Abstract
Aims:
Salivary gland intraductal carcinoma (IDC) is a complex ductal neoplasm surrounded by a layer of myoepithelial cells. Recent insights have shown that there are three different types: intercalated duct-like, with frequent NCOA4–RET fusions; apocrine, with salivary duct carcinoma-like mutations; and mixed intercalated duct-like/apocrine, with RET fusions, including TRIM27–RET. In addition, an oncocytic IDC has been described, but it remains unclear whether it represents a fourth variant or simply oncocytic metaplasia of another IDC type. Our aim was to more completely characterize oncocytic IDC.
Methods and results:
Six IDCs with oncocytic changes were retrieved from the authors’ archives, from three men and three women ranging in age from 45 to 75 years (mean, 63 years). Five arose in the parotid gland, with one in an accessory parotid gland. Four patients with follow-up were free of disease after 1–23 months. Several immunostains (S100, mammaglobin, androgen receptor, and p63/p40) and molecular tools (RNA sequencing, RET fluorescence in-situ hybridisation, BRAF V600E VE1 immunohistochemistry, and Sanger sequencing) were applied. Histologically, the tumours were variably cystic with solid intracystic nodules often difficult to recognise as intraductal. In all, tumour ducts were positive for S100 and mammaglobin, negative for androgen receptor, and completely surrounded by myoepithelial cells positive for p63/p40. Molecular analysis revealed TRIM33–RET in two of six cases, NCOA4–RET in one of six cases, and BRAF V600E in two of six cases. One case had no identifiable alterations.
Conclusions:
Oncocytic IDC shares similarities with intercalated duct-like IDC. Although additional verification is needed, the oncocytic variant appears to be sufficiently unique to be now regarded as the fourth distinct subtype of IDC. Because of its indolent nature, oncocytic IDC should be distinguished from histological mimics.
Keywords: BRAF V600E, intraductal carcinoma, NCOA4–RET, salivary gland neoplasms, TRIM33–RET
Introduction
Intraductal carcinoma (IDC) is an uncommon salivary gland neoplasm that has previously been referred to as ‘low-grade salivary duct carcinoma’ and ‘low-grade cribriform cystadenocarcinoma’.1–5 Recent molecular analysis has shown that there are at least three distinctive IDC variants: (i) intercalated duct-like IDC, which is positive for S100 and SOX10 and often has NCOA4–RET fusion; (ii) apocrine IDC, which is negative for S100 and SOX10, is positive for androgen receptor, and has complex genetics, including HRAS and PIK3CA hotspot mutations; and (iii) hybrid or mixed IDC, which has both intercalated duct-like and apocrine features, and often harbours RET fusions, especially TRIM27–RET.6–13 Apocrine IDC may be cytologically low-grade or high-grade, whereas the other two forms are usually low-grade. Regardless of this, when IDC is completely intraductal—i.e. surrounded by myoepithelial cells—it has an excellent prognosis, whereas widely invasive examples without myoepithelial cells are much more aggressive.10–12,14
In 2018, Nakaguro et al. described a form of IDC with prominent oncocytic features.15 Oncocytic metaplasia is common in various salivary gland tumours, so it is currently unclear whether oncocytic IDC represents a variant of one of the well-established forms or a distinct tumour subtype. To answer this question, we performed molecular analyses on a series of oncocytic IDC cases.
Materials and methods
CASE SELECTION
With institutional review board approval (STU 112017-073), we identified cases of IDC with prominent oncocytic features from the authors’ consultation files. Three cases had been previously included in the original Nakaguro et al. series.15 All cases were reviewed by two or more of the authors, and were confirmed to meet the diagnostic criteria for IDC detailed in the 2017 World Health Organization classification of head and neck tumours.5
RNA SEQUENCING
Five of six cases were subjected to targeted RNA sequencing for fusions, as previously described.16 Briefly, whole-slide tissue sections were cut at 10 μm, and Qiagen AllPrep kits (Qiagen, Germantown, MD, USA) were used for RNA isolation. A sequencing library was made by use of a modified TruSight RNA Pan-Cancer kit (Illumina, San Diego, CA, USA) with 1425 genes. Sequencing was performed on the Next-Seq 550 (Illumina) with a minimum of 6 000 000 mapped reads. Fusions were called by use of the Star-Fusion algorithm.17 All fusions were manually reviewed via the Integrated Genomics Viewer (Broad Institute, Cambridge, MA, USA).
IMMUNOHISTOCHEMISTRY (IHC)
We performed IHC for S100 (Ventana Medical Systems, Tucson, AZ, USA), smooth muscle actin (Ventana), androgen receptor (Ventana), mammaglobin (Dako, Glostrup, Denmark), anti-mitochondria (Abcam, Cambridge, UK), anti-BRAF V600E VE1 (Ventana), and either p40 (BioCare Medical, Concord, CA, USA) or p63 (BioCare Medical). Staining was performed, with appropriate controls, on 4-μm whole-slide sections by the use of standardised automated protocols on Ventana BenchMark Ultra autostainers (Ventana).
FLUORESCENCE IN-SITU HYBRIDIZATION (FISH)
For five of six cases, we performed FISH for RET rearrangement according to the manufacturer’s instructions, utilising a dual-colour DNA probe set (Agilent Technologies, Santa Clara, CA, USA) that hybridised to each end of RET in chromosome band 10q11.21. A total of 200 interphase nuclei within areas of tumour were manually enumerated, with separation of the 5′ and 3′ signals in >10% of cells considered to indicate positivity for RET rearrangement.
BRAF MUTATIONAL ANALYSIS
BRAF V600E mutations were detected with polymerase chain reaction (PCR) followed by Sanger sequencing. Briefly, DNA was extracted from unstained slides of the formalin-fixed paraffin-embedded (FFPE) tissue and extracted with a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). PCR products were purified with a QIAquick Spin Kit (Qiagen). Each purified product was directly sequenced by use of a forward primer with a BigDye Terminator v3.1 Cycle Sequencing Kit on an ABI 3730 instrument (Applied Biosystems, Foster City, CA, USA). A mutation analysis was carried out to detect BRAF (exon 15), with the following primers: 5′-TCCTTTACTTACTACACCTCAGAT-3′ (BRAF-Exon15-F), and 5′-AGTGGAAAAATAGCCT-CAAT-3′ (BRAF-Exon15-R).
Results
Six oncocytic IDCs were identified. The clinical information and demographic information for these cases are summarised in Table 1. The tumours arose in three men and three women, ranging in age from 45 to 75 years (mean, 63 years). Each patient presented with a mass or swelling in the parotid region, and underwent surgical resection. One patient also received external beam radiation because of a positive surgical margin. Five of the cases arose in the parotid gland, and one case arose in an accessory parotid gland. The tumours had an average size of 21 mm (range, 8–35 mm). Four patients with follow-up information available had not developed recurrence or metastasis after an average of 14.5 months (range, 1–23 months).
Table 1.
Clinical and demographic information
| Case | Age (years) | Sex | Site | Size (mm) | Follow-up | Reference |
|---|---|---|---|---|---|---|
| 1 | 74 | M | Right parotid gland | 35 | NED at 23 months following surgery | Nakaguro et al.15 |
| 2 | 45 | M | Left accessory parotid gland | 33 | NED at 23 months following surgery | Nakaguro et al.15 |
| 3 | 63 | M | Right parotid gland | 8 | NA | None |
| 4 | 50 | F | Right parotid gland | 18 | NED at 1 month following surgery, plan for radiotherapy because of positive margins | None |
| 5 | 69 | F | Right parotid gland | 20 | NA | None |
| 6 | 75 | F | Right parotid gland | 12 | NED at 11 months following surgery | Nakaguro et al.15 |
F, female; M, male; NA, not available; NED, no evidence of disease.
Histologically, five of six tumours had a prominent cystic tumour component. Whereas one case was entirely cystic and lined by micropapillary to flattened epithelium, the remaining four cystic cases had an intracystic solid nodule at low power (Figure 1A). The cysts were filled with eosinophilic secretions, and were often haemorrhagic. The remaining case was almost entirely solid and lobulated, lacking any macrocystic growth (Figure 1B). In the nodular tumour areas, the IDCs were characterised predominantly by compact collections of solid nodules, punctuated by scattered ductal spaces, and separated by thin strands of fibrosis (Figure 2A,B). Focal cribriform and/or papillary growth, typical of other forms of IDC, was also seen (Figure 2C,D). Pink intraluminal secretions were seen in each case; in one case they calcified, forming psammomatoid calcifications. Myoepithelial cells were difficult to discern on routine histology, especially around the compact, rounded nests in the more solid areas of the tumours. Oncocytic cellular features, defined as granular, eosinophilic cytoplasm and round nuclei with prominent nucleoli, were seen in all cases (Figure 3A). The extent of oncocytic change in the tumours ranged from 40% to 100% (mean, 85%). In the two cases that were not completely oncocytic, the non-oncocytic cellular component resembled intercalated ductal cells: small, cuboidal cells with minimal, pale cytoplasm, and small oval nuclei with open chromatin (Figure 3B). All of the IDCs were histologically low-grade, with no significant pleomorphism, low mitotic rates, and no necrosis. There were no irregular nests or desmoplasia to suggest overt stromal invasion.
Figure 1.
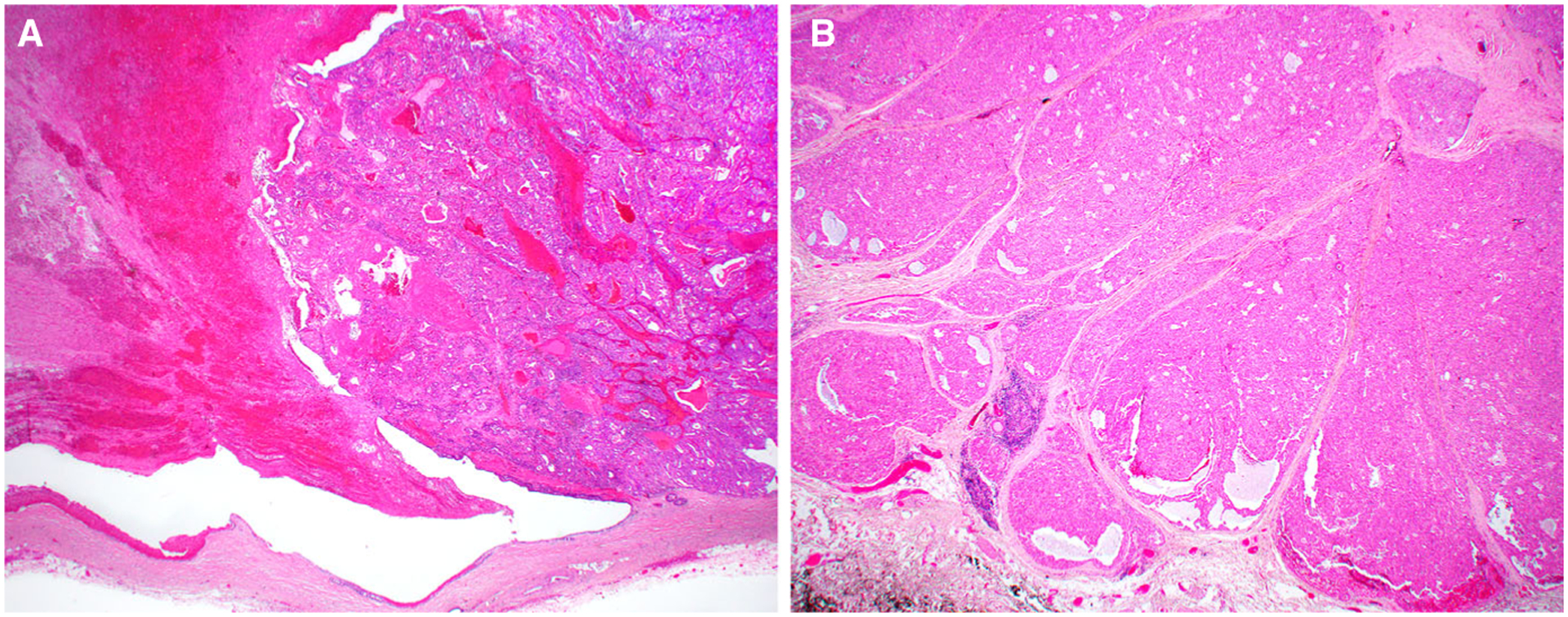
Most oncocytic intraductal carcinomas (case 2 is shown) were partly macrocystic with intracystic secretions and haemorrhage (left), and intracystic solid tumour nodules (right) (A). Case 4, however, had no significant cystic growth and instead appeared as a solid, nodular tumour (B).
Figure 2.
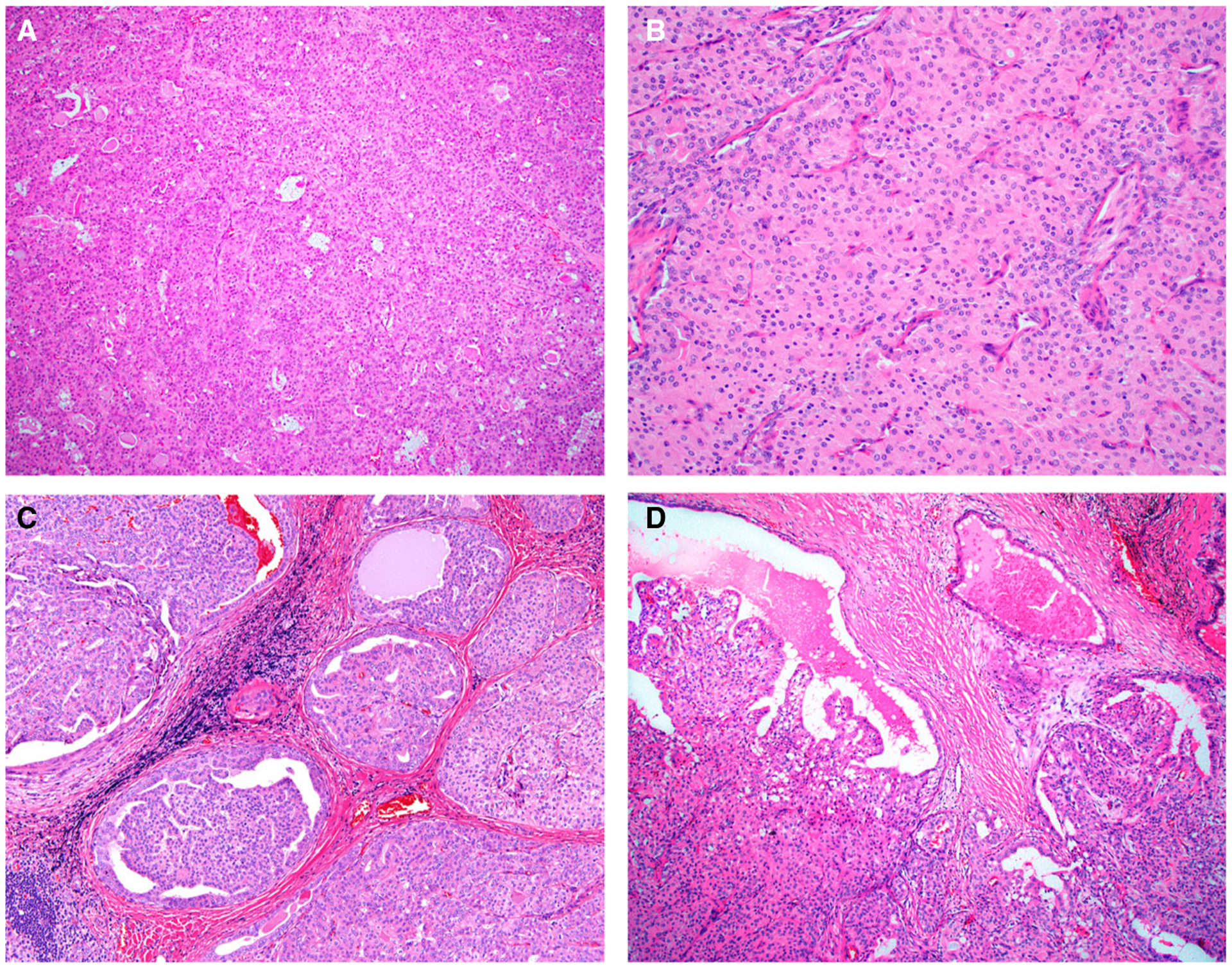
The solid areas of oncocytic intraductal carcinoma (IDC) consisted of back-to-back solid nodules, punctuated by scattered ducts (cases 4 and 1 are shown) (A, B). Most cases also showed areas that were more typical of IDC, with cribriform nodules (case 4 is shown) (C) and papillary cystic growth (case 1 is shown) (D).
Figure 3.
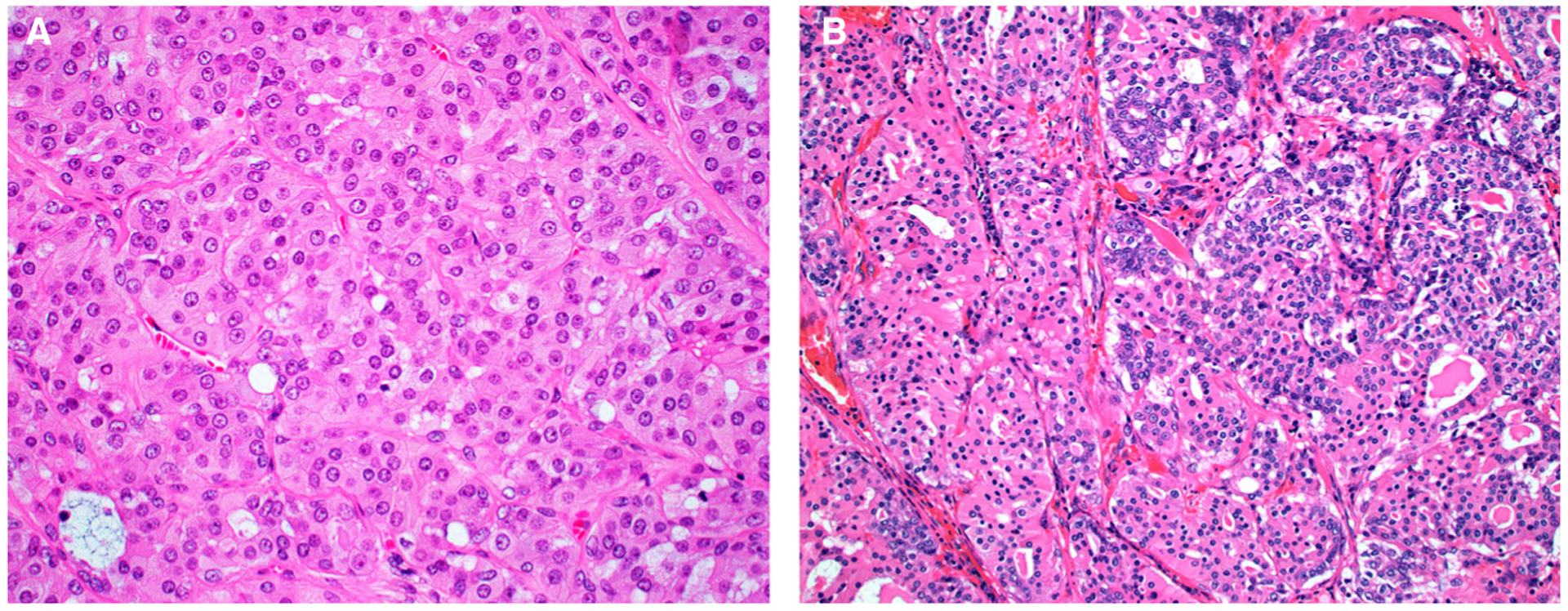
Oncocytes in the oncocytic intraductal carcinomas had abundant, eosinophilic, granular cytoplasm, with round nuclei that have prominent nucleoli (case 4 is shown) (A). Two cases had mixed oncocytic (left) and intercalated duct-like (right) cellular features. The intercalated duct-like cells had a more basophilic appearance, with less cytoplasm and more oval, pale nuclei (case 2 is shown) (B).
The immunohistochemical findings are summarised in Table 2. The oncocytic IDCs were diffusely positive for S100 and mammaglobin (Figure 4A,B), and negative for androgen receptor. In all cases, the proliferative ducts were completely surrounded by a layer of myoepithelial cells that were positive for p63 and/or p40. These myoepithelial cells were present not only around the large cysts and small nests, but also in the back-to-back nests of the solid tumour components (Figure 4C,D). Anti-mitochondria staining was positive in four of four cases tested.
Table 2.
Immunohistochemical testing
| Case | S100 | Mammaglobin | p63/p40 | Androgen receptor | Mitochondria | Molecular alteration |
|---|---|---|---|---|---|---|
| 1 | Diffuse | Diffuse | Peripheral | Negative | Positive | BRAF V600E mutation |
| 2 | Diffuse | Diffuse | Peripheral | Negative | Positive | TRIM33–RET fusion |
| 3 | Diffuse | Diffuse | Peripheral | Negative | Positive | None found |
| 4 | Diffuse | Diffuse | Peripheral | Negative | NA | TRIM33–RET fusion |
| 5 | Diffuse | Diffuse | Peripheral | Negative | NA | NCOA4–RET fusion |
| 6 | Diffuse | Diffuse | Peripheral | Negative | Positive | BRAF V600E mutation |
NA, not available.
Figure 4.
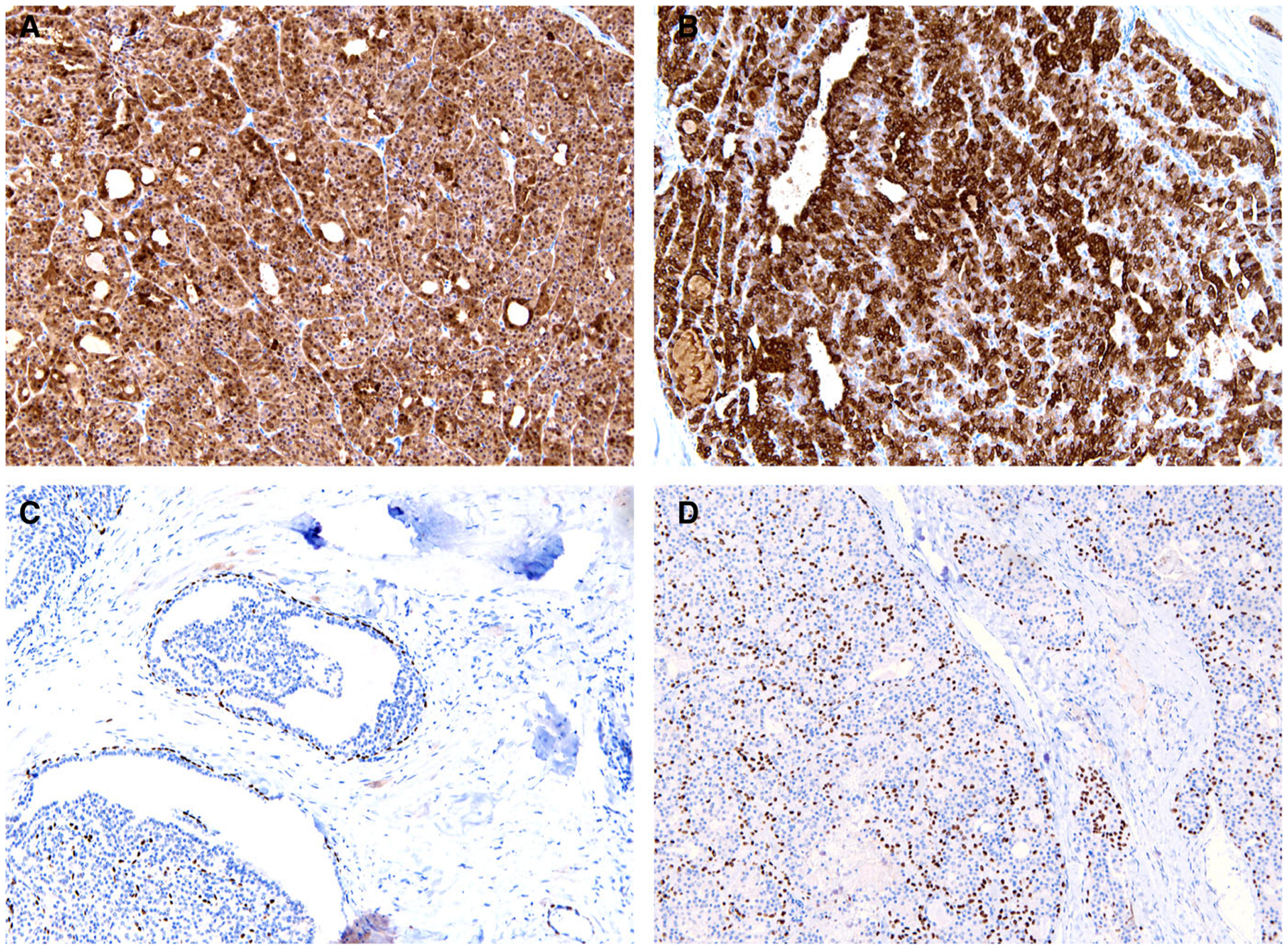
Oncocytic intraductal carcinoma was diffusely positive for S100 (A) and mammaglobin (B). The myoepithelial marker p63 was positive around cysts and cribriform nests (C) and also within the solid tumour components (D). Case 4 is shown.
The molecular findings are summarised in Table 3. RNA sequencing identified a TRIM33–RET fusion in two cases (exon 11 of TRIM33 and exon 12 of RET), with both showing a classic split signal on RET FISH. RNA sequencing also demonstrated NCOA4–RET fusion in one case; FISH was not performed in this case. RNA sequencing incidentally identified a probable BRAF V600E mutation in one of the fusion-negative cases, but because RNA sequencing is not the ideal method for detecting mutations, it was confirmed with IHC and Sanger sequencing (Figure 5). One additional oncocytic IDC with BRAF V600E mutation was subsequently found by the use of IHC and Sanger sequencing. Finally, one case—the one IDC that was highly cystic, with no solid growth—had no detectable genetic alterations. There were no obvious molecular–histological correlations with the identified genetic alterations.
Table 3.
Molecular analysis
| Case | Summary of histological findings | RNA sequencing | RET FISH | BRAF V600E IHC | BRAF V600E Sanger sequencing | Summary of molecular results |
|---|---|---|---|---|---|---|
| 1 | 100% oncocytic; 50% cystic, with solid, cribriform and papillary patterns | No fusions, probable BRAF V600E mutation | Negative | Positive | Positive | BRAF V600E mutation |
| 2 | 40% oncocytic; 60% cystic, with solid, cribriform and tubular patterns | TRIM33–RET fusion | Positive, classic split | ND | ND | TRIM33–RET fusion |
| 3 | 70% oncocytic; 100% cystic, lined by flattened or micropapillary patterns | No fusions | Negative | ND | ND | None found |
| 4 | 100% oncocytic; solid and cribriform patterns, not cystic | TRIM33–RET fusion | Positive, classic split | ND | ND | TRIM33–RET fusion |
| 5 | 100% oncocytic; 70% cystic with cribriform and tubular patterns | NCOA4–RET fusion | ND | ND | ND | NCOA4–RET fusion |
| 6 | 100% oncocytic; 80% cystic with solid and papillary patterns | ND | Negative | Positive | Positive | BRAF V600E mutation |
FISH, fluorescence in-situ hybridisation; IHC, immunohistochemistry; ND, not done.
Figure 5.
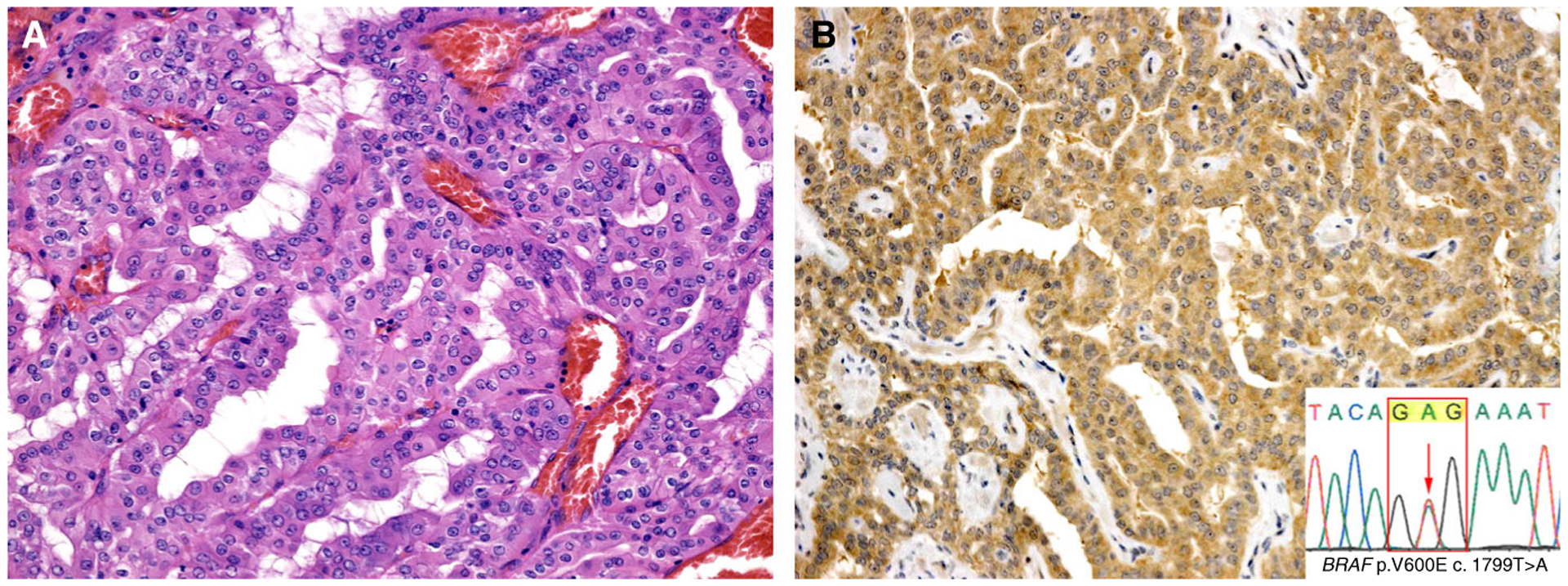
Two cases of oncocytic intraductal carcinoma (A) were positive for anti-BRAF V600E VE1 immunohistochemistry (B), with mutations confirmed by Sanger sequencing (inset). Case 6 is shown.
To summarise the oncocytic IDC molecular results: two cases had TRIM33–RET fusions, two had BRAF V600E mutations, one had NCOA4–RET fusion, and one had no molecular alterations detected.
Discussion
In recent years, molecular testing has considerably altered our understanding of salivary gland tumours in general, and, in particular, the neoplasm known as IDC. These emerging studies have shown that IDC exists in at least three variants, each with unique histological, immunophenotypic and molecular features. Intercalated duct-type IDC is the most common. This variant is diffusely positive for S100 and SOX10, negative for androgen receptor, and has NCOA4–RET fusions in approximately half of reported cases.10–12 Rarely, this variant may have alternative fusions like STRN–ALK, TUT1–ETV5, and KIAA1217–RET, and some cases have been fusion-negative.10,18 The apocrine form of IDC is negative for S100 and SOX10 but strongly positive for androgen receptor. Apocrine IDC has not been found to harbour fusions; instead this variant has a complex mutational profile (e.g. PIK3CA and HRAS mutations, TP53 loss) reminiscent of salivary duct carcinoma.6,8,11,12 The mixed intercalated duct/apocrine form of IDC has hybrid histological and immunophenotypic features of the other IDC types. Like intercalated duct-like IDC, mixed IDC commonly harbours RET fusions, although TRIM27–RET is more common than NCOA4–RET in this variant.9–11 More recently, it has been shown that the myoepithelial cells are probably neoplastic in fusion-positive IDCs, raising interesting questions about the staging and terminology for this enigmatic tumour.19 Indeed, the term ‘intraductal’ may be inaccurate and could be abandoned in future classification schemes. Importantly, all forms of IDC are very indolent when they are low-grade and contain myoepithelial cells.
In 2018, Nakaguro et al. reported a form of low-grade IDC with oncocytic features.15 Although these authors believed that it represented a unique form of IDC, at the time it was difficult to exclude the possibility of oncocytic metaplastic change in intercalated duct-like IDCs.15 After all, oncocytic metaplasia can be seen in almost all salivary gland tumour types, perhaps most notably in the oncocytic variant of mucoepidermoid carcinoma.20,21 In most salivary gland tumours, oncocytic variants are genetically identical to their non-oncocytic counterparts, so we performed genetic analysis to determine whether oncocytic IDC is molecularly distinct from the other three forms of this tumour.
We found that oncocytic IDC has some features that are reminiscent of intercalated duct-type IDC. Both types of IDC predominate in the parotid gland of adults and show indolent behaviour, and they have almost identical immunophenotypes. Moreover, our genetic analysis showed that RET fusions were present in half of the oncocytic IDCs, which is similar to the rate reported for intercalated duct-type IDC, and one case harboured the NCOA4–RET fusion, which is most often seen in that more common variant. The fact that two of six cases had a component of typical intercalated duct-like morphology also supports the notion that these are related tumours.
On the other hand, we also demonstrated that oncocytic IDC is unique in many respects. Aside from the obvious distinctively oncocytic cytomorphology, the compact, solid growth seen in most cases is also unusual. This growth, especially when it is not accompanied by a cystic component, can make it difficult to recognise the intraductal nature of oncocytic IDC. Also, TRIM33–RET is a fusion that has not been previously reported in IDC or any other salivary gland tumour. The fact that it was found in two of six oncocytic IDCs points to the uniqueness of this variant. This relationship is similar to that seen with TRIM27–RET, which is not always found in mixed intercalated duct/apocrine IDCs (occasional cases have NCOA4–RET), but does appear to be specific for that variant. Finally, the BRAF V600E mutation, which was also identified in two oncocytic IDCs, has also never previously been reported in IDCs. Table 4 summarises an updated classification of IDC.
Table 4.
Current classification of salivary gland intraductal carcinoma
| Variant | Frequency | Grade | S100 | Androgen receptor | Genetics | Associated invasion |
|---|---|---|---|---|---|---|
| Intercalated duct | Most common | Low | ++ | − | Usually NCOA4–RET Rare STRN–ALK, TUT1–ETV5, and KIAA1217–RET |
Rare |
| Apocrine | Uncommon | Low or high | − | ++ | Complex, with multiple mutations (HRAS, PIK3CA, and others) No fusions |
Common |
| Mixed intercalated duct/apocrine | Rare | Low | ++ in intercalated duct-like areas | ++ in apocrine areas | Usually TRIM27–RET Occasionally NCOA4–RET |
Rare |
| Oncocytic | Rare | Low | ++ | − |
TRIM33–RET BRAF V600E mutation Occasionally NCOA4–RET |
Not reported |
The finding of two oncocytic IDCs with TRIM33–RET expands the list of salivary gland tumours shown to have RET fusions, which includes not only the intercalated duct and hybrid forms of IDC, but also occasional secretory carcinomas and, possibly, rare salivary duct carcinomas.7,22–24 TRIM33 is located on chromosome 1p13.2. Like the gene product of TRIM27, TRIM33 is a member of the tripartite motif-containing (TRIM) family of proteins with E3 ubiquitin ligase activity, which are involved in numerous crucial biological processes.25 Although TRIM33–RET has never been reported in salivary gland tumours, it has rarely been described as a presumed driver in papillary thyroid carcinoma and lung adenocarcinoma.26,27 We found that TRIM33–RET is positive on break-apart RET FISH, like TRIM27–RET but unlike NCOA4–RET (a subtle inversion that is difficult to visualise), which simplifies testing, as RET FISH is more readily available than next-generation sequencing.
Although they have not previously been seen in IDC, BRAF V600E mutations have been described in salivary gland tumours. Rare cases of salivary duct carcinoma have shown this mutation.28–30 Although this link is intriguing, the lack of high-grade features or androgen receptor positivity in oncocytic IDC makes it unlikely that this tumour is related to salivary duct carcinoma. A rare but distinctive salivary duct tumour known as sialadenoma papilliferum harbours the BRAF V600E mutation in approximately 70–75% of cases.31,32 Like IDC, sialadenoma papilliferum is an indolent, low-grade ductal proliferation that is surrounded by a layer of basal cells. On the other hand, sialadenoma papilliferum almost always occurs in the oral cavity, and has a component of papillary or verrucoid squamous proliferation that is lacking in IDC. Moreover, most sialadenoma papilliferum cases are not oncocytic. Although there is an oncocytic form of sialadenoma papilliferum, this variant, surprisingly, does not actually harbour the BRAF V600E mutation.3 Accordingly, although sialadenoma papilliferum and oncocytic IDC may well belong to a family of low-grade intraductal BRAF-mutated neoplasms, they are distinct from each other.
Although it is not entirely clear whether IDC is a form of carcinoma in situ or has a pushing invasive front, what is evident is that, in their pure form (i.e. with myoepithelial cells and without frank stromal invasion), all variants of IDC are very indolent tumours. Accordingly, they should be distinguished from other salivary gland carcinomas that have an increased capacity for aggressive behaviour. The prominent solid growth in most IDCs may make it difficult to recognise, and more likely to be confused with histological mimics. Secretory carcinoma, in particular, may resemble IDC, with its oncocytic appearance, mixed cystic, papillary and solid growth patterns, and consistent S100 and mammaglobin positivity. Recognising focal areas of more typical IDC growth (isolated, cribriform, or micropapillary nests) is helpful. In addition, although secretory carcinoma may have very focal intraductal growth, it lacks the diffuse network of myoepithelial cells seen in IDC. Another potential pitfall is mistaking oncocytic IDC for epithelial–myoepithelial carcinoma, especially the oncocytic/apocrine variant. Again, identifying typical IDC foci is helpful. Although both tumours have a myoepithelial cell component, the myoepithelial cells of epithelial–myoepithelial carcinoma tend to be much larger and more prominent, and they often have a clear cell appearance. Finally, both secretory carcinoma (ETV6 fusions) and epithelial–myoepithelial carcinoma (frequent HRAS mutations), which are tumours with some morphological and architectural overlap with oncocytic IDC, appear to have discernible genetic differences from IDC.33,34
In summary, oncocytic IDC is a variant of IDC that has unique histological and immunophenotypic features. It may show prominent solid growth and variable genetic alterations (TRIM33–RET and BRAF V600E) that have not previously been described in IDC. As with other salivary tumours, a single distinct molecular profile is not seen. However, much like other reported IDC variants, oncocytic IDC appears to be an indolent tumour. Given the heterogeneous molecular findings and small number of cases, additional cases will be needed for confirmation of these findings.
Funding
This study was funded in part by the Jane B. and Edwin P. Jenevein, MD Endowment for Pathology at UT Southwestern Medical Center.
Footnotes
Conflict of interest
The authors state that they have no conflicts of interest.
Ethics approval and/or informed consent
Institutional review board approval was received (STU 112017–073). Informed content was not required for this study.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Brandwein-Gensler M, Gnepp DR. Low-grade cribriform cystadenocarcinoma. In Barnes L, Eveson JW, Reichart P, Sidransky D eds. Pathology and genetics of head and neck tumours. World Health Organization classification of tumours. Lyon: IARC Press, 2005; 170–171. [Google Scholar]
- 2.Brandwein-Gensler M, Hille J, Wang BY et al. Low-grade salivary duct carcinoma: description of 16 cases. Am. J. Surg. Pathol 2004; 28; 1040–1044. [DOI] [PubMed] [Google Scholar]
- 3.Chen KT. Intraductal carcinoma of the minor salivary gland. J. Laryngol. Otol 1983; 97; 189–191. [DOI] [PubMed] [Google Scholar]
- 4.Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer 1996; 78; 958–967. [DOI] [PubMed] [Google Scholar]
- 5.Loening T, Leivo I, Simpson RHW, Weinreb I. Intraductal carcinoma. In El-Naggar A, Chan JK, Grandis JR, Takata T, Slootweg PJ eds. World Health Organization classification of head and neck tumours. Lyon: IARC Press, 2017; 170–171. [Google Scholar]
- 6.Bishop JA, Gagan J, Krane JF, Jo VY. Low-grade apocrine intraductal carcinoma: expanding the morphologic and molecular spectrum of an enigmatic salivary gland tumor. Head Neck Pathol. 2020; 14; 869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilmette J, Dias-Santagata D, Nose V, Lennerz JK, Sadow PM. Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum. Pathol 2019; 83; 50–58. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh MS, Lee YH, Jin YT, Kuo YJ. Clinicopathological study of intraductal carcinoma of the salivary gland, with emphasis on the apocrine type. Virchows Arch. 2020; 477; 581–592. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Graham RP, Seethala R, Chute D. Intraductal carcinoma of salivary glands harboring TRIM27-RET fusion with mixed low grade and apocrine types. Head Neck Pathol. 2020; 14; 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skalova A, Ptakova N, Santana T et al. NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors: is ‘intraductal’ correct? Am. J. Surg. Pathol 2019; 43; 1303–1313. [DOI] [PubMed] [Google Scholar]
- 11.Skalova A, Vanecek T, Uro-Coste E et al. Molecular profiling of salivary gland intraductal carcinoma revealed a subset of tumors harboring NCOA4-RET and novel TRIM27-RET fusions: a report of 17 cases. Am. J. Surg. Pathol 2018; 42; 1445–1455. [DOI] [PubMed] [Google Scholar]
- 12.Weinreb I, Bishop JA, Chiosea SI et al. Recurrent RET gene rearrangements in intraductal carcinomas of salivary gland. Am. J. Surg. Pathol 2018; 42; 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinreb I, Tabanda-Lichauco R, Van der Kwast T, Perez-Ordonez B. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am. J. Surg. Pathol 2006; 30; 1014–1021. [DOI] [PubMed] [Google Scholar]
- 14.Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology 2008; 53; 416–425. [DOI] [PubMed] [Google Scholar]
- 15.Nakaguro M, Urano M, Suzuki H et al. Low-grade intraductal carcinoma of the salivary gland with prominent oncocytic change: a newly described variant. Histopathology 2018; 73; 314–320. [DOI] [PubMed] [Google Scholar]
- 16.Bishop JA, Gagan J, Baumhoer D et al. Sclerosing polycystic ‘adenosis’ of salivary glands: a neoplasm characterized by PI3K pathway alterations more correctly named sclerosing polycystic adenoma. Head Neck Pathol. 2020; 14; 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas BJ, Dobin A, Li B, Stransky N, Pochet N, Regev A. Accuracy assessment of fusion transcript detection via read-mapping and de novo fusion transcript assembly-based methods. Genome Biol. 2019; 20; 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooper LM, Thompson LDR, Gagan J, Oliai BR, Weinreb I, Bishop JA. Salivary intraductal carcinoma arising within intra-parotid lymph node: a report of 4 cases with identification of a novel STRN-ALK fusion. Head Neck Pathol. 2020. E-pub ahead of print, 13July. 10.1007/s12105-020-01198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop JA, Rooper LM, Sangoi AR, Gagan J, Thompson LDR, Inagaki H. The myoepithelial cells of salivary intercalated duct-type intraductal carcinoma are neoplastic: a study using combined whole-slide imaging, immunofluorescence, and RET fluorescence in situ hybridization. Am. J. Surg. Pathol 2020. E-pub ahead of print, 20October. 10.1097/PAS.0000000000001605. [DOI] [PubMed] [Google Scholar]
- 20.Garcia JJ, Hunt JL, Weinreb I et al. Fluorescence in situ hybridization for detection of MAML2 rearrangements in oncocytic mucoepidermoid carcinomas: utility as a diagnostic test. Hum. Pathol 2011; 42; 2001–2009. [DOI] [PubMed] [Google Scholar]
- 21.Weinreb I, Seethala RR, Perez-Ordonez B, Chetty R, Hoschar AP, Hunt JL. Oncocytic mucoepidermoid carcinoma: clinicopathologic description in a series of 12 cases. Am. J. Surg. Pathol 2009; 33; 409–416. [DOI] [PubMed] [Google Scholar]
- 22.Skalova A Mammary analogue secretory carcinoma of salivary gland origin: an update and expanded morphologic and immunohistochemical spectrum of recently described entity. Head Neck Pathol. 2013; 7(Suppl. 1); S30–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skalova A, Baneckova M, Thompson LDR et al. Expanding the molecular spectrum of secretory carcinoma of salivary glands with a novel VIM-RET fusion. Am. J. Surg. Pathol 2020; 44; 1295–1307. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Russell JS, McDermott JD et al. Profiling of 149 salivary duct carcinomas, carcinoma ex pleomorphic adenomas, and adenocarcinomas, not otherwise specified reveals actionable genomic alterations. Clin. Cancer Res 2016; 22; 6061–6068. [DOI] [PubMed] [Google Scholar]
- 25.Hatakeyama S TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem. Sci 2017; 42; 297–311. [DOI] [PubMed] [Google Scholar]
- 26.Drilon A, Wang L, Hasanovic A et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov. 2013; 3; 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabes HM, Demidchik EP, Sidorow JD et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-Chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin. Cancer Res 2000; 6; 1093–1103. [PubMed] [Google Scholar]
- 28.Dalin MG, Desrichard A, Katabi N et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin. Cancer Res 2016; 22; 4623–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiosea SI, Williams L, Griffith CC et al. Molecular characterization of apocrine salivary duct carcinoma. Am. J. Surg. Pathol 2015; 39; 744–752. [DOI] [PubMed] [Google Scholar]
- 30.Gargano SM, Senarathne W, Feldman R et al. Novel therapeutic targets in salivary duct carcinoma uncovered by comprehensive molecular profiling. Cancer Med. 2019; 8; 7322–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh MS, Bishop JA, Wang YP et al. Salivary sialadenoma papilliferum consists of two morphologically, immunopheno-typically, and genetically distinct subtypes. Head Neck Pathol. 2020; 14; 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakaguro M, Urano M, Ogawa I et al. Histopathological evaluation of minor salivary gland papillary-cystic tumours: focus on genetic alterations in sialadenoma papilliferum and intraductal papillary mucinous neoplasm. Histopathology 2020; 76; 411–422. [DOI] [PubMed] [Google Scholar]
- 33.Skalova A, Vanecek T, Sima R et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol 2010; 34; 599–608. [DOI] [PubMed] [Google Scholar]
- 34.Urano M, Nakaguro M, Yamamoto Y et al. Diagnostic significance of HRAS mutations in epithelial-myoepithelial carcinomas exhibiting a broad histopathologic spectrum. Am. J. Surg. Pathol 2019; 43; 984–994. [DOI] [PubMed] [Google Scholar]


