Abstract
Mutations to lamins in skeletal muscle cells have been shown to reduce nuclear stability, increase nuclear envelope rupture, and induce DNA damage and cell death. New research shows that limiting mechanical loads can rescue myofibre function and viability.
Within the inner nuclear membrane, the nuclear genome is enclosed by a fibrous meshwork formed by a special class of intermediate filament proteins, primarily A-type and B-type lamins. Lamins are in intimate contact with chromatin, nuclear envelope proteins and nuclear pore complexes, and also with the cytoskeleton via proteins that transverse the nuclear membrane. Laminopathies are a subgroup of rare diseases caused by mutations in the Lmna gene encoding for A-type lamins1, which include muscular dystrophies such as Emery–Dreifuss muscular dystrophy type 2 and limb girdle muscular dystrophy type 1B, dilated cardiomyopathy, lipodystrophies, neuropathies, and premature ageing syndromes such as Hutchinson–Gilford progeria syndrome and atypical Werner syndrome. While the gene is expressed in nearly all differentiated cell types, most cases of Lmna mutations are dominated by pathologies of cardiac and skeletal muscle. The underlying mechanism for the pathophysiology of laminopathies has been much debated but there are a number of hypotheses proposed so far2. One such hypothesis is based on mechanical stress and it posits that mutant lamins undermine the architectural support for nuclei, compromising their structural integrity in the face of cellular forces. In this issue, Jan Lammerding and colleagues3 present strong evidence for the mechanical stress hypothesis. Their findings explain the peculiar sensitivity of contractile tissues to Lmna mutations and point to directions for potential therapeutic intervention.
Lammerding and colleagues exploit three mouse models of striated muscle laminopathies with varying degrees of severity and an in vitro muscle differentiation strategy to dissect functional relationships between skeletal muscle mechanics, nuclear integrity and DNA damage. They find that Lmna gene mutations result in mechanically weaker and more deformable myonuclei, with transient nuclear envelope rupture, chromatin protrusions and DNA damage, ultimately triggering cell death. Importantly, chimaeric myofibres comprising wild-type and Lmna knockout nuclei show that the defects are intrinsic to the genotype of the nuclei and not due to changes in the shared cytoplasm containing both types of nuclei.
Nuclear envelope rupture is shown by the binding of the protein cyclic GMP-AMP synthase (cGAS) to DNA exposed to the cytoplasm. To test for nuclear envelope rupture in vivo, the authors generate a Lmna knockout mouse expressing a fluorescently tagged cGAS, which was then bred with the lamin-mutant strains. They found a large fraction of Lmna-deficient myonuclei in hindlimb muscle fibres that are positive for fluorescent cGAS foci, which were absent in wild-type mice. Another cytoplasmic marker, the chaperone protein Hsp90, also accumulated in the nucleus of Lmna knockout mice, confirming nuclear envelope rupture.
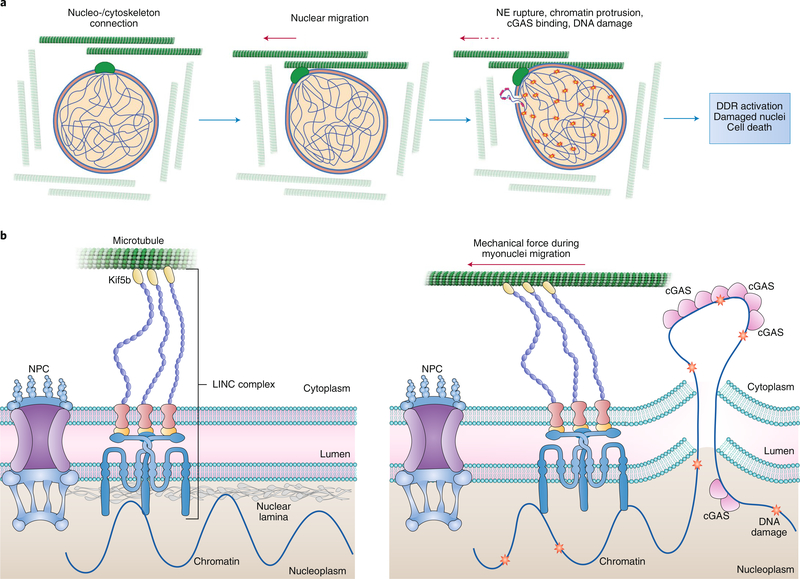
The etiology of nuclear rupture in differentiating myocytes seems to be the mechanical force that the cytoskeleton microtubules and motor proteins impose on a weak nucleoskeleton-dysfunctional lamina via the linker of nucleoskeleton and cytoskeleton (LINC) complex during nuclei migration along the length of myotubes. As such, disruption of the LINC complex or depletion of Kif5b, a protein necessary for nuclear migration, inhibits the appearance of chromatin protrusions, cGAS foci and DNA damage accumulation in lamin-deficient muscle cells/fibres (Fig. 1). These data support other studies that suggest a role for cytoskeletal forces such as kinesin motors and actin–myosin contraction4. In striated muscle cells, the microtubule network forms a cage-like structure around the myonuclei during differentiation. Lmna mutant myoblasts show no nuclear abnormalities prior to differentiation. A previous study implicated microtubule nucleation from the nuclear envelope in the mechanism of myotube nuclear spreading5. Lammerding and colleagues also show that treating in vitro differentiated Lmna knockout myoblasts with low doses of the microtubule-stabilizing drug, paclitaxel, significantly reduces nuclear deformation and fragility in response to cytoplasmic mechanical load.
Fig. 1 |. Nuclear envelope rupture during migration of myonuclei along microtubules in the course of differentiation.
a, Myonuclei are surrounded by a cage-like structure of microtubules, which are used as a railroad for migration of the nuclei during differentiation. Lammerding and colleagues show that mechanical forces imposed by migration cause nuclear deformation, which in lamin A/C-deficient cells elicits nuclear envelope (NE) rupture, chromatin protrusion into the cytoplasm, binding of cGAS to chromatin at the site of rupture (pink), and DNA damage (stars). Activation of the DNA damage response (DDR) and the accumulation of damaged nuclei leads to the loss/death of muscle cells in laminopathies. b, Left: the interaction of the nucleoskeleton (nuclear lamina) and the cytoskeleton (microtubules and motor proteins) via the LINC complex which can transverse the NE. Right: illustration of how the mechanical force on migrating nuclei through the motor protein Kif5b and the LINC complex results in NE rupture, protrusion of chromatin or cGAS entry into the nucleus, cGAS binding and DNA damage.
The researchers also found that DNA damage plays a major role in muscle decline. Chemical induction of DNA damage did not further reduce viability of Lmna knockout muscle cells. Inhibitors of the damage-signalling proteins DNA-protein kinase (DNA-PK) and ataxia telangiectasis mutated (ATM) reduced viability in wild-type myofibres to the levels of Lmna-deficient myofibres, suggesting that DNA damage or inefficient repair contributes to loss of myofibre viability. Lastly, the authors show that skeletal muscle biopsies from patients with lamin-related muscular dystrophies exhibit high levels of DNA damage. Importantly, they rule out an intrinsic DNA repair defect in Lmna-deficient nuclei, which can respond to ionizing radiation and repair DNA damage as efficiently as wild-type nuclei.
The finding that the underlying muscle pathology in laminopathies is a by-product of cytoskeletal forces on muscle nuclei involving kinesin motors has important therapeutic implications. The findings point to a role of microtubules in the formation of nuclear envelope protrusions and breaks in nuclei of Lmna mutant cells. As the authors note, this suggests that muscle pathology could be ameliorated by treatment with microtubule-stabilizing drugs and/or nuclease inhibitors.
References
- 1.Maggi L, Carboni N & Bernasconi P Cells 5, 33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozzetta C & Tedesco FS J. Cell Biol. 218, 2826–2828 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earle AJ et al. Nat. Mater. 10.1038/s41563-019-0563-5 (2019). [DOI] [Google Scholar]
- 4.Cho S et al. Dev. Cell 49, 920–935 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimpel P et al. Curr. Biol. 27, 2999–3009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]