PURPOSE
Patients with metastatic urothelial carcinoma (mUC) who progress on platinum-based combination chemotherapy (PLT) and checkpoint inhibitors (CPIs) have limited options that offer objective response rates (ORRs) of approximately 10% with a median overall survival (OS) of 7-8 months. Sacituzumab govitecan (SG) is a TROP-2–directed antibody-drug conjugate with an SN-38 payload that has shown preliminary activity in mUC.
METHODS
TROPHY-U-01 (ClinicalTrials.gov identifier: NCT03547973) is a multicohort, open-label, phase II, registrational study. Cohort 1 includes patients with locally advanced or unresectable or mUC who had progressed after prior PLT and CPI. Patients received SG 10 mg/kg on days 1 and 8 of 21-day cycles. The primary outcome was centrally reviewed ORR; secondary outcomes were progression-free survival, OS, duration of response, and safety.
RESULTS
Cohort 1 included 113 patients (78% men; median age, 66 years; 66.4% visceral metastases; median of three [range, 1-8] prior therapies). At a median follow-up of 9.1 months, the ORR was 27% (31 of 113; 95% CI, 19.5 to 36.6); 77% had decrease in measurable disease. Median duration of response was 7.2 months (95% CI, 4.7 to 8.6 months), with median progression-free survival and OS of 5.4 months (95% CI, 3.5 to 7.2 months) and 10.9 months (95% CI, 9.0 to 13.8 months), respectively. Key grade ≥ 3 treatment-related adverse events included neutropenia (35%), leukopenia (18%), anemia (14%), diarrhea (10%), and febrile neutropenia (10%), with 6% discontinuing treatment because of treatment-related adverse events.
CONCLUSION
SG is an active drug with a manageable safety profile with most common toxicities of neutropenia and diarrhea. SG has notable efficacy compared with historical controls in pretreated mUC that has progressed on both prior PLT regimens and CPI. The results from this study supported accelerated approval of SG in this population.
INTRODUCTION
Patients with metastatic urothelial carcinoma (mUC) with disease progression after combination platinum-based chemotherapy and immune checkpoint inhibitors (CPIs) have limited treatment options.1 Following progression, the only widely available agents indicated per NCCN and ESMO guidelines have been taxanes and vinflunine (approved in the European Union). These agents have response rates of approximately 10% with a median overall survival (OS) of 7-8 months.2-7 The therapeutic landscape for mUC in the United States has been expanded by the accelerated US Food and Drug Administration (FDA) approvals of erdafitinib, a pan-fibroblast growth factor receptor inhibitor for patients with tumors harboring FGFR2- or FGFR3-activating mutation or fusion (following platinum-based chemotherapy), and enfortumab vedotin (EV), a nectin-4–directed antibody-drug conjugate (ADC) following platinum-based chemotherapy and CPI.8-10 Although both EV and erdafitinib have objective response rates (ORRs) of approximately 40%, most patients progress on these therapies. Moreover, erdafitinib is limited to patients with FGFR2/3 mutation or fusion (15%-20% of patients depending on cancer type).11 Hence, new agents are still needed.
CONTEXT
Key Objective
Patients with advanced or metastatic urothelial cancer (mUC) have limited treatment options after progression on platinum or checkpoint inhibitors (CPI). The TROPHY-U-01 study evaluated sacituzumab govitecan (SG), a trophoblast cell surface antigen 2–directed antibody-drug conjugate, in patients with locally advanced or unresectable or mUC who had progressed after prior platinum and CPI.
Knowledge Generated
Of 113 patients who received SG, central review confirmed an objective response rate (ORR) of 27% with six complete responses and 25 partial responses, confirming results from the prior phase I/II study demonstrating that SG is generally well tolerated and has significant anticancer activity in heavily pretreated patients with mUC who had progressed on platinum and CPI.
Relevance
The ORR of 27%, median duration of response of 7.2 months, and median overall survival of 10.9 months compare favorably with single-agent chemotherapy in this population, where ORR is approximately 10% and overall survival is 7 to 8 months.
Trophoblast cell surface antigen 2 (Trop-2) is a transmembrane glycoprotein that is highly expressed on the surface of most epithelial cancer cells.12-16 Elevated Trop-2 expression is associated with poor prognosis for several cancer types, including mUC.12-21 Trop-2 also plays a key role in cell transformation and proliferation.18,22-24 Sacituzumab govitecan (SG) is a novel Trop-2–directed ADC composed of an anti–Trop-2 humanized monoclonal antibody hRS7 IgG1κ coupled to SN-38, the active metabolite of the topoisomerase 1 inhibitor irinotecan with a high drug-to-antibody ratio (7.6 molecules of SN-38 per antibody).16,25 This coupling is achieved using a hydrolyzable, proprietary linker, CL2A, that permits a dual mechanism of action.16,25-29 Internalization of Trop-2–bound SG delivers SN-38 inside tumor cells, thereby killing the tumor cells,26 while the hydrolyzable linker enables SN-38 to be released into the tumor microenvironment, killing adjacent tumor cells (bystander effect).16,27,28 The activity of SG, initially assessed in a phase I/II trial (IMMU-132-01; ClinicalTrials.gov identifier: NCT01631552) in patients with advanced epithelial cancers who had received at least one prior therapy for metastatic disease,28,30 showed encouraging clinical activity across various solid tumors.31 SG demonstrated clinical activity in patients with relapsed or refractory mUC (ORR, 31%), including a 27% ORR in patients with prior CPI and platinum therapy.31,32 The TROPHY-U-01 phase II trial was designed to confirm this initial signal in patients with mUC. We hypothesized that SG would have significant antitumor activity, as measured by ORR, comparing favorably to historical controls of cytotoxic chemotherapy. Here, we report the primary results from the full cohort 1 of the TROPHY-U-01 study in patients with mUC who progressed after prior platinum-based and CPI-based therapies.
METHODS
Study Participants
TROPHY-U-01 is a phase II study assessing the activity of SG in patients with locally advanced unresectable or mUC (Appendix Fig A1, online only). In cohort 1, eligible patients included adults with histologically confirmed, locally advanced UC or mUC who had disease progression following a platinum-containing regimen and CPI therapy. Patients who recurred within 12 months after completion of platinum therapy in the neoadjuvant or adjuvant setting were considered refractory to platinum therapy and permitted to enroll if they progressed after subsequent CPI therapy. All patients also were required to have measurable disease by RECIST v1.1,33 an Eastern Cooperative Oncology Group performance status of 0 to 1, adequate hepatic, renal, and hematologic function, and no known Gilbert syndrome. Patients must have recovered from all acute toxicities (except grade ≤ 2 neuropathy or alopecia) from prior therapy with a minimum washout period of 4 weeks from prior monoclonal antibody therapy and 2 weeks from prior chemotherapy, small-molecule therapy, or radiotherapy, and patients with treated, nonprogressive brain metastases were allowed to enroll. There was no requirement for tumor Trop-2 expression for enrollment (Appendix, online only).
Treatment
SG 10 mg/kg was administered intravenously on days 1 and 8 in a 21-day treatment cycle, until unacceptable toxicity, loss of clinical benefit, or withdrawal of consent. Hematopoietic growth factors or blood transfusions were allowed as clinically indicated. Pre-medication with a 2-drug antiemetic was recommended (followed by a 3-drug antiemetic for persistent nausea and vomiting), with premedication for infusion-related reactions and other supportive or palliative care recommended based on institution policy. The scheduled day 1 and day 8 infusions may have been delayed for up to 1 week for recovery of treatment-related toxicities with a maximum dose delay of 5 weeks permitted for any reason.
Assessments
For efficacy evaluations, computed tomography or magnetic resonance imaging scans were obtained at baseline and at 6-week intervals from the initiation of treatment until completion of 12 cycles of therapy, after which the interval could be lengthened to every 9 weeks. Confirmatory computed tomography or magnetic resonance imaging scans were to be obtained 4 to 6 weeks after first evidence of response. Response was evaluated by blinded independent central review (BICR) using RECIST v1.1.
Safety evaluations included adverse events (AEs), standard laboratory safety evaluations, physical examinations, and vital signs. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v5.0. Additional safety analyses examined the impact of UGT1A1 genotype status on the incidence of AEs in evaluable patients.
End Points
The primary objective of this phase II study was to determine the ORR per BICR. Secondary objectives included assessments of duration of response (DOR) and progression-free survival (PFS), both centrally reviewed, investigator-assessed ORR, OS, and safety.
Trial Oversight
All patients provided written informed consent. The Protocol (online only) was approved by the institutional review boards or independent ethics committees at the participating institutions and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines and other applicable local regulatory requirements and laws.
Statistical Analysis
Target enrollment was approximately 100 patients, based on a Simon two-stage design for 90% power to reject the null hypothesis of ORR ≤ 12%. A sample size of 100 provided sufficient power to ensure the lower boundary of the 95% CI calculated from the Clopper-Pearson exact method would exclude an ORR of ≤ 15%, assuming a 24% ORR (24 out of 100 responders). There was a preplanned interim analysis based on investigator assessment of data per RECIST v1.1 from cohort 1 after 35 response-evaluable patients were enrolled, with continued enrollment if four or more responses were observed. The initial stage demonstrated that 10 of 35 evaluable patients responded, which surpassed futility criteria to continue enrollment.34 Final analysis was based on BICR assessment of data per RECIST v1.1 from cohort 1. ORR, defined as a best overall response of complete response (CR) or partial response (PR), was calculated with 95% CI estimated by the Clopper-Pearson method.35 DOR, PFS, and OS were analyzed by the Kaplan-Meier method with medians and corresponding 95% CIs determined according to the Brookmeyer and Crowley formula with log-log transformation. Descriptive statistics were used to characterize and present treatment-related AEs (TRAEs).
RESULTS
Study Participants
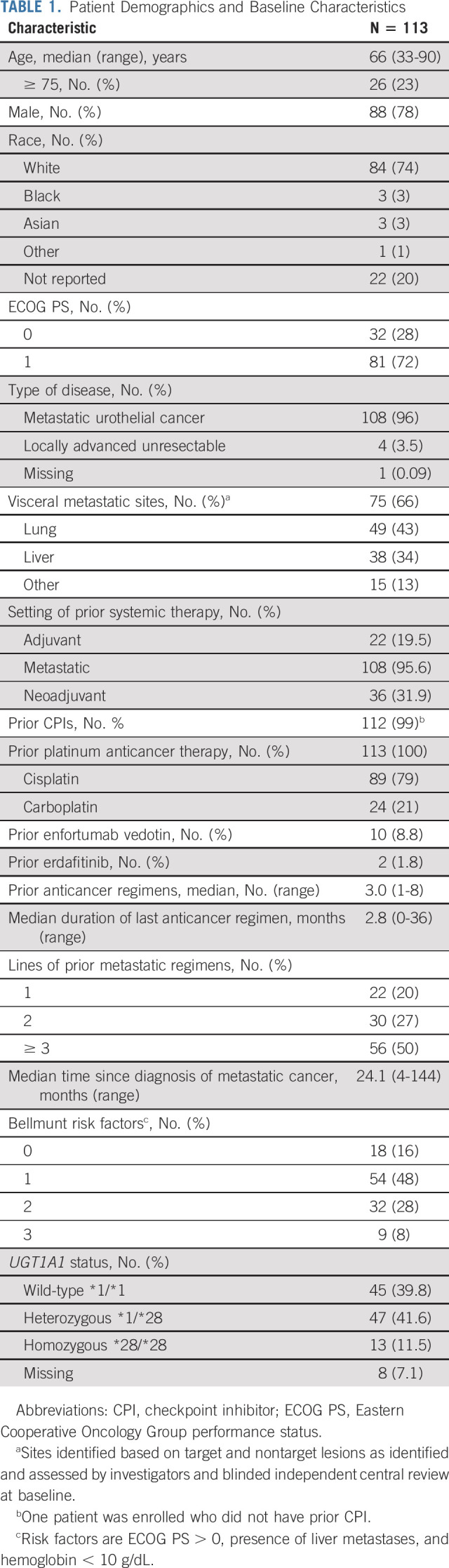
From August 2018 to November 2019, 113 patients were enrolled and treated in cohort 1 (Fig 1); these patients form the population for all analyses with a data cutoff of September 18, 2020. Patients were predominantly men (78%), with a median age of 66 years (range, 33-90 years) (Table 1). Visceral disease was present in 75 patients (66.4%) and 38 (33.6%) patients had liver metastases. Of the 113 patients enrolled, 112 previously received CPI therapy. Patients received a median of three prior anticancer regimens (range, 1-8), with 21% (n = 24) receiving combination chemotherapy with carboplatin or 79% (n = 89) with cisplatin. The majority (84%) had at least one adverse Bellmunt prognostic risk factor (including performance status, hemoglobin < 10 g/dL, and the presence of liver metastases).36 Patients received a median of 6 cycles of SG (11 doses; range, 1-56 doses), with median treatment duration of 3.7 months (range, 0-20 months). The median relative dose intensity was 96.9% despite 31.0% requiring a single dose reduction. Only four (3.5%) patients required an infusion interruption. Most patients (n = 103) discontinued treatment, primarily because of cancer progression (n = 81) (Fig 1). As of the data cutoff date, 16 patients continued to receive study therapy.
FIG 1.
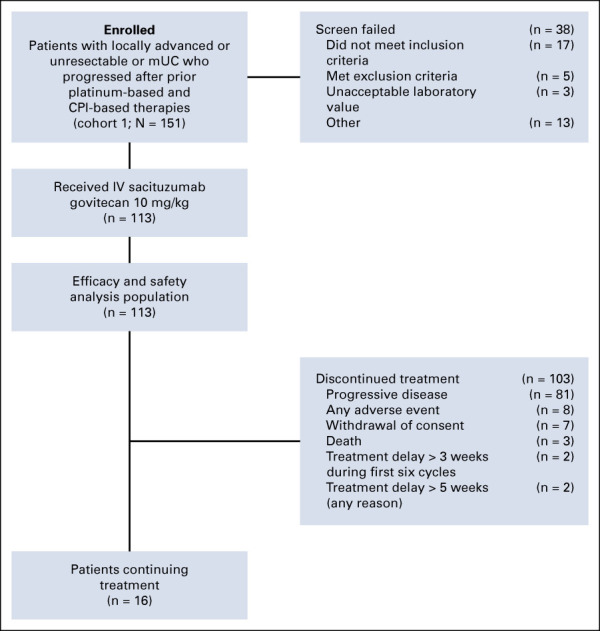
CONSORT diagram. CPI, checkpoint inhibitor; IV, intravenous; mUC, metastatic urothelial cancer.
TABLE 1.
Patient Demographics and Baseline Characteristics
Efficacy
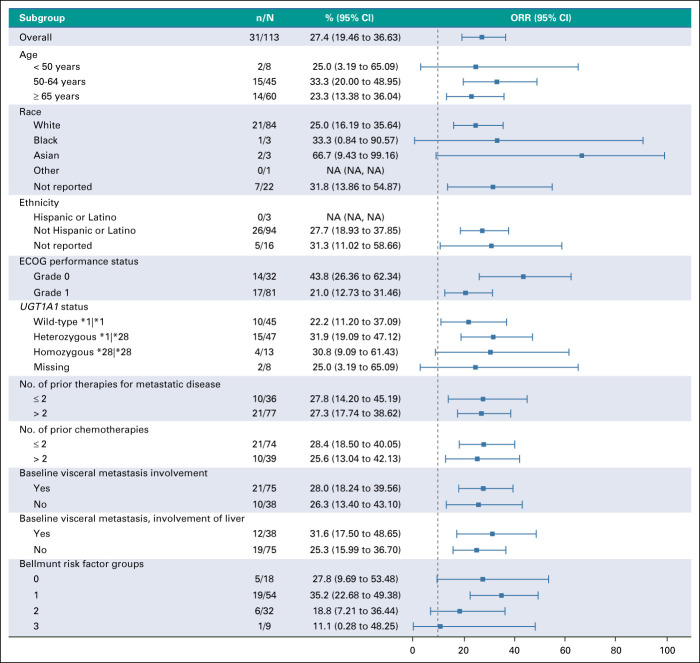
Clinical activity (based on BICR) was observed with an ORR of 27.4% (31 of 113) (95% CI, 19.5 to 36.6; Table 2) including six confirmed CR (5.3%) and 25 confirmed PR (22.1%) in an intent-to-treat analysis. The clinical benefit rate (defined as CR plus PR plus stable disease ≥ 6 months) was 37.2% (95% CI, 28.3 to 46.8; Table 2). Stable disease as best response was observed in 33.6% (38 of 113) of patients and 18.6% (21 of 113) had progressive disease as best response at data cutoff. SG showed efficacy in all evaluated subgroups, including patients with ≥ 2 prior lines of therapy, visceral and liver metastases at baseline, and by Bellmunt risk factor (Appendix Fig A2, online only). Interestingly, in the small subgroup of patients who received prior EV therapy (n = 10), three patients achieved PR, with 30% ORR (95% CI, 6.7 to 65.3). Of those three patients with PR, two had a best response of progressive disease with prior EV.
TABLE 2.
Summary of Treatment Efficacy
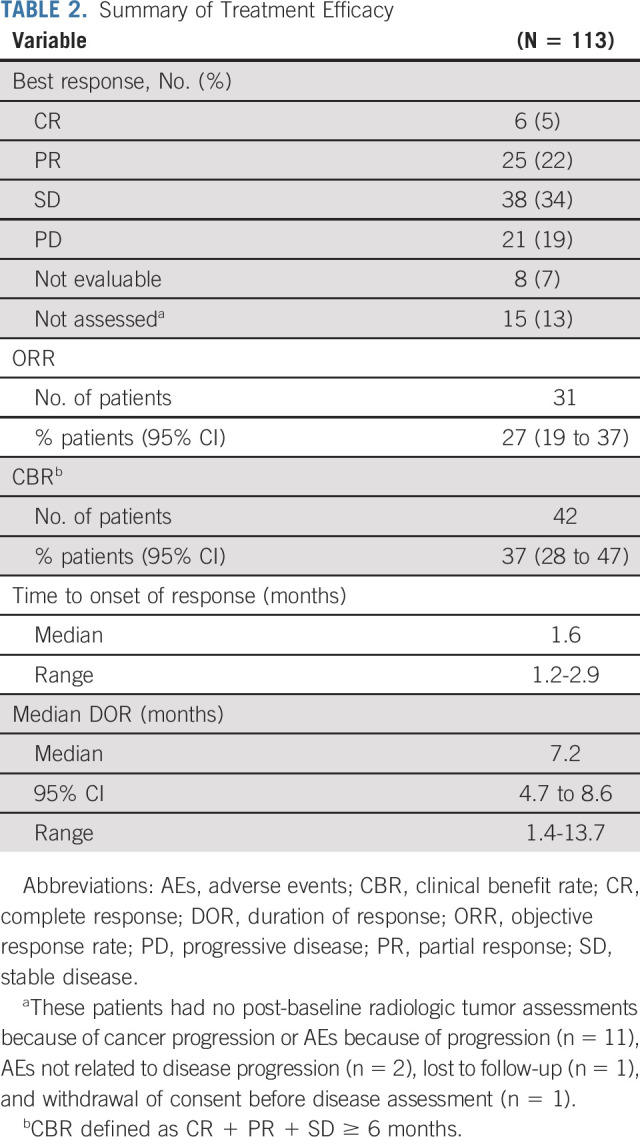
With a median follow-up duration of 9.1 months (range, 0-19.9 months), the median DOR was 7.2 months (95% CI, 4.7 to 8.6 months) (Table 2). The median time to objective response was 1.6 months (range, 1.2-5.5 months). Six (5.3%) patients achieved CR with DOR ranging from 1.4 to 13.7 months. A reduction in the size of target lesions was achieved by 77% (72 of 94) of patients with at least 1 post-baseline target lesion measurement by BICR (Fig 2A). The spider plot by BICR of best percent change from baseline in the sum of the diameters of the target lesions (Fig 2B) shows the reduction in the size of target lesions was durable in most patients, including many of those who did not have a documented confirmed response. The onset of response and DOR for responders (CR or PR) is summarized in the swimmer plot by BICR (Fig 2C), with 30 of 31 patients still alive at the time of data cutoff and four patients with ongoing response at the time of data cutoff. Median PFS was 5.4 months (95% CI, 3.5 to 7.2 months; range, 2.4-8.9 months), and median OS was 10.9 months (95% CI, 9.0 to 13.8 months; range, 3.8-19.8 months) (Fig 3).
FIG 2.
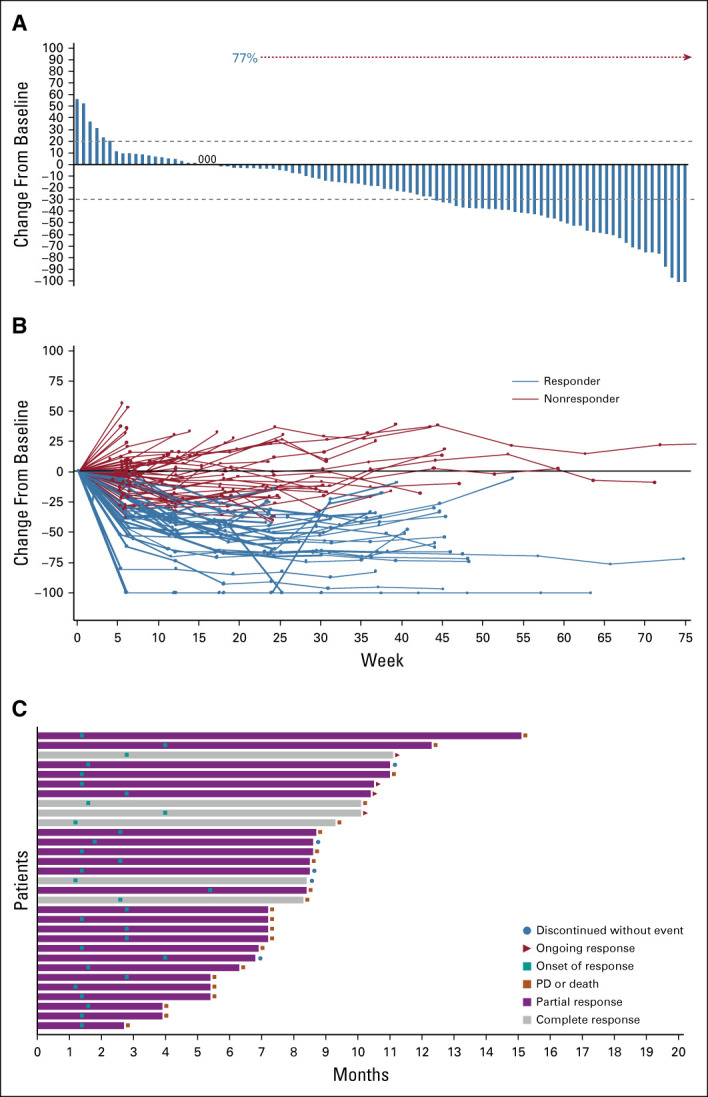
Tumor response to sacituzumab govitecan. (A) Waterfall plot showing best percent change from baseline in the sum of the diameters of the target lesions (longest for non-nodal and short axis for nodal lesions) in 94 patients (excludes 19 patients; 15 patients did not have post-baseline radiologic assessments and four patients lacked or had unevaluable target lesions at baseline or post-baseline). The dashed lines at +20% and −30% indicate thresholds for PD and partial response, respectively, according to RECIST v1.1. Target lesions were reduced in 77% of patients (72 of 94) with at least 1 post-baseline target lesion measurement. (B) Spider plot of tumor response by week. (C) Swimmer plot of response and duration. PD, progressive disease.
FIG 3.
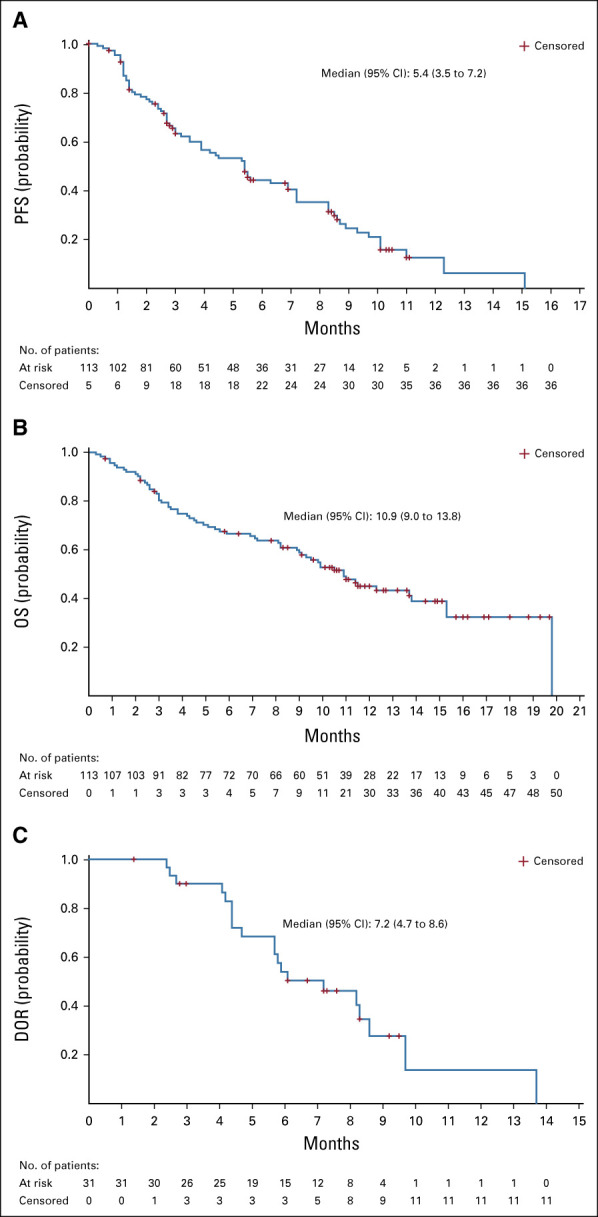
Kaplan-Meier analysis of (A) PFS, (B) OS, and (C) DOR. DOR, duration of response; OS, overall survival; PFS, progression-free survival.
Safety
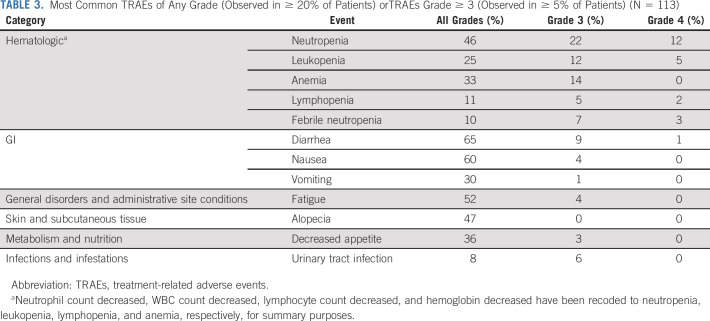
Almost all patients (111 of 113; 98.2%) experienced at least 1 AE during the study, and 107 of 113 (94.7%) experienced a TRAE. The most common any-grade TRAEs that occurred in ≥ 20% of patients included diarrhea (65%), nausea (60%), fatigue (52%), alopecia (47%), neutropenia (46%), decreased appetite (36%), anemia (33%), vomiting (30%), and leukopenia (25%) (Table 3). These AEs were primarily managed with routine supportive care, including antidiarrheal, antiemetics, hydration, and growth factor support, and/or dose reduction or delay. There was a low rate of treatment-related skin rash (6%), maculopapular rash (7%), ocular disorders (4%), peripheral neuropathy (4%; grade ≤ 2), and hyperglycemia (< 1%; grade ≤ 2). About a third (39%) of patients had dose reduction because of TRAEs primarily for neutropenia, diarrhea, and fatigue. Dose interruption or delay because of TRAEs occurred in 45% of patients, most commonly because of neutropenia, leukopenia, and anemia. TRAEs led to discontinuations in 6% (n = 7) of patients primarily due to neutropenia or associated complications (ie, febrile neutropenia and sepsis).
TABLE 3.
Most Common TRAEs of Any Grade (Observed in ≥ 20% of Patients) orTRAEs Grade ≥ 3 (Observed in ≥ 5% of Patients) (N = 113)
Most common grade ≥ 3 TRAEs that occurred in ≥ 5% of patients included neutropenia (35%), leukopenia (18%), anemia (14%), diarrhea (10%), febrile neutropenia (10%), lymphopenia (7%), and urinary tract infection (6%) (Table 3). Notably, although treatment-related neutropenia of any grade occurred in almost half of the patients (46%), febrile neutropenia was relatively infrequent (n = 11; 10%). Neutropenia was managed through use of dose reductions or interruptions, while 30.1% of patients received growth factor support (18% received granulocyte colony-stimulating factor [G-CSF] in cycle 1 and the remainder received G-CSF in cycle 2 or later). Most cases of treatment-related diarrhea were grade 1 (n = 45; 40%), with 15% (n = 17) grade 2, 9% (n = 10) grade 3, and < 1% (n = 1) grade 4.
Grade ≥ 3 serious TRAEs that occurred in more than 1 patient included febrile neutropenia (n = 10), diarrhea (n = 4), urinary tract infection (n = 4), sepsis (n = 2), and thrombocytopenia (n = 2). A single case of grade 2 interstitial lung disease occurred in a 76-year-old woman with ischemic cardiomyopathy who had discontinued avelumab 2 months before enrolling in the trial; the patient recovered, and her condition resolved. There was one treatment-related death because of sepsis as a result of febrile neutropenia in a 65-year-old man with mUC, stage III chronic kidney disease, and medical history of lung cancer. Four days after receiving the last dose (cycle 3, day 1) of SG, the patient developed severe sepsis, with grade 4 febrile neutropenia and grade 3 thrombocytopenia. The patient was treated with broad-spectrum antibiotics and G-CSF; however, he was transitioned to inpatient hospice and subsequently died.
There were 105 (93%) evaluable patients for whom UGT1A1 genotype analysis was performed (Table 1). Neutropenia (all grade) was numerically more frequent in homozygous (*28/*28) patients (54%) and heterozygous (*1/*28) patients (51%) compared with wild-type (*1/*1) patients (38%). Similarly, grade ≥ 3 neutropenia occurred more frequently in homozygous patients (54%) compared with heterozygous (34%) and wild-type (31%) patients. The frequency of diarrhea was generally not higher in homozygous patients versus the other groups (69%, 75%, and 53%, for homozygous, heterozygous, and wild-type patients, respectively). The incidence of discontinuation was similar across homozygous, heterozygous, and wild-type patients (8%, 6%, and 7%, respectively); however, treatment interruption was more common numerically in homozygous patients compared with heterozygous or wild-type patients (69%, 36%, and 42%, respectively).
DISCUSSION
In this study, SG has demonstrated a clinically and statistically significant ORR (27%) in patients with pretreated locally advanced unresectable or mUC when administered after progression on platinum-based chemotherapy and immunotherapy compared with historical controls.6 The ORR reported here is also consistent with the 27% ORR seen in the earlier phase I/II study in the cohorts of patients with mUC who were treated with both CPI and platinum (n = 15).31 Responses lasted for a median of 7.2 months, with the longest ongoing response of 9.5 months at the time of data cutoff (September 18, 2020). The median PFS (5.4 months) and median OS (10.9 months) observed with SG compare favorably to that of single-agent chemotherapy (median 2.7-3.3 months PFS and approximately 7 months OS).3,5 Benefit with SG was also seen across multiple subgroups (including the small subgroup with prior exposure to EV), although some subgroups were small and warrant further investigation. Although the numbers are very small, responses in patients previously treated with EV highlight the different antigen target, linker, and payload delivered by SG, and support the hypothesis of nonoverlapping mechanisms of action and resistance.
Patients with mUC who have had disease progression after platinum-based chemotherapy and CPI therapy have poor outcomes and limited treatment options.6,7,37 Several single and combination therapies have been investigated to improve the safety and efficacy of currently available options. Single and combination chemotherapy (pemetrexed, vinflunine, nab-paclitaxel, docetaxel, and ifosfamide) have resulted in ORRs of approximately 5.0%-25.0% and median OS of only 4.0-7.5 months.38-41 Novel agents, such as oral mocetinostat (class I/IV histone deacetylase inhibitor) and rucaparib (PARP inhibitor) did not have notable clinical activity,42,43 whereas erdafitinib, the first FGFR2/3-targeting agent, achieved a 40% ORR in a single-arm phase II trial, and significantly exceeded historical controls in a biomarker-selected platinum refractory population.10
For those who do not receive maintenance immunotherapy, a CPI is now standard second-line treatment with a significant OS advantage over single-agent chemotherapy, such as taxane or vinflunine; however, only about 13%-21% of patients exhibit a response.5,44-47 Recent data indicate that the combination of the CPIs nivolumab and ipilimumab resulted in improved ORR compared with nivolumab alone in a nonrandomized trial; however, this combination remains investigational in UC.48 Furthermore, outcomes with single-agent chemotherapy after progression on CPI therapy remain short with no apparent difference compared with historic pre-CPI era data.49 ADCs represent a promising therapeutic modality for patients with refractory UC.7,28,37,50 One such ADC, EV, received accelerated FDA approval in patients who have received prior platinum-based chemotherapy and CPI therapy based on the EV-201 phase II trial, and most recently demonstrated OS survival benefit over single-agent taxane or vinflunine in the EV-301 trial.51 EV was associated with fatigue, skin toxicities, peripheral neuropathy, and hyperglycemia, among other toxicities, and cannot be used in those with baseline uncontrolled hyperglycemia and neuropathy.37,51 Erdafitinib has accelerated approval in the United States, but is appropriate only for patients harboring activating mutation or fusion in FGFR2 or FGFR3 genes.10
SG was found to be tolerable, and despite dose interruptions and delays, the dose intensity remained 96%. The AEs most commonly associated with SG were neutropenia and diarrhea, consistent with its SN-38 payload (irinotecan metabolite). These AEs are predictable and manageable, resulting in a low rate of treatment discontinuation (6%; n = 7). Few patients discontinued because of TRAEs (n = 7); very few discontinued because of neutropenia (n = 4) and no patients discontinued because of diarrhea, possibly because of the systemic rather than localized release of SN-38 metabolite. Proactive management using established guidelines is recommended for both neutropenia and diarrhea as well as common AEs such as nausea and vomiting.52 Other common toxicities associated with ADC therapy were quite low. AEs of rash, ocular toxicity, and peripheral neuropathy were infrequent and all were grade ≤ 2. Patients with known UGT1A1 homozygous *28/*28 genotype are at increased risk of neutropenia, and while prescreening is not required, close monitoring is advised. It is theoretically possible that heterozygotes have lower enzymatic activity and higher risk of neutropenia, but this small, nonrandomized data set is not able to address this question.
Study limitations include moderate sample size, lack of biomarker analysis, and single-arm, open-label study design. While there were a limited number of UGT1A1 *28 homozygous patients to make any statistically valid observations, and despite the lack of a comparator arm, the final results for cohort 1 of this study confirm the interim findings and prior phase I/II results of SG as a tolerable and clinically active agent in patients with mUC.31,34 The safety results reported here are also consistent with previous reports in other cancers.28,30,53-55
SG (Trodelvy) has recently been approved by the FDA for the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer who have received two or more prior systemic therapies, at least one of them for metastatic disease.56 The phase III confirmatory ASCENT trial that compared SG with chemotherapy of physician's choice in triple-negative breast cancer reported a highly significant benefit for SG in all end points including ORR (35% v 5%), PFS (5.6 v 1.7 months), and OS (12.1 v 6.7 months).57 The clinically meaningful activity and safety profile of SG demonstrated in cohort 1 of the TROPHY-U-01 mUC trial led to the accelerated FDA approval of SG58 for patients with locally advanced or mUC who previously received a platinum-containing chemotherapy and either a programmed death-1 or a programmed death-ligand 1 inhibitor. The results will be corroborated in the ongoing phase III confirmatory trial of SG versus taxane or vinflunine in mUC (TROPiCS-04; ClinicalTrials.govidentifier: NCT04527991). Additional cohorts of TROPHY-U-01 continue to evaluate the role of SG in mUC. Cohort 2 is investigating the role of SG in platinum-ineligible patients with mUC who progressed after CPI therapy. Cohort 3 is evaluating SG in combination with pembrolizumab in patients with mUC who are CPI-naive and progressed after prior platinum-based chemotherapies. Both cohorts 4 and 5 are evaluating SG as induction and maintenance therapy in platinum-naïve patients with mUC who are not refractory to platinum-based therapy in the neoadjuvant setting either as a cisplatin combination (cohort 4) or in addition to both cisplatin and avelumab (cohort 5) during induction. Both cohorts 4 and 5 will also receive SG in addition to avelumab as maintenance therapy. In conclusion, the results of cohort 1 of the TROPHY-U-01 trial supported fast-track designation and accelerated FDA approval of SG for the treatment of mUC previously treated with platinum-based chemotherapy and CPI by the FDA.
ACKNOWLEDGMENT
The authors thank the patients and their caregivers for helping them realize the possibilities of this research. They thank the dedicated clinical trial investigators and their devoted team members participating in the TROPHY-U-01 trial. They thank Drs Usman Aziz and Charu Kanwal for their role in trial management and data analysis. The study was sponsored by Immunomedics, Inc, a subsidiary of Gilead Sciences, Inc, and was designed through a collaboration of the sponsor and the lead investigators. Medical writing and editorial assistance were provided by Peloton Advantage, an OPEN Health company, Parsippany, NJ, and was funded by Immunomedics, Inc, a subsidiary of Gilead Sciences, Inc.
APPENDIX
List of TROPHY cohort 1 investigators
The following investigators (listed by country) participated in the TROPHY-U-01 Cohort 1 study:
United States: Clarence Adoo, Neeraj Agarwal, Arjun V. Balar, Pranshu Bansal, Manojkumar Bupathi, Bradley Carthon, Christopher Chen, Mary Crow, Jorge Darcourt, Saby George, Petros Grivas, Elisabeth Heath, Rohit K. Jain, Christos E. Kyriakopoulos, Luke Nordquist, Rami Owera, Phillip Palmbos, Chandler Park, Daniel Petrylak, Joseph Pizzalato, Arash Rezazedeh, Scott Tagawa, Eddie Thara, Nicholas Vogelzang, and Shenhong Wu. France: Philippe Barthelemy, Philippe Beuzeboc, Aude Fléchon, Yohann Loriot, and Damien Pouessel.
SUPPLEMENTARY RESULTS
Efficacy by Investigator Assessment
Clinical activity (based on investigator's assessment) was demonstrated with an objective response rate of 23% (26 of 113) (95% CI, 15.6 to 31.9) including six confirmed complete responses (CRs) (5.3%) and 20 confirmed partial responses (PRs) (17.7%). The clinical benefit rate (defined as CR plus PR plus stable disease [SD] ≥ 6 months) was 38.9% (95% CI, 29.9 to 48.6). SD as best response was observed in 43.4% (49 of 113) of patients and 20.4% (23 of 113) had progressive disease at data cutoff. Sacituzumab govitecan demonstrated efficacy in all subgroups evaluated, including patients with ≥ 2 prior lines of therapy, visceral and liver metastases at baseline, and by Bellmunt risk factor. Interestingly, in the small subgroup of patients who received prior therapy with enfortumab vedotin (n = 10), there was 1 responder who achieved a PR, with an objective response rate of 10% (95% CI, 0.25 to 44.5), six who had SD, and three who had a best response of progressive disease with sacituzumab govitecan.
With a median follow-up duration of 9.1 months, the median duration of response was 7.7 months (95% CI, 4.4 to 9.0 months). The median time to objective response was 1.6 months (range, 1.2-2.9 months). Six subjects achieved a CR with a duration of response ranging from 2.7 to 15.8 months. A reduction in the size of target lesions was achieved by 71% (70 of 99) of patients with at least one post-baseline target lesion measurement by investigator assessment. The median progression-free survival and median overall survival were 4.4 months (95% CI, 2.9 to 5.7 months) and 10.9 months (95% CI, 9.0 to 13.8 months), respectively.
FIG A1.
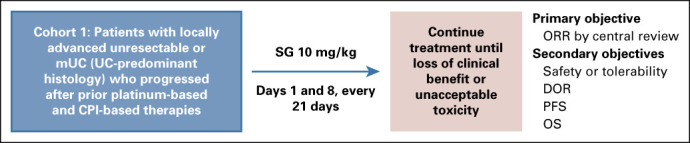
TROPHY-U-01 study design. EudraCT Number: 2018-001167-23; ClinicalTrials.gov identifier: NCT03547973; IMMU-132-06 study. CPI, checkpoint inhibitor; DOR, duration of response; mUC, metastatic urothelial cancer; ORR, objective response rate; OS, overall survival; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; SG, sacituzumab govitecan.
FIG A2.
Forest plot showing ORR in different subgroups. Horizontal line represents CI. ECOG, Eastern Cooperative Oncology Group; NA, not available; ORR, objective response rate.
Scott Tagawa
Consulting or Advisory Role: Medivation, Astellas Pharma, Dendreon, Janssen, Bayer, Genentech, Endocyte, Immunomedics, Karyopharm Therapeutics, Abbvie, Tolmar, QED Therapeutics, Amgen, Sanofi, Pfizer, Clovis Oncology, Novartis, Genomic Health, POINT Biopharma, Blue Earth Diagnostics, Seattle Genetics, AIkido Pharma
Research Funding: Lilly, Sanofi, Janssen, Astellas Pharma, Progenics, Millennium, Amgen, Bristol-Myers Squibb, Dendreon, Rexahn Pharmaceuticals, Bayer, Genentech, Newlink Genetics, Inovio Pharmaceuticals, AstraZeneca, Immunomedics, Novartis, AVEO, Boehringer Ingelheim, Merck, Stem CentRx, Karyopharm Therapeutics, Abbvie, Medivation, Endocyte, Exelixis, Clovis Oncology
Travel, Accommodations, Expenses: Sanofi, Immunomedics, Amgen
Uncompensated Relationships: Telix Pharmaceuticals, ATLAB Pharma, Phosplatin Therapeutics
Arjun Balar
Honoraria: Merck, Genentech/Roche, AstraZeneca/MedImmune
Consulting or Advisory Role: Immunomedics, Bristol Myers Squibb, Genentech/Roche, Merck, Cerulean Pharma, AstraZeneca/MedImmune, Pfizer/EMD Serono, Incyte, Seattle Genetics/Astellas, Nektar, Dragonfly Therapeutics, GlaxoSmithKline
Research Funding: Immunomedics, Merck, Genentech/Roche, AstraZeneca/MedImmune, Seattle Genetics
Daniel Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, TYME
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seattle Genetics, Urogen pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics
Research Funding: Progenics, Sanofi, Endocyte, Genentech, Merck, Astellas Medivation, Novartis, AstraZeneca, Bayer, Lilly, Innocrin Pharma, MedImmune, Pfizer, Roche, Seattle Genetics, Clovis Oncology, Bristol Myers Squibb, Advanced Accelerator Applications, Agensys, BioXCel therapeutics, Eisai, Mirati Therapeutics, Replimune
Expert Testimony: Celgene, sanofi
Arash Rezazadeh
Stock and Other Ownership Interests: ECOM Medical
Consulting or Advisory Role: Exelixis, AstraZeneca, Bayer, Pfizer, Novartis, Genentech, Bristol Myers Squibb, EMD Serono, Immunomedics, Gilead Sciences
Speakers' Bureau: Janssen, Astellas Medivation, Pfizer, Novartis, Sanofi, Genentech/Roche, Eisai, AstraZeneca, Bristol Myers Squibb, Amgen, Exelixis, Exelixis, EMD Serono, Merck, Seattle Genetics/Astellas, Seattle Genetics/Astellas, Myovant Sciences, Gilead Sciences, AVEO
Research Funding: Genentech, Exelixis, Janssen, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Macrogenics, Astellas Pharma, BeyondSpring Pharmaceuticals, BioClin Therapeutics, Clovis Oncology, Bavarian Nordic, Seattle Genetics, Immunomedics, Epizyme
Travel, Accommodations, Expenses: Genentech, Prometheus, Astellas Medivation, Janssen, Eisai, Bayer, Pfizer, Novartis, Exelixis, AstraZeneca
Yohann Loriot
Honoraria: Sanofi, Pfizer
Consulting or Advisory Role: Janssen, Astellas Pharma, Roche, AstraZeneca, MSD Oncology, MSD Oncology, Seattle Genetics, Bristol Myers Squibb, Immunomedics, Taiho Pharmaceutical
Research Funding: Sanofi, Janssen Oncology, MSD Oncology, AstraZeneca, Clovis Oncology, Exelixis, Boehringer Ingelheim, Incyte, Pfizer, Oncogenex, Medivation, CureVac, Nektar
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology, Roche, MSD Oncology, AstraZeneca, Seattle Genetics
Aude Flechon
Honoraria: MSD Oncology, AstraZeneca, Bristol Myers Squibb, Janssen-Cilag, Astellas Pharma, Pfizer, Sanofi/Aventis, Roche/Genentech, Bayer, Ipsen, AAA HealthCare
Travel, Accommodations, Expenses: Astellas Pharma, Sanofi/Aventis, Janssen-Cilag, Bayer, Pfizer, Ipsen, Bristol Myers Squibb, AstraZeneca, MSD Oncology, Roche/Genentech, AAA HealthCare
Rohit Jain
Honoraria: Alphasights
Consulting or Advisory Role: Taiho Oncology, Pfizer, Seattle Genetics/Astellas, Gilead Sciences, EMD Serono
Speakers' Bureau: Seattle Genetics/Astellas
Neeraj Agarwal
Consulting or Advisory Role: Pfizer, Medivation/Astellas, Bristol Myers Squibb, AstraZeneca, Nektar, Lilly, Bayer, Foundation One Inc, Pharmacyclics, Foundation Medicine, Astellas Pharma, Lilly, Exelixis, AstraZeneca, Pfizer, Merck, Novartis, Lilly, Eisai, Seattle Genetics, EMD Serono, Janssen Oncology, AVEO, Calithera Biosciences, MEI Pharma, Genentech, Astellas Pharma
Research Funding: Bayer, Bristol Myers Squibb, GlaxoSmithKline, Takeda, Novartis, Pfizer, BN ImmunoTherapeutics, Exelixis, TRACON Pharma, Rexahn Pharmaceuticals, Amgen, AstraZeneca, Active Biotech, Bavarian Nordic, Calithera Biosciences, Celldex, Eisai, Genentech, Immunomedics, Janssen, Merck, Newlink Genetics, Prometheus, Sanofi
Manojkumar Bupathi
Honoraria: Bristol Myers Squibb, Exelixis, AstraZeneca, Pfizer, Astellas Pharma
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Exelixis
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Pfizer, Exelixis, Astellas Pharma
Philippe Barthelemy
Honoraria: Ipsen, Bristol Myers Squibb, MSD, Astellas Pharma, Janssen-Cilag, Pfizer, Merck KGaA
Consulting or Advisory Role: Ipsen, Bristol Myers Squibb, MSD Oncology, Pfizer, Janssen-Cilag, AstraZeneca, Amgen
Travel, Accommodations, Expenses: Bristol Myers Squibb, Pfizer, Janssen-Cilag, Astellas Pharma, MSD, Ipsen
Phillip Palmbos
Research Funding: Roche, Immunomedics
Christos Kyriakopoulos
Consulting or Advisory Role: Exelixis, Sanofi, AVEO, EMD Serono, Janssen Oncology
Research Funding: Sanofi
Damien Pouessel
Honoraria: Ipsen, Janssen Oncology, Bristol Myers Squibb, AstraZeneca, Merck, Astellas Pharma
Consulting or Advisory Role: Astellas Pharma, AstraZeneca, Janssen Oncology, Pfizer, Sanofi
Research Funding: Incyte, Merck Sharp & Dohme, Roche, Bristol Myers Squibb, AstraZeneca, Janssen Oncology, Seattle Genetics
Travel, Accommodations, Expenses: Janssen Oncology, AstraZeneca, Pfizer
Cora Sternberg
Consulting or Advisory Role: BMS, MSD, Pfizer, Roche-Genentech, Incyte, AstraZeneca, Merck, Medscape, UroToday, Astellas Pharma, Sanofi-Genzyme, Immunomedics, now Gilead, Foundation Medicine, CCO Clinical, Janssen, NCI
Research Funding: Pfizer, MSD, Astellas, BMS, Immunomedics, now Gilead, Arvinas, Mirati
Quan Hong
Employment: Immunomedics, Gilead Sciences
Leadership: Immunomedics
Stock and Other Ownership Interests: Immunomedics
Trishna Goswami
Employment: Immunomedics/Gilead
Leadership: Immunomedics/Gilead
Stock and Other Ownership Interests: Immunomedics/Gilead
Patents, Royalties, Other Intellectual Property: Patents around combinations with Trodelvy
Travel, Accommodations, Expenses: Immunomedics/Gilead
Loretta Itri
Employment: Immunomedics/Gilead
Leadership: Immunomedics/Gilead
Stock and Other Ownership Interests: Immunomedics
Consulting or Advisory Role: Immunomedics
Travel, Accommodations, Expenses: Immunomedics
Petros Grivas
Consulting or Advisory Role: Merck, Bristol Myers Squibb, AstraZeneca, Clovis Oncology, EMD Serono, Seattle Genetics, Foundation Medicine, Pfizer, Janssen, Bayer, Genzyme, Mirati Therapeutics, Exelixis, Roche, GlaxoSmithKline, Genentech, Immunomedics, Dyania Health, Infinity Pharmaceuticals, QED Therapeutics, 4D Pharma PLC
Research Funding: Pfizer, Clovis Oncology, Bavarian Nordic, Immunomedics, Bristol Myers Squibb, Debiopharm Group, Merck, QED Therapeutics, Kure It Cancer Research, GlaxoSmithKline, Mirati Therapeutics
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the European Society for Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, September 27-October 1, 2019 and ESMO 2020 Virtual Meeting, September 19-21, 2020.
SUPPORT
Supported by Immunomedics Inc, a subsidiary of Gilead Sciences, Inc.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Scott T. Tagawa, Arjun V. Balar, Yohann Loriot, Neeraj Agarwal, Cora N. Sternberg, Trishna Goswami, Loretta M. Itri, Petros Grivas
Provision of study materials or patients: Scott T. Tagawa, Arjun V. Balar, Daniel P. Petrylak, Yohann Loriot, Aude Fléchon, Rohit K. Jain, Neeraj Agarwal, Phillip Palmbos, Christos E. Kyriakopoulos, Damien Pouessel, Cora N. Sternberg, Trishna Goswami, Petros Grivas
Collection and assembly of data: Scott T. Tagawa, Daniel P. Petrylak, Arash Rezazadeh Kalebasty, Yohann Loriot, Aude Fléchon, Rohit K. Jain, Neeraj Agarwal, Manojkumar Bupathi, Philippe Barthelemy, Philippe Beuzeboc, Phillip Palmbos, Christos E. Kyriakopoulos, Damien Pouessel, Cora N. Sternberg, Quan Hong, Trishna Goswami, Loretta M. Itri
Data analysis and interpretation: Scott T. Tagawa, Arjun V. Balar, Daniel P. Petrylak, Arash Rezazadeh Kalebasty, Yohann Loriot, Rohit K. Jain, Neeraj Agarwal, Philippe Barthelemy, Christos E. Kyriakopoulos, Damien Pouessel, Cora N. Sternberg, Quan Hong, Trishna Goswami, Loretta M. Itri, Petros Grivas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Scott Tagawa
Consulting or Advisory Role: Medivation, Astellas Pharma, Dendreon, Janssen, Bayer, Genentech, Endocyte, Immunomedics, Karyopharm Therapeutics, Abbvie, Tolmar, QED Therapeutics, Amgen, Sanofi, Pfizer, Clovis Oncology, Novartis, Genomic Health, POINT Biopharma, Blue Earth Diagnostics, Seattle Genetics, AIkido Pharma
Research Funding: Lilly, Sanofi, Janssen, Astellas Pharma, Progenics, Millennium, Amgen, Bristol-Myers Squibb, Dendreon, Rexahn Pharmaceuticals, Bayer, Genentech, Newlink Genetics, Inovio Pharmaceuticals, AstraZeneca, Immunomedics, Novartis, AVEO, Boehringer Ingelheim, Merck, Stem CentRx, Karyopharm Therapeutics, Abbvie, Medivation, Endocyte, Exelixis, Clovis Oncology
Travel, Accommodations, Expenses: Sanofi, Immunomedics, Amgen
Uncompensated Relationships: Telix Pharmaceuticals, ATLAB Pharma, Phosplatin Therapeutics
Arjun Balar
Honoraria: Merck, Genentech/Roche, AstraZeneca/MedImmune
Consulting or Advisory Role: Immunomedics, Bristol Myers Squibb, Genentech/Roche, Merck, Cerulean Pharma, AstraZeneca/MedImmune, Pfizer/EMD Serono, Incyte, Seattle Genetics/Astellas, Nektar, Dragonfly Therapeutics, GlaxoSmithKline
Research Funding: Immunomedics, Merck, Genentech/Roche, AstraZeneca/MedImmune, Seattle Genetics
Daniel Petrylak
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, TYME
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Roche, Astellas Pharma, AstraZeneca, Lilly, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Incyte, Janssen, Pharmacyclics, Seattle Genetics, Urogen pharma, Advanced Accelerator Applications, Ipsen, Bicycle Therapeutics, Mirati Therapeutics, Monopteros Therapeutics
Research Funding: Progenics, Sanofi, Endocyte, Genentech, Merck, Astellas Medivation, Novartis, AstraZeneca, Bayer, Lilly, Innocrin Pharma, MedImmune, Pfizer, Roche, Seattle Genetics, Clovis Oncology, Bristol Myers Squibb, Advanced Accelerator Applications, Agensys, BioXCel therapeutics, Eisai, Mirati Therapeutics, Replimune
Expert Testimony: Celgene, sanofi
Arash Rezazadeh
Stock and Other Ownership Interests: ECOM Medical
Consulting or Advisory Role: Exelixis, AstraZeneca, Bayer, Pfizer, Novartis, Genentech, Bristol Myers Squibb, EMD Serono, Immunomedics, Gilead Sciences
Speakers' Bureau: Janssen, Astellas Medivation, Pfizer, Novartis, Sanofi, Genentech/Roche, Eisai, AstraZeneca, Bristol Myers Squibb, Amgen, Exelixis, Exelixis, EMD Serono, Merck, Seattle Genetics/Astellas, Seattle Genetics/Astellas, Myovant Sciences, Gilead Sciences, AVEO
Research Funding: Genentech, Exelixis, Janssen, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Macrogenics, Astellas Pharma, BeyondSpring Pharmaceuticals, BioClin Therapeutics, Clovis Oncology, Bavarian Nordic, Seattle Genetics, Immunomedics, Epizyme
Travel, Accommodations, Expenses: Genentech, Prometheus, Astellas Medivation, Janssen, Eisai, Bayer, Pfizer, Novartis, Exelixis, AstraZeneca
Yohann Loriot
Honoraria: Sanofi, Pfizer
Consulting or Advisory Role: Janssen, Astellas Pharma, Roche, AstraZeneca, MSD Oncology, MSD Oncology, Seattle Genetics, Bristol Myers Squibb, Immunomedics, Taiho Pharmaceutical
Research Funding: Sanofi, Janssen Oncology, MSD Oncology, AstraZeneca, Clovis Oncology, Exelixis, Boehringer Ingelheim, Incyte, Pfizer, Oncogenex, Medivation, CureVac, Nektar
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology, Roche, MSD Oncology, AstraZeneca, Seattle Genetics
Aude Flechon
Honoraria: MSD Oncology, AstraZeneca, Bristol Myers Squibb, Janssen-Cilag, Astellas Pharma, Pfizer, Sanofi/Aventis, Roche/Genentech, Bayer, Ipsen, AAA HealthCare
Travel, Accommodations, Expenses: Astellas Pharma, Sanofi/Aventis, Janssen-Cilag, Bayer, Pfizer, Ipsen, Bristol Myers Squibb, AstraZeneca, MSD Oncology, Roche/Genentech, AAA HealthCare
Rohit Jain
Honoraria: Alphasights
Consulting or Advisory Role: Taiho Oncology, Pfizer, Seattle Genetics/Astellas, Gilead Sciences, EMD Serono
Speakers' Bureau: Seattle Genetics/Astellas
Neeraj Agarwal
Consulting or Advisory Role: Pfizer, Medivation/Astellas, Bristol Myers Squibb, AstraZeneca, Nektar, Lilly, Bayer, Foundation One Inc, Pharmacyclics, Foundation Medicine, Astellas Pharma, Lilly, Exelixis, AstraZeneca, Pfizer, Merck, Novartis, Lilly, Eisai, Seattle Genetics, EMD Serono, Janssen Oncology, AVEO, Calithera Biosciences, MEI Pharma, Genentech, Astellas Pharma
Research Funding: Bayer, Bristol Myers Squibb, GlaxoSmithKline, Takeda, Novartis, Pfizer, BN ImmunoTherapeutics, Exelixis, TRACON Pharma, Rexahn Pharmaceuticals, Amgen, AstraZeneca, Active Biotech, Bavarian Nordic, Calithera Biosciences, Celldex, Eisai, Genentech, Immunomedics, Janssen, Merck, Newlink Genetics, Prometheus, Sanofi
Manojkumar Bupathi
Honoraria: Bristol Myers Squibb, Exelixis, AstraZeneca, Pfizer, Astellas Pharma
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Exelixis
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Pfizer, Exelixis, Astellas Pharma
Philippe Barthelemy
Honoraria: Ipsen, Bristol Myers Squibb, MSD, Astellas Pharma, Janssen-Cilag, Pfizer, Merck KGaA
Consulting or Advisory Role: Ipsen, Bristol Myers Squibb, MSD Oncology, Pfizer, Janssen-Cilag, AstraZeneca, Amgen
Travel, Accommodations, Expenses: Bristol Myers Squibb, Pfizer, Janssen-Cilag, Astellas Pharma, MSD, Ipsen
Phillip Palmbos
Research Funding: Roche, Immunomedics
Christos Kyriakopoulos
Consulting or Advisory Role: Exelixis, Sanofi, AVEO, EMD Serono, Janssen Oncology
Research Funding: Sanofi
Damien Pouessel
Honoraria: Ipsen, Janssen Oncology, Bristol Myers Squibb, AstraZeneca, Merck, Astellas Pharma
Consulting or Advisory Role: Astellas Pharma, AstraZeneca, Janssen Oncology, Pfizer, Sanofi
Research Funding: Incyte, Merck Sharp & Dohme, Roche, Bristol Myers Squibb, AstraZeneca, Janssen Oncology, Seattle Genetics
Travel, Accommodations, Expenses: Janssen Oncology, AstraZeneca, Pfizer
Cora Sternberg
Consulting or Advisory Role: BMS, MSD, Pfizer, Roche-Genentech, Incyte, AstraZeneca, Merck, Medscape, UroToday, Astellas Pharma, Sanofi-Genzyme, Immunomedics, now Gilead, Foundation Medicine, CCO Clinical, Janssen, NCI
Research Funding: Pfizer, MSD, Astellas, BMS, Immunomedics, now Gilead, Arvinas, Mirati
Quan Hong
Employment: Immunomedics, Gilead Sciences
Leadership: Immunomedics
Stock and Other Ownership Interests: Immunomedics
Trishna Goswami
Employment: Immunomedics/Gilead
Leadership: Immunomedics/Gilead
Stock and Other Ownership Interests: Immunomedics/Gilead
Patents, Royalties, Other Intellectual Property: Patents around combinations with Trodelvy
Travel, Accommodations, Expenses: Immunomedics/Gilead
Loretta Itri
Employment: Immunomedics/Gilead
Leadership: Immunomedics/Gilead
Stock and Other Ownership Interests: Immunomedics
Consulting or Advisory Role: Immunomedics
Travel, Accommodations, Expenses: Immunomedics
Petros Grivas
Consulting or Advisory Role: Merck, Bristol Myers Squibb, AstraZeneca, Clovis Oncology, EMD Serono, Seattle Genetics, Foundation Medicine, Pfizer, Janssen, Bayer, Genzyme, Mirati Therapeutics, Exelixis, Roche, GlaxoSmithKline, Genentech, Immunomedics, Dyania Health, Infinity Pharmaceuticals, QED Therapeutics, 4D Pharma PLC
Research Funding: Pfizer, Clovis Oncology, Bavarian Nordic, Immunomedics, Bristol Myers Squibb, Debiopharm Group, Merck, QED Therapeutics, Kure It Cancer Research, GlaxoSmithKline, Mirati Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer Version 5.2020. https://www.nccn.org/professionals/physician_gls/ [Google Scholar]
- 2.Petrylak DP de Wit R Chi KN, et al. : Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): A randomised, double-blind, phase 3 trial. Lancet 390:2266-2277, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Raggi D Miceli R Sonpavde G, et al. : Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: A systematic review and meta-analysis. Ann Oncol 27:49-61, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Niegisch G Gerullis H Lin SW, et al. : A real-world data study to evaluate treatment patterns, clinical characteristics and survival outcomes for first- and second-line treatment in locally advanced and metastatic urothelial cancer patients in Germany. J Cancer 9:1337-1348, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fradet Y Bellmunt J Vaughn DJ, et al. : Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of > 2 years of follow-up. Ann Oncol 30:970-976, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Lorenzo G Buonerba C Bellelli T, et al. : Third-line chemotherapy for metastatic urothelial cancer: A retrospective observational study. Medicine (Baltimore) 94:e2297, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlachostergios PJ Jakubowski CD Niaz MJ, et al. : Antibody-drug conjugates in bladder cancer. Bladder Cancer 4:247-259, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padcev [Package Insert]. Northbrook, IL, Astellas Pharma US, 2019 [Google Scholar]
- 9.FDA Grants Accelerated Approval to Enfortumab Vedotin-ejfv for Metastatic Urothelial Cancer [Press Release]. Silver Spring, MD: US Food and Drug Administration, December 19, 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-enfortumab-vedotin-ejfv-metastatic-urothelial-cancer [Google Scholar]
- 10.Loriot Y Necchi A Park SH, et al. : Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 381:338-348, 2019 [DOI] [PubMed] [Google Scholar]
- 11.de Almeida Carvalho LM de Oliveira Sapori Avelar S Haslam A, et al. : Estimation of percentage of patients with fibroblast growth factor receptor alterations eligible for off-label use of erdafitinib. JAMA Netw Open 2:e1916091, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trerotola M Cantanelli P Guerra E, et al. : Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene 32:222-233, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Avellini C Licini C Lazzarini R, et al. : The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget 8:58642-58653, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shvartsur A, Bonavida B: Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 6:84-105, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stepan LP Trueblood ES Hale K, et al. : Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: Potential implications as a cancer therapeutic target. J Histochem Cytochem 59:701-710, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldenberg DM, Stein R, Sharkey RM: The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 9:28989-29006, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muhlmann G Spizzo G Gostner J, et al. : TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol 62:152-158, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Ohmachi T Tanaka F Mimori K, et al. : Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res 12:3057-3063, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Fang YJ Lu ZH Wang GQ, et al. : Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis 24:875-884, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Fong D Spizzo G Gostner JM, et al. : TROP2: A novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol 21:186-191, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Fong D Moser P Krammel C, et al. : High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer 99:1290-1295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fornaro M Dell'Arciprete R Stella M, et al. : Cloning of the gene encoding Trop-2, a cell-surface glycoprotein expressed by human carcinomas. Int J Cancer 62:610-618, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Stoyanova T Goldstein AS Cai H, et al. : Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via beta-catenin signaling. Genes Dev 26:2271-2285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arafat W Stahlfeld C Sperger JM, et al. : Intra-patient heterogeneity in urothelial cancer (UC) circulating tumor cells (CTC) and PDL1 expression to identify biomarkers of response and new therapeutic targets: A pilot study. J Clin Oncol 35:4537, 2017. (15 suppl; abstr) [Google Scholar]
- 25.Goldenberg DM Cardillo TM Govindan SV, et al. : Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 6:22496-22512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardillo TM Govindan SV Sharkey RM, et al. : Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: Preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res 17:3157-3169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardillo TM Govindan SV Sharkey RM, et al. : Sacituzumab govitecan (IMMU-132), an anti-trop-2/SN-38 antibody-drug conjugate: Characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem 26:919-931, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Starodub AN Ocean AJ Shah MA, et al. : First-in-human trial of a novel anti-trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res 21:3870-3878, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Govindan SV, Cardillo TM, Goldenberg DM: Topoisomerase inhibitors as antibody-drug conjugate payloads, in Thurston DE, Jackson PJM. (eds): Cytotoxic Payloads for Antibody-Drug Conjugates. London, UK: The Royal Society of Chemistry, 2019, pp 166-186 [Google Scholar]
- 30.Ocean AJ Starodub AN Bardia A, et al. : Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics. Cancer 123:3843-3854, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Tagawa ST Faltas BM Lam ET, et al. : Sacituzumab govitecan (IMMU-132) in patients with previously treated metastatic urothelial cancer (mUC): Results from a phase I/II study. J Clin Oncol 37:354, 2019 [Google Scholar]
- 32.Faltas B Goldenberg DM Ocean AJ, et al. : Sacituzumab govitecan, a novel antibody—drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer 14:e75-79, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Therasse P Arbuck SG Eisenhauer EA, et al. : New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Tagawa ST Balar A Petrylak DP, et al. : A phase 2 open-label study of sacituzumab govitecan in patients with metastatic urothelial cancer (mUC) after failure of platinum-based regimens or immunotherapy [presentation]. Annual Meeting of the European Society for Medical Oncology. Barcelona, Spain, 2019
- 35.Clopper CJ, Pearson ES: The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404-413, 1934 [Google Scholar]
- 36.Bellmunt J Choueiri TK Fougeray R, et al. : Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 28:1850-1855, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg JE O'Donnell PH Balar AV, et al. : Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 37:2592-2600, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellmunt J Theodore C Demkov T, et al. : Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 27:4454-4461, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Krege S Rembrink V Börgermann C, et al. : Docetaxel and ifosfamide as second line treatment for patients with advanced or metastatic urothelial cancer after failure of platinum chemotherapy: A phase 2 study. J Urol 165:67-71, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Sridhar SS Blais N Tran B, et al. : Cctg BL12: Randomized phase II trial comparing nab-paclitaxel (Nab-P) to paclitaxel (P) in patients (pts) with advanced urothelial cancer progressing on or after a platinum containing regimen (NCT02033993). J Clin Oncol 36:4505, 2018. (15 suppl; abstr) [Google Scholar]
- 41.Bambury RM Benjamin DJ Chaim JL, et al. : The safety and efficacy of single-agent pemetrexed in platinum-resistant advanced urothelial carcinoma: A large single-institution experience. Oncologist 20:508-515, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grivas P Mortazavi A Picus J, et al. : Mocetinostat for patients with previously treated, locally advanced/metastatic urothelial carcinoma and inactivating alterations of acetyltransferase genes. Cancer 125:533-540, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grivas P Loriot Y Feyerabend S, et al. : Rucaparib for recurrent, locally advanced or metastatic urothelial carcinoma: Results from ATLAS, a phase 2, open-label trial [oral presentation], Annual Genitourinary Cancers Symposium. San Francisco, CA, 2020
- 44.Rosenberg JE Hoffman-Censits J Powles T, et al. : Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 387:1909-1920, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powles T O'Donnell PH Massard C, et al. : Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol 3:e172411, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel MR Ellerton J Infante JR, et al. : Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN solid tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 19:51-64, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellmunt J de Wit R Vaughn DJ, et al. : Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376:1015-1026, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma P Siefker-Radtke A de Braud F, et al. : Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: CheckMate 032 nivolumab 1 mg/kg plus ipilimumab 3 mg/kg expansion cohort results. J Clin Oncol 37:1608-1616, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barata PC Gopalakrishnan D Koshkin VS, et al. : Atezolizumab in metastatic urothelial carcinoma outside clinical trials: Focus on efficacy, safety, and response to subsequent therapies. Target Oncol 13:353-361, 2018 [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg JE Sridhar SS Zhang J, et al. : Mature results from EV-101: a phase I study of enfortumab vedotin in patients with metastatic urothelial cancer (mUC). J Clin Oncol 37:377, 2019. (suppl 7S; abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seattle Genetics and Astellas Announce PADCEV® (Enfortumab Vedotin-ejfv) Significantly Improved Overall Survival in Phase 3 Trial in Previously Treated Locally Advanced or Metastatic Urothelial Cancer [Press Release]. Bothell, WA: Seattle Genetics, 2020. https://www.astellas.com/en/news/16026 [Google Scholar]
- 52.Trodelvy [package Insert]. Morris Plains, NJ, Immunomedics,, 2020 [Google Scholar]
- 53.Bardia A Mayer IA Vahdat LT, et al. : Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med 380:741-751, 2019 [DOI] [PubMed] [Google Scholar]
- 54.Gray JE Heist RS Starodub AN, et al. : Therapy of small cell lung cancer (SCLC) with a topoisomerase-I-inhibiting antibody-drug conjugate (ADC) targeting Trop-2, sacituzumab govitecan. Clin Cancer Res 23:5711-5719, 2017 [DOI] [PubMed] [Google Scholar]
- 55.Heist RS Guarino MJ Masters G, et al. : Therapy of advanced non-small-cell lung cancer with an SN-38-anti-Trop-2 drug conjugate, sacituzumab govitecan. J Clin Oncol 35:2790-2797, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Trodelvy [package insert]. Morris Plains, NJ, Immunomedics, Inc, 2021. [Google Scholar]
- 57.Bardia A Hurvitz SA Tolaney SM, et al. : Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med (in press) [DOI] [PubMed] [Google Scholar]
- 58.FDA grants accelerated approval to sacituzumab govitecan for advanced urothelial cancer [press release]. Silver Spring, MD: US Food and Drug Administration, April 13, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sacituzumab-govitecan-advanced-urothelial-cancer [Google Scholar]