Abstract
Background
It has been consistently shown in several meta-analyses that infants born after ART have an excess of birth defects compared with those after spontaneous conception, however, the prevalence of birth defects among ART offspring in China is incompletely studied. Moreover, it is unclear to what extent the risk of birth defects is associated with parental infertility characteristics, specific ART procedures and twinning.
Methods
In the prospective cohort study, we included women who participated in the cohort, and had pregnancies of at least 20 gestational weeks between August 2016 and May 2019, and followed them until their children reached 1 year of age. Exposures of interest were ART, as well as infertility-related characteristics, certain ART procedures and specific medication usage. The primary outcome was birth defects including both major and minor defects, which we analysed with logistic generalized estimating equations to investigate the association with ART and certain ART characteristics.
Findings
A total of 1,825 women with ART-pregnancy and 3,483 women with spontaneous-pregnancy were included in the analysis. The prevalence of any defects was significantly higher among ART-births than their non-ART counterparts at each follow-up, specifically at prenatal screening (2•2% vs. 1•2%), at delivery (4•9% vs. 2•9%), at 6 months (10•4% vs. 5•3%) and 1 year of age (13•9% vs. 7•0%), and the associations between ART and increased risk of birth defects at each follow-up were similarly robust. Among ART-births, GnRH antagonist regimen for ovulation induction in women was associated with an increased risk of birth defects in their offspring after taking into account potential influencing factors (Multivariable model: adjusted risk ratio [aRR] 1•47, 1•04–2•07). Additionally, mediation through twinning accounted for 31•1% of the risk of ART-associated birth defects.
Interpretation
The results suggest that ART confers an increased risk for birth defects in offspring. The risk is partly attributable to infertility characteristics, certain ovulation induction regimen, and to some extent mediated by twinning. Our findings highlight the importance of long-term follow-up of children conceived via ART for health conditions.
Funding
National Key Research and Development Program of China, National Natural Science Foundation of China and Natural Science Foundation of Jiangsu Province.
1. Introduction
Since the first infant conceived with assisted reproductive technology (ART) was born in 1988 in China, ART has become a standard and common practice to treat infertility. At present, an average of 700,000 ART treatments are performed in China annually, with upward trends in recent years[1]. It has been consistently shown in several meta-analyses that infants born after ART had an excess of birth defects when compared with those after spontaneous conception[2], [3], [4], [5]. Of note, present findings on this topic are primarily from researches conducted outside of China, and the prevalence of birth defects among ART pregnancies in China is incompletely studied.
Parental factors, such as age, socioeconomic status, obesity, infertility diagnosis, and pre-existing health problems have been demonstrated to impact the risk of birth defects, and the associations between ART and birth defects tended to be reduced after adjustment for these factors[2,4,[6], [7], [8]]. In addition, the increased risk of birth defects in offspring might also be attributable to ovulation induction medications or micromanipulation involved in ART procedures, but the results were inconsistent and the effect could not be distinguished from the influence from subfertility[9]. The majority of earlier studies with large number of participants were registry-based retrospective cohort studies[10], [11], [12], which commonly lacked information on ART processes for individual cycle. Additionally, it remained inconclusive whether the association between ART and birth defects was due mainly to the higher incidence of multiple pregnancy after ART. Findings from a recent study demonstrated that the risk for congenital heart defects associated with ART was dominantly mediated by twinning[13], whereas several other studies suggested that the associations appeared to be more pronounced for singleton births[12,[14], [15], [16]]. In general, to which extent the impact of ART on the risk of birth defects mediated by multiple births is less clear.
Given that many factors are potentially underlying the associations between ART use and birth defects, sufficient and accurate information collections are needed to evaluate the associations with comprehensive considerations of possible influencing factors. Therefore, we performed a large prospective cohort study in Jiangsu, China, recruiting women who received ART and those who conceived spontaneously, and followed them until their children reached 1 year of age. We aimed to assess the prevalence of birth defects among births conceived using ART compared with those who were conceived spontaneously, and to investigate the risk of birth defects associated with parental characteristics relating to infertility, and with specific ART procedures among ART-conceived births.
2. Methods
2.1. Study design and study population
We conducted this study within the Jiangsu Birth Cohort Study, a prospective and longitudinal study recruiting women who are going to receive ART and who are at the first trimester of spontaneous conception at clinics in Jiangsu, China. The overarching goal of the cohort is to elucidate heterogeneity between ART-conceived and spontaneous-conceived pregnancies regarding to their birth outcomes and pregnancy complications, and to systematically evaluate how clinical, environmental and genetic factors may influence the outcomes. In the present study, we included all women who had pregnancies up to 20 weeks’ gestation (1825 ART- and 3483 spontaneous-pregnancies) between August 2016 and May 2019 from the Women's Hospital of Nanjing Medical University and Suzhou Affiliated Hospital of Nanjing Medical University. In the ART group, 35 were excluded from the analysis (2 were lost to follow-up of birth outcomes, 2 were conceived with triplets or higher-order of births, 18 were transferred with embryos from multiple oocyte retrieval cycles, 13 had in vitro fertilization with donated semen). In the spontaneous conception group, 83 participants were excluded due to loss to follow-up of birth outcomes (appendix p 2).
Baseline characteristics and the history of infertility treatment were collected from standardized questionnaires by face-to-face interviews. Women who conceived spontaneously were recruited in the spontaneous conception group regardless of the history of infertility treatment. We identified 107 families with the self-reported history of infertility treatment in the spontaneous conception group. All pregnant women were followed up with the same procedure during gestation, regardless of ART or non-ART. Questionnaire surveys were conducted by telephone or face-to-face. Clinical information, including infertility diagnoses, specific ART procedures at each cycle and health status during pregnancy were extracted from medical records. During parturition, birth outcomes and pregnancy complications were collected from electronic medical records (EMR). Neonates were examined at obstetric clinics and their anthropometric parameters were measured. During the postnatal period, all mothers and their children were followed up for their health-related status after 6 months via telephone. Once reached 1 year of age, all cohort children received invitation to have systematic physical examination at child health care clinics by licensed clinicians at the hospitals where they were born. Follow-up rates reached over 80%. All methods and protocols for information collection were approved by the institutional review board of Nanjing Medical University, China NJMUIRB (2017) 002. Informed, written consents were obtained from all participants.
2.2. Outcomes
Pregnancy outcomes were obtained from medical records. Termination of pregnancy refers to induced ending of pregnancy after 20 weeks of gestation. Stillbirth is defined as foetal deaths at 20 gestational weeks or later. We were authorized to access their health related information from EMR in the maternal and child care hospitals where they received antenatal examinations and gave births. Birth defects were diagnosed by licensed clinicians and the diagnoses were coded and categorized into 13 subgroups by them according to the International Classification of Diseases, 10th edition (ICD-10 codes). Detailed diagnoses and ICD-10 codes during prenatal follow-ups, at birth and at early infancy were obtained from EMR. Six months after birth, any new diagnosed birth defects in the children were reported by the parents via telephone interview. When reached 1 year of age, children with suspected birth defects and those who were reported have birth defects at 6 months by their parents were referred to specialists for diagnoses, and the ICD-10 codes were provided by the specialists as well. We obtained the diagnoses and ICD-10 codes from EMR. One clinician was responsible for identifying cases through ICD-10 codes in the dataset, and another clinician was responsible for validating cases through chart review. The birth defects assessed in this study included both major and minor defects. Major defects, referring to those have surgical, medical, or serious cosmetic importance, were defined according to the European Surveillance of Congenital Anomalies (EUROCAT, website: http://www.eurocat-network.eu/accessprevalencedata/prevalencetables). The rest undefined defects were classified according to the severity classification used by the South Australian Birth Defects Registry (BDR) and the Chinses National BDR.
2.3. Exposures
ART was the exposure in the study. The participants in the ART group received either in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) therapy. Infertility-related characteristics (i.e. duration of infertility and infertility diagnosis), certain ART procedures and specific medication usage were also exposures of interest. Infertility diagnoses in the ART group included polycystic ovary syndrome (PCOS), ovarian dysfunction, endometriosis, pelvic or tubal factor, uterine factor, thyroid dysfunction, sperm disorder and others. If couples had multiple infertility diagnoses, they were counted and analysed in each related subgroup analyses and therefore could appear more than once. ART procedures included cycle type (fresh/frozen), number of embryo transferred (one/two or more), stage of embryo transferred (cleavage/blastocyst), fertilization method (IVF/ICSI), preimplantation genetic diagnosis (PGD, no/yes), assisted hatching (no/yes) and regimens of ovulation induction, i.e. gonadotropin-releasing hormone agonist (GnRH-a) regimen, GnRH antagonist (GnRHant) regimen and microstimulation regimen.
2.4. Confounders or mediator
Potential confounding variables accounted for in the study were maternal age (<35/≥35 years), parity (nulliparous/multiparous), maternal pre-pregnancy BMI (<18•5/18•5–23•9/24–27•9/≥28 kg/m2), household income (<50,000/50,000–100,000/100,000–200,000/≥200,000 China yuan (CNY)), maternal education (<12/≥12 years), tobacco use (yes/no) and alcohol intake (yes/no) during pregnancy, diseases during pregnancy (diabetes/non-diabetes, and hypertension/non-hypertension). Given that the association between ART and birth defects has been proved mediated by twinning[13], twin status was considered as a potential mediator rather than confounder in the association analysis of ART and birth defects in this study.
2.5. Statistical analysis
We examined the distribution of maternal characteristics for all ART- and spontaneously-conceived pregnancies. All analyses were conducted by plurality (singleton births and twins). To ensure the completeness of data collection, the prevalence of birth defects diagnosed prenatally or by 7 days after delivery was presented as the number of birth defects per 100 births born at least one month before the end of follow-up (December 31, 2019). The cumulative prevalence of birth defects by 6 months and by 1 year after delivery was calculated using the number of cases of at least 7 months and 13 months of age, respectively. Children who had not reached 6 months and 1 year of age were not involved when analysing the associations between ART use and risks for birth defects diagnosed at 6 months and by 1 year after delivery, respectively. Chi-square test was used to compare the differences of birth defects between ART- and spontaneous-conceived groups. Fisher exact test was applied when the expected number in any of the groups was <5. False discovery rate approach was used to adjust for the comparison of prevalence of birth defects by organ system and P value <0•05 after adjustment was considered as the level of statistical significance. Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated by comparing the prevalence of birth defects between groups. To adjust for the effect of potential correlations of risk between clustering of births (twins and siblings) within women, we constructed all models with the use of logistic generalized estimating equations. After examining possible non-linear associations by modeling birth defect with maternal age and maternal pre-pregnancy BMI using restricted cubic splines, RRs were derived after adjustment for maternal age (continuous variable), parity, maternal pre-pregnancy BMI (continuous variable), diseases during pregnancy (diabetes and hypertension) in the adjusted analyses. Due to the small size of the spontaneous conception families who had the history of infertility treatment (n = 107), we could not take it as a separated group and perform robust association analyses. We investigated the risk of birth defects in ART-births as compared to control population after excluding them as sensitivity analyses. We did not adjust for multiple births, as multiple gestations might exert effects on the causal pathway between exposure ART and birth defects. Further, we restricted the study in the ART-births whose mothers received frequently-used ovulation induction regimens, to evaluated adjusted RRs for the association between certain ART characteristic and birth defects. Multivariable logistic regression analyses were performed to assess the independent effects of specific ART characteristic on birth defects. Records containing missing values were omitted by default in multivariable analysis. Additionally, in order to evaluate the possible role of multiple births in the association between ART and birth defects, we restricted the research in singletons and assessed potential mediation effect of twin status[17,18]. The total effect was composed of natural direct effect (NDE) and indirect effect (NIE). Two multivariable adjusted regression models were established. The outcome models treated ART and twin status as exposures and birth defects as an outcome, while the mediator model treated ART as an exposure and twin status as an outcome. Both models were adjusted for confounders. We restricted the analysis in fresh cycles so as to further evaluate the possible role of regimen of ovulation induction and medication usage in the endometrial implantation environment and in turn possible birth defects. Data analysis was conducted with the use of R software (Version 3.6.1, 2019-07-05; R Foundation for Statistical Computing, http://www.cran.r-project.org/). Statistical significance was interpreted as P-value <0•05.
2.6. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Baseline characteristics
A total of 1790 ART- and 3400 spontaneous-pregnancies were included in final analysis. The twin rate differed between groups, with 24•6% (n = 441) of ART treated women and 1•0% (n = 33) of non-ART women being pregnant with twins (Table 1). Women who conceived by ART were more common to be over 35 years old, to be experiencing their first pregnancy, to be overweight or obese, and to have a lower socioeconomic and education status, but were less common to engage in alcohol consumption during pregnancy. Additionally, both the prevalence of diabetes (chronic or gestational diabetes) and hypertension (chronic or pregnancy-induced hypertension) were much higher among women using ART than those among non-ART women (Table 1). When only singleton births were considered, terminated pregnancies and stillbirths were more common in the ART group. In addition, more ART singletons were born by caesarean section and before term, and had birth weight of less than 2500 g or even less than 1500 g. In contrast to singletons, ART-twins were less common to undergo pregnancy termination, and the frequencies of stillbirth and low birth weight were comparable between ART and non-ART twins (appendix p 3).
Table 1.
Characteristics of 1790 women who conceived by ART and 3400 women who conceived spontaneously.
| Characteristicsa | ART-conception group (N = 1790) | Spontaneous-conception group (N = 3400) |
|---|---|---|
| Maternal age, year, No. (%) | ||
| <35 | 1447 (80•8) | 2952 (86•8) |
| ≥35 | 343 (19•2) | 448 (13•2) |
| Parity, No. (%) | ||
| Nulliparous | 1615 (90•2) | 2254 (66•3) |
| Multiparous | 175 (9•8) | 1145 (33•7) |
| Plurality, No. (%) | ||
| Singleton | 1349 (75•4) | 3367 (99•0) |
| Twins | 441 (24•6) | 33 (1•0) |
| Maternal pre-pregnancy BMI, kg/m2, No. (%) | ||
| <18•5 | 148 (8•3) | 465 (13•7) |
| 18•5–23•9 | 1185 (66•2) | 2284 (67•2) |
| 24–27•9 | 360 (20•1) | 411 (12•1) |
| ≥28 | 97 (5•4) | 87 (2•6) |
| Household income, CNY, No. (%) | ||
| <50,000 | 107 (6•0) | 135 (4•0) |
| 50,000–100,000 | 564 (31•5) | 638 (18•8) |
| 100,000–200,000 | 655 (36•6) | 1482 (43•6) |
| ≥200,000 | 464 (25•9) | 1110 (32•6) |
| Maternal education, year, No. (%) | ||
| <12 | 526 (29•4) | 335 (9•9) |
| ≥12 | 1264 (70•6) | 2937 (86•4) |
| Tobacco use during pregnancy, No. (%) | 1 (0•1) | 12 (0•4) |
| Alcohol intake during pregnancy, No. (%) | 5 (0•3) | 59 (1•7) |
| Diseases during pregnancy | ||
| Diabetes, No. (%)b | 502 (28•0) | 781 (23•0) |
| Hypertension, No. (%)c | 172 (9•6) | 97 (2•9) |
Abbreviation: ART, assisted reproductive technology; BMI, body mass index; CNY, China yuan.
a Missing data less than 5% for parity, maternal pre-pregnancy BMI, household income, maternal education, tobacco use during pregnancy, alcohol intake during pregnancy, diabetes and hypertension during pregnancy. No missing for maternal age and plurality.
b Includes chronic and gestational diabetes.
c Includes chronic and pregnancy-induced hypertension.
3.2. Risk of birth defects associated with ART by plurality
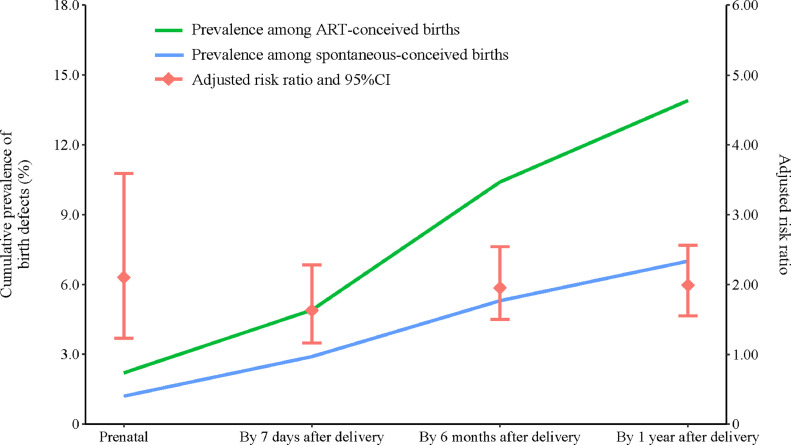
Prenatally, 2•2% (n = 50) of the foetuses conceived by ART vs. 1•2% (n = 42) of the spontaneously conceived foetuses were diagnosed with birth defects. By 7 days after delivery, the number of children being identified with birth defects were doubled in both ART (n = 109, 4•9%) and spontaneous-conception (n = 100, 2•9%) groups. After adjusting for maternal age, parity, maternal pre-pregnancy BMI, diabetes and hypertension, ART use was associated with a 2•10-fold [adjusted risk ratio (aRR) 2•10, 95% CI 1•23–3•59] and a 1•63-fold (1•16–2•28) higher risk for birth defects when diagnosed prenatally and by 7 days after birth. Thereafter, all births were followed up for their health-related status at age of 6 months and 1 year, and the number of births diagnosed with birth defects largely increased by over 2 folds as compared to 7 days after birth regardless of ART treatment. The prevalence of 1 or more defects was 13•9% (n = 217) among ART-births and 7•0% (n = 194) among spontaneously conceived births. Those conceived with ART were 1•99-fold (1•55–2•56) as likely as spontaneously conceived births to have 1 or multiple birth defects diagnosed by one year old after the adjustment for maternal characteristics (Table 2; Fig. 1). After excluding the 107 spontaneous conception families who had the history of infertility treatment, the similarly robust risk of birth defects was observed associated with ART use at each follow-up (Prenatal: aRR 2•01; By 7 days after delivery: aRR 1•63; By 6 months after delivery: aRR 1•95; By 1 year after delivery: aRR 2•02) (appendix p 4). The associations remained significant when restricted to singleton births (Table 2). In contrast, an inverse pattern was observed in twin births, with ART-twins having a lower prevalence of birth defects relative to spontaneously conceived twins, though the associations were not statistically significant (Table 2). Notably, only a small percentage of birth defects were identified prenatally (<10%) or within 1 week after birth (~ 30%) in twin births.
Table 2.
Cumulative prevalence of birth defects according to age at diagnosis and plurality.
| Age at diagnosis | ART-conceived | Spontaneous-conceived | Crude RR (95%CI) | aRR (95%CI)a | ||
|---|---|---|---|---|---|---|
| No. of birth | No. of birth defects (%) | No. of birth | No. of birth defects (%) | |||
| All Births | ||||||
| Prenatal | 2231 | 50 (2•2) | 3433 | 42 (1•2) | 1•89 (1•24–2•86) | 2•10 (1•23–3•59) |
| By 7 days after delivery | 2231 | 109 (4•9) | 3433 | 100 (2•9) | 1•74 (1•31–2•31) | 1•63 (1•16–2•28) |
| By 6 months after delivery | 1921 | 200 (10•4) | 3118 | 164 (5•3) | 2•09 (1•67–2•62) | 1•95 (1•50–2•54) |
| By 1 year after delivery | 1566 | 217 (13•9) | 2768 | 194 (7•0) | 2•17 (1•75–2•69) | 1•99 (1•55–2•56) |
| Singleton Births | ||||||
| Prenatal | 1349 | 42 (3•1) | 3367 | 40 (1•2) | 2•74 (1•77–4•25) | 2•97 (1•67–5•29) |
| By 7 days after delivery | 1349 | 73 (5•4) | 3367 | 95 (2•8) | 1•99 (1•46–2•72) | 1•82 (1•25–2•65) |
| By 6 months after delivery | 1118 | 107 (9•6) | 3059 | 153 (5•0) | 2•02 (1•56–2•61) | 1•88 (1•38–2•55) |
| By 1 year after delivery | 873 | 112 (12•8) | 2713 | 182 (6•7) | 2•05 (1•60–2•63) | 1•85 (1•38–2•48) |
| Twins | ||||||
| Prenatal | 882 | 8 (0•9) | 66 | 2 (3•0) | 0•29 (0•06–1•43) | 0•24 (0•06–1•03) |
| By 7 days after delivery | 882 | 36 (4•1) | 66 | 5 (7•6) | 0•52 (0•17–1•60) | 0•50 (0•13–1•92) |
| By 6 months after delivery | 803 | 93 (11•6) | 59 | 11 (18•6) | 0•56 (0•24–1•29) | 0•45 (0•18–1•10) |
| By 1 year after delivery | 693 | 105 (15•2) | 55 | 12 (21•8) | 0•64 (0•28–1•50) | 0•51 (0•20–1•31) |
a Analyses were adjusted for maternal age, parity, maternal pre-pregnancy BMI, diabetes and hypertension.
Fig. 1.
Cumulative prevalence of birth defects according to age at diagnosis.
3.3. Associations of ART with birth defects by severity and by organ system
We classified birth defects as major or minor according to the severity of conditions. Significant associations were detected between ART and major defects at all follow-ups. By 1 year of age, the prevalence of major birth defects was 8•0% among ART-infants vs. 4•6% among their counterparts conceive spontaneously, and the aRR was 1•91 (1•39–2•63) (appendix p 5). Regarding to minor defects, we did not find significant association until 6 months after birth, which was probably due to the relatively low identification rate of minor conditions prenatally or at birth. Overall, by 1 year after birth, ART infants were at 1•94-fold (1•31–2•86) greater risk of having minor defects as compared to spontaneously conceived infants (6•9% of the ART infants vs. 2•7% of the spontaneous-conceived infants), while the associations were not significant when restricted to singletons or twins (appendix p 5). In the analyses of birth defects by organ system, increased risk was detected for the association between ART and circulatory malformations by 1 year of age (appendix p 6–9). Chromosomal abnormalities, e.g. trisomy 21, trisomy 18, trisomy X and 47, XYY, were rare in our cohort (appendix p 6–9), and the findings remained unchanged after excluding these conditions.
3.4. Associations of birth defects with specific infertility diagnoses and ART procedures
Among ART-births, we assessed whether any specific infertility diagnosis or ART procedure was associated with the risk of birth defects. We observed null associations of birth defects with duration of infertility, so as with 6 infertility diagnoses including polycystic ovary syndrome (PCOS), ovarian dysfunction, infertility due to pelvic or tubal factor, uterine factor, thyroid factor, and due to sperm disorder in the adjusted analyses. Children born to parents with the 6 above-mentioned diagnoses were all at higher risk of developing birth defects vs. those born to spontaneously conceived parents, whereas no individual subfertility cause displayed excess risk as compared with other causes (appendix p 10). Only ART-births born to mothers with endometriosis showed a comparable prevalence of birth defects as those spontaneously conceived births (appendix p 10), and a lower risk (aRR 0•45, 0•22–0•95) relative to the ART-births born to parents with other diagnoses (Table 3).
Table 3.
Association between ART characteristics and birth defects among ART-conceived births.
| Characteristics | Levels | No. of birth | No. of birth defects (%) | aRR (95%CI)a | P valuea |
|---|---|---|---|---|---|
| Infertility Diagnosis | |||||
| Duration of infertility, month | <36 | 753 | 105 (13•9) | 1•00 | |
| ≥36 | 799 | 112 (14•0) | 1•06 (0•77–1•46) | 0•728 | |
| PCOS | No | 1368 | 193 (14•1) | 1•00 | |
| Yes | 184 | 24 (13•0) | 0•91 (0•53–1•56) | 0•728 | |
| Ovarian dysfunction | No | 1404 | 189 (13•5) | 1•00 | |
| Yes | 148 | 28 (18•9) | 1•52 (0•93–2•50) | 0•095 | |
| Endometriosis | No | 1441 | 209 (14•5) | 1•00 | |
| Yes | 111 | 8 (7•2) | 0•45 (0•22–0•95) | 0•037 | |
| Pelvic or tubal factor | No | 344 | 49 (14•2) | 1•00 | |
| Yes | 1208 | 168 (13•9) | 0•96 (0•66–1•38) | 0•814 | |
| Uterine factor | No | 1061 | 152 (14•3) | 1•00 | |
| Yes | 491 | 65 (13•2) | 0•85 (0•60–1•20) | 0•347 | |
| Thyroid factor | No | 1492 | 205 (13•7) | 1•00 | |
| Yes | 60 | 12 (20•0) | 1•74 (0•87–3•48) | 0•115 | |
| Sperm disorder | No | 622 | 100 (16•1) | 1•00 | |
| Yes | 930 | 117 (12•6) | 0•84 (0•61–1•15) | 0•275 | |
| ART Procedure | |||||
| Cycle type | Fresh | 295 | 38 (12•9) | 1•00 | |
| Frozen | 1257 | 179 (14•2) | 1•07 (0•71–1•61) | 0•756 | |
| Number of embryos transferred | One | 367 | 57 (15•5) | 1•00 | |
| Two or more | 1185 | 160 (13•5) | 0•87 (0•62–1•22) | 0•427 | |
| Stage of embryo transferred | Cleavage | 671 | 82 (12•2) | 1•00 | |
| Blastocyst | 881 | 135 (15•3) | 1•17 (0•85–1•62) | 0•327 | |
| Fertilization method | IVF | 1143 | 162 (14•2) | 1•00 | |
| ICSI | 395 | 55 (13•9) | 0•98 (0•69–1•41) | 0•934 | |
| PGD | No | 1521 | 215 (14•1) | 1•00 | |
| Yes | 31 | 2 (6•5) | 0•38 (0•09–1•61) | 0•187 | |
| Assisted hatching | No | 466 | 52 (11•2) | 1•00 | |
| Yes | 1086 | 165 (15•2) | 1•26 (0•88–1•82) | 0•208 | |
| Regimen of ovulation induction | GnRH-a | 712 | 80 (11•2) | 1•00 | |
| GnRHant | 750 | 119 (15•9) | 1•48 (1•05–2•09) | 0•024 | |
| Microstimulation | 90 | 18 (20•0) | 2•11 (1•13–3•93) | 0•019 | |
| Medication Usage | |||||
| GnRH agonist doses | – | 1552 | 217 (14•0) | 0•91 (0•79–1•05) | 0•176 |
| GnRHant doses | – | 1552 | 217 (14•0) | 1•13 (0•87–1•47) | 0•351 |
| Gn doses, per 1000IU | – | 1552 | 217 (14•0) | 1•01 (0•78–1•31) | 0•956 |
| Gn type | FSH only | 767 | 107 (14•0) | 1•00 | |
| FSH+LH | 126 | 17 (13•5) | 0•88 (0•48–1•62) | 0•685 | |
| hMG | 656 | 92 (14•0) | 1•10 (0•79–1•53) | 0•581 | |
| Clomiphene | No | 1494 | 205 (13•7) | 1•00 | |
| Yes | 58 | 12 (20•7) | 1•65 (0•83–3•26) | 0•153 | |
| Growth hormone | No | 1483 | 202 (13•6) | 1•00 | |
| Yes | 69 | 15 (21•7) | 1•71 (0•88–3•33) | 0•117 |
Abbreviation: PCOS, polycystic ovary syndrome; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; PGD, preimplantation genetic diagnosis; GnRH-a, gonadotropin-releasing hormone agonist; GnRHant, gonadotropin-releasing hormone antagonist; Gn, gonadotropin; FSH, follicle stimulating hormone; LH, luteinizing hormone; hMG, human chorionic gonadotropin.
a Analyses were adjusted for maternal age, parity, maternal pre-pregnancy BMI, diabetes and hypertension.
When assessing specific ART procedures, births born to mothers receiving diverse regimens of ovulation induction displayed differential risk of birth defects, with the prevalence of birth defects increased for births born to mothers using regimens of GnRH antagonist (aRR 1•48, 1•05–2•09) and microstimulation (aRR 2•11, 1•13–3•93) as compared to those born to mothers using agonist regimen. We did not observe significant associations between birth defects and other procedures (fresh vs. frozen embryo-transfer cycle, cleavage vs. blastocyst embryo-transfer cycle, transferring two or more embryos vs. one embryo, conventional IVF vs. ICSI, PGD vs. no PGD, assisted hatching vs. no assisted hatching) or maternal medication usage (Gn, Clomiphene and Growth hormone) (Table 3). In the multivariable model, when maternal characteristics and potential influencing factors were considered, regimen of GnRH antagonist remained significant association with increased risk of birth defects in the ART-births (Multivariable Model: aRR 1•47, 1•04–2•07) (Table 4). We observed a similar, but not statistically significant, trend in the singletons born to treated couples (appendix p 11). In fresh cycles, regimen of GnRH antagonist showed similar, but not statistically significant, association with increased risk of birth defects in the ART-births, and we did not observe significant associations between birth defects and medication usage (appendix p 12).
Table 4.
Multivariable logistic regression analysis of ART characteristics on birth defects among ART-conceived births.
| Characteristics | Levels | No. of birth | No. of birth defects (%) | aRR (95%CI) | P value |
|---|---|---|---|---|---|
| Maternal age, year | – | 1552 | 217 (14•0) | 1•01 (0•96–1•05) | 0•776 |
| Parity | Nulliparous | 1394 | 194 (13•9) | 1•00 | |
| Multiparous | 158 | 23 (14•6) | 1•03 (0•59–1•80) | 0•907 | |
| Maternal pre-pregnancy BMI, kg/m2 | – | 1552 | 217 (14•0) | 0•99 (0•94–1•04) | 0•764 |
| Diabetes | No | 1062 | 153 (14•4) | 1•00 | |
| Yes | 435 | 62 (14•3) | 0•97 (0•67–1•39) | 0•850 | |
| Hypertension | No | 1322 | 187 (14•1) | 1•00 | |
| Yes | 170 | 27 (15•9) | 1•07 (0•65–1•76) | 0•804 | |
| Ovarian dysfunction | No | 1404 | 189 (13•5) | 1•00 | |
| Yes | 148 | 28 (18•9) | 1•22 (0•66–2•25) | 0•528 | |
| Regimen of ovulation induction | GnRH-a | 712 | 80 (11•2) | 1•00 | |
| GnRHant | 750 | 119 (15•9) | 1•47 (1•04–2•07) | 0•029 | |
| Microstimulation | 90 | 18 (20•0) | 1•85 (0•86–3•99) | 0•117 |
3.5. Association between ART and birth defects mediated by twinning
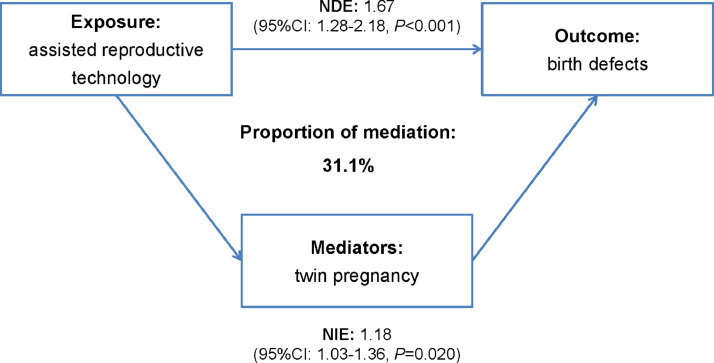
One of the most important consequences of increased use of ART is a substantially increased rate of twin pregnancies. Therefore, we assessed the association between ART and birth defects mediated by twinning. Through mediation analysis, we observed the adjusted RR was 1•97 (1•56–2•48) for the total effect, 1•67 (1•28–2•18) for the natural direct effect of ART, and 1•18 (1•03–1•36) for the indirect effect of twinning. Twin pregnancies after ART explained 31•1% of the relative effect of ART on birth defects in our data (Fig. 2).
Fig. 2.
Analysis of assisted reproductive technology and birth defects association with twin pregnancy as a mediator.
4. Discussion
Based on the prospective and longitudinal cohort study comparing ART- and spontaneous-births, we found that births born to mothers who conceived with ART were at a higher risk of birth defects, and both infertility characteristics and certain ovulation induction regimen contributed to the increased risk. Additionally, twinning, to some extent, mediated the association between ART use and the risk of birth defects.
Large meta-analyses had shown that children born after ART had a 30–40% increased risk of birth defects compared with spontaneous conceptions [2,19,20], but most large studies to date reported the prevalence of birth defects using data from birth registry databases. For instance, the prevalence of any birth defects among births resulting from ART was 34 per 1000 in Canada [21], 32 per 1000 in Netherlands[22] and 82 per 1000 in Australia[23]. One register-based study in Shanghai, China has suggested that ART might increase the risk of birth defects in children in Chinese population, whereas the prevalence of birth defects among ART offspring presented in that study was 19•73 per 1000 live births, much lower than that in many countries[24]. In fact, birth registry systems often record birth defects detected at birth or during the early neonatal period (within 7 days after birth), and minor defects may not be recorded in complete as some disorders are unapparent at birth and do not manifest clinical signs until several months of age such as minor congenital circulatory system defects, or that some may be misdiagnosed in the first week after delivery such as genitourinary system defects or gastrointestinal system defects[25,26], which leads to less consideration of minor conditions and defects. Yin et al. followed up children conceived by ART, and reported that the cases of birth defects had doubled after 3 years relative to those detected at birth (5•16% at 3 years vs. 2•22% at birth)[25]. Our cohort study displayed consistent findings that births resulting from ART are predisposed to increased risk of birth defects. We presented the rate of birth defects detected at prenatal screening, at birth, 6 months and 1 year after birth among children conceived by ART vs. those conceived spontaneously, and demonstrated the similarly robust risk associated with ART at each follow-up. Additionally, we confirmed that the number of children diagnosed with birth defects increased by over 2 folds at 1 year of age as compared to those at birth. Consistently, we observed a slight increase in the magnitude of association between ART use and birth defects at 1 year of age versus at birth, which might due to the lag in the manifestation and identification of minor conditions. In our cohort, the high follow-up rate (approximately 90% at 1 year after birth) and a variety of sources for case identification (i.e. follow-up via telephone, physical examination at clinic and discharge diagnosis from local children's hospital) largely reduced the rate of missed identification of birth defects. Moreover, the high rates of birth defects we reported in both ART and spontaneously conceived children were attributable to the considerable number of cases with minor defects, which were often not recorded in birth registry databases.
It has long been debated whether the excess of birth defects after ART is due to parental characteristics, underlying subfertility, or the ART technique itself, and whether the risk is similar across different ART approaches and related micromanipulations. Evidence from animal models suggested that ART procedures may influence the development of foetal and placenta, leading to adverse pregnancy outcomes[27]. In human studies, ovulation induction alone has also been identified to be associated with increased risk of birth defects in retrospective cohort study settings[21]. The safety of two most common ART approaches, IVF and ICSI, has long been evaluated with inconsistent findings. Some studies demonstrated an increased risk of birth defects in children conceived by ICSI but not by IVF[12]. In comparison, a meta-analysis reported the risk of birth defect was not significantly different between children conceived by IVF and ICSI[28]. Cleavage (Day 3) versus blastocyst (Day 5) stage transfer of embryos has also been discussed for their influence on foetal development with blastocyst transfer being associated with higher risk of birth defects[29]. But inconsistent with these findings on ART procedures, Parazzini et al. suggested the increased risk of birth defects in children was largely due to confounding factors, e.g. advanced maternal age, which often indicates poor oocyte quality, mitochondrial dysfunction, aneuploidy and epigenetic alteration[30]. Boulet et al. demonstrated the association between birth defects with both underlying infertility (ovulation disorders) and ART procedures (assisted hatching) through birth registry system[10]. In the present study, with detailed information collected prospectively, we collectively assessed the effects of maternal characteristic, infertility diagnoses as well as ART procedures on the risk of birth defects. Our findings suggested that GnRH antagonist regimen for ovulation induction may predispose offspring to increased risk of birth defects in comparison with agonist regimen, and the association remained true after taking maternal characteristics and other potential influencing factors into account. We are aware of only one study in human investigating the risk of birth defects of children born after ART with GnRH antagonist regimen, but women receiving antagonist regimen were compared with non-ART women instead of those using other regimens[31]. Regards parental characteristics, we could not attribute the increased risk to any specific underlying infertility causes. Infants born to parents diagnosed with PCOS, ovarian dysfunction, pelvic or tubal factor, uterine factor, thyroid factor, and sperm disorder displayed comparable risks with each other but higher risks than spontaneously conceived infants. It indicates that some of the determinants of infertility may share a common causal path with mechanisms that cause congenital malformations in children.
The well-documented consequence of ART is a substantially increased rate of multiple gestation pregnancy[32,33]. Association between ART and birth defects among twin births was not significant in the present study, in agreement with prior findings[12,15,24]. Overall, compared with singletons, the risks of adverse pregnancy outcomes, including birth defects, largely increase in multiple births[34,35]. To improve the conception rate, it is a common practice to transfer 2 to 3 embryos in one cycle, which leads to the high rate of multiple pregnancy among women conceived by ART[1]. In our cohort, the proportion of twin births resulting from ART was 39•5%, which was slightly higher than that reported in a recent study in China (38•6%)[24], but was lower than that in a U.S. study (43•2%)[10]. In the present study, the prevalence of birth defects among multiple births was twice that among singleton births, and mediation analysis demonstrated that twinning contributed up to 31•1% of the association between ART and birth defects, which was in consistent with findings from several recent studies[13,36]. With the improvement of pregnancy rate in ART treatment, the policy of single-embryo transfer (SET) has become more popular nowadays, which resulted in a marked decline in the multiple pregnancy rate in many countries[23]. One Canada study reported the twin rate among ART pregnancies was as low as 18•5% since SET was supported by government funding since 201513. With an increasing implementation of SET, improvements in perinatal outcomes including lower birth defects rate are expected.
Our study has several notable strengths. The prospective and rich data collection allowed a thorough adjustment for confounding, and assessment of subfertility related characteristic as well as detailed ART procedures, including medication usage. Further, the notification of minor defects is often incomplete at birth, therefore being excluded from the registry databases. The present cohort study followed up all children up to 1 year of age, and provided systematic physical examination at clinics regardless of conception status, which ascertained accurate identification of cases with birth defects, including major and minor conditions. The robustness of the associations at each follow-up demonstrated the accuracy of data collected in this study. Additionally, some research suggested that the higher risk of birth defects associated with ART could be due to the ART-infants being more intensely and carefully monitored for health conditions. Our setting could rule out this possible bias as identical follow-up schedules were applied to ART- and spontaneous-births by investigators and physicians without knowing their conception mode.
Some potential limitations of our study merit discussion. First, despite significant associations were observed between ART use and birth defects, we cannot exclude the possibility of underestimating the significance of true associations due to lack of diagnoses of malformations for miscarriages and induced abortions in early pregnancy. We cannot exclude the possibility that subfertility couples were included in the control population as the infertility related diagnoses and time to conception were not investigated among spontaneously conceived families. It might cause underestimation of the RR of birth defects in children born after ART when comparing to those born to parents in well reproductive status. Second, infertile couples are more likely to request multiple prenatal screening after they conceive by treatment, which may contribute to the greater likelihood of birth defects being identified prenatally among ART-pregnancies. However, we obtained similar results after excluding Down's syndrome and neural tube defects (the most common defects diagnosed at screening). Third, previous data has demonstrated that chorionicity in twin pregnancies would largely increase the risks for adverse perinatal outcomes and birth defects[37]; however, we do not have information on chorionicity available for this cohort. Future studies are warranted to investigate the relative contributions of each type of twins to the risk of birth defects and the influencing factors associated with the odds of conceiving each type of twins. Finally, the present cohort study was limited by moderate sample size, such that classification of birth defects by organ system yielded rare cases in absolute terms. With the recruitment of participants in the ongoing cohort study, it may gain enough statistical power to evaluate rare malformations or subcategories in the future analysis.
In conclusion, these results suggest that ART use confers an increased risk for birth defects. This is at least partly due to the underlying infertility, and may be further increased by ovulation induction with regimens of antagonist. In addition, we demonstrate that the risk of ART use on birth defects is, to some extent, mediated by twinning. Our findings highlight the importance of longitudinal monitoring children conceived via ART for health conditions including both major and minor birth defects. With the continuous increase in ART use, it is critical to determine the safety of ART approaches, including the number of embryo transfer and the regimen of ovulation induction.
Contributors
HS and ZH initiated, conceived and supervised the study. ZH, HLv and YL prepared the manuscript. HLv, YL, FD, JD, TC and QM were involved in study design, conduct of the cohort study, long-term follow-up with XLing, HLi, QX, ST, LH, MW, MP, CL, QL, YH, YY, BX, XH and KZ. HLv, JD, TC, QM, CS and YXu organized the clinical information with YJ and XLiu. HLv and YL performed statistical analysis with TJ and YZ. HM, GJ, YXia and JL proofread the manuscript. All authors critically reviewed and provided feedback on drafts and approved of the final version.
Data sharing statement
After publication, the data collected for the study (deidentified participant data) could be accessed on reasonable request to the corresponding author. A proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability of requests. Additional, relevant documents might also be required during the process of evaluation.
Declaration of Competing Interests
We declare no competing interests.
Acknowledgements
We are grateful to all the families for participating this study, and the whole Jiangsu Birth Cohort team. This work was funded by the National Key Research and Development Program of China (2018YFC1004200, 2016YFC1000200), National Natural Science Foundation of China (81830100, 82003527), Natural Science Foundation of Jiangsu Province (BK20200669).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2020.100090.
Contributor Information
Yuan Lin, Email: yuanlin@njmu.edu.cn.
Zhibin Hu, Email: zhibin_hu@njmu.edu.cn.
Hongbing Shen, Email: hbshen@njmu.edu.cn.
Appendix. Supplementary materials
Translated abstract
References
- 1.Yang X., Li Y., Li C., Zhang W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril. 2014;101(2):385–391. doi: 10.1016/j.fertnstert.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Hansen M., Kurinczuk J.J., Milne E., de Klerk N., Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. 2013;19(4):330–353. doi: 10.1093/humupd/dmt006. [DOI] [PubMed] [Google Scholar]
- 3.Qin J., Wang H., Sheng X., Liang D., Tan H., Xia J. Pregnancy-related complications and adverse pregnancy outcomes in multiple pregnancies resulting from assisted reproductive technology: a meta-analysis of cohort studies. Fertil Steril. 2015;103(6) doi: 10.1016/j.fertnstert.2015.03.018. 1492-508 e1-7. [DOI] [PubMed] [Google Scholar]
- 4.Giorgione V., Parazzini F., Fesslova V. Congenital heart defects in IVF/ICSI pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(1):33–42. doi: 10.1002/uog.18932. [DOI] [PubMed] [Google Scholar]
- 5.Wen J., Jiang J., Ding C. Birth defects in children conceived by in vitro fertilization and intracytoplasmic sperm injection: a meta-analysis. Fertil Steril. 2012;97(6) doi: 10.1016/j.fertnstert.2012.02.053. 1331-7 e1-4. [DOI] [PubMed] [Google Scholar]
- 6.Hansen M., Kurinczuk J.J., Bower C., Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 7.Tararbit K., Houyel L., Bonnet D. Risk of congenital heart defects associated with assisted reproductive technologies: a population-based evaluation. Eur Heart J. 2011;32(4):500–508. doi: 10.1093/eurheartj/ehq440. [DOI] [PubMed] [Google Scholar]
- 8.Tararbit K., Lelong N., Thieulin A.C. The risk for four specific congenital heart defects associated with assisted reproductive techniques: a population-based evaluation. Hum Reprod. 2013;28(2):367–374. doi: 10.1093/humrep/des400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinborg A., Henningsen A.K., Malchau S.S., Loft A. Congenital anomalies after assisted reproductive technology. Fertil Steril. 2013;99(2):327–332. doi: 10.1016/j.fertnstert.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Boulet S.L., Kirby R.S., Reefhuis J. Assisted reproductive technology and birth defects among live born infants in Florida, Massachusetts, and Michigan, 2000-2010. JAMA Pediatr. 2016;170(6) doi: 10.1001/jamapediatrics.2015.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halliday J.L., Ukoumunne O.C., Baker H.W. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25(1):59–65. doi: 10.1093/humrep/dep364. [DOI] [PubMed] [Google Scholar]
- 12.Davies M.J., Moore V.M., Willson K.J. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 13.Wen S.W., Miao Q., Taljaard M. Associations of assisted reproductive technology and twin pregnancy with risk of congenital heart defects. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2019.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert R.D. Safety issues in assisted reproduction technology: the children of assisted reproduction confront the responsible conduct of assisted reproductive technologies. Hum Reprod. 2002;17(12):3011–3015. doi: 10.1093/humrep/17.12.3011. [DOI] [PubMed] [Google Scholar]
- 15.Reefhuis J., Honein M.A., Schieve L.A. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009;24(2):360–366. doi: 10.1093/humrep/den387. [DOI] [PubMed] [Google Scholar]
- 16.Ericson A., Kallen B. Congenital malformations in infants born after IVF: a population-based study. Hum Reprod. 2001;16(3):504–509. doi: 10.1093/humrep/16.3.504. [DOI] [PubMed] [Google Scholar]
- 17.Sobel M.E. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 18.Valeri L., Vanderweele T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen M., Bower C., Milne E., de Klerk N., Kurinczuk J.J. Assisted reproductive technologies and the risk of birth defects–a systematic review. Hum Reprod. 2005;20(2):328–338. doi: 10.1093/humrep/deh593. [DOI] [PubMed] [Google Scholar]
- 20.McDonald S.D., Murphy K., Beyene J., Ohlsson A. Perinatel outcomes of singleton pregnancies achieved by in vitro fertilization: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2005;27(5):449–459. doi: 10.1016/s1701-2163(16)30527-8. [DOI] [PubMed] [Google Scholar]
- 21.El-Chaar D., Yang Q., Gao J. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil Steril. 2009;92(5):1557–1561. doi: 10.1016/j.fertnstert.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 22.Anthony S., Buitendijk S.E., Dorrepaal C.A., Lindner K., Braat D.D., den Ouden A.L. Congenital malformations in 4224 children conceived after IVF. Hum Reprod. 2002;17(8):2089–2095. doi: 10.1093/humrep/17.8.2089. [DOI] [PubMed] [Google Scholar]
- 23.Hansen M., Kurinczuk J.J., de Klerk N., Burton P., Bower C. Assisted reproductive technology and major birth defects in Western Australia. Obstet Gynecol. 2012;120(4):852–863. doi: 10.1097/AOG.0b013e318269c282. [DOI] [PubMed] [Google Scholar]
- 24.Yu H.T., Yang Q., Sun X.X. Association of birth defects with the mode of assisted reproductive technology in a Chinese data-linkage cohort. Fertil Steril. 2018;109(5):849–856. doi: 10.1016/j.fertnstert.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Yin L., Hang F., Gu L.J., Xu B., Ma D., Zhu G.J. Analysis of birth defects among children 3 years after conception through assisted reproductive technology in China. Birth Defects Res A Clin Mol Teratol. 2013;97(11):744–749. doi: 10.1002/bdra.23116. [DOI] [PubMed] [Google Scholar]
- 26.Zhu J.L., Basso O., Obel C., Bille C., Olsen J. Infertility, infertility treatment, and congenital malformations: danish national birth cohort. BMJ. 2006;333(7570):679. doi: 10.1136/bmj.38919.495718.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloise E., Feuer S.K., Rinaudo P.F. Comparative intrauterine development and placental function of ART concepti: implications for human reproductive medicine and animal breeding. Hum Reprod Update. 2014;20(6):822–839. doi: 10.1093/humupd/dmu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massaro P.A., MacLellan D.L., Anderson P.A., Romao R.L. Does intracytoplasmic sperm injection pose an increased risk of genitourinary congenital malformations in offspring compared to in vitro fertilization? A systematic review and meta-analysis. J Urol. 2015;193(5 Suppl):1837–1842. doi: 10.1016/j.juro.2014.10.113. [DOI] [PubMed] [Google Scholar]
- 29.Dar S., Lazer T., Shah P.S., Librach C.L. Neonatal outcomes among singleton births after blastocyst versus cleavage stage embryo transfer: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(3):439–448. doi: 10.1093/humupd/dmu001. [DOI] [PubMed] [Google Scholar]
- 30.Parazzini F., Cipriani S., Bulfoni G. The risk of birth defects after assisted reproduction. J Assist Reprod Genet. 2015;32(3):379–385. doi: 10.1007/s10815-014-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig M., Riethmuller-Winzen H., Felberbaum R.E. Health of 227 children born after controlled ovarian stimulation for in vitro fertilization using the luteinizing hormone-releasing hormone antagonist cetrorelix. Fertil Steril. 2001;75(1):18–22. doi: 10.1016/s0015-0282(00)01632-0. [DOI] [PubMed] [Google Scholar]
- 32.Thurin A., Hausken J., Hillensjo T. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351(23):2392–2402. doi: 10.1056/NEJMoa041032. [DOI] [PubMed] [Google Scholar]
- 33.Toner J.P., Coddington C.C., Doody K. Society for assisted reproductive technology and assisted reproductive technology in the United States: a 2016 update. Fertil Steril. 2016;106(3):541–546. doi: 10.1016/j.fertnstert.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Dawson A.L., Tinker S.C., Jamieson D.J. Twinning and major birth defects, national birth defects prevention study, 1997-2007. J Epidemiol Community Health. 2016;70(11):1114–1121. doi: 10.1136/jech-2015-206302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardin J., Carmichael S.L., Selvin S., Lammer E.J., Shaw G.M. Increased prevalence of cardiovascular defects among 56,709 California twin pairs. Am J Med Genet A. 2009;149A(5):877–886. doi: 10.1002/ajmg.a.32745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liberman R.F., Getz K.D., Heinke D. Assisted reproductive technology and birth defects: effects of subfertility and multiple births. Birth Defects Res. 2017;109(14):1144–1153. doi: 10.1002/bdr2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawwass J.F., Badell M.L. Maternal and fetal risk associated with assisted reproductive technology. Obstet Gynecol. 2018;132(3):763–772. doi: 10.1097/AOG.0000000000002786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Translated abstract