m, 3H, HAr), 7.50 (s, 1H, H4), 7.39 (m, 6H, HAr), 6.50 (dd, J=5.2, 1.8 Hz, 1H, H2′), 6.34 (dd, J=7.2, 5.2 Hz, 1H, H3′), 6.25 (d, J=1.8 Hz, 1H, H1′), 4.94–4.90 (m, 1H, H4′), 4.74 (dd, J=12.2, 3.8 Hz, 1H, H5′), 4.53 (dd, J=12.2, 5.1 Hz, 1H, H5′), 3.60–3.48 (m, 2H, CH2CH2CH2Cl), 3.03–2.88 (m, 2H, CH2CH2CH2Cl), 2.20–2.09 (m, 2H, CH2CH2CH2Cl); 13C NMR (CDCl3): δ 166.0 (C(O)), 165.1 (C(O)), 165.0 (C(O)), 136.7 (C5), 133.7 (CAr), 133.5 (CAr), 133.1 (CAr), 132.6 (C4), 129.7 (CAr), 129.6 (CAr), 129.2 (CAr), 128.6 (2C, CAr), 128.5 (CAr), 128.3 (2C, CAr), 87.8 (C1′), 80.8 (C4′), 75.2 (C2′), 71.8 (C3′), 63.5 (C5′), 43.4 (CH2CH2CH2Cl), 30.7 (CH2CH2CH2Cl), 19.9 (CH2CH2CH2Cl); MS (ESI): m/z [M+Na]+ calcd for C31H28ClN3NaO7: 612.6, found: 612.5.
5.3.7. 2′,3′,5′-Tri-O-benzoyl-1′-[5-tert-butyl-[1,2,3]triazol-1-yl]ribofuranose (10g)
Prepared from compound 8 with the typical procedure described before to give 10g (see Table 1) as a brown oil. IR: 1721, 1602, 1451, 1250, 1092, 1069, 706 cm−1; 1H NMR (CDCl3): δ 8.09 (d, J=8.5 Hz, 2H, HAr), 8.01 (d, J=8.5 Hz, 2H, HAr), 7.97 (d, J=8.5 Hz, 2H, HAr), 7.61–7.54 (m, 3H, HAr), 7.47–7.36 (m, 7H, HAr and H4), 6.45 (d, J=3.1 Hz, 1H, H2′), 6.19–6.15 (m, 2H, H3′ and H1′), 4.89–4.80 (m, 2H, H4′ and H5′), 4.61 (dd, J=11.9, 4.1 Hz, 1H, H5′), 1.27 (s, 9H, C(CH3)3); 13C NMR (CDCl3): δ 166.1 (C(O)), 165.1 (C(O)), 165.0 (C(O)), 146.5 (C5), 133.7 (CAr), 133.4 (CAr), 133.0 (CAr), 131.2 (C4), 129.8 (CAr), 129.7 (CAr), 129.4 (CAr), 128.8 (2C, CAr), 128.5 (CAr), 128.4 (CAr), 128.3 (CAr), 117.4 (C4), 89.2 (C1′), 80.7 (C4′), 72.4 (C2′), 63.8 (C5′), 30.3 (C(CH3)3), 30.1 (C(CH3)3); MS (ESI): m/z [M+Na]+ calcd for C32H31N3NaO7: 592.6, found: 592.0.
5.3.8. 2′,3′,5′-Tri-O-benzoyl-1′-[5-O-ethoxy-[1,2,3]triazol-1-yl]ribofuranose (10h)
Prepared from compound 8 with the typical procedure described before to give 10h (see Table 1) as a brown oil. IR: 1720, 1577, 1451, 1261, 1091, 1026, 705 cm−1; 1H NMR (CDCl3): δ 8.07–7.98 (m, 4H, HAr), 7.96–7.90 (m, 2H, HAr), 7.55 (m, 3H, HAr), 7.44–7.31 (m, 6H, HAr), 7.07 (s, 1H, H4), 6.34 (dd, J=5.3, 2.4 Hz, 1H, H2′), 6.30–6.25 (m, 2H, H3′ and H1′), 4.88–4.82 (m, 1H, H4′), 4.72 (dd, J=12.0, 4.1 Hz, 1H, H5′), 4.60 (dd, J=12.0, 5.4 Hz, 1H, H5′), 4.23–4.16 (dq, J=7.1, 1.4 Hz, 2H, OCH2CH3), 1.46 (t, J=7.1 Hz, 3H, OCH2CH3); 13C NMR (CDCl3): δ 166.2 (C(O)), 165.0 (2C, C(O)), 152.0 (C5), 133.7 (CAr), 133.5 (CAr), 133.1 (CAr), 129.8 (CAr), 129.7 (CAr), 129.4 (CAr), 128.7 (2C, CAr), 128.5 (CAr), 128.4 (CAr), 128.3 (CAr), 113.8 (C4), 86.7, 80.2, 74.5, 71.8, 69.3, 63.9, 14.4; MS (ESI): m/z [M+Na]+ calcd for C30H27N3NaO8: 580.6, found: 580.5.
5.4. General procedure for sugar deprotection
To the protected compounds (9a–h and 10a–h) was added a solution of 7 N NH3/MeOH. The reaction was followed by TLC. After evaporation of the solvent under reduced pressure, the crude product was purified by liquid chromatography on silica gel (methanol/ethyl acetate, 1:9, v/v) to give the desired pure product.
5.4.1. 1′-[4-Phenyl-[1,2,3]triazol-1-yl]ribofuranose (12a)
Prepared from compound 9a with the typical procedure described above to give 12a (>98%) as an oil. IR: 3368, 1661, 1447, 1191, 1135, 799, 723 cm−1; 1H NMR (CD3OD): δ 8.58 (s, 1H, H5), 7.82 (d, J=8.3 Hz, 2H, HAr), 7.43 (t, J=7.5 Hz, 2H, HAr), 7.35 (t, J=7.4 Hz, 1H, HAr), 6.09 (d, J=4.0 Hz, 1H, H1′), 4.57 (t, J=4.5 Hz, 1H, H2′), 4.37 (t, J=5.1 Hz, 1H, H3′), 4.17 (m, 1H, H4′), 3.86 (dd, J=12.3, 3.2 Hz, 1H, H5′), 3.73 (dd, J=12.3, 4.2 Hz, 1H, H5′); 13C NMR (CD3OD): δ 149.0 (C4), 131.6 (CAr), 130.0 (CAr), 129.5 (CAr), 126.7 (CAr), 120.9 (C5), 94.5 (C1′), 87.2 (C4′), 77.1 (C2′), 71.9 (C3′), 62.8 (C5′); HRMS (ESI): m/z [M+H]+ calcd for C13H16N3NO4: 278.1141, found: 278.1148. Known product CAS: 26295-54-5.
5.4.2. 1′-[4–4-Fluoro-3-methylphenyl-[1,2,3]triazol-1-yl]ribofuranose (12b)
Prepared from compound 9b with the typical procedure described above to give 121b (>98%) as white solid. Mp: 158 °C (MeOH); IR: 3344, 2929, 1493, 1233, 1082, 1043, 817 cm−1; UV (MeOH) λmax: 241 nm; −76.4 (c 1.45, MeOH); 1H NMR (CD3OD): δ 8.53 (s, 1H, H5), 7.71 (d, J=7.3 Hz, 1H, HAr), 7.67–7.62 (m, 1H, HAr), 7.10 (t, J=9.1 Hz, 1H, HAr), 6.08 (d, J=4.0 Hz, 1H, H1′), 4.55 (t, J=4.5 Hz, 1H, H2′), 4.35 (t, J=5.0 Hz, 1H, H3′), 4.18–4.13 (m, 1H, H4′), 3.85 (dd, J=12.2, 3.2 Hz, 1H, H5′), 3.71 (dd, J=12.2, 4.2 Hz, 1H, H5′), 2.32 (s, 3H, CH3); 13C NMR (CDCl3): δ 148.3 (C4), 130.0 (CAr), 126.1 (CAr), 120.7 (C5), 116.6 (CAr), 116.4 (CAr), 94.6 (C1′), 87.3 (C4′), 77.2 (C2′), 71.9 (C3′), 62.9 (C5′), 14.6 (CH3); HRMS (ESI): m/z [M+H]+ calcd for C14H17FN3O4: 310.1203, found: 310.1218.
5.4.3. 1′-[4-Methylbenzen-[1,2,3]triazol-1-yl]ribofuranose (12c)
Prepared from compound 9c with the typical procedure described above to give 12c (>98%) as white solid. Mp: 100 °C (MeOH); IR: 3355, 2928, 2360, 1454, 1230, 1047, 725 cm−1; UV (MeOH) λmax: 212 nm; −58.0 (c 0.59, MeOH); 1H NMR (CD3OD): δ 7.97 (s, 1H, H5), 7.30–7.16 (m, 5H, HAr), 6.00 (d, J=4.1 Hz, 1H, H1′), 4.48 (t, J=4.6 Hz, 1H, H2′), 4.30 (t, J=5.0 Hz, 1H, H3′), 4.14–4.09 (m, 1H, H4′), 4.04 (s, 2H, CH2Ph), 3.79 (dd, J=12.2, 3.2 Hz, 1H, H5′), 3.67 (dd, J=12.2, 4.3 Hz, 1H, H5′); 13C NMR (CD3OD): δ 148.6 (C4), 140.3 (CAr), 129.6 (2C, CAr), 127.5 (CAr), 122.6 (C5), 94.8 (C1′), 87.1 (C4′), 77.0 (C2′), 71.9 (C3′), 62.9 (C5′), 32.6 (CH2Ph); HRMS (ESI): m/z [M+H]+ calcd for C14H18N3O4: 292.1297, found: 292.1283.
5.4.4. 1′-[4-Methylcyclopentyl-[1,2,3]triazol-1-yl]ribofuranose (12d)
Prepared from compound 9d with the typical procedure described above to give 12d (>98%) as a white solid. Mp: 87 °C (MeOH); IR: 3349, 1298, 2868, 1452, 1228, 1048 cm−1; UV (MeOH) λmax: 223 nm; −51.5 (c 1.37, MeOH); 1H NMR (CD3OD): δ 8.00 (s, 1H, H5), 6.00 (d, J=4.0 Hz, 1H, H1′), 4.47 (t, J=4.5 Hz, 1H, H2′), 4.30 (t, J=5.1 Hz, 1H, H3′), 4.15–4.09 (m, 1H, H4′), 3.81 (dd, J=12.2, 3.2 Hz, 1H, H5′), 3.69 (dd, J=12.2, 4.3 Hz, 1H, H5′), 2.70 (d, J=7.4 Hz, 2H, CH2–CHCH2CH2), 2.24–2.12 (m, 1H, CH2–CHCH2CH2), 1.82–1.72 (m, 2H, CH2–CHCH2CH2), 1.71–1.62 (m, 2H, CH2–CHCH2CH2), 1.61–1.51 (m, 2H, CH2–CHCH2CH2), 1.31–1.18 (m, 2H, CH2–CHCH2CH2); 13C NMR (CD3OD): δ 148.9 (C4), 122.2 (C5), 94.3 (C1′), 87.1 (C4′), 77.1 (C2′), 71.9 (C3′), 62.9 (C5′), 41.3 (CH2–CHCH2CH2), 33.4 (CH2–CHCH2CH2), 32.4 (CH2–CHCH2CH2), 26.1 (CH2–CHCH2CH2); HRMS (ESI): m/z [M+H]+ calcd for C13H22N3O4: 284.1610, found: 284.1608.
5.4.5. 1′-[4-Butyl-[1,2,3]triazol-1-yl]ribofuranose (12e)
Prepared from compound 9e with the typical procedure described above to give 12e (>98%) as a white solid. Mp: 105 °C (MeOH); IR: 3316, 2930, 2870, 1456, 1043, 826 cm−1; UV (MeOH) λmax: 223 nm; −50.7 (c 0.56, MeOH); 1H NMR (CD3OD): δ 8.00 (s, 1H, H5), 6.00 (d, J=4.1 Hz, 1H, H1′), 4.48 (t, J=4.5 Hz, 1H, H2′), 4.32 (t, J=5.0 Hz, 1H, H3′), 4.15–4.11 (m, 1H, H4′), 3.82 (dd, J=12.2, 3.2 Hz, 1H, H5′), 3.70 (dd, J=12.2, 4.3 Hz, 1H, H5′), 2.71 (t, J=7.6 Hz, 2H, CH2CH2CH2CH3), 1.70–1.61 (m, 2H, CH2CH2CH2CH3), 1.45–1.34 (m, 2H, CH2CH2CH2CH3), 0.95 (t, J=7.4 Hz, 3H, CH2CH2CH2CH3); 13C NMR (CD3OD): δ 149.4 (C4), 121.8 (C5), 94.3 (C1′), 87.1 (C4′), 77.0 (C2′), 71.9 (C3′), 62.9 (C5′), 32.7 (CH2CH2CH2CH3), 26.0 (CH2CH2CH2CH3), 23.3 (CH2CH2CH2CH3), 14.2 (CH2CH2CH2CH3); HRMS (ESI): m/z [M+Na]+ calcd for C11H19N3NaO4: 280.1273, found: 280.1269.
5.4.6. 1′-[4-(3-Chloropropyl)-[1,2,3]triazol-1-yl]ribofuranose (12f)
Prepared from compound 9f with the typical procedure described above to give 12f (>98%) as colorless crystals. Mp: 91 °C (MeOH); IR: 3316, 2928, 2361, 1546, 1229, 1048 cm−1; UV (MeOH) λmax: 223 nm; −46.7 (c 0.89, MeOH); 1H NMR (CD3OD): δ 8.05 (s, 1H, H5), 6.01 (d, J=4.1 Hz, 1H, H1′), 4.49 (t, J=4.5 Hz, 1H, H2′), 4.31 (t, J=5.0 Hz, 1H, H3′), 4.15–4.11 (m, 1H, H4′), 3.81 (dd, J=12.2, 3.2 Hz, 1H, H5′), 3.69 (dd, J=12.2, 4.3 Hz, 1H, H5′), 3.61 (t, J=6.5 Hz, 2H, CH2CH2CH2Cl), 2.87 (t, J=7.5 Hz, 2H, CH2CH2CH2Cl), 2.17–2.08 (m, 2H, CH2CH2CH2Cl); 13C NMR (CD3OD): δ 147.9 (C4), 122.2 (C5), 94.3 (C1′), 87.1 (C4′), 77.0 (C2′), 71.9 (C3′), 62.9 (C5′), 44.8 (CH2CH2CH2Cl), 33.3 (CH2CH2CH2Cl), 23.6 (CH2CH2CH2Cl); HRMS (ESI): m/z [M+H]+ calcd for C10H17ClN3O4: 278.0908, found: 278.0902.
5.4.7. 1′-[4-tert-Butyl-[1,2,3]triazol-1-yl]ribofuranose (12g)
Prepared from compound 9g with the typical procedure described above to give 12g (>98%) as a white solid. Mp: 114 °C (MeOH); IR: 3355, 2964, 2361, 1461, 1365, 1232, 1104, 1047 cm−1; UV (MeOH) λmax: 221 nm; −55.7 (c 0.88, MeOH); 1H NMR (400 MHz, CD3OD): δ 8.01 (s, 1H, H5), 6.00 (d, J=4.2 Hz, 1H, H1′), 4.49 (t, J=4.6 Hz, 1H, H2′), 4.30 (t, J=5.0 Hz, 1H, H3′), 4.14–4.09 (m, 1H, H4′), 3.82 (dd, J=12.2, 3.2 1H, Hz, H5′), 3.69 (dd, J=12.2, 4.3 Hz, 1H, H5′), 1.34 (s, 9H, C(CH3)3); 13C NMR (101 MHz, CD3OD): δ 158.7 (C4), 119.9 (C5), 94.2 (C1′), 87.1 (C4′), 77.0 (C2′), 71.9 (C3′), 62.9 (C5′), 31.7 (C(CH3)3), 30.7 (C(CH3)3); HRMS (ESI): m/z [M+H]+ calcd for C11H20N3O4: 258.1454, found: 258.1457.
5.4.8. 1′-[4-O-Ethoxy-[1,2,3]triazol-1-yl]ribofuranose (12h)
Prepared from compound 9h with the typical procedure described above to give 12h (>98%) as colorless crystals. Mp: 127 °C (MeOH); IR: 3348, 2927, 2361, 1667, 1571, 1381, 1340, 1045 cm−1; UV (MeOH) λmax: 233 nm; −63.1 (c 1.12, MeOH); 1H NMR (400 MHz, CD3OD): δ 7.71 (s, 1H, H5), 5.91 (d, J=4.1 Hz, 1H, H1′), 4.46 (t, J=4.6 Hz, 1H, H2′), 4.29 (t, J=5.0 Hz, 1H, H3′), 4.17 (q, J=7.1 Hz, 2H, OCH2CH3), 4.13–4.09 (m, 1H, H4′), 3.81 (dd, J=12.2, 3.2 Hz, 1H, H5′), 3.69 (dd, J=12.2, 4.2 Hz, 1H, H5′), 1.38 (t, 3H, J=7.1 Hz, OCH2CH3); 13C NMR (101 MHz, CD3OD): δ 162.4 (C4), 106.4 (C5), 95.0 (C1′), 87.2 (C4′), 76.9 (C2′), 71.9 (C3′), 67.9 (OCH2CH3), 62.8 (C5′), 15.1 (OCH2CH3); HRMS (ESI): m/z [M+Na]+ calcd for C9H15N3NaO5: 268.0909, found: 268.0915.
5.4.9. 1′-[5-Phenyl-[1,2,3]triazol-1-yl]ribofuranose (13a)
Prepared from compound 10a with the typical procedure described above to give 13a (>98%) as a white solid. Mp: 151 °C (MeOH); IR: 3332, 2930, 1486, 1454, 1243, 1058, 765, 699 cm−1; UV (MeOH) λmax: 242 nm; −116.5 (c 0.83, MeOH); 1H NMR (CD3OD): δ 7.84 (s, 1H, H4), 7.64–7.60 (m, 2H, HAr), 7.58–7.52 (m, 3H, HAr), 5.81 (d, J=3.7 Hz, 1H, H1′), 4.91 (dd, J=5.1, 3.7 Hz, 1H, H2′), 4.51 (t, J=5.1 Hz, 1H, H3′), 4.16–4.12 (m, 1H, H4′), 3.80 (dd, J=12.1, 3.9 Hz, 1H, H5′), 3.66 (dd, J=12.1, 5.8 Hz, 1H, H5′); 13C NMR (CD3OD): δ 141.2 (C5), 133.4 (CAr), 131.0 (CAr), 130.3 (2C, CAr), 127.4 (C4), 91.4 (C1′), 87.3 (C4′), 75.9 (C2′), 72.6 (C3′), 63.7 (C5′); HRMS (ESI): m/z [M+H]+ calcd for C13H16N3O4: 278.1141, found: 278.1142.
5.4.10. 1′-[5–4-Fluoro-3-methylphenyl-[1,2,3]triazol-1-yl]ribofuranose (13b)
Prepared from compound 10b with the typical procedure described above to give 13b (>98%) as yellow crystals. Mp: 69 °C (MeOH); IR: 3332, 2929, 1493, 1243, 1124, 1059, 824 cm−1; UV (MeOH) λmax: 243 nm; −91.3 (c 0.67, MeOH); 1H NMR (CD3OD): δ 7.86 (s, 1H, H4), 7.57–7.49 (m, 2H, HAr), 7.30–7.23 (t, J=9.0 Hz, 1H, HAr), 5.83 (d, J=3.7 Hz, 1H, H1′), 4.95 (dd, J=4.9, 3.8 Hz, 1H, H2′), 4.55 (t, J=5.2 Hz, 1H, H3′), 4.22–4.17 (m, 1H, H4′), 3.84 (dd, J=12.1, 3.8 Hz, 1H, H5′), 3.70 (dd, J=12.1, 5.9 Hz, 1H, H5′), 2.40 (d, J=1.8 Hz, 3H, CH3); 13C NMR (CD3OD): δ 164.8 (CAr), 162.4 (CAr), 140.4 (C5), 133.7 (2C, CAr), 133.5 (CAr), 129.9 (CAr), 129.8 (CAr), 127.4 (C4), 127.2 (C4), 123.4 (2C, CAr), 116.9 (CAr), 116.7 (CAr), 91.3 (C1′), 87.4 (C4′), 75.9 (C2′), 72.6 (C3′), 63.7 (C5′), 14.4 (2C, CH3); HRMS (ESI): m/z [M+H]+ calcd for C14H17FN3O4: 310.1203, found: 310.1216.
5.4.11. 1′-[5-Benzyl-[1,2,3]triazol-1-yl]ribofuranose (13c)
Prepared from compound 10c with the typical procedure described above to give 13c (>98%) as an yellow oil. IR: 3341, 2928, 2361, 1455, 1242, 1058, 720. UV (MeOH) λmax: 214 nm; −50.6 (c 0.51, MeOH); 1H NMR (CD3OD): δ 7.38 (s, 1H, H4), 7.36–7.31 (m, 2H, HAr), 7.29–7.21 (m, 3H, HAr), 5.88 (d, J=3.6 Hz, 1H, H1′), 4.82 (dd, J=5.0, 3.6 Hz, 1H, H2′), 4.42 (t, J=5.2 Hz, 1H, H3′), 4.18 (d, J=2.7 Hz, 2H, CH2Ph), 4.13–4.08 (m, 1H, H4′), 3.74 (dd, J=12.1, 3.7 Hz, 1H, H5′), 3.58 (dd, J=12.1, 5.6 Hz, 1H, H5′); 13C NMR (CD3OD): δ 139.8 (C5), 137.8 (CAr), 134.1 (C4), 129.9 (CAr), 129.7 (CAr), 128.2 (CAr), 91.5 (C1′), 87.2 (C4′), 75.7 (C2′), 72.3 (C3′), 63.6 (C5′), 29.6 (CH2Ph); HRMS (ESI): m/z [M+H]+ calcd for C14H18N3O4: 292.1297, found: 292.1298.
5.4.12. 1′-[5-Methylcyclopentyl-[1,2,3]triazol-1-yl]ribofuranose (13d)
Prepared from compound 10d with the typical procedure described above to give 13d (>98%) as an yellow oil. IR: 3315, 2946, 2867, 1449, 1241, 1050, 830 cm−1; UV (MeOH) λmax: 221 nm; −62.1 (c 1.04, MeOH); 1H NMR (CD3OD): δ 7.55 (s, 1H, H4), 5.89 (d, J=4.0 Hz, 1H, H1′), 4.83 (dd, J=5.0, 4.0 Hz, 1H, H2′), 4.43 (t, J=5.0 Hz, 1H, H3′), 4.14–4.10 (m, 1H, H4′), 3.74 (dd, J=12.0, 3.9 Hz, 1H, H5′), 3.60 (dd, J=12.0, 5.6 Hz, 1H, H5′), 2.79 (d, J=7.5 Hz, 2H, CH2–CHCH2CH2), 2.29–2.16 (m, 1H, CH2–CHCH2CH2), 1.88–1.77 (m, 2H, CH2–CHCH2CH2), 1.75–1.64 (m, 2H, CH2–CHCH2CH2), 1.64–1.53 (m, 2H, CH2–CHCH2CH2), 1.31–1.19 (m, 2H, CH2–CHCH2CH2); 13C NMR (CD3OD): δ 140.4 (C5), 133.3 (C4), 91.1 (C1′), 87.4 (C4′), 75.9 (C2′), 72.5 (C3′), 63.6 (C5′), 40.3 (CH2–CHCH2CH2), 33.5 (CH2–CHCH2CH2), 29.7 (CH2–CHCH2CH2), 26.0 (CH2–CHCH2CH2); HRMS (ESI): m/z [M+H]+ calcd for C13H22N3O4: 284.1610, found: 284.1600.
5.4.13. 1′-[5-Butyl-[1,2,3]triazol-1-yl]ribofuranose (13e)
Prepared from compound 10e with the typical procedure described above to give 13e (>98%) as an yellow oil. IR: 3331, 2932, 2871, 1428, 1243, 1056, 832 cm−1; UV (MeOH) λmax: 221 nm; −64.4 (c 0.70, MeOH); 1H NMR (CD3OD): δ 7.53 (s, 1H, H4), 5.88 (d, J=3.9 Hz, 1H, H1′), 4.84 (dd, J=5.0, 3.9 Hz, 1H, H2′), 4.33 (t, J=5.0 Hz, 1H, H3′), 4.14–4.11 (m, 1H, H4′), 3.74 (dd, J=12.1, 3.8 Hz, 1H, H5′), 3.59 (dd, J=12.1, 5.6 Hz, 1H, H5′), 2.79 (t, J=7.8 Hz, 2H, CH2CH2CH2CH3), 1.73–1.64 (m, 2H, CH2CH2CH2CH3), 1.48–1.39 (m, 2H, CH2CH2CH2CH3), 0.98 (t, J=7.4 Hz, 3H, CH2CH2CH2CH3); 13C NMR (CD3OD): δ 140.9 (C5), 132.9 (C4), 91.1 (C1′), 87.3 (C4′), 75.8 (C2′), 72.5 (C3′), 63.7 (C5′), 31.7 (CH2CH2CH2CH3), 23.3 (2C, CH2CH2CH2CH3 and CH2CH2CH2CH3), 14.0 (CH2CH2CH2CH3); HRMS (ESI): m/z [M+H]+ calcd for C11H20N3O4: 258.1454, found: 2258.1448.
5.4.14. 1′-[5-(3-Chloropropyl)-[1,2,3]triazol-1-yl]ribofuranose (13f)
Prepared from compound 10f with the typical procedure described above to give 13f (>98%) as an yellow oil. IR: 3315, 2927, 2360, 1717, 1446, 1276, 1242, 1058, 833 cm−1; UV (MeOH) λmax: 220 nm; −50.5 (c 0.39, MeOH); 1H NMR (CD3OD): δ 7.59 (s, 1H, H4), 5.91 (d, J=3.9 Hz, 1H, H1′), 4.88 (dd, J=5.0, 3.9 Hz, 1H, H2′), 4.43 (t, J=5.0 Hz, 1H, H3′), 4.15–4.10 (m, 1H, H4′), 3.73 (dd, J=12.1, 3.8 Hz, 1H, H5′), 3.67–3.60 (m, 2H, CH2CH2CH2Cl), 3.58 (dd, J=12.1, 5.6 Hz, 1H, H5′), 3.01–2.95 (t, J=7.6 Hz, 2H, CH2CH2CH2Cl), 2.20–2.11 (m, 2H, CH2CH2CH2Cl); 13C NMR (CD3OD): δ 139.5 (C5), 133.2 (C4 ), 91.2 (C1′), 87.3 (C4′), 75.7 (C2′), 72.4 (C3′), 63.6 (C5′), 44.6 (CH2CH2CH2Cl), 32.4 (CH2CH2CH2Cl), 21.0 (CH2CH2CH2Cl); HRMS (ESI): m/z [M+H]+ calcd for C10H17ClN3O4: 278.0908, found: 278.0892.
5.4.15. 1′-[5-tert-Butyl-[1,2,3]triazol-1-yl]ribofuranose (13g)
Prepared from compound 10g with the typical procedure described above to give 13g (>98%) as an yellow oil. IR: 3346, 2969, 2360, 1371, 1057 cm−1; UV (MeOH) λmax: 221 nm; −70.9 (c 1.21, MeOH); 1H NMR (CD3OD): δ 7.49 (s, 1H, H4), 6.20 (d, J=3.8 Hz, 1H, H1′), 4.85 (dd, J=5.0, 3.8 Hz, 1H, H2′), 4.47 (t, J=5.1 Hz, 1H, H3′), 4.16–4.08 (m, 1H, H4′), 3.76 (dd, J=12.0, 4.0 Hz, 1H, H5′), 3.64 (dd, J=12.0, 5.7 Hz, 1H, H5′), 1.44 (d, J=6.2 Hz, 9H, C(CH3)3); 13C NMR (CD3OD): δ 148.7 (C5), 131.5 (C4), 92.4 (C1′), 87.4 (C4′), 76.5 (C2′), 72.6 (C3′), 63.8 (C5′), 31.4 (C(CH3)3), 30.5 (C(CH3)3); HRMS (ESI): m/z [M+H]+ calcd for C11H20N3O4: 258.1454, found: 258.1451.
5.4.16. 1′-[5-O-Ethoxy-[1,2,3]triazol-1-yl]ribofuranose (13h)
Prepared from compound 10h with the typical procedure described above to give 13h (>98%) as a white solid (MeOH). Mp: 149 °C; IR: 3357, 2936, 1579, 1458, 1325, 1056, 982 cm−1; UV (MeOH) λmax: 231 nm; −56.2 (c 0.55, MeOH); 1H NMR (CD3OD): δ 7.24 (s, 1H, H4), 5.83 (d, J=3.9 Hz, 1H, H1′), 4.68 (dd, J=5.0, 3.9 Hz, 1H, H2′), 4.39 (t, J=5.0 Hz, 1H, H3′), 4.25 (q, J=7.5 Hz, 2H, OCH2CH3), 4.10–4.05 (m, 1H, H4′), 3.75 (dd, J=12.0, 3.9 Hz, 1H, H5′), 3.62 (dd, J=12.0, 5.8 Hz, 1H, H5′), 1.45 (t, J=7.5 Hz, 3H, OCH2CH3); 13C NMR (CD3OD): δ 153.9 (C5), 114.8 (C4), 90.9 (C1′), 86.9 (C4′), 75.2 (C2′), 72.3 (C3′), 70.6 (OCH2CH3), 63.7 (C5′), 14.8 (OCH2CH3); HRMS (ESI): m/z [M+Na]+ calcd for C9H15N3NaO5: 268.0909, found: 268.0909.
Acknowledgements
This work was supported by an ‘ANR-05-BLAN-0368–03’ grant (LAA), NIH CFAR grant 5P30-AI-50409 (RFS) and the Department of Veterans Affairs (RFS).
References and notes
- 1.(a) Simons C Nucleoside Mimetics: their Chemistry and Biological Properties; Gordon and Breach Science: Amsterdam, 2001; Vol. 3, p 192; [Google Scholar]; (b) Agrofoglio LA; Challand SR Acyclic, Carbocyclic and l-Nucleosides; Kluwer Academic: Dordrecht, 1998; [Google Scholar]; (c) Agrofoglio LA; Amblard F; Broggi J; Garnier E In Modern Approaches to the Synthesis of O- and N-Heterocyles; Kaufman TS, Larghi EL, Eds.; Research Signpost: Trivandrum, India, 2007; Vol. 2, pp 195–246; [Google Scholar]; (e) De Clercq E. Biochem. Pharmacol 2007, 73, 911. [DOI] [PubMed] [Google Scholar]
- 2.(a) Lau JY; Tam RC; Liang TJ; Hong Z Hepatology 2002, 35, 1002; [DOI] [PubMed] [Google Scholar]; (b) Borowski P; Lang M; Niebuhr A; Haag A; Schmitz H; Schulze zur Wiesch J; Choe J; Siwecka MA; Kulikowski T Acta Biochim. Pol 2001, 48, 739; [PubMed] [Google Scholar]; (c) Contreras AM; Hiasa Y; He W; Terella A; Schmidt EV; Chung RT J. Virol 2002, 76, 8505; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Crotty S; Cameron CE; Andino R Proc. Natl. Acad. Sci. U.S.A 2001, 98, 6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Hollecker L; Choo H; Chong Y; Chu CK; Lostia S; McBrayer TR; Stuyver LJ; Mason JC; Du J; Rachakonda S; Shi J; Schinazi RF; Watanabe KA Antiviral Chem. Chemother 2004, 15, 43; [DOI] [PubMed] [Google Scholar]; (b) Stuyver LJ; Whitaker T; McBrayer TR; Hernandez-Santiago BI; Liosta S; Tharnish PM; Ramesh M; Chu CK; Jordan R; Shi J; Rachakonda S; Watanabe KA; Otto MJ; Schinazi RF Antimicrob. Agents Chemother 2003, 47, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Carroll SS; Tomassini JE; Bosserman M; Getty K; Stahlhut MW; Eldrup AB; Bhat B; Hall D; Simcoe AL; LaFemina R; Rutkowski CA; Wolanski B; Yang Z; Migliaccio G; De Franceso R; Kuo LC; MacCoss M; Olsen DB J. Biol. Chem 2003, 278, 11979; [DOI] [PubMed] [Google Scholar]; (b) Eldrup AB; Allerson CR; Bennett CF; Bera S; Bhat B; Bhat N; Bosserman MR; Brooks J; Burlein C; Carroll SS; Cook PD; Getty KL; MacCoss M; McMasters DR; Olsen DB; Prakash TP; Prhavc M; Song Q; Tomassini JE; Xia JJ Med. Chem 2004, 47, 2283. [DOI] [PubMed] [Google Scholar]
- 5.Smith KL; Lai VCH; Prigaro BJ; Ding Y; Gunic E; Girardet J-L; Zhong W; Hong Z; Lang S; An H Bioorg. Med. Chem. Lett 2004, 14, 3517. [DOI] [PubMed] [Google Scholar]
- 6.Wang P; Hollecker L; Pankiewicz KW; Patterson SE; Whitaker T; McBrayer TR; Tharnish PM; Sidwell RW; Stuyver LJ; Otto MJ; Schinazi RF; Watanabe KA J. Med. Chem 2004, 47, 6100. [DOI] [PubMed] [Google Scholar]
- 7.(a) El Akri K; Bougrin K; Balzarini J; Faraj A; Benhida R Bioorg. Med. Chem. Lett 2007, 17, 6656; [DOI] [PubMed] [Google Scholar]; (b) Guezguez R; Bougrin K; El Akri K; Benhida R Tetrahedron Lett. 2006, 47, 4807. [Google Scholar]
- 8.Saito Y; Escuret V; Durantel D; Zoulim F; Schinazi RF; Agrofoglio LA Bioorg. Med. Chem 2003, 11, 3633. [DOI] [PubMed] [Google Scholar]
- 9.(a) Huisgen R Angew. Chem., Int. Ed. Engl 1963, 2, 565; [Google Scholar]; (b) Huisgen R In 1,3-Di-polar Cycloaddition Chemistry; Padwa A, Ed.; Wiley: New York, NY, 1984; Vol. 1. [Google Scholar]
- 10.(a) Mock WL; Irra TA; Wepsiec JP; Manimaran TL J. Org. Chem 1983, 48, 3619; [Google Scholar]; (b) Lewis WG; Green LG; Grynszpan F; Radic Z; Carlier PR; Taylor P; Finn MG; Sharpless KB Angew. Chem., Int. Ed 2002, 41, 1053. [DOI] [PubMed] [Google Scholar]
- 11.(a) Gilchrist TL; Gymer GE Adv. Heterocycl. Chem 1974, 16, 33; [Google Scholar]; (b) Finley T; Montgomery JA The Chemistry of Heterocyclic Compounds. Triazoles 1,2,3; Wiley: New York, NY, 1981; Vol. 39; [Google Scholar]; (c) Katritzky AR; Zhang Y; Singh SK Heterocycles 2003, 60, 1225; [Google Scholar]; (d) Rostovtsev VV; Green LG; Fokin VV; Sharpless KB Angew. Chem., Int. Ed 2002, 41, 2596; [DOI] [PubMed] [Google Scholar]; (e) Wu P; Feldman AK; Nugent AK; Hawker CJ; Scheel A; Voit B; Pyun J; Frechet JM; Sharpless KB; Fokin VV Angew. Chem., Int. Ed 2004, 43, 3928; [DOI] [PubMed] [Google Scholar]; (f) Broggi J; Díez-González S; Petersen JL; Berteina-Raboin S; Nolan SP; Agrofoglio LA Synthesis 2008, 1, 141. [Google Scholar]
- 12.(a) Stimac A; Kobe J Carbohydr. Res 2000, 324, 149; [DOI] [PubMed] [Google Scholar]; (b) Camarasa MJ; Alonso R; De Las Heras FG Carbohydr. Res 1980, 83, 152. [Google Scholar]
- 13.(a) Zhang L; Chen X; Xue P; Sun HHY; Williams ID; Sharpless KB; Fokin VV; Jia GJ Am. Chem. Soc 2005, 127, 15998; [DOI] [PubMed] [Google Scholar]; (b) Majireck MM; Weinreb SM J. Org. Chem 2006, 71, 8680; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Oppilliart S; Mousseau G; Zhang L; Jia G; Thuéry P; Rousseau B; Cintrat J-C Tetrahedron 2007, 63, 8094; [Google Scholar]; (d) Tam A; Arnold U; Soellner MB; Raines RT J. Am. Chem. Soc 2007, 129, 12670; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Imperio D; Pirali T; Galli U; Pagliai F; Cafici L; Canonico PL; Sorba G; Genazzani AA; Tron GC Bioorg. Med. Chem 2007, 15, 6748. [DOI] [PubMed] [Google Scholar]
- 14.Krasinski A; Fokin VV; Sharpless KB Org. Lett 2004, 6, 1237. [DOI] [PubMed] [Google Scholar]
- 15.Coats SJ; Link JS; Gauthier D; Hlasta DJ Org. Lett 2005, 7, 1469. [DOI] [PubMed] [Google Scholar]
- 16.Fokin V; Jia G; Sharpless KB Patent WO 2007041451; CAN; 146:401986. [Google Scholar]
- 17.During the course of our investigations, Kokin et al. reported a RuAAC of aryl azides under microwave irradiation with [Cp*RuCl]4 but under drastic anhydrous conditions and in DMF Rasmussen LK; Boren BC; Fokin VV Org. Lett 2007, 9, 5337. [DOI] [PubMed] [Google Scholar]
- 18.For reviews on microwave-assisted reactions see:; (a) Caddick S Tetrahedron 1995, 51, 10403; [Google Scholar]; (b) Perreux L; Loupy A Tetrahedron 2001, 57, 9199; [Google Scholar]; (c) Loupy A Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, 2002; [Google Scholar]; (d) Kappe CO Angew. Chem., Int. Ed 2004, 43, 6250. [DOI] [PubMed] [Google Scholar]
- 19.Schinazi RF; Peters J; Williams CC; Chance D; Nahmias AJ Antimicrob. Agents Chemother 1982, 22, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schinazi RF; Chou TC; Scott RJ; Yao X; Nahmias AJ Antimicrob. Agents Chemother 1986, 30, 491. [DOI] [PMC free article] [PubMed] [Google Scholar]


