Abstract
Inflammation has been implicated in the mechanisms responsible for preterm and term parturition, as well as fetal injury. Out of all of the suspected causes of preterm labour and delivery, infection and/or inflammation is the only pathological process for which both a firm causal link with preterm birth has been established and a molecular pathophysiology defined. Inflammation has also been implicated in the mechanism of spontaneous parturition at term.
Most cases of histopathological inflammation and histological chorioamnionitis, both in preterm and term labour, are sub-clinical in nature. The isolation of bacteria in the amniotic fluid, known as microbial invasion of the amniotic cavity, is a pathological finding; the frequency of which is dependent upon the clinical presentation and gestational age. There is a window of time during which it may be possible to detect a ‘molecular signature of inflammation’ by analysis of the transcriptome before histological evidence is observed.
This article reviews the role of inflammation in preterm and term parturition. It is possible that modulation of inflammation using anti-inflammatory cytokines, corticoids, antioxidants and/or other factors may complement antibiotic therapy and limit fetal injury.
Keywords: Cytokines, Preterm Premature Rupture of Membranes, Microbial Invasion of the Amniotic Cavity, Fetal Inflammatory Response Syndrome, Innate Immunity, Chemokines
Introduction
Inflammation has been implicated in the mechanisms responsible for preterm and term parturition, as well as fetal injury. Of all suspected causes of preterm labor (PTL) and delivery, infection and/or inflammation is the only pathologic process for which both a firm causal link with preterm birth has been established and a molecular pathophysiology defined.1 Inflammation has also been implicated in the mechanism of spontaneous parturition at term. This article will review the role of inflammation in preterm and term parturition.
The spectrum of inflammation: clinical, histopathological and molecular definitions
Clinical inflammation has been classically defined by heat, pain, redness, swelling and impaired function, which reflect the effects of inflammatory mediators on local blood vessels and tissues. In contrast, histologic inflammation, the gold standard for diagnosis, is defined by the infiltration of tissue by neutrophils, macrophages, and lymphocytes. Chemotactic signals must be present for white blood cells (WBC) to migrate to the site of injury/infection. Thus, there is a window of time in which it may be possible to detect a “molecular signature of inflammation” by analysis of the transcriptome (below)2 before histologic evidence is observed.
There is a common misconception that the absence of systemic clinical signs, such as fever, leukocytosis, etc., makes inflammation/infection unlikely. In reality, the evidence suggests that the opposite holds true. Most cases of histopathological inflammation and chorioamnionitis, both in preterm3,4 and term labor (Yoon and Romero, unpublished observations), are sub-clinical.
Inflammation accomplishes three main goals: 1) to deliver cells and molecules to suppress infection; 2) to generate a physical barrier to the spread of infection; and 3) to promote repair of injured tissue.
Cells recruited to the site of injury release molecules during the course of inflammation including antimicrobial peptides, cytokines, and other inflammatory mediators. Some of these molecules alter macrophage and neutrophil activation so that microbial killing is enhanced. A second major goal of inflammation is to prevent the spread of microorganisms, and this is often accomplished by activation of the coagulation system and formation of thrombi in blood vessels draining the site.
Injury can be the result of exposure to microorganisms or non-microbial-related insults. The immune system has evolved to identify non-self using pattern recognition receptors (PRR), which identify patterns of molecular structure common to most microorganisms. It is now realized that PRR can also be used to identify “danger signals.” The basic premise of the “danger model” is that the immune system is more concerned with damage than with foreignness (“non-self”), and that an immune reaction is initiated by “alarm signals” from injured tissues, rather than by recognition of non-self.
Inflammation is part of the innate immune response which acts immediately, is non-specific, and lacks memory. Inflammation maintains tissue homeostasis; however, exaggerated inflammation or lack of a response can lead to disease.
Infection as a cause of premature labor
Evidence of causality
Infection is a frequent and important mechanism of disease in PTL and delivery.5;6 The evidence in support of this includes: 1) intrauterine infection or systemic administration of microbial products to pregnant animals can result in PTL and delivery;7–10 2) extrauterine maternal infections, such as pyelonephritis, have been associated with premature parturition; 3) sub-clinical intrauterine infections are associated with PTL and delivery;11 4) patients with intraamniotic infection17;18 or inflammation (defined as an elevation of amniotic fluid (AF) concentrations of cytokines12) in the midtrimester are at risk for subsequent preterm delivery; 5) antibiotic treatment of ascending intrauterine infections can prevent prematurity in experimental models of chorioamnionitis;13 and 6) treatment of asymptomatic bacteriuria prevents prematurity.
The frequency and clinical significance of intrauterine infection
Intrauterine infections caused by bacteria are considered to be the leading cause of infection-associated preterm birth. Less than 1% of AF of women not in labor at term will contain bacteria. Therefore, isolation of bacteria in AF is a pathologic finding, defined as microbial invasion of the amniotic cavity (MIAC). Most of these infections are sub-clinical and are detected without AF analysis. The frequency of MIAC depends upon the clinical presentation and gestational age. MIAC occurs in 9% of asymptomatic patients with a short cervix (<25mm sonographically) in the mid-trimester.14 In patients with PTL with intact membranes, the rate of positive (+) cultures is 12.8%.6 However, among patients who have PTL with intact membranes and deliver preterm, the frequency is 22%. Among women with preterm premature rupture of membranes (PROM), the rate of +AF cultures at admission is 32.4%;6 however, at the time of the onset of labor, as many as 75% of patients will have MIAC,15 suggesting that microbial invasion occurs during the latency period. An interesting and consistent observation is that the lower the gestational age at presentation of PTL, the higher the frequency of +AF cultures.16
Microbiology of intrauterine infection
The most common microorganisms found in the amniotic cavity are genital Mycoplasma species, in particular, Ureaplasma urealyticum. However, other microorganisms found include Mycoplasma hominis, Streptococcus agalactiae, Escherichia coli, Fusobacterium species, and Gardnerella vaginalis.
Significance of MIAC detected by molecular microbiology techniques
The prevalence of MIAC is based on the results of standard microbiologic methods (i.e., cultivation), dependent upon culture conditions and whether growth requirements of microorganisms are known. Of note, only 1% of the microbial world can be detected by cultivation (“the great plate count anomaly”). Consequently, the frequency of MIAC reported represents minimum estimates. In fact, several investigators have demonstrated that the prevalence of MIAC is higher when molecular techniques are used to detect conserved sequences in prokaryotes.17;18
Patients with a positive polymerase chain reaction (+PCR) for U. urealyticum but negative cultures have similar adverse outcomes as patients with a +AF culture and have worse outcomes than patients with sterile AF and a negative PCR.19;20 Moreover, patients with a +PCR but a negative culture have the same degree of inflammation (AF IL-6, histologic chorioamnionitis/funisitis) as those with a +AF culture.20 Collectively, this evidence suggests that the presence of microbial footprints detected by PCR is associated with adverse outcomes.
Microorganisms in the chorioamniotic membranes – always pathological?
The amniotic cavity is normally considered sterile for bacteria, even with molecular techniques. In contrast, fluorescent in situ hybridization with a DNA probe specific for bacterial DNA has detected bacteria in the membranes of up to 70% of women undergoing term elective cesarean delivery.21 Bacteria are often present in membranes of patients with PTL and intact membranes, as well as patients with preterm PROM.21 These findings suggest that the presence of bacteria alone is not sufficient to cause PTL and delivery, and that microbial colonization of chorioamniotic membranes may not always elicit a fetal or maternal inflammatory response.
MIAC as a chronic process
Although chorioamnionitis is traditionally considered an acute process, evidence that MIAC exists for an extended period is mounting. Multiple observations suggest that microbial invasion could be clinically silent in the midtrimester and that pregnancy loss/preterm delivery could take weeks to occur.22
Pathways of intra-amniotic infection
Microorganisms may gain access to the amniotic cavity and fetus using any of the following pathways: 1) ascending from the vagina/cervix, (most commonly); 2) hematogenous dissemination through the placenta; 3) retrograde seeding from the peritoneal cavity; and 4) accidental introduction during invasive procedures (Figure 1).23
Figure 1. The ascending route of intrauterine infection.

(I) Vaginal infection; (II) Inflammation of fetal membranes; (III) Microbial invasion of the amniotic cavity; and (IV) Fetal inflammation.24
There is some evidence to support a relationship between periodontal disease and PTL and delivery.24 The mechanism underlying this association is not definitively established; however, microorganisms found in the gingival crevice can be isolated from AF, suggesting that maternal bacteremia and transplacental passage could account for some of these infections (see below).
Microbial products in the amniotic cavity
The adverse events associated with microbial invasion can be due to proliferation of intact microorganisms or bacterial products. For example, the cell walls of Gram-negative bacteria contain lipopolysaccharide (LPS) or endotoxin. Gram-positive bacteria cell walls contain peptidoglycans (PGN) and lipoteichoic acid, while those of Mycoplasma have lipoglycans. Many of the effects of microorganisms are mediated by products, released during bacterial death. Consequently, nonviable bacteria may exert deleterious effects if their products are recognized by PRR and elicit an inflammatory response. Indeed, concentrations of bacterial endotoxin in AF were found to be significantly higher in women with preterm PROM in labor than in women with PROM not in labor (Figure 225).
Figure 2. The concentration of microbial products in women with preterm PROM in labor vs. not in labor.
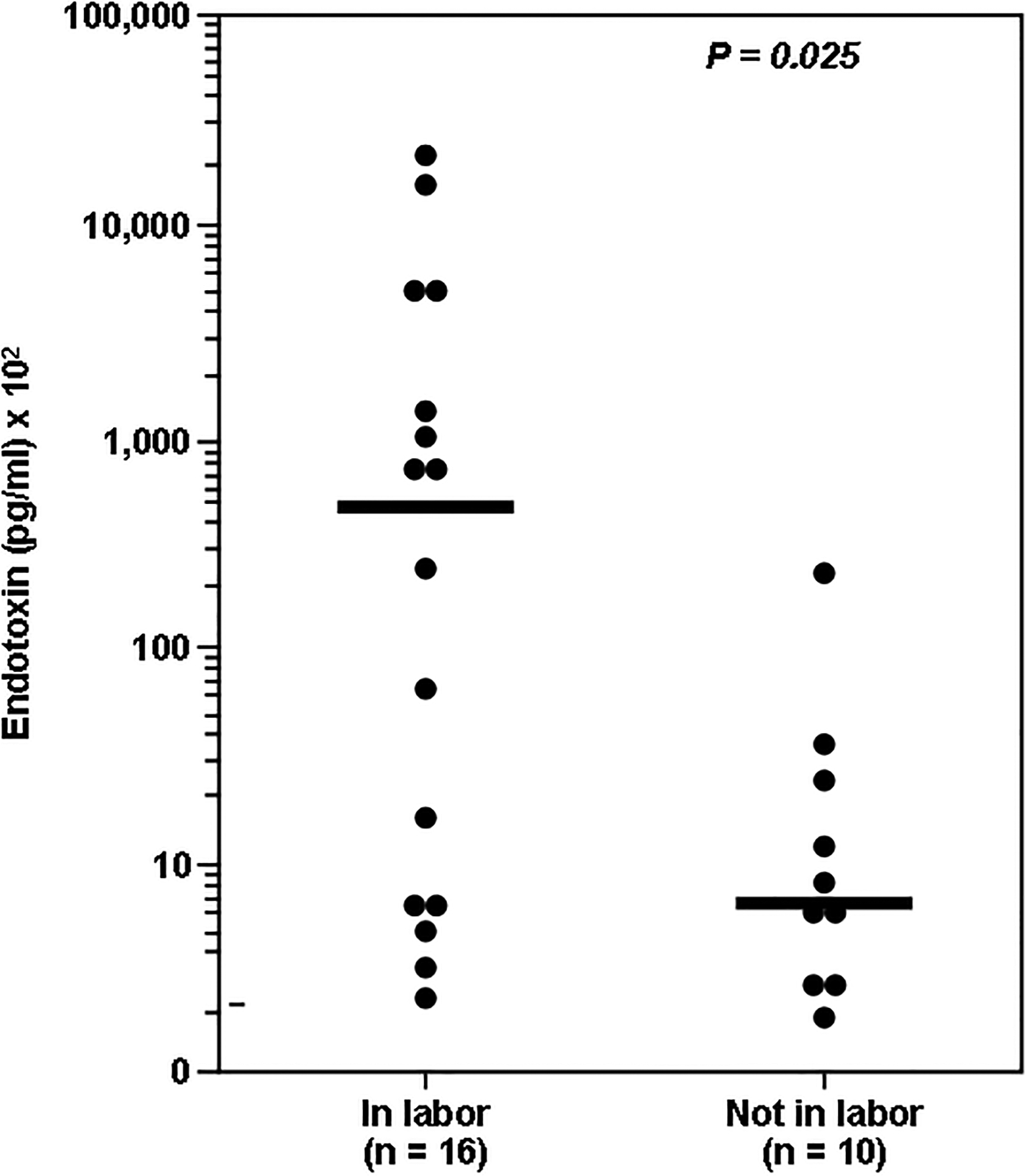
Concentrations of microbial products were significantly higher in women with preterm PROM in labor.25
Detection of viral antigen in AF and adverse pregnancy outcome
Viral antigen is detected in the AF of up to 15% of asymptomatic low-risk pregnancies at the time of genetic amniocentesis26 and 41% of pregnancies with suspected viral infection based on ultrasound abnormalities.27 The most common viral DNA isolates are adenovirus, cytomegalovirus (CMV), and enterovirus. Burguete et al.28 determined the prevalence of adeno-associated parvovirus by PCR analysis in midtrimester AF was 27%. Moreover, it has been found that among structurally normal fetuses, those with a +AF PCR for viral genome were more likely to die in utero and have both a lower gestational age and birth weight.
Inflammation as a mechanism for preterm parturition
An overview of the inflammatory response
Multiple anti-infective mechanisms protect normal pregnancy. Evidence suggests that bacteria cross intact chorioamniotic membranes.29 Epithelium, however, represents more than a physical barrier against microorganisms. Most epithelia produce natural anti-microbial peptides (e.g., defensins, surfactant proteins), which can kill bacteria by damaging their cell membrane or facilitating phagocytosis.
Another mechanism of host defense derives from the metabolic products of bacteria. For example, lactobacilli, which colonize the vagina shortly after birth, produce lactic acid and antimicrobial products.
The innate component of the immune system also provides immediate protection from microbial challenge by recognizing the presence of microorganisms, thus preventing tissue invasion and/or eliciting a host response to limit proliferation/inflammation. One mechanism by which innate immunity recognizes microorganisms is by using PRRs. PRRs, which are classified based on function and sub-cellular localization, include: 1) soluble PRRs, such as “the acute phase proteins” Mannan Binding Lectin and C-Reactive Protein (CRP), which act as opsonins to neutralize and clear pathogens; 2) transmembrane PRRs, which include scavenger receptors, C-type lectins and Toll-like receptors (TLRs); and 3) intracellular PRRs, including Nod1 and Nod2, which mediate recognition of intracellular pathogens. Ten different TLRs, which recognize various ligands such as LPS, PGN, double-stranded RNA, and flagellin, have been recognized.
TLR-1, -2, -3, -5, and -6, have been identified in the epithelia from vagina, ecto- and endocervix, endometrium, and uterine tubes. TLR-4 has only been demonstrated in the endocervix, endometrium, and uterine tubes, but not in vagina and ectocervix. Similarly, trophoblast cells are able to recognize and respond to pathogens through expression of TLRs, which may directly promote trophoblast cell death30 observed in pregnancy complications.
The importance of TLRs in preterm parturition
Mice with a spontaneous mutation for TLR-4 are less likely to deliver preterm after intrauterine inoculation of heat killed bacteria or LPS than wild type.31 Moreover, spontaneous labor at term and preterm delivery with histologic chorioamnionitis, regardless of membrane status, are associated with increased mRNA expression of TLR-2 and TLR-4 in membranes.32 These observations suggest that the innate immune system plays a role in parturition.
The role of pro-inflammatory cytokines (IL-1, TNF-α)
A solid body of evidence indicates that cytokines, play a central role in the mechanisms of inflammation/infection-induced preterm parturition.11;33 Evidence in support of the participation of IL-1 includes: 1) production by human decidua in response to bacterial products; 2) increased concentration and bioactivity in AF of women with PTL and infection;34 3) stimulation of myometrial contractions (Bulletti C, personal communication, 2002); and 4) induction of PTL and delivery by administration to pregnant animals, which was blocked by administration of antagonist.35
Similarly, evidence supporting the role of TNF-α in mechanisms of preterm parturition includes: 1) TNF-α stimulates prostaglandin production by the amnion, decidua, and myometrium; 2) human decidua can produce TNF-α in response to bacterial products;36 3) AF TNF-α bioactivity and immunoreactive concentrations are elevated in women with PTL and intraamniotic infection;36 4) in women with preterm PROM and intraamniotic infection, TNF-α concentrations are higher in the presence of labor;37 5) TNF-α application in the cervix induces changes that resemble cervical ripening;38 and 6) TNF-α can induce preterm parturition when administered systemically to pregnant animals.
Redundancy in the cytokine network
Additional cytokines (interleukins, colony stimulating factors, etc.) and chemokines have also been implicated in the mechanisms of PTL and delivery. With the redundancy of the cytokine network implicated in parturition, a blockade of a single factor is insufficient to prevent preterm delivery in the context of infection.39
Anti-inflammatory cytokines and PTL
IL-10 is thought to be a key cytokine for the maintenance of pregnancy. Indeed, IL-10 production is significantly reduced in term not in labor placenta compared with that from first- and second-trimesters, suggesting that downregulation of IL-10 is a physiologic event favoring an inflammatory state temporal to the onset of labor.40 IL-10 has also been implicated in the control of preterm parturition associated with inflammation. Finally, the administration of IL-10 in animal models of infection has been associated with improved pregnancy outcome.41
Thrombin, inflammation and PTL and delivery
Thrombin, a serine protease, plays a central role in blood coagulation by catalyzing conversion of fibrinogen into fibrin. This enzyme has also been implicated in platelet aggregation, endothelial cell and neutrophil activation, monocyte chemotaxis, lymphocyte proliferation and smooth muscle proliferation/contractility. With these multiple functions, thrombin has been proposed to play a central role in regulation of inflammation and coagulation.
Accumulating evidence supports the role of thrombin in mechanisms responsible for preterm parturition. Evidence in support of this includes: 1) decidua is a source of tissue factor, the primary initiator of coagulation; 2) intrauterine administration of whole blood to pregnant rats stimulates myometrial contractility in a dose-dependent manner, while heparinized blood does not;42 3) thrombin/anti-thrombin (TAT) complexes, a marker of thrombin generation, are increased in plasma43 and AF44 of patients with PTL and preterm PROM; 4) an elevation of plasma TAT complex concentration in the second trimester is associated with subsequent preterm PROM;45 and 5) the presence of retroplacental hematoma detected by first trimester ultrasound or vaginal bleeding in the first or second trimester are associated with adverse pregnancy outcomes.46;47 The mechanisms responsible for increased generation of thrombin in PTL have not been determined.
The fetal inflammatory response syndrome
While the traditional definition of inflammation describes a local phenomenon, it is now recognized that inflammation may be present in circulating blood. Such a state, referred to as the “Systemic Inflammatory Response Syndrome” (SIRS), is characterized by fever, tachycardia, hyperventilation, and an elevated WBC count. These symptoms have been attributed to the effects of pro-inflammatory mediators. In 2001, certain mediators, such as IL-6, were found to be associated with SIRS, an observation that may bring about a new, more specific, definition of SIRS. We defined the fetal counterpart of SIRS, the “Fetal Inflammatory Response Syndrome” (FIRS), in 1997, using the same parameters as those proposed for adults: an elevated fetal blood IL-6 concentration of IL-6 >11 pg/ml.48;49
FIRS was originally described in pregnancies complicated by PTL and preterm PROM. Fetuses with FIRS had a higher rate of severe neonatal morbidity (e.g., respiratory distress syndrome, suspected/proven neonatal sepsis, pneumonia, bronchopulmonary dysplasia, intraventricular hemorrhage, periventricular leukomalacia, or necrotizing enterocolitis)49 and a shorter cordocentesis-to-delivery interval.
Many findings have since been confirmed using umbilical cord blood at birth, including the relationship between pro-inflammatory cytokines and the likelihood of clinical and suspected sepsis. Pathological examination of the umbilical cord is an alternative approach to determine whether fetal inflammation was present before birth. Funisitis and chorionic vasculitis are the histopathologic hallmarks of FIRS.50 Funisitis is associated with endothelial activation, a key mechanism in the development of organ damage; and neonates with funisitis are at increased risk for neonatal sepsis4 and long-term handicaps, such as bronchopulmonary dysplasia and cerebral palsy (CP).51 In addition, since neutrophils in the AF are predominantly of fetal origin, the AF WBC count can be used as an indirect index of fetal inflammation.52 Intra-amniotic inflammation is a risk factor for impending preterm delivery and adverse perinatal outcome in women with preterm PROM, even in the absence of documented intra-amniotic infection.53
Fetal microbial invasion or other insults resulting in FIRS can progress toward multiple organ dysfunction, shock, and perhaps death in the absence of timely delivery. Several fetal organs, including the hematopoietic system, the adrenals, heart, brain, lungs and skin, have been proposed to be target organs during FIRS (Figure 3; Romero R. et al., unpublished results).
Figure 3. Fetal organs proposed to be target organs during the Fetal Inflammatory Response Syndrome (FIRS):

the hematopoietic system, adrenals, heart, brain, lungs and skin (Romero R. et al., unpublished results). CSFs, colony stimulating factors; MMPs, matrix metalloproteinases.
The role of the fetus in the onset of PTL
Among women with preterm PROM, FIRS is associated with the onset of PTL, regardless of the AF inflammatory state (Figure 454). This suggests that the human fetus plays a role in initiating labor; however, maternal cooperation must occur. Thus, it is possible that systemic fetal inflammation may occur in the absence of labor when the inflammatory process does not involve the chorioamniotic membranes and decidua, particularly in the context of hematogenous infections or other disease processes (i.e., alloimmunization).
Figure 4. The association of the Fetal Inflammatory Response Syndrome (FIRS) and preterm labor.
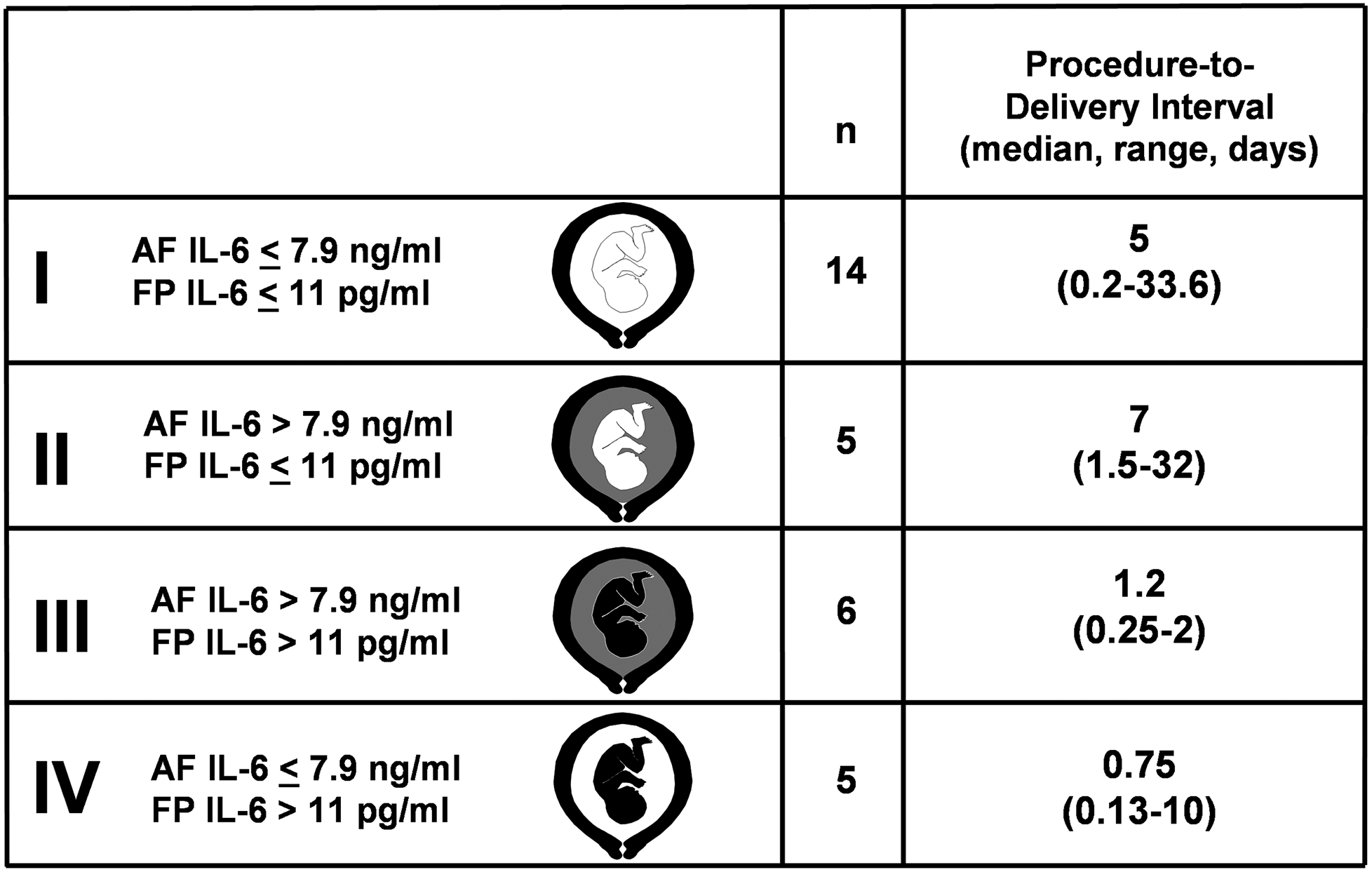
Among women with preterm PROM, FIRS, as measured by fetal serum (FP) concentration of IL-6 > 11 pg/ml, is associated with the impending onset of preterm labor, regardless of the inflammatory state of the amniotic fluid.54
Fetal death and maternal/fetal systemic inflammation
The rate of maternal inflammation is nine times more frequent than that of fetal inflammation in stillbirth. This could have two potential explanations. First, it is possible that fetal infection occurs, but fails to trigger a fetal inflammatory response and labor. In this case, in utero fetal death would represent failure of the host response to infection. Second, inflammation of placental membranes may occur after fetal death and be unrelated to the demise.
Infection in term labor
Microbial invasion of the amniotic cavity may be detected in approximately 18% of the patients in labor at term55 and in 30% of patients with PROM at term.56 Although the microbial isolates are similar to the ones observed in PTL, no difference has been observed in the rates of chorioamnionitis, cervical dilatation, and neonatal outcome between culture-positive and culture-negative patients. The frequency of fetal invasion is extremely low as is the intensity of the cytokine response to microorganisms and their products in the amniotic cavity.55
Inflammation in term labor
Parturition as an inflammatory process
Liggins was the first to liken cervical ripening with an inflammatory response. Since then accumulating evidence supports the idea that inflammation can be detected in the cervix, myometrium, chorioamniotic membranes and amniotic cavity of women in labor. Spontaneous labor at term is associated with infiltration of inflammatory cells in these tissues and increased production of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, and IL-8)33;34 and chemokines (GROα, GCSF, GMCSM, neutrophil attractant/activating peptide-1/IL-8, etc.).33 Recently, using a genome wide screen, we have been able to demonstrate that genes involved in the control of inflammation are upregulated in the chorioamniotic membranes after labor at term, even in the absence of histologic chorioamnionitis. Of interest is that an inflammatory signature was not observed in the maternal circulation of patients with spontaneous labor at term.2 This suggests that an inflammatory process is localized to the membranes. These findings may reflect that spontaneous parturition is associated with increased pro-inflammatory cytokine and chemokine response.33
The Fetal Inflammatory Response Syndrome at term
In the context of MIAC, a fetal cytokine response, as assessed by IL-6 concentrations in umbilical cord plasma, has been demonstrated to be more intense in preterm than term neonates. This has been observed even among neonates born to mothers with a negative AF culture.57 There are two possible explanations for this: 1) the fetal attack rate by microorganisms in the AF is higher in preterm than term gestation; and/or 2) there are differences in the cytokine response to microbial products in these fetuses. Congenital sepsis is more frequent in the preterm than the term neonate, and this may be related to an increased susceptibility to infection by the preterm fetus. The other possibility is that MIAC leading to PTL and preterm PROM may be chronic in nature and prolonged exposure to microorganisms increases the likelihood of fetal infection and response. Indeed, there is evidence that patients with a positive culture for microorganisms at the time of mid-trimester genetic amniocentesis are at increased risk for prematurity and pregnancy loss,23 as are patients with evidence of intra-amniotic inflammation.
Recent observations indicate that funisitis can occur in the absence of clinical chorioamnionitis (Yoon et al. unpublished results). In a study of 832 consecutive patients who delivered term singleton neonates within 72 hours of amniocentesis, the prevalence of funisitis was 4% (30/832). Most patients with funisitis [83% (25/30)] also had histologic chorioamnionitis, suggesting that, when funisitis occurs, a maternal inflammatory response has been deployed. Of note, 17% of fetuses had funisitis without histologic chorioamnionitis. Potential explanations for this include a systemic transplacental infection, which affects the fetus without the membranes. Alternatively a sampling bias may occur if chorioamnionitis does not involve the entire membrane. The first explanation is unlikely, because villitis would be expected in cases of isolated funisitis, which was not observed. In addition, although funisitis was associated with MIAC, histologic chorioamnionitis and intraamniotic inflammation, none of the patients with funisitis (n=30) had clinical chorioamnionitis. Thus, funisitis at term appears to have different clinical significance than preterm funisitis.
A role of the fetus in the onset of labor
Recently, it has been demonstrated that the fetus plays a role in initiation of mouse parturition. Evidence supporting this view includes: 1) intra-amniotic administration of SP-A to mice resulted in labor; 2) treatment with anti-SP-A antibody prolonged gestation; 3) the concentration of SP-A in AF increased with advancing gestation from 17 days postcoitum until term; and 4) AF macrophages migrate to the uterus with increasing AF SP-A. The authors proposed that production of SP-A by fetal lung near term causes activation/migration of fetal macrophages to the maternal uterus, where increased IL-1β leads to labor.58
The maternal systemic inflammatory response in normal pregnancy and PTL and delivery
Normal pregnancy has been proposed to involve physiologic activation of the innate limb of the immune response. Preterm parturition with intact or ruptured membranes is associated with phenotypic and metabolic changes in maternal monocytes and granulocytes that are consistent with the presence of intravascular maternal inflammation.59;60
Conclusions
The evidence reviewed indicates that parturition at term is an inflammatory process. Intraamniotic infection/inflammation is causally linked to preterm parturition, the development of FIRS in a subset of patients, and fetal injury. Moreover, preterm parturition (with intact or ruptured membranes) is associated with a maternal systemic inflammatory response characterized by phenotypic and metabolic changes of maternal monocytes and granulocytes. It is possible that modulation of inflammation with anti-inflammatory cytokines, corticoids, antioxidants, and/or other factors may complement antibiotic therapy and limit fetal injury.
Practice Points.
Of all suspected causes of preterm labor and delivery, infection and/or inflammation is the only pathologic process for which both a firm causal link with preterm birth has been established and a molecular pathophysiology defined.
Most cases of histopathological inflammation and histologic chorioamnionitis, both in preterm and term labor, are sub-clinical in nature.
The immune system has evolved to identify the non-self using pattern recognition receptors, e.g., Toll-like Receptors, which identify patterns of molecular structure common to most microorganisms.
The isolation of bacteria in the amniotic fluid, microbial invasion of the amniotic cavity (MIAC), is a pathologic finding; the frequency of which is dependent upon the clinical presentation and gestational age.
The presence of microbial footprints detected by PCR is associated with adverse outcomes.
The most common pathway which microorganisms use to gain access to the amniotic cavity and fetus is ascending from the vagina/cervix.
Epithelia represent more than a physical barrier against microorganisms by the production of natural anti-microbial peptides (e.g., defensins and surfactant proteins), which can kill bacteria by damaging their cell membrane or facilitating phagocytosis.
A solid body of evidence indicates that cytokines, including IL-1, play a central role in the mechanisms of inflammation/infection-induced preterm parturition.
Thrombin has been proposed to play a role in preterm parturition through the regulation of both inflammation and coagulation.
Funisitis and chorionic vasculitis are the histopathologic hallmarks of FIRS.
Funisitis is associated with endothelial cell activation, a key mechanism in the development of organ damage.
Neonates with funisitis are at increased risk for neonatal sepsis and long-term handicap, such as bronchopulmonary dysplasia and cerebral palsy.
MIAC may be detected in approximately 18% of the patients in labor at term and in 30% of patients with PROM at term. Although the microbial isolates are similar to those observed in PTL, no difference has been observed in the rates of chorioamnionitis, cervical dilatation, and neonatal outcome between culture-positive and culture-negative patients.
The frequency of fetal invasion at term in patients with MIAC is extremely low as is the intensity of the cytokine response to microorganisms and their products in the amniotic cavity.
Research Directions.
Use of a molecular signature for prediction/risk assessment of those patients destined to have preterm labor and preterm premature rupture of membranes.
Enhance detection of sub-clinical infection/inflammation, possibly using transcriptomics/proteomics, since early treatment may improve neonatal/maternal outcomes.
Manipulate innate immune system to improve surveillance/suppress infection, keeping balance with its protective role.
Explore the mechanisms by which epithelia actively inhibit infection and mechanisms to enhance their action/potential.
Manipulate of cytokine balance to promote successful pregnancy.
Elucidate of the fetal role in initiation perpetuation of preterm and term labor and delivery.
Acknowledgement:
This research was supported by the Intramural Program of the National Institute of Child Health and Human Development, NIH, DHHS.
References
- 1.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann.N.Y.Acad.Sci 1994;734:414–29. [DOI] [PubMed] [Google Scholar]
- 2.Haddad R, Tromp G, Kuivaniemi H, et al. Spontaneous labor at term is characterized by a genomic signature of acute inflammation in the chorioamniotic membranes but not in the systemic circulation. Am J Obstet Gynecol 2004;191:S138. [Google Scholar]
- 3.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. American Journal of Obstetrics and Gynecology 2001;185:1130–36. [DOI] [PubMed] [Google Scholar]
- 4.Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am.J.Obstet.Gynecol 2000;183:1124–29. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am.J.Obstet.Gynecol 1989;161:817–24. [DOI] [PubMed] [Google Scholar]
- 6.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment.Retard.Dev.Disabil.Res.Rev 2002;8:3–13. [DOI] [PubMed] [Google Scholar]
- 7.McDuffie RS Jr., Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol 1992;167:1583–88. [DOI] [PubMed] [Google Scholar]
- 8.Fidel PL Jr., Romero R, Wolf N, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994;170:1467–75. [DOI] [PubMed] [Google Scholar]
- 9.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J.Obstet.Gynecol 1994;171:1660–67. [DOI] [PubMed] [Google Scholar]
- 10.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol.Metab 2004;15:479–87. [DOI] [PubMed] [Google Scholar]
- 11.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin.Perinatol 1995;22:281–342. [PubMed] [Google Scholar]
- 12.Romero R, Munoz H, Gomez R, et al. Two thirds of spontaneous abortion/fetal deaths after genetic amniocentesis are the result of a pre-existing sub-clinical inflammatory process of the amniotic cavity. Am.J.Obstet.Gynecol 1995;172:S261. [Google Scholar]
- 13.Fidel P, Ghezzi F, Romero R, et al. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern.Fetal Neonatal Med 2003;14:57–64. [DOI] [PubMed] [Google Scholar]
- 14.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J.Perinat.Med 2006;34:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am.J.Obstet.Gynecol 1988;159:661–66. [DOI] [PubMed] [Google Scholar]
- 16.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet.Gynecol 1992;79:351–57. [DOI] [PubMed] [Google Scholar]
- 17.Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin.Infect.Dis 1997;24:1228–32. [DOI] [PubMed] [Google Scholar]
- 18.Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am.J.Perinatol 2004;21:319–23. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Romero R, Kim M, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am.J.Obstet.Gynecol 2000;183:1130–37. [DOI] [PubMed] [Google Scholar]
- 20.Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am.J.Obstet.Gynecol 2003;189:919–24. [DOI] [PubMed] [Google Scholar]
- 21.Steel JH, O’donoghue K, Kennea NL, Sullivan MH, Edwards AD. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta 2005;26:672–77. [DOI] [PubMed] [Google Scholar]
- 22.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG: An International Journal of Obstetrics and Gynaecology 2002;109:527–33. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Mazor M, Morrotti R, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am.J.Obstet.Gynecol 1992;166:129–33. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Mazor M. Infection and preterm labor. Clin.Obstet.Gynecol 1988;31:553–84. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Roslansky P, Oyarzun E, et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am.J.Obstet.Gynecol 1988;158:1044–49. [DOI] [PubMed] [Google Scholar]
- 26.Wenstrom KD, Andrews WW, Bowles NE, Towbin JA, Hauth JC, Goldenberg RL. Intrauterine viral infection at the time of second trimester genetic amniocentesis. Obstet.Gynecol 1998;92:420–24. [DOI] [PubMed] [Google Scholar]
- 27.Van den Veyver I, Ni J, Bowles N, et al. Detection of intrauterine viral infection using the polymerase chain reaction. Mol.Genet.Metab 1998;63:85–95. [DOI] [PubMed] [Google Scholar]
- 28.Burguete T, Rabreau M, Fontanges-Darriet M, et al. Evidence for infection of the human embryo with adeno-associated virus in pregnancy. Hum.Reprod 1999;14:2396–401. [DOI] [PubMed] [Google Scholar]
- 29.Galask RP, Varner MW, Petzold CR, Wilbur SL. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol 1984;148:915–28. [DOI] [PubMed] [Google Scholar]
- 30.Abrahams VM, Bole-Aldo P, Kim YM, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–96. [DOI] [PubMed] [Google Scholar]
- 31.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YM, Romero R, Chaiworapongsa T, et al. Toll-like receptor-2 and −4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004;191:1346–55. [DOI] [PubMed] [Google Scholar]
- 33.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta 2003;24 Suppl A:S33–S46. [DOI] [PubMed] [Google Scholar]
- 34.Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–23. [DOI] [PubMed] [Google Scholar]
- 35.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J.Obstet.Gynecol 1992;167:1041–45. [DOI] [PubMed] [Google Scholar]
- 36.Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J.Clin.Invest 1989;83:430–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero R, Manogue KR, Mitchell MD, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. American Journal of Obstetrics and Gynecology 1989;161:336–41. [DOI] [PubMed] [Google Scholar]
- 38.Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1[beta] and tumour necrosis factor [alpha] in guinea-pigs. Hum.Reprod 1994;9:2173–81. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch E, Filipovich Y. The IL-1 type I and the TNF type I receptors are essential mediators of bacterially induced preterm labor in a murine model. J Soc Gynecol Inv 2004;11. [Google Scholar]
- 40.Hanna N, Hanna I, Hleb M, et al. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164:5721–28. [DOI] [PubMed] [Google Scholar]
- 41.Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN Jr., Bennett WA. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol 2001;98:476–80. [DOI] [PubMed] [Google Scholar]
- 42.Elovitz MA, Saunders T, Ascher-Landsberg J, Phillippe M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am J.Obstet.Gynecol 2000;183:799–804. [DOI] [PubMed] [Google Scholar]
- 43.Chaiworapongsa T, Espinoza J, Yoshimatsu J, et al. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J.Matern.Fetal Neonatal Med 2002;11:368–73. [DOI] [PubMed] [Google Scholar]
- 44.Gomez R, Athayde N, Pacora P, Mazor M, Yoon BH, Romero R. Increased Thrombin in Intrauterine Inflammation. Am.J.Obstet.Gynecol 1998;178:S62. [Google Scholar]
- 45.Rosen T, Kuczynski E, O’Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern.Fetal Med 2001;10:297–300. [DOI] [PubMed] [Google Scholar]
- 46.Signore CC, Sood AK, Richards DS. Second-trimester vaginal bleeding: correlation of ultrasonographic findings with perinatal outcome. Am.J.Obstet.Gynecol 1998;178:336–40. [DOI] [PubMed] [Google Scholar]
- 47.Williams MA, Mittendorf R, Lieberman E, Monson RR. Adverse infant outcomes associated with first-trimester vaginal bleeding. Obstet.Gynecol 1991;78:14–18. [PubMed] [Google Scholar]
- 48.Gomez R, Ghezzi F, Romero R, et al. Two thirds of human fetuses with microbial invasion of the amniotic cavity have a detectable systemic cytokine response before birth. Am J.Obstet.Gynecol 1997;176:514. [Google Scholar]
- 49.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am.J.Obstet.Gynecol 1998;179:194–202. [DOI] [PubMed] [Google Scholar]
- 50.Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J.Matern.Fetal Neonatal Med 2002;11:18–25. [DOI] [PubMed] [Google Scholar]
- 51.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am.J.Obstet.Gynecol 2000;182:675–81. [DOI] [PubMed] [Google Scholar]
- 52.Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am.J.Obstet.Gynecol 1997;176:77–81. [DOI] [PubMed] [Google Scholar]
- 53.Shim SS, Yoon BH, Romero R, et al. The frequency and clinical significance on intra-amniotic inflammation in patients with preterm premature rupture of the membranes. Am J.Obstet.Gynecol 2003;189:S83. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am.J.Obstet.Gynecol 1998;179:186–93. [DOI] [PubMed] [Google Scholar]
- 55.Romero R, Nores J, Mazor M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod.Med 1993;38:543–48. [PubMed] [Google Scholar]
- 56.Romero R, Mazor M, Morretti R, et al. Infection and labor VII: Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol 1992;166:129. [DOI] [PubMed] [Google Scholar]
- 57.Yoon BH, Romero R, Moon J, et al. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. J.Matern.Fetal Neonatal Med 2003;13:32–38. [DOI] [PubMed] [Google Scholar]
- 58.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc.Natl.Acad.Sci.U.S.A 2004;101:4978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gervasi MT, Chaiworapongsa T, Naccasha N, et al. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol 2001;185:1124–29. [DOI] [PubMed] [Google Scholar]
- 60.Gervasi MT, Chaiworapongsa T, Naccasha N, et al. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern.Fetal Neonatal Med 2002;11:171–75. [DOI] [PubMed] [Google Scholar]


