Abstract
Purpose.
Caspase-associated recruitment domain-9 (CARD9) deficiency is an inborn error of immunity that typically predisposes otherwise healthy patients to single fungal infections and the occurrence of multiple invasive fungal infections is rare. It has been described as the first known condition that predisposes to extrapulmonary Aspergillus infection with preserved lungs. We present a patient that expands the clinical variability of CARD9 deficiency.
Materials and methods.
Genetic analysis was performed by Sanger sequencing. Neutrophils and mononuclear phagocyte response to fungal stimulation were evaluated through luminol-enhanced chemiluminescence and whole blood production of the proinflammatory mediator IL-6 respectively.
Results.
We report a 56-year-old Argentinean woman, whose invasive Exophiala spinifera infection at the age of 32 years was unexplained and reported in year 2004. At the age of 49 years, she presented with chronic pulmonary disease due to Aspergillus nomius. After partial improvement following treatment with caspofungin and posaconazole, right pulmonary bilobectomy was performed. Despite administration of multiple courses of antifungals, sustained clinical remission could not be achieved. We recently found that the patient’s blood showed an impaired production of interleukin (IL)-6 when stimulated with zymosan. We also found that she is homozygous for a previously reported CARD9 loss-of-function mutation (Q289*).
Conclusions.
This is the first report of a patient with inherited CARD9 deficiency and chronic invasive pulmonary aspergillosis (IPA) due to A. nomius. Inherited CARD9 deficiency should be considered in otherwise healthy children and adults with one or more invasive fungal diseases.
Keywords: Inherited CARD9 deficiency, Exophiala spinifera, Aspergillus nomius, chronic invasive pulmonary aspergillosis, phaeohyphomycosis
Introduction
Exophiala is the major genus of black yeasts and includes numerous ubiquitous species. Melanin deposited in their cell walls is responsible for their dark color and is believed to be a virulence factor; therefore, black fungi are overrepresented as etiologic agents of opportunistic infection. It has been reported as a causative agent of both phaeohyphomycosis and rare cases of chromoblastomycosis, classically featured by the presence of muriform cells [1]. Clinical pathology of phaeohyphomycosis due to E. spinifera develops almost exclusively from traumatic introduction in or beneath the skin deriving in a suppurative foreign body reaction. Hematogenous spread of the fungus to one or more distant sites may result in bone, joints, lymphatics, eyes, and visceral organ locations. Secondary cutaneous lesions emerging on all parts of the body are characterized by hyperproliferation rather than necrosis [1]. Overall, two patient groups have been described [2]. On the one hand, otherwise healthy children and adolescents at risk of developing fatal disseminated infection failing in response to standard antifungal agents and on the other, elderly patients with mild disease symptoms and successful recovery. Possibly several of the disseminated cases were patients with undisclosed congenital immune defects. Inherited susceptibility to invasive Exophiala infection has been so far described only in patients with chronic granulomatous disease (CGD) or inherited CARD9 deficiency [3,4].
Aspergillus nomius is an aflatoxin-producing member of Aspergillus section Flavi that shows a cosmopolitan distribution. It has been described so far as a human pathogen in a case of breakthrough pneumonia in a patient with acute myeloid leukemia [5]. In parallel, A. nomius has also been isolated from single cases of keratitis after ocular injury and onychomycosis in otherwise healthy patients [6,7]. As far as we know, invasive pulmonary aspergillosis (IPA), whose most frequent causal agent is A. fumigatus, has not been reported to be caused by A. nomius [8]. As general consideration, chronic IPA progresses from bronchi to respiratory tree and bronchial wall and finally into the adjacent lung parenchyma. Single or multiple pulmonary cavities, fibrosis, and epithelioid granuloma with giant cells surrounding the invasive fungal hyphae can be observed [9,10]. The risk of IPA is predicted by the intensity and duration of neutropenia particularly in patients with hematologic malignancies and myelodysplastic syndrome [11,12]. Angioinvasion is involved in the pathogenesis in neutropenic hosts and is responsible for the higher frequency of dissemination to other organs such as skin, brain or eyes. It is not commonly seen in patients with normal neutrophils count [8]. Chronic invasive pulmonary infections by the ubiquitous inhaled mold Aspergillusis associated with defects in immune host responses and/or alterations of pulmonary architecture [8]. Patients with malignancies, organ transplantation, autoimmune or inflammatory conditions, as well as patients with chronic obstructive pulmonary disease appear to be at an increased risk [8]. Invasive aspergillosis in patients with inherited defects of innate immunity is rare, and pulmonary involvement has been mainly associated to CGD, autosomal dominant (AD) hyper-IgE syndrome due to STAT3 deficiency, or AD GATA binding protein 2 (GATA2) deficiency [13]. Invasive A. fumigatus disease in patients with autosomal recessive (AR) CARD9 deficiency has been referred to show predilection for non-pulmonary sites with little impact on the lungs [14,15]. We report here a case of a 56-year-old woman who developed invasive chronic pulmonary aspergillosis caused by A. nomius after suffering for 21 years with adult-onset disseminated phaeohyphomycosis caused by E. spinifera with normal basic immunophenotyping and fatal evolution [16].
Materials and methods
Patient
We studied an otherwise healthy 56-year-old woman from Argentina with invasive fungal disease. Informed consent for participation in this study was obtained in accordance with local regulations, with approval from the IRB.
Blood cell culture and stimulation
Whole blood samples were diluted 1/2 with RPMI 1640 (GIBCO BRL) and stimulated with unopsonized zymosan, (5 μg/ml, InvivoGen), LPS from Salmonella minnesota (100 ng/ml, Sigma-Aldrich), PMA (10−7 M; Sigma-Aldrich) with Ionomycin (10−5 M; Sigma-Aldrich). Supernatants were recovered after 48 hours and they were used for ELISA. Patient samples have been run in parallel to two age-matched healthy controls.
Polymorphonuclear leukocytes (PMNs) separation
Suspension of PMNs was prepared from heparinized peripheral blood by gravity sedimentation with Ficol-Hypaque centrifugation and Dextran. PMN preparations contained >95% neutrophils.
Reactive Oxygen Species (ROS) production by chemiluminescence
Luminol-enhanced chemiluminescence (CL) response of purified neutrophils (4 × 106/ml) in HBSS without phenol red (GIBCO BRL) to unopsonized zymosan (Z) (5μg/ml, InvivoGen) were monitored for 60 min using a BioTek FLx800 microplate reader. The instrument was programmed with a 2-min count per well, it was controlled at room temperature and the plate was not shaken during the run except for automatic movement of the plate between counting cycles.
Cytokine analysis
IL-6 concentrations in supernatants were measured by enzyme-linked immunosorbent assay (ELISA), according to the kit manufacturer’s instructions (Pierce Biotechnology, Thermo Scientific). Cytokine production was normalized according to the number of PBMCs in the individual tested.
DNA sequencing
Genomic DNA was isolated from whole blood. Exon 6 of CARD9was amplified with specific primers (Forward primer sequence: 5’ GGCACACCTCATCTGCATGC 3’ and reverse primer sequence: 5’ CACTGTGCCTCCAGGAGTGG 3’; Tm = 59°C) with DreamTaq™ from Thermofisher Scientific. PCR products were analyzed by electrophoresis in 1% agarose gels and then sequenced with the Big Dye Terminator cycle sequencing kit V3.1™ (Applied Biosystems, Foster City, Calif) using an ABI Prism 3700 apparatus (Applied Biosystems, Foster City, Calif). Sequences were analyzed with Sequence Scanner V1.0 software (Applied Biosystems, Foster City, Calif).
Results
Case report
We studied an otherwise healthy 56-year-old woman from Santiago del Estero, Argentina, born to healthy non-consanguineous parents (Fig 1A). She has been previously reported as an extremely rare case of invasive infection with Exophiala spinifera at the age of 32 years. At that time, she was referred to the mycology unit of Francisco Javier Muñiz Infectious Diseases Hospital, Buenos Aires, Argentina [16,17]. On admittance, her medical history consisted in congenital kidney agenesis and intermittent asthma episodes. The patient had phaeohyphomycosis caused by E.spinifera with involvement of skin and lymph nodes (Fig 1B and Fig 1C). The fungal pathogen was identified by examination of a biopsy specimen from carotid lymph node based on both macroscopic appearance and microscopic examination of fungal morphology (Fig 1C) and further molecular studies enabled a confirmatory diagnosis. Right side of forehead showed a light-brown plaque with raised borders measuring 3 cm in diameter, similar to a granuloma annulare. Physical examination revealed right-sided, hard-elastic, 3 cm in diameter submandibular lymphadenopathy attached to the skin, and several little tubercles in thighs and on the abdomen. Chest X-ray revealed diffuse lung infiltrate in the left upper lobe. Counter-immunoelectrophoresis searching for antibodies against a metabolic antigen of E. spinifera presented two anodic and two cathodic bands and immunodiffusion test showed two precipitation bands. She was orally treated with 400-mg itraconazole/day plus 100-mg 5-fluorocytosine/kg/day for 6 months. Cutaneous lesions disappeared and lymphadenopathy diminished. At age 35, she was in good clinical condition; submandibular lymphadenopathy was persistent and had developed vegetating cutaneous lesions with serohematic scabs measuring 7 cm in diameter on the arms, and cervical lymph nodes enlargement. At this time, lesions failed to ameliorate when the previously successful combined antifungal regimen was administered. Over the next six years, various antifungal agents were indicated: amphotericin B deoxycholate, liposomal amphotericin B, griseofulvin, and 500-mg terbinafine/day alone or associated with 400-mg itraconazole/day. However, slow progression of cutaneous lesions and cervical lymph node swelling could not be prevented. At age 41 and during pregnancy, she presented lymphadenopathies, anemia, fever, loss of body weight and deterioration of existing skin lesions as well as the appearance of new cutaneous tubercles. At 32 weeks of gestation, in order to administrate a new antifungal treatment, a preterm cesarean delivery and interrupting breastfeeding were indicated. After childbirth, she presented sepsis, fever, anemia, leukocytosis and eosinophilia, suppurative axillary and inguinal lymphadenopathies, and bilateral scrofuloderma in the neck. Right knee showed tumefaction, erythema, pain, limited movement and positive patellar tap test. Right knee X-ray revealed two isolated 2 × 4 cm osteolytic lesions located at both tibia’s proximal region (Fig 1B and Fig 1C) and right femoral condyle. Fluid accumulation was evidenced by knee joint ultrasound. She received 800-mg posaconazole oral suspension /day for 18 months; this treatment was well tolerated and showed good clinical response. The patient improved her general condition, and blood cell counts normalized. All cutaneous and almost all lymph node lesions disappeared. Submandibular lymphadenopathy diminished in size, but tibia osteolytic lesion persisted. Negative fungal culture from bone biopsy determined the cessation of the treatment. Three months later, lesions recurred and the same antifungal treatment regimen was indicated. This time a very slow progression of all lesions was observed. At age 47, regular treatment indication was changed to 400-mg voriconazole/day since posaconazole provision had been discontinued. After 4 months of transient improvement, lesions started to grow again. 800-mg posaconazole oral suspension /day was then indicated as before. Between the ages of 49 and 53 years, the patient experienced three episodes of abscessed pneumonia. Fresh preparations of three broncho-alveolar lavage specimens examined by direct microscopy showed hyaline, septate hyphae and an Aspergillus section Flavi was isolated in cultures (Fig 1D), it was identified as A. nomius by molecular biology-base techniques. Immunodiffusion and counter-immunoelectrophoresis tests searching for antibodies against an A. nomius metabolic antigen presented positive results. Fifty mg caspofungin acetate/day by intravenous infusion in association with oral 800-mg posaconazole/day were administered. She had a favorable clinical response; however, a dense pulmonary infiltrate with pseudo-tumoral aspect persisted at the middle and lower lobes of the right lung (Fig 1B). At age 54, removal of right middle and lower lobes was performed, after which there was no evidence of aspergillosis recurrence. Histopathological study of surgical specimen was compatible with invasive chronic pulmonary aspergillosis with blood vessels involvement (Fig 1C). Thin septate hyphae with dichotomic branching within epithelioid granuloma with giant cells inside arterioles were observed. Hyphae were also present in necrotic areas. The lung architecture was preserved in extensive areas and an intense mononuclear inflammatory response lacking polymorphonuclear neutrophils located near blood vessels was observed. At age 55, phaeohyphomycosis was following a chronic and progressive course when overt worsening of cutaneous, lymph nodes, eyes and osteoarticular lesions occurred, besides loss of weight and deterioration of the general condition. At age 56, recombinant interferon gamma-1b 100 micrograms/vial was subcutaneously administered three times a week, in two three-month periods each, in association with posaconazole. Evident reduction of the skin lesions after the first course was achieved; in contrast, in the second phase of the treatment the patient showed only a modest improvement. Besides eosinophilia (18% - 2280/mm3), blood cell counts were within normal ranges. Classical immunological examination showed normal IgG, IgA and IgM levels, CD4+ and CD8+ T cell counts. A dihydrorhodamine123 (DHR) assay on neutrophils stimulated with Phorbol 12- Myristate 13-Acetate (PMA) showed that the respiratory burst was normal. HIV infection was ruled out by laboratory testing. At age 57, the homozygous CARD9 mutation was identified. At that time there was a decline in her general condition and a worsening of all original lesions; she became blind and cachectic, and died at 58 years.
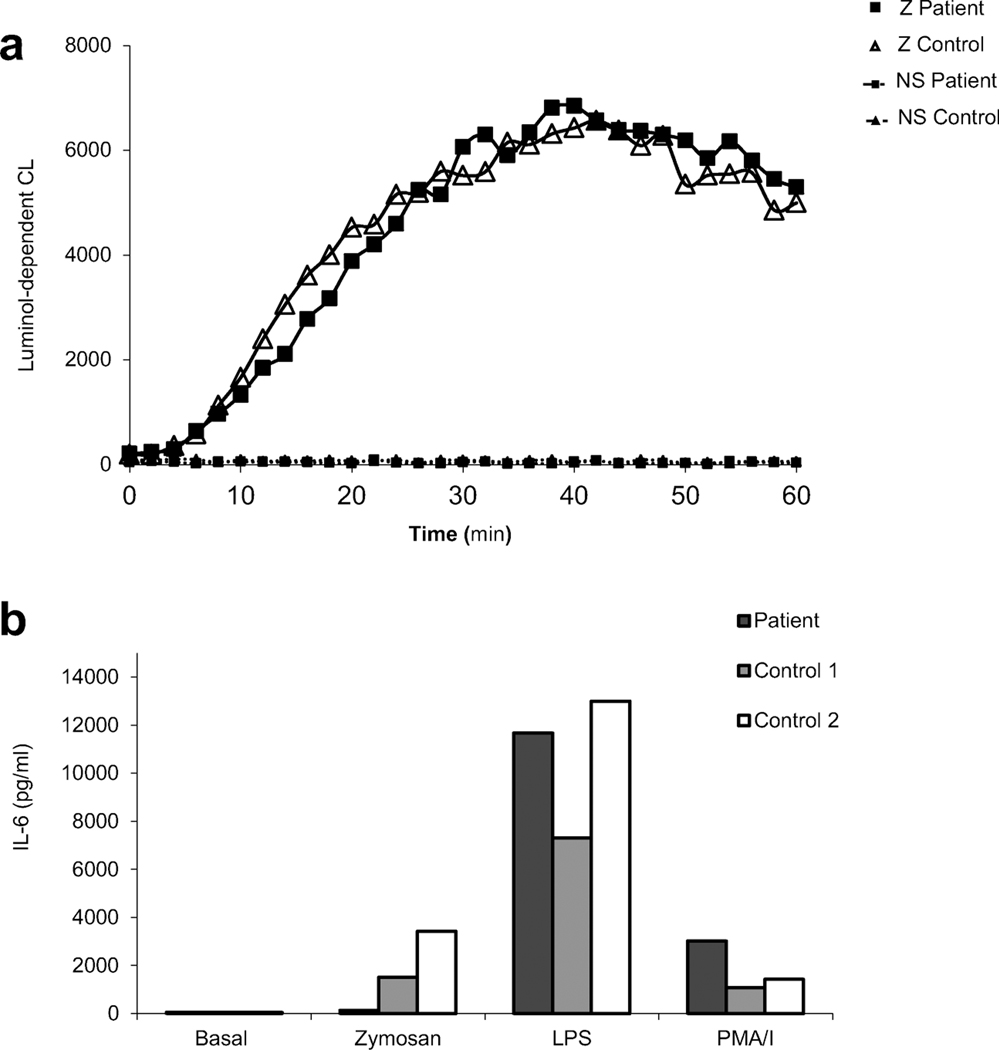
Figure 1. Identification and characterization of CARD9 deficiency.
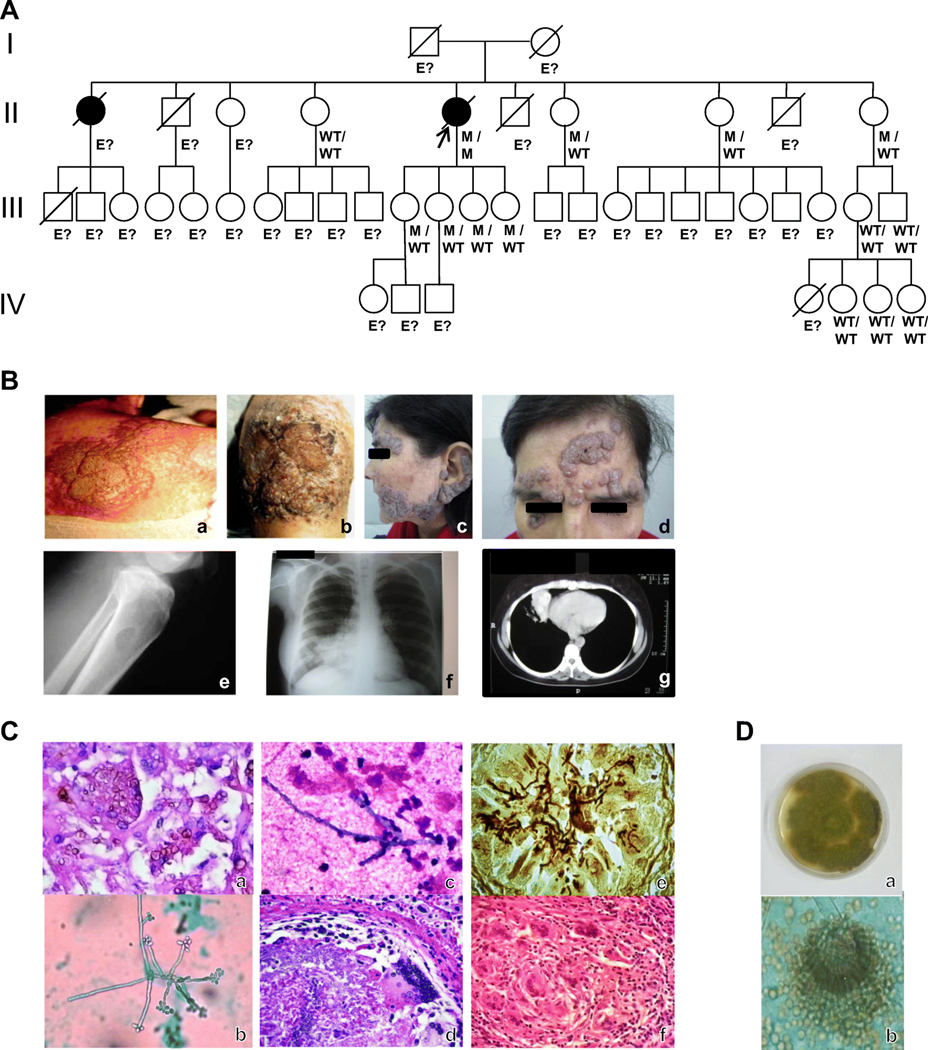
A. Pedigree of the family. I-IV (left margin) indicate generations; arrow indicates proband; black symbols indicate patients with phaeohyphomycosis. M, mutant; WT. wild-type; “E?” indicates those whose genetic status could not be evaluated. (Collection of pedigree information may be incomplete). B. Clinical and radiological features of the patient. Vegetating, verrucous skin lesions due to E. spinifera (a-d): on the back (a), on the knee (b), on the cheek and outer ear (c), and on the forehead (d). Phaeohyphomycosis involving the bone: osteolytic lesion at tibia’s proximal region (e). Imaging findings in pulmonary aspergillosis (f,g): chest radiography showing dense pulmonary infiltrate in the middle lobe of right lung (f). Chest computed tomography scan showing pseudotumoral lesion in the right lung (g). C. Microscopic and histological features of E. spinifera (a,b,c) and A. nomius (d,e,f) infection. Histopathological study of a lymph node biopsy specimen showing numerous giant cells with round brown bodies inside (H&E stain; original magnification, X400) (a). Microculture of Exophiala spinifera (Lactophenol cotton blue stain; original magnification, X1000) (b). Giemsa stained smear taken from the draining fistula arising from the osteolytic lesion in the tibia to the skin, showing neutrophilic accumulation around Exophiala hyphae (c). Histopathological study of lung surgical piece showing an epithelioid granuloma with a giant cell surrounding fungal hyphae within an arteriole (H&E stain; original magnification, X400) (d). Histopathological preparation of tissue sections of lung. Fungal hyphae within an arteriole are observed (Grocottś methenamine-silver stain, original magnification, X400) (e). Histopathological study of lung surgical piece showing an epithelioid granuloma with giant cells. No neutrophils are seen (H&E stain, original magnification, X200) (f). D. Macro- and micromorphological aspects of Aspergillus nomius. Rough colonies of yellowish-green color and wooly texture (a). Radiate heads with uniseriate and biseriate conidiogenous cells, hyaline rough-walled conidiophores and echinulate, spherical conidia (b).
“In vitro” antifungal susceptibility tests revealed that voriconazole, posaconazole and caspofungin were the most active drugs against the isolated strain of E. spinifera, showing a Minimum Inhibitory Concentration (MIC) value of 0.03 μg/ml. They were followed by amphotericin B and itraconazole in order of activity. On the other hand, A. nomius was susceptible to terbinafine (MIC value of 0.03 μg/ml) and resistant to amphotericin B (MIC value of 2μg/ml). Patient’s family history included a sister suffering from phaeohyphomycosis caused by Alternaria tenuis. Her disease evolved to destruction of the facial bones resembling malignant midline granulomatosis. She died under not well documented circumstances at 38 years of age, after controlling the fungal infection with griseofulvin.
Genetic analysis
By Sanger sequencing, the patient was found to be homozygous for the already reported c.865C>T loss-of-function (LOF) mutation located in exon 6 (Fig S1) of CARD9. This mutation results in a premature termination codon in position 289, p.Q289*, in the region encoding the coiled-coil domain of CARD9 protein (18). This mutation has been reported in other patients with CARD9 deficiency. The family members for whom genetic material was available were found to be homozygous for the wild-type (WT) allele or heterozygous (Fig 1A).
ROS production and cytokine analysis
CARD9 is mainly expressed in myeloid cells [19]. Neutrophil functions have been studied in patients with CARD9 deficiency and the results suggest the involvement of this protein in the recruitment of neutrophils to the site of infection and to some degree in the selective killing of some few fungi [20]. Production of hydrogen peroxide (H2O2) induced by several stimuli was evaluated through DHR and Amplex Red assays, and normal NADPH oxidase function has been found in the so far studied patients [15,21–25]. Considering that none of them carried p.Q289* mutation, and since inherited susceptibility to invasive Exophiala infection and IPA has been described in patients with reduced ROS generation that occurs in chronic granulomatous disease (CGD) (3,13), we decided to evaluate NADPH oxidase function through luminol-enhanced chemiluminescence, a common technique to measure both intracellular and extracellular ROS, including superoxide anion and H2O2 [26]. When stimulated with unopsonized yeast-derived particle zymosan, ROS amounts produced by patient’s neutrophils were essentially identical to that produced by neutrophils from healthy control (Fig 2A). At the same time, we investigated the production of IL-6 in whole blood activation assay. Production of IL-6 was similar to healthy control in response to LPS and phorbol ester (PMA)/Ionomycin (I) (Fig 2B). However, mononuclear phagocyte response was affected with an impaired whole blood production of the pro-inflammatory mediator IL-6 upon fungal-specific stimulation (Fig 2B). Whole blood production of IL-8 as a read-out of neutrophils response to zymosan was not evaluated.
Figure 2. ROS and IL-6 production in blood cells of CARD9 deficient patient.
A. Normal NADPH oxidase activity in the patient’s neutrophils after activation with unopsonized zymosan (Z) (5μg/ml), as assessed by the monitoring of luminol-dependent chemiluminescence for 60 minutes at room temperature. B. Interleukin 6 (IL-6) production in the supernatants of cultured whole blood cells stimulated for 48 hours with unopsonized zymosan (Z) (5 μg/ml); LPS from Salmonella Minnesota (100ng/ml) or phorbol 12-myristate 13-acetate (PMA) – Ionomycin (I), 10−7 M and 10−5 M), as measured by enzyme-linked immunosorbent assay (ELISA). The results are representative from one experiment performed using duplicate samples involving two healthy age matched controls.
Discussion
Inherited susceptibility to invasive Exophiala infection (ie, noncontiguous extracutaneous localization) has been reported in two patients with CARD9 deficiency in the past [4]. Invasive E. spinifera disease was recognized in an Iranian woman with CARD9 deficiency who presented with subcutaneous tissue, bone and lung infection at 18 years of age [4]. We describe here an adult-onset patient that has shown sustained infection by E. spinifera for 26 years. The disease involved skin, lymph nodes, bones, joints and eyes. She presented extensive lesions, blindness, cachexia and sepsis at the time of her death. Despite administration of multiple courses of standard antifungals, as well as the immunomodulatory agent IFN-γ, sustained clinical remission has never been achieved. MIC for the administered antifungal drugs showed low values. However, these “in vitro” antifungal susceptibility testing results were inconsistent with the clinical response of the patient. Even when basic immunological evaluation appeared normal, an inherited immunodeficiency condition has never been excluded since her sister had developed a phaeohyphomycosis by A. tenuis with destruction of the facial bones at 37 years of age.
Regarding the features of chronic IPA developed by the patient, some peculiarities deserve to be highlighted, as the recurrent abscessed pneumopathy at presentation under posaconazole administration, the favorable response to caspofungin associated to posaconazole as a treatment, the “in vitro” amphotericin B-resistance of the isolated strain of Aspergillus, and remarkably, the angioinvasive nature of the fungal infection with epithelioid granulomas at blood vessel lumen observed by histopathological examination. Strikingly, no neutrophil infiltration was seen in the Aspergillus-infected lung, which is unexpected considering their central role in controlling this fungal infection. This finding represents the mirror image of infected tissue described in extrapulmonary Aspergillus infection in patients with CARD9 deficiency [15]. Humans constantly inhale mold conidia and experience lifelong asymptomatic clearance. Development of invasive aspergillosis suggests a failure of neutrophils and alveolar macrophages in preventing the transition from these asexual spores to tissue-invasive filaments. Data from mouse models showed that CARD9 controls neutrophil function during respiratory A. fumigatus infection mediated by regulating neutrophil recruitment and phagocytic and conidiacidal activity [27]. However, lack of an association between pulmonary mold infections and CARD9 deficiency has been described in mouse and human and it has been shown that CARD9 deficient mice susceptibility to pulmonary disease caused by A. fumigatus requires a highly supra-physiologic number of conidia [19]. Concurrently, Aspergillus in human CARD9 deficiency has been referred as a fungal agent that shows predilection for non-pulmonary sites with little impact on the lungs [14,15]. Therefore, the clinical case we report here reveals that Aspergillus can show tropism for lung, expanding clinical spectrum of CARD9 deficiency. Patient’s peripheral blood neutrophil count was normal, as well as was the production of ROS upon stimulation with unopsonized zymosan (an agonist of at least Dectin-1 and Toll-like receptor 2). However, we could detect a defect in IL-6 production induced after 48 hours of stimulation with the same agonist in a whole blood assay as a functional consequence of the CARD9 mutation.
CARD9 is an adaptor protein positioned downstream of multiple fungal sensors expressed on myeloid cells that belong to the selective group of C-type lectin receptors (CLRs), including at least Dectin-1, Dectin-2, Dectin-3 and Mincle. Following CLRs ligation, CARD9 complexes with BCL10 and MALT1, a signalosome that is critical for NF-κB and MAPK activation [19]. CARD9 deficiency has been associated with invasive infection due to mostly a single fungus, including Candida spp. [15,21,22,24,28–36], Phialophora verrucosa [37], Trichophyton spp. [15,18,23,30,36,38,39], Exophiala spp. [4,40], Corynespora cassiicola [40–42], Aureobasidium pullulans [35], Ochroconis musae [40], and its growing clinical spectrum has included extrapulmonary Aspergillus infection, related to an inability to recruit neutrophils to the site of infection [15]. All the above mentioned fungi belong to the phylum Ascomycota that contains most of the fungi causing human diseases. Infections caused by Mucor irregularis [43] and Prototheca zopffi, an achlorophyllic microalgae containing Dectin-1 ligands β-glucans [44] have recently been described. Intriguingly, only two patients with AR CARD9 deficiency have been reported so far to suffer from invasive fungal diseases caused by two different fungi [15,35]. The patient we describe here belongs to this narrow category for suffering from invasive infections due to both E. spiniphera and A. nomius. To date, the homozygous CARD9 p.Q289*mutation is the most frequently reported and mainly prevalent in patients from North Africa (Algeria, Egypt, Morocco and Tunisia), showing association with increased risk of deep dermatophytosis [20]. Infections due to Exophiala or Aspergillus geni have not been so far described in patients carrying this mutation.
In summary, this is the first patient with inherited CARD9 deficiency and chronic IPA to be described. The patient suffered from disseminated invasive E. spinifera infection followed by chronic pulmonary aspergillosis due to A. nomius, both developed at the adulthood, with fatal outcome. Despite administration of multiple courses of standard antifungals followed by posaconazole, sustained clinical remission has never been achieved. CARD9 deficiency must therefore be investigated in children and adults with severe idiopathic fungal diseases.
Supplementary Material
Acknowledgments
We would like to thank the patient’s family for consenting to this publication. The Laboratory of Human Genetics of Infectious Diseases is supported in part by institutional grants from INSERM, Paris Descartes University, The Rockefeller University and the St. Giles Foundation, the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health grant # R01AI127564, and grants from the French National Research Agency (ANR) under the “Investments for the future” program (ANR-10-IAHU-01) and ANR-HGDIFD (ANR-14-CE15-006-01 for AP).
Footnotes
Compliance with ethical standards
Research involving human participants. Informed consent for participation in this study was obtained in accordance with local regulations, with approval from the IRB. The experiments described here were performed in Argentina and in France, in accordance with local regulations, and with the approval of the IRB of Necker Hospital for Sick Children, France.
Informed consent. Written informed consent was obtained from the patient’s family.
Conflict of interest: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1 -.Chowdhary A, Perfect J, de Hoog GS. Black Molds and Melanized Yeasts Pathogenic to Humans. Cold Spring Harb Perspect Med. 2014. November 10;5(8): a019570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 -.Song Y, Laureijssen-van de Sande WWJ, Moreno LF, Gerrits van den Ende B, Li R, de Hoog S. Comparative Ecology of Capsular Exophiala Species Causing Disseminated Infection in Humans. Front Microbiol. 2017. December 19;8:2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3 -.Kenney RT, Kwon-Chung KJ, Waytes AT, Melnick DA, Pass HI, Merino MJ, Gallin JI. Successful treatment of systemic Exophiala dermatitidis infection in a patient with chronic granulomatous disease. Clin Infect Dis. 1992. January;14(1):235–42. [DOI] [PubMed] [Google Scholar]
- 4 -.Lanternier F, Barbati E, Meinzer U, Liu L, Pedergnana V, Migaud M, Héritier S, Chomton M, Frémond ML, Gonzales E, Galeotti C, Romana S, Jacquemin E, Angoulvant A, Bidault V, Canioni D, Lachenaud J, Mansouri D, Mahdaviani SA, Adimi P, Mansouri N, Jamshidi M, Bougnoux ME, Abel L, Lortholary O, Blanche S, Casanova JL, Picard C, Puel A. Inherited CARD9 deficiency in 2 unrelated patients with invasive Exophiala infection. J Infect Dis. 2015. April 15;211(8):1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5 -.Caira M, Posteraro B, Sanguinetti M, de Carolis E, Leone G, Pagano L. First case of breakthrough pneumonia due to Aspergillus nomius in a patient with acute myeloid leukemia. Med Mycol. 2012. October;50(7):746–50. [DOI] [PubMed] [Google Scholar]
- 6 -.Manikandan P, Varga J, Kocsubé S, Samson RA, Anita R, Revathi R, Dóczi I, Németh TM, Narendran V, Vágvölgyi C, Manoharan C, Kredics L. Mycotic keratitis due to Aspergillus nomius. J Clin Microbiol. 2009. October;47(10):3382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7 -.Zotti M, Machetti M, Persi A, Barabino G, Parodi A. Onychomycosis: first case due to Aspergillus nomius. Acta DermVenereol. 2011. September;91(5):591–2. [DOI] [PubMed] [Google Scholar]
- 8 -.Kousha M, Tadi R, Sobani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011. September 1;20(121):156–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9 -.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015. March;70(3):270–7. [DOI] [PubMed] [Google Scholar]
- 10 -.Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016. January;47(1):45–68. [DOI] [PubMed] [Google Scholar]
- 11 -.Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016. August 15; 63(4):e1–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12 -.Vázquez E, Messina F, Santiso G, Metta H, Negroni R. [Focal brain lesion due to cerebral aspergillosis in a patient with AIDS. Case report and literature review]. Rev Chilena Infectol. 2017. October;34(5):502–506. [DOI] [PubMed] [Google Scholar]
- 13 -.Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, Puel A. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013. December;25(6):736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14 -.De Bruyne M, Hoste L, Bogaert DJ, Van den Bossche L, Tavernier SJ, Parthoens E, Migaud M, Konopnicki D, Yombi JC, Lambrecht BN, van Daele S, Alves de Medeiros AK, Brochez L, Beyaert R, De Baere E, Puel A, Casanova JL, Goffard JC, Savvides SN, Haerynck F, Staal J, Dullaers M. A CARD9 Founder Mutation Disrupts NF-κB Signaling by Inhibiting BCL10 and MALT1 Recruitment and Signalosome Formation. Front Immunol. 2018. October 31;9:2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15 -.Rieber N, Gazendam RP, Freeman AF, Hsu AP, Collar AL, Sugui JA, Drummond RA, Rongkavilit C, Hoffman K, Henderson C, Clark L, Mezger M, Swamydas M, Engeholm M, Schüle R, Neumayer B, Ebel F, Mikelis CM, Pittaluga S, Prasad VK, Singh A, Milner JD, Williams KW, Lim JK, Kwon-Chung KJ, Holland SM, Hartl D, Kuijpers TW, Lionakis MS. Extrapulmonary Aspergillus infection in patients with CARD9 deficiency. JCI Insight. 2016. October 20;1(17):e89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16 -.Negroni R, Helou SH, Petri N, Robles AM, Arechavala A, Bianchi MH. Case study: posaconazole treatment of disseminated phaeohyphomycosis due to Exophiala spinifera. Clin Infect Dis. 2004. February 1;38(3):e15–20. [DOI] [PubMed] [Google Scholar]
- 17 -.Negroni R, López Daneri G, Robles AM, Arechavala A, Bianchi MH, Santiso G.[Clinical cases in medical mycology. Case no. 21].Rev Iberoam Micol. 2006. June;23(2):119–21. [DOI] [PubMed] [Google Scholar]
- 18 -.Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, Migaud M, Taibi L, Ammar-Khodja A, Stambouli OB, Guellil B, Jacobs F, Goffard JC, Schepers K, Del Marmol V, Boussofara L, Denguezli M, Larif M, Bachelez H, Michel L, Lefranc G, Hay R, Jouvion G, Chretien F, Fraitag S, Bougnoux ME, Boudia M, Abel L, Lortholary O, Casanova JL, Picard C, Grimbacher B, Puel A. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med.2013. October 31;369(18):1704–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19 -.Drummond RA, Lionakis MS. Mechanistic Insights into the Role of C-Type Lectin Receptor/CARD9 Signaling in Human Antifungal Immunity. Front Cell Infect Microbiol. 2016. April 5;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.-Corvilain E, Casanova JL, Puel A. Inherited CARD9 Deficiency: Invasive Disease Caused by Ascomycete Fungi in Previously Healthy Children and Adults. J Clin Immunol. 2018. August;38(6):656–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.-Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, Fink DL, Hsu AP, Zhai B, Karauzum H, Mikelis CM, Rose SR, Ferre EM, Yockey L, Lemberg K, Kuehn HS, Rosenzweig SD, Lin X, Chittiboina P, Datta SK, Belhorn TH, Weimer ET, Hernandez ML, Hohl TM, Kuhns DB, Lionakis MS. CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog. 2015. December 17;11(12):e1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.-Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, van Leeuwen EM, Roos D, Scalais E, de Beaufort C, Janssen H, van den Berg TK, Kuijpers TW. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013. March 28;121(13):2385–92. [DOI] [PubMed] [Google Scholar]
- 23.-Grumach AS, de Queiroz-Telles F, Migaud M, Lanternier F, Filho NR, Palma SM, Constantino-Silva RN, Casanova JL, Puel A. A homozygous CARD9 mutation in a Brazilian patient with deep dermatophytosis. J Clin Immunol. 2015. July;35(5):486–90. [DOI] [PubMed] [Google Scholar]
- 24.-Herbst M, Gazendam R, Reimnitz D, Sawalle-Belohradsky J, Groll A, Schlegel PG, Belohradsky B, Renner E, Klepper J, Grimbacher B, Kuijpers T, Liese J. Chronic Candida albicans Meningitis in a 4-Year-Old Girl with a Homozygous Mutation in the CARD9 Gene (Q295X). Pediatr Infect Dis J. 2015. September;34(9):999–1002. [DOI] [PubMed] [Google Scholar]
- 25.-Liang P, Wang X, Wang R, Wan Z, Han W, Li R. CARD9 deficiencies linked to impaired neutrophil functions against Phialophora verrucosa. Mycopathologia. 2015. June;179(5–6):347–57. [DOI] [PubMed] [Google Scholar]
- 26.-Bylund J, Brown KL, Movitz C, lgren C, Karlsson A. Intracellular generation of superoxide by the phagocyte NADPH oxidase: how, where, and what for? Free Radic Biol Med. 2010. December 15;49(12):1834–45. [DOI] [PubMed] [Google Scholar]
- 27.-Jhingran A, Mar KB, Kumasaka DK, Knoblaugh SE, Ngo LY, Segal BH, Iwakura Y, Lowell CA, Hamerman JA, Lin X, Hohl TM. Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung. Cell Rep. 2012. December 27;2(6):1762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.-Lanternier F, Mahdaviani SA, Barbati E, Chaussade H, Koumar Y, Levy R, Denis B, Brunel AS, Martin S, Loop M, Peeters J, de Selys A, Vanclaire J, Vermylen C, Nassogne MC, Chatzis O, Liu L, Migaud M, Pedergnana V, Desoubeaux G, Jouvion G, Chretien F, Darazam IA, Schäffer AA, Netea MG, De Bruycker JJ, Bernard L, Reynes J, Amazrine N, Abel L, Van der Linden D, Harrison T, Picard C, Lortholary O, Mansouri D, Casanova JL, Puel A. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol. 2015. June;135(6):1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.-Gavino C, Hamel N, Zeng JB, Legault C, Guiot MC, Chankowsky J, Lejtenyi D, Lemire M, Alarie I, Dufresne S, Boursiquot JN, McIntosh F, Langelier M, Behr MA, Sheppard DC, Foulkes WD, Vinh DC. Impaired RASGRF1/ERK-mediated GM-CSF response characterizes CARD9 deficiency in French-Canadians. J Allergy Clin Immunol. 2016. April;137(4):1178–1188. [DOI] [PubMed] [Google Scholar]
- 30.-Alves de Medeiros AK, Lodewick E, Bogaert DJ, Haerynck F, Van Daele S, Lambrecht B, Bosma S, Vanderdonckt L, Lortholary O, Migaud M, Casanova JL, Puel A, Lanternier F, Lambert J, Brochez L, Dullaers M. Chronic and Invasive Fungal Infections in a Family with CARD9 Deficiency. J Clin Immunol. 2016. April;36(3):204–9. [DOI] [PubMed] [Google Scholar]
- 31.-Cetinkaya PG, Ayvaz DC, Karaatmaca B, Gocmen R, Söylemezoğlu F, Bainter W, Chou J, Chatila TA, Tezcan I. A young girl with severe cerebral fungal infection due to CARD9 deficiency. Clin Immunol. 2018. June;191:21–26. [DOI] [PubMed] [Google Scholar]
- 32.-Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschläger N, Gross O, Ruland J, Grimbacher B.A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009. October 29;361(18):1727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.-Gavino C, Cotter A, Lichtenstein D, Lejtenyi D, Fortin C, Legault C, Alirezaie N, Majewski J, Sheppard DC, Behr MA, Foulkes WD, Vinh DC. CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin Infect Dis. 2014. July 1;59(1):81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.-Jones N, Garcez T, Newman W, Denning D. Endogenous Candida endophthalmitis and osteomyelitis associated with CARD9 deficiency. BMJ Case Rep. 2016. March 3;2016. pii: bcr2015214117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.-Gavino C, Mellinghoff S, Cornely OA, Landekic M, Le C, Langelier M, Golizeh M, Proske S, Vinh DC. Novel bi-allelic splice mutations in CARD9 causing adult-onset Candida endophthalmitis. Mycoses. 2018. January;61(1):61–65. [DOI] [PubMed] [Google Scholar]
- 36.-Celmeli F, Oztoprak N, Turkkahraman D, Seyman D, Mutlu E, Frede N, Köksoy S, Grimbacher B. Successful Granulocyte Colony-stimulating Factor Treatment of Relapsing Candida albicans Meningoencephalitis Caused by CARD9 Deficiency. Pediatr Infect Dis J. 2016. April;35(4):428–31. [DOI] [PubMed] [Google Scholar]
- 37.-Wang X, Wang W, Lin Z, Wang X, Li T, Yu J, Liu W, Tong Z, Xu Y, Zhang J, Guan L, Dai L, Yang Y, Han W, Li R. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17cell deficiencies. J Allergy Clin Immunol. 2014. March;133(3):905–8. [DOI] [PubMed] [Google Scholar]
- 38.-Jachiet M, Lanternier F, Rybojad M, Bagot M, Ibrahim L, Casanova JL, Puel A, Bouaziz JD. Posaconazole treatment of extensive skin and nail dermatophytosis due to autosomal recessive deficiency of CARD9. JAMA Dermatol. 2015. February;151(2):192–4. [DOI] [PubMed] [Google Scholar]
- 39.-Boudghene Stambouli O, Amrani N, Boudghéne Stambouli K, Bouali F. Dermatophytic disease with deficit in CARD9: A new case with a brain impairment. J Mycol Med. 2017. June;27(2):250–253. [DOI] [PubMed] [Google Scholar]
- 40.-Wang X, Zhang R, Wu W, Song Y, Wan Z, Han W, Li R. Impaired Specific Antifungal Immunity in CARD9-Deficient Patients with Phaeohyphomycosis. J Invest Dermatol. 2018. March;138(3):607–617 [DOI] [PubMed] [Google Scholar]
- 41.-Yan XX, Yu CP, Fu XA, Bao FF, Du DH, Wang C, Wang N, Wang SF, Shi ZX, Zhou GZ, Tian HQ, Liu H, Zhang FR. CARD9 mutation linked to Corynespora cassiicola infection in a Chinese patient. Br J Dermatol. 2016. January;174(1):176–9. [DOI] [PubMed] [Google Scholar]
- 42.-Arango-Franco CA, Moncada-Vélez M, Beltrán CP, Berrío I, Mogollón C, Restrepo A, Trujillo M, Osorio SD, Castro L, Gómez LV, Muñoz AM, Molina V, Del Río Cobaleda DY, Ruiz AC, Garcés C, Alzate JF, Cabarcas F, Orrego JC, Casanova JL, Bustamante J, Puel A, Arias AA, Franco JL. Early-Onset Invasive Infection Due to Corynespora cassiicola Associated with Compound Heterozygous CARD9 Mutations in a Colombian Patient. J Clin Immunol. 2018. October;38(7):794–803. [DOI] [PubMed] [Google Scholar]
- 43.-Wang X, Wang A, Wang X, Li R, Yu J. Cutaneous mucormycosis caused by Mucor irregularis in a patient with CARD9 deficiency. Br J Dermatol. 2019. January;180(1):213–214. [DOI] [PubMed] [Google Scholar]
- 44.-Sari S, Dalgic B, Muehlenbachs A, De Leon-Carnes M, Goldsmith CS, Ekinci O, Jain D, Keating MK, Vilarinho S. Prototheca zopfii Colitis in Inherited CARD9 Deficiency. J Infect Dis. 2018. July 2;218(3):485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.