Abstract
Both stroke and smoking continue to be major public health crises in the United States, with stroke being the third and fourth leading cause of death among women and men, respectively. The goal of this review will be to provide clinicians a succinct overview regarding the epidemiology, economics, and biology of stroke in the setting of smoking and electronic cigarette use. Special attention will be given to the escalating public health crisis of electronic cigarette use, emphasizing mechanistic relationships of stroke and lung injury. Readers will be made aware of the need for continued scientific advancement and study regarding these relationships, as well as the need for improved governmental and public health efforts to curb these ongoing public health crises.
Keywords: Stroke, Ischemia, Smoking, Vaping, Risk, Evali, Covid-19
INTRODUCTION
Cigarette smoking is a well-established risk factor for stroke and has been shown to be responsible for up to 18% of all strokes in Western populations including the United States of America (USA), with even higher Population Attributable Risks (PAR) in younger stroke populations [1–3]. While public health measures over the last decade have led to a reduction in the prevalence of smoking in the USA from ~20 to ~14%, a new and similarly detrimental risk factor has emerged during this same time-period, namely electronic cigarettes, also known as e-cigarettes or vaping. In this review we will discuss the relationships between ischemic stroke, cigarette smoking and e-cigarettes, emphasizing why these habits are so detrimental to the health of the individual and are economically harmful to society at-large.
Stroke (the No-Good): The Epidemiology
Stroke is the third and fourth leading cause of death among women and men respectively behind cancer, heart disease, and lung disease and is the leading cause of long-term disability in the USA [4]. Despite these well-established facts, knowledge and education regarding stroke risk and prevention remain lacking in both the general population as well as the medical community.
In one USA Gallup study conducted by the National Stroke Association, 19% of respondents were unaware that stroke was preventable, in addition to another 38% were unaware of the location that a stroke occurs in the body [5]. In a similar study with a sample size of 1880 participants, ~27% of the respondents could not list a common symptom associated with stroke, with another 24% listing dizziness and 16% listing headache as a common symptom of stroke, with numbness, weakness, and speech alteration being listed as 19%, 15%, and 8% respectively [6]. Despite the pneumonic “FAST” for Face, Arm, Speech and Time as promoted by the American Heart Association, 67% of the respondents did not know any of these symptoms, with only 42% being able to list one symptom [7]. Similarly, stroke education is underrepresented in United States (US) medical schools, with only 81% and 55% of medical schools requiring rotations in neurology and emergency medicine, respectively [8]. Notably medical students participating in these rotations are not guaranteed exposure to a patient presenting with acute stroke or for post stroke care [9]. Therefore, with poor knowledge in the public sector, as well as limited exposure in formal medical training, it remains of utmost importance to improve strategies to disseminate stroke-related information across both public and medical-training domains.
Key to the prevention of stroke is an awareness that patients and physicians must address the numerous preventable risk factors that cause stroke. These risk factors include hypertension, diabetes, dyslipidemia, smoking status and being overweight, among others [10,11]. These risk factors are highly prevalent in the US population with hypertension being the most common, seen in 29% of subjects. Notably, hypertension prevalence increases with age: 7.5% among 18-39yo, 33.2% among 40-59yo, and 63% among those >60years old as per a 2015-2016 study [12]. A similar trend is true of diabetes, found in 13% of the US population with an increasing prevalence by age [13]. While hyperlipidemia does not follow a similar age-related prevalence trend, it is highly prevalent in the US population with elevated total cholesterol (≥240mg/dL) seen in 12.4% US adults and low HDL (<40mg/dL), also known as ‘good’ cholesterol, only seen in 18% of the US population [14]. While rates of obesity do not increase with age, data from 2017-2018 demonstrate its high prevalence in the USA with 42% of US adults considered obese (BMI>30), with 9.2% classified as severe obesity (BMI>40) [15]. Smoking, the focus of this review, continues to be highly prevalent in US society despite well-established risks. Importantly, not only are these risk factors highly prevalent in the US population, numerous studies have demonstrated that these risk factors act synergistically with one another to further increase risk. In other words, in the presence of one another, these risk factors increase stroke risk in a multiplicative or exponential fashion, rather than contributing to risk in an additive fashion. For example, evaluating the Framingham Stroke Risk Profile of a 70-year-old male with isolated HTN as a risk factor on no medications had an 8% chance of stroke within 10 years, but that same patient 70 year old patient on medications, with diabetes, daily smoking, and with cardiovascular disease has a 40% chance of stroke within 10 years, which is greater than the sum of the individual risks [16]. Further highlighting these synergistic relationships, (Figure 1) [16] demonstrates greater-than-additive increasing risk in a 55-year-old with multiple risk factors. Given the high prevalence of these common vascular risk factors throughout the US population and worldwide, a continued focus on risk factor control in the primary care setting as well in the public health sector remains critical.
Figure 1.
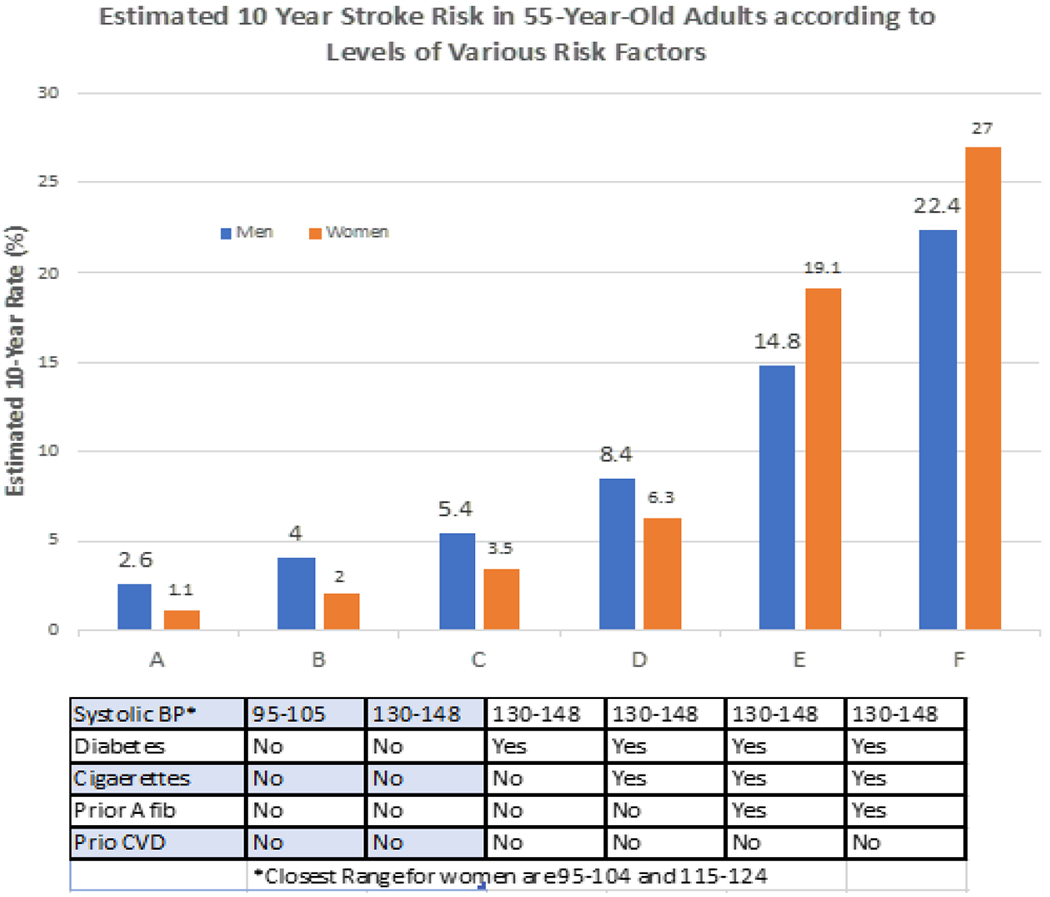
Estimated 10-year stroke risk in 55-year-old adults according to levels of various risk factors.
Adapted from: D’Agostino RB, Wolf PA, Be langer AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. Te Framingham Study. Stroke. 1994; 25: 40-43.
Stroke: The Biology
In general, vascular risk factors increase stroke risk through alterations of the blood, the blood vessels, and/or the heart. Such detrimental changes can occur in isolation or in combination. Blood can become more coagulopathic, larger blood vessels can develop atherosclerosis or arteriosclerosis or both, while smaller brain-specific vessels undergo a wall thickening process known as hyalinosis. Risk factors injure the endothelial cells of all-sized blood vessels and those lining the heart, thereby leading to an altered coagulatory and inflammatory response. Reduced blood vessel compliance forces the heart to work harder with each heartbeat leading to hypertrophy and subsequent cardiac arrythmias [17–19]. Over time, flow limiting atherosclerotic blood-vessel stenosis can develop, reducing blood flow and the ability of the brain to ensure that it is adequately perfused, leading to a mismatch between energy supply and demand (i.e. altered cerebral autoregulation) [20–23]. In the setting of severe intracranial or extracranial stenosis, acute embolic occlusions can lead to insufficient intravascular pressures within the brain and compromised collateral flow from non-ischemic vascular territories. Recall that such collateral flow patterns are critical for the survival and viability of the penumbral pre-infarcted tissue [24]. Not only can risk factors ultimately lead to altered flow patterns, some risk factors, such as aging and diabetes, seem to independently increase the susceptibility of brain tissue to injury in the setting of decreased blood flow [25]. While arterial occlusions typically result from an acute thrombus or embolus, medical practitioners must always ask themselves, “what was the cause of this acute occlusion?” We emphasize that: Understanding the etiology of a stroke is critical to preventing a re-occurrence in the future. Both atherosclerosis and smoking having been shown to play key roles in blood clot formation. Atherosclerosis can cause a clot to form when a friable plaque ruptures allowing an interaction of the plaque material with circulating platelets and coagulation factors, leading to platelet activation and aggregation with subsequent formation of an atherothrombus [26]. Smoking can lead to clot formation by inducing and accelerating atherosclerosis, by increasing the coagulability of blood, and by increasing the risk of cardiac disease, among other etiologies [27,28]. Given this basic framework regarding stroke biology, we will now focus on arguably the most preventable, prevalent, and well-established stoke risk factor in the US and worldwide, namely smoking.
Smoking (the Bad): The Epidemiology
Smoking remains a critical ongoing threat to US public health. Despite user prevalence reductions over the last decade, smoking remains highly prevalent in the USA with ~14 out of every 100 US adults smoking cigarettes regularly. This correlates to 34.2 million Americans or 13.7% of the USA population that regularly smoke [29]. Smoking is dangerous to the individual user and those nearby via passive second-hand smoke exposure. Per CDC studies, tobacco use is the leading cause of preventable disease, disability and death in the USA, causing approximately one in five deaths [30]. Specific to this review, smoking is highly associated with stroke risk. One large meta-analysis of 22 studies indicated an approximate doubling of the relative risk of stroke comparing smokers to non-smokers [31]. Many other studies have demonstrated that stroke risk increases with the daily amount of smoking. Hence, a dose-response relationship exists, that can be succinctly stated, “the more you smoke the more you stroke”. For example, comparing women who had never smoked, to those who smoked 1 to 14 cigarettes per day, the smokers had an age-adjusted relative stroke risk of 2.2 (95% confidence interval (CI) = 1.5 - 3.3), whereas those who smoked 25 or more cigarettes per day had a relative risk of 3.7 (95%CI = 2.7 - 5.1) [32]. Similar data obtained from a study looking at young men under the age of 50 found that the odds ratio comparing smokers to never smokers was 1.88, with an increasing odds ratio with increasing amount of daily cigarette use [33]. (Figure 2) [34] demonstrates a linear increase in stroke risk with increased number of cigarettes per day and that any smoking increases risk. Therefore, with smoking being such a threat to the public health due to its relatively high prevalence, and its association with morbidity and mortality including stroke, it becomes paramount to better understand who smokes and to evaluate the economic impact of smoking on society at-large.
Figure 2.
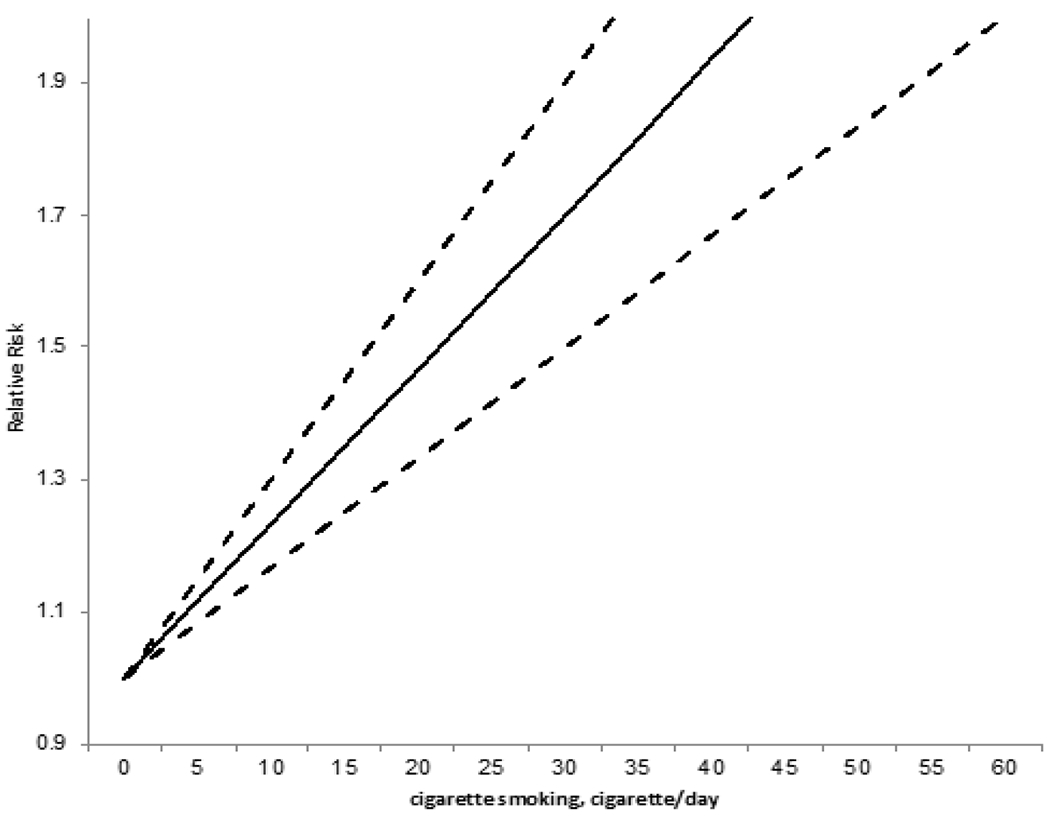
Increasing stroke risk with increased number of cigarettes per day.
Adapted from: Pan B, Jin X, Jun L, Qiu S, Zheng Q, Pan M. The relationship between smoking and stroke: A meta-analysis Medicine. 2019; 98.
Evaluating smoking epidemiologically, there are clear differences in the amount of cigarette smoking based upon gender, age, ethnicity, education level, economic prosperity, history of drug abuse and or psychological illness, military status, and by geographical region within the USA. A study from 2015 demonstrated smoking was more prevalent among males compared to females, 16.7% versus 13.6% respectively [35]. Regarding age, smoking was most prevalent in 25-44 yo at 14.8%, with it being the lowest in those greater than or equal to 65yo [35]. Racial differences were also noted, with smoking prevalence greatest among American-Indian/Alaska-Natives at 21.9% and lowest among US Asians at 7.0% [31]. Location of residence also plays a role, with a 16.2% prevalence of smoking in the US Midwest compared to 11% in the US West [36]. Education level and socioeconomic status demonstrate marked disparities, with those having lower education levels (to include a GED) as well as individuals below the poverty line having higher smoking rates at 34.1% and 26.1% respectively, as compared to those with graduate degrees and being above the poverty line at 3.6% and 13.9% respectively [36]. Those with substance abuse and psychological disorders disproportionately smoke. Broadly defined, this group makes up approximately 25% of the population but consumes almost 40% of all cigarettes [37]. Military service also shows a disparity with 21.6% of service members smoking in the last 30 days compared to the civilian estimate of ~14% as described above [38]. This is thought to be partially related to lower cigarettes prices on military installations, found to be ~13% less than nearby commercial outlets [39,40]. Considering these clear epidemiologic disparities, smoking cessation efforts focusing on these groups seems an obvious intervention opportunity.
Smoking: The Economics
Even with extensive public health measures targeting vulnerable groups, and by addressing established stroke risk factors, the economics driving tobacco-use make intervening in this public health crisis extremely challenging. First, the amount of money tobacco companies annually spend on advertising dwarfs the amount spent on smoking prevention. In 2018 alone, cigarette and smokeless tobacco companies spent more than $9 billion on advertising, with $8.4 billion promoting cigarettes and ~$660 million promoting smokeless tobacco [41,42]. Against such massive expenditures, it becomes very difficult for private and public anti-smoking initiatives to counterbalance such efforts. For example, in 2008 tobacco companies outspent tobacco prevention 23 to 1 [43]. As part of these huge corporate expenditures there are many marketing ‘ploys and tricks’ implemented to increase sales within specifically targeted and susceptible groups. For example, young adults are often targeted using advertising demonstrating ‘good looking’ young adults having fun in interesting social and environmental settings, with the goal to influence young people to start using tobacco [44]. In addition, tobacco companies target women with specific brands ‘made for women’, with themes in advertisements dominated by ideas of social desirability, empowerment, and independence [45,46]. Not only are specific age groups and sexes targeted, but also ethnic groups. Hispanic and Native-American groups have been targeted with brand names such as Rio, Dorado, and American Spirit [47]. African-Americans are targeted specifically regarding menthol cigarettes, with tobacco companies using urban culture and language to promote these cigarettes, organizing tobacco sponsored hip-hop bar nights, or using targeted direct mail promotions [44,46]. Asians have also been targeted, with direct sponsorships of Chinese and/or Vietnamese New Year’s Festivals, extensive billboard and instore advertising in predominantly Asian communities, and by financial contributions to both Asian community and business associations [46,47]. Given the extensive advertising and expenditures promoting tobacco use, private associations as well as federal and state governments working to counterbalance these efforts have experienced limited success.
One successful strategy implemented by state agencies, is to increase the cost of cigarettes to the consumer. It has been shown that a 10% increase in the price of cigarettes decreases overall cigarette consumption by ~4%, this, while increasing tax revenues [48,49]. (Figure 3) [50] demonstrates this concept from 1970-2015, with progressively increasing price per pack leading to decreasing sales over this same time period. One might think that both a decrease in amount of smoking and an increase in state revenue would be a win-win situation, but this is not necessarily the case. Despite these increased revenues, many states do not use these funds specifically for tobacco prevention and the treatment of tobacco related disease. Further, most states spend significantly less than what is recommend by the Centers for Disease Control (CDC) for tobacco prevention programs. For example, in the state of Maryland in 2020, the state made over $500 million in tobacco revenue and only spent $10.5 million on tobacco prevention programs, which is only 21.8% of the $48 million the CDC recommended for the state [50–52]. The amount spent by each state varies greatly, with California as an example spending 93.7% of the amount the CDC recommends, while others like Texas spend only 1.8% of what the CDC recommends [50–52]. With this wide-range of state-specific expenditures, additional pressures need to be placed upon state legislatures and/or the enactment of federal legislation to provide more consistent nationwide tobacco prevention efforts.
Figure 3.
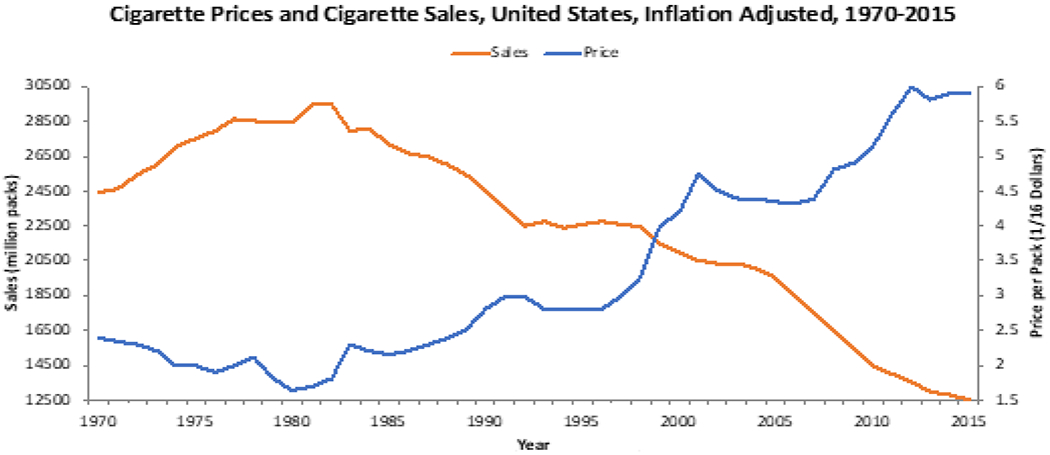
US Cigarette Prices and Cigarette Sales (inflation adjusted) from 1970-2015.
Adapted from: Orzechowski W, Walker R. The Tax Burden on Tobacco, Historical Compilation, 1970-2018.
Arlington, VA: Orzechowski & Walker Consulting. 2018 with calculations from Chaloupka FJ and Tobacconomic.
Since States are not spending enough on prevention, one needs to then ask if the tax revenues obtained from tobacco sales are at least are covering the health care costs of the diseases caused by tobacco use? The simple answer to this question is no. In 2019 the US government received $12.5 billion dollars in revenue from tobacco taxes [53]. This amount of money is significantly less than the estimated US health care costs of tobacco related illness. Per the CDC, smoking-related illness in the US costs ~$300 billion annually with ~$170 billion for direct medical costs. Smoking-related disease cost the US government $39.6 billion in Medicaid care payments with the federal government’s share being $22.6 billion and States being $17.0 billion. Regarding Medicare, expenditures were $45 million, with other tobacco-related health care costs to include VA health care being $32.8 billion [30]. Notably, not only are there direct healthcare costs, but also nation-wide costs associated with lost productivity estimated at $151 billion annually [30]. (Figure 4) [30] summarizes the monetary costs of smoking on the US health care system and society in general. With an estimated 2.5 billion packs of cigarettes sold in the US in 2019, each pack ultimately costs society $19.16 in healthcare costs, lost wages, disability, etc. These numbers indicate that the revenues obtained from tobacco sales by the State and National Governments, is substantially less than the direct and indirect costs of tobacco use. Given that a pack of cigarettes costs on average $7 to $10 in most US States, there is a substantial shortfall in the revenues required to cover smoking related healthcare costs, this, at both the State and National levels. Hence, these economic numbers clearly indicate that the non-smoking individuals in our society substantially monetarily contribute to the healthcare, lost wages and disability costs induced by smoking among the smoking populace. While this economic situation is both disturbing and disappointing, the primary concern remains on the individual user, who will inevitably experience the dire health-related outcomes associated with smoking-related diseases.
Figure 4.
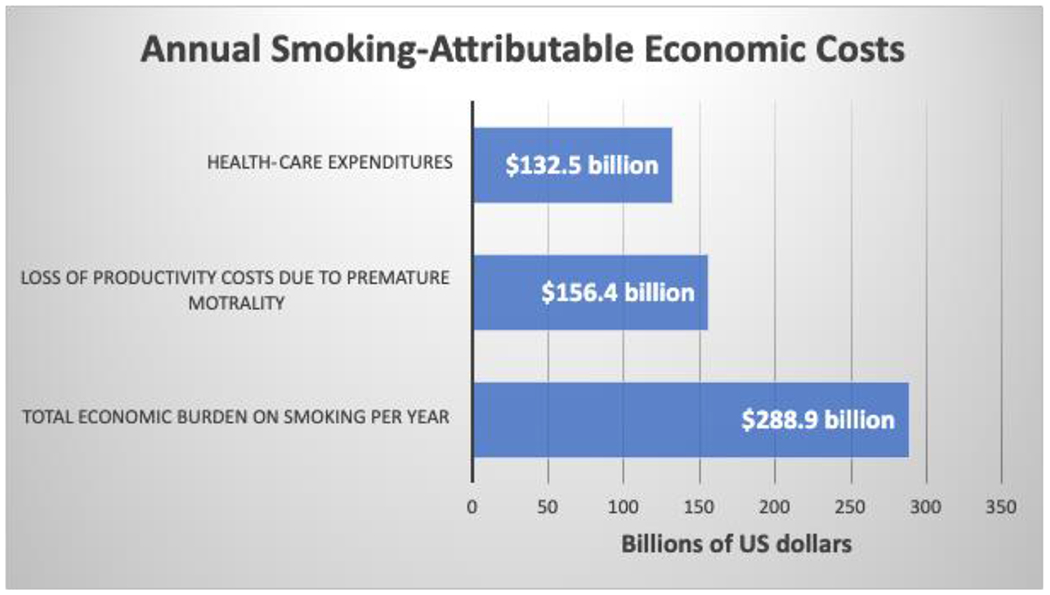
Annual Smoking-Attributable Economic Costs.
Adapted from: US Department of Health and Human Services. The health Consequence of Smoking-50 years of progress: a report of the Surgeon General.
Smoking: The Addiction
Simply put, smokers are addicted to nicotine, and healthcare providers must work to compassionately treat these individuals as such. After an individual smokes a cigarette, the active ingredient nicotine is absorbed into patient’s pulmonary venous circulation followed by transition to the arterial circulation and then crosses the blood-brain barrier where it binds to nicotinic cholinergic receptors, thereby stimulating them [54,55]. The stimulation of these receptors causes the release of many neurotransmitters [55,56]. This includes dopamine, which contributes to perceived pleasure, thereby reinforcing the use of tobacco [57]. Additionally, nicotine stimulates the release of glutamate, an excitatory neurotransmitter, which is associated with learning and memory [58,59]. Nicotine also indirectly stimulates the release of GABA, which is associated with a modulatory reduction of anxiety [59–61]. Not only does the use of nicotine promote these positive responses with continued use, it also promotes an increase in the number of high-affinity nicotine binding sites in the brain [57]. In order to avoid withdraw symptoms patients must keep these receptors occupied with nicotine, therefore reinforcing the need to continue to smoke [62]. In addition to the nicotine itself, there are genetic associations related to smoking addiction. Studies of twins have shown a high degree of heritability of smoking [59]. There also have been studies that have demonstrated strong genetic associations between specific genes, genetic variants and addictive cigarette smoking behavior [63]. Considering the complex pharmacology and genetic contributions to smoking behaviors, moving forward it becomes important to consider and develop targeted treatments to aid in smoking cessation, and potentially, treatments for primary smoking prevention.
Over the last several decades nicotine dependence and rates of cigarette smoking have decreased. In data released by the CDC, the rate of smoking in USA adults greater to or equal to 18 years of age has declined from 20.9 percent in 2005 to 15.5% in 2016. An even greater decrease when evaluating from 1965 through 2017, from 42% to 14% [64]. In addition, recent data from a survey taken by the National Institute of Health showed that among adults who have ever used cigarettes the percent of those that quit increased from 50.8% in 2005 and 59% in 2016 [65]. With these positive trends, one may ask is “smoking still the chief preventable cause of death in our society”, as stated succinctly in 1983 by former US Surgeon General C. Everett Koop. Unfortunately, with the recent advent of electronic cigarettes and vaping, the answer to this question is highly uncertain.
Electronic Cigarettes and Vaping (the Ugly).
Electronic cigarettes or e-cigarette use, also known as vaping, implements a battery-operated device that delivers nicotine to users by aerosolizing a nicotine containing solution [66]. While there remains some thought that these devices may be useful smoking cessation tools, this has not been scientifically proven or approved by the US Food and Drug Administration (FDA). Nonetheless, the prevalence of e-cigarette use has markedly increased over recent years [66,67]. At this time, both the long-term safety of these devices and their efficacy as a tool to quit smoking remain uncertain [66]. In an analysis of multiple studies Ghosh et al. concluded that e-cigarettes are not superior to approved nicotine replacement therapy, but also concluded that these devices are not clearly inferior to approved nicotine replacement therapies [66]. Given the nebulous aura surrounding e-cigarette cessation efficacy, and the uncertainty of their cardiovascular safety, we now summarize the epidemiology, marketing, science and technology of e-cigarettes, culminating with a discussion of their cardiovascular risks.
E-cigarettes and Vaping: The Epidemiology
E-cigarette use has increased over the recent years, especially among youth (<18yo) and young adults (18-24yo), who use e-cigarettes more than any other age groups. In 2018, the rapid rise of electronic tobacco products led the USA Surgeon General to issue an advisory about the “youth e-cigarette epidemic” [67]. Since then, rates have continued to increase with e-cigarettes use now being more prevalent than standard tobacco products among these younger age groups. In 2017, 11% of high school students had used an e-cigarette in the past 30 days. By 2018, that number had risen to 21%, and by 2019, 27.5% of high school students had used e-cigarettes in the past month [68–70]. Compared with the very small amount of youth use in 2011 (1.5%), this represents an alarming increase of more than 1,800% in just eight years, with a substantial increase occurring between 2013 and 2015, when use rose from 4.5% to 16%, coinciding with the emergence of the JUUL© product. Despite a January 2020 Federal intervention requiring JUUL© and other companies to remove all flavor pod-based cigarettes except for menthol and tobacco flavor, these companies were still able to exploit loopholes to increase rates of e-cigarette use [69,71,72]. Research suggests that mint and menthol, which remain available for sale, have since increased in popularity with 2019 National Youth Tobacco Survey (NYTS) data demonstrating use of these flavors rising over 6% in 2018 among high school current users [73].
Like youth, young adults aged 18-24 are also using e-cigarettes at increasing rates. Young adult use of e-cigarettes every day or some days increased from 2.4% in 2012 and 2013, to 5.2% in 2017, and further increased to 7.6% in 2018 [74–76].
Among adults, e-cigarette use has remained relatively low and stable since 2012. Between 2012 and 2013, 2.4% of adults aged 25-44 and 2% of adults aged 45-64 used e-cigarettes. By 2016, the rates had moderately increased to 4.2% and 2.8%, respectively. Data for the year 2018 show current use of e-cigarettes remaining at 4.2% among adults 25-44 and further reduced to 2.1% among adults aged 45-64 [75,77].
Rates of e-cigarette use not only vary among age groups, but also by US state ranging from 16.2% in Washington DC to 28.4% in Arkansas. An increased rate of e-cigarette use does not necessarily correlate with the prevalence of current cigarette smoking in a state, with some states having low levels of cigarette smoking having higher levels of e-cigarette use [78].
Dual Use of Tobacco Products (combined smoking and vaping use)
Among all age groups, e-cigarettes are most commonly used by those who also use other tobacco products, including standard cigarettes. This pattern is commonly referred to as “dual use” or “poly tobacco use.” Among adult users, this is a troubling pattern because it suggests that some e-cigarette use may be simply supplementing smoking instead of replacing it. While some individuals using e-cigarettes to quit cigarette smoking may experience a period of dual use, this as they transition between products, such a situation must be carefully monitored and often leads to varying levels of success.
Among youth, the data tell a different narrative. Taken in aggregate, the evidence indicates that e-cigarette use increases risk of ever using combustible tobacco cigarettes among youth and young adults, suggesting that e-cigarette use itself is a risk factor for smoking, not just a correlate with smoking. Young e-cigarette users have been shown to be four times more likely to begin smoking tobacco-based cigarettes compared to their peers who do not use e-cigarettes [79]. The study also estimated that e-cigarettes are likely responsible for 22% of new ‘ever cigarette use’ (trying a cigarette) and 15.3% of current cigarette use for the same group-totaling nearly 200,000 new cigarette initiators. (Figure 5) [80] clearly summarizes the disturbing trends regarding the use of electronic cigarettes and any tobacco product in isolation or combination among middle and high school students [80].
Figure 5.
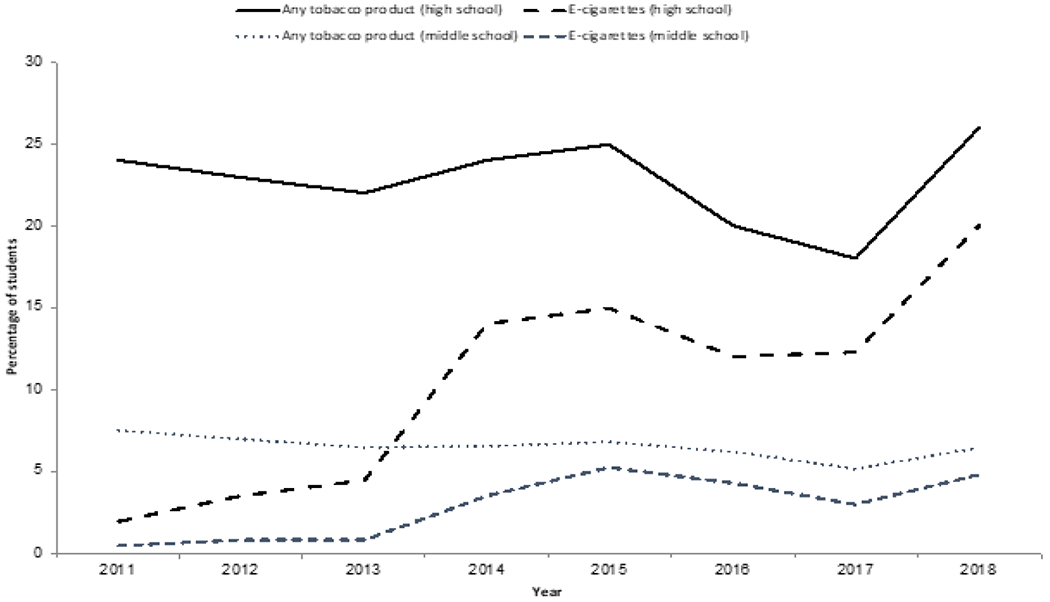
Recent trends regarding the use of electronic Cigarettes and any tobacco product in isloation or combination among middle and high school students.
Adapted from: Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students-United states, 2011-2018. Morbidity and Mortality Weekly Report. 2018; 67: 1276.
E-cigarettes and Vaping: The Marketing
The increase in electronic cigarette use among youth is clearly related to marketing tactics put forth by many of the manufacturers of electronic cigarettes, including the now prohibited fruity and candy-like flavors [81]. E-cigarette manufacturers also use social media influences to market their products on various platforms including Instagram, Twitter, Facebook, blogs, and vlogs. In addition to using social media influences e-cigarette manufacturers will market their products towards youth-oriented groups by holding sampling events with young, attractive, healthy-appearing spokespeople. Similar tactics are employed in print and online advertising campaigns [82]. Other companies have used similar marketing tools as well as purporting ‘advanced technology’ in their advertisements describing the ‘recharge ability’ of their devices [82]. Targeting these younger consumers is further concerning in lieu of the current lack of knowledge regarding risks associated with both short- and long-term e-cigarette use.
E-cigarettes and Vaping: The Technology
E-cigarettes come in a variety of designs and complexity, but typically these devices contain a battery, heating coil, an atomizer that transforms the nicotine containing liquid into an aerosol, a cartridge containing the liquid, and a mouthpiece. When a user wants to take ‘a puff’ of an e-cigarette the heating element is activated either manually by pressing a button or by the change in air pressure as associated with taking in a breath. Following this the heating element vaporizes a fixed quantity of the nicotine containing e-liquid, which then is drawn into the user’s mouth, then lungs and then blood stream to be distributed throughout their body [83]. Some of the chemicals, beyond nicotine, including propylene glycol and/or vegetable glycerine have been shown to induce lung and airway inflammation [83]. In addition, these volatile organic compounds can cause damage to the liver, kidney, and nervous system [84]. These chemicals not only travel into the lungs of the e-smoker, but also are released as secondhand smoke, with long term effects of secondhand e-cigarette smoke being completely unknown [85].
E-cigarette and Vaping: Stroke Risk
Not only do vaping products lead to airway and lung injury, they also have been shown to affect patients’ vasculature, specifically the endothelium (i.e. the inner lining of blood vessels). A recent study demonstrated that after inhalation by healthy nonsmokers of e-cigarette vapor there was an acute change in flow dynamics consistent with endothelial dysfunction [86]. This study further demonstrated a decrease in hemoglobin saturation suggesting microvascular dysfunction, as well as stiffening of the aorta [86]. All of these changes are significant, since alterations in blood vessel function can be associated with stroke. However, given the paucity of data on this new topic, the risk relationship between e-cigarette use and stroke remains uncertain.
A recent study by Parekh et al. claimed that there was an increased risk of stroke in current e-cigarette users with concomitant use and/or former use of combustible cigarettes compared to solely to combustible cigarette use [87]. Interestingly, this study did not demonstrate an increased risk of stroke in sole e-cigarette users compared to non-smokers, and additionally demonstrated that the risk of stroke was lower in sole e-cigarette users compared to combustible cigarette use [87]. Notably, the validity of these findings was called into question because of the statistical methods used, where the authors failed to control for smoking duration, as well as current and past consumption [88]. However, given these significant short term affects, as well as lack of knowledge about long term effects, and the unclear link between e-cigarette use and stroke, increased public heath efforts are needed to curb the use of these devices and further research is required to better understand risks.
E-cigarette or Vaping product use-Associated Lung Injury (EVALI)
The chemical thought to be the primary contributor to e-cigarette or Vaping Associated Lung Injury (EVALI) is Vitamin E Acetate. In a recent study, 48 of 51 patients with confirmed or presumed EVALI had Vitamin E Acetate collected from their Bronchoalveolar Lavages (BAL), whereas the control group had no Vitamin E Acetate in their BALs [89]. Another study evaluated mice exposed to Vitamin E Acetate and found that those exposed had pulmonary injury evaluated by albumin in BAL fluid as well as increased White Blood Cell (WBC) counts [90]. As of February 2020, the total number of hospitalized e-cigarette/vaping associated lung injuries or deaths was 2807, with 68 of these being deaths. Other studies of EVALI patients demonstrated that 96% of such patients required hospitalization with 26% requiring intubation [91,92].
E-cigarette and Vaping product use and COVID-19
A recent study demonstrated a strong association between vaping and an increased incidence of COVID-19 infection [93]. The angiotensin-converting-enzyme-2 (ACE2) receptor is expressed on respiratory epithelium has been shown to be an effective site for host entry of the severe-acute-respiratory-syndrome coronavirus-2 (SARS-CoV2), the virus that causes COVID-19 infection [94]. ACE2 expression has been shown to be elevated among individuals who smoke and is similarly hypothesized to be elevated those who use nicotine-containing e-cigarettes [95]. High concentrations of ACE2 receptors are found on the lung epithelium, resulting in increased absorption of COVID-19 particles within the small airways. In addition, like smoking, vaping triggers oxidative stress and decreases the body’s ability to initiate disease inflammatory responses in the lungs, increasing the risk of bacterial and viral infections. Oxidative stress damages the epithelial membrane increasing cell permeability resulting in decreased ability to clear secretions, thus increasing susceptibility to respiratory compromise [96].
Special consideration needs to be paid to the association between youth who smoke or use e-cigarettes and COVID-19. A recently reported national population-based on-line survey of adolescents and young adults (n=4,351) 13-24 years of age demonstrated significant public health concerns indicating vaping is a risk factor of COVID-19 infection [93]. Multivariable logistic regression was used to assess relationships between COVID 19-symptoms, testing, diagnosis, cigarette use, e-cigarette use, and dual cigarette use, socioeconomic factors and compliance with sheltering in place. Significant positive results revealed a positive COVID-19 diagnosis was 5 times more likely among ever users of e-cigarettes (95% CI 1.82-13.96) and that dual users were 7 times more likely to have a positive COVID-19 diagnosis (95% CI: 1.98-24.55) [93]. This creates an ‘urgent call to action’ for health care providers to educate youth on the risks of smoking, vaping and COVID-19.
E-cigarette and Vaping: Where are we going?
Currently the FDA, as well as the CDC, are working to address the e-cigarette/vaping epidemic as well as curb the use of these devices. Unfortunately, this remains a challenge due to the classification of these devices, as some use nicotine, while others do not. Tobacco products are categorized by their nicotine status and therefore their regulations are based on this classification [97]. The status of e-cigarettes, some with nicotine and some without, makes classifying these products nebulous in certain instances, thereby limiting the possibility of complete all-encompassing regulations.
For the health benefit of its citizens, the US Federal Government should work to limit e-cigarette use as well as traditional cigarette use, but responsibility also rests on the public and health-care professionals. Every year ~40% of smokers will attempt to quit, with only 4-6% being successful [98,99]. Success normally involves a two-step process. The first step is motivation by both the smoker and their clinicians to stop smoking that can be implemented using tools to include the US Public Health Service-National Tobacco Cessation Toolkit (5A&5Rs), motivational interviewing, and stages of change models [100–103]. These approaches have been shown to be effective in randomized trials in decreasing smoking rates with as little as three minutes of daily motivation, increasing the quit rate from a factor of 1.3 to 1.7 [101,104]. The second step focuses on treatments helping them try to quit [101]. This includes both pharmacotherapy as well as psychosocial treatments. Pharmacotherapy consists of proven regiments including: nicotine gums, inhalers, lozenges, patches, as well as non-nicotine medications including bupropion, nortriptyline, and clonidine, which have all been shown to be roughly equally effective, increasing quit rates by a factor of 1.5 to 2.7 [100]. Psychosocial therapies include behavioral therapy as well as finding social supports, with such therapies increasing quit rates by a factor of 1.5 to 2.1 and 1.3 to 1.5, respectively [100,105,106]. Given the ever-changing landscape between nicotine delivery devices, their risks, and public perception, it remains of the utmost importance to maintain an active collaboration between both public and governmental organizations to curb the use of both traditional tobacco products as well as e-cigarettes.
CONCLUSION
The use of traditional cigarettes, and now e-cigarettes, is an ongoing large-scale public health crisis inducing extensive morbidity, mortality and socio-economic detriments to our society. Only with continued research, scientific advancements, and governmental and public health efforts and initiatives can we overcome this seemingly unrelenting crisis.
ACKNOWLEDGEMENT
J.W. Cole is partially supported by an American Heart Association-Bayer Discovery Grant (grant 17IBDG33700328), the AHA Cardiovascular Genome-Phenome Study (grant 15GPSPG23770000), the NIH (grants R01-NS114045, R01-NS100178, R01-NS105150), and the US Department of Veterans Affairs.
ABBREVIATIONS
- TIA
Transient Ischemic Attack
- CDC
Centers for Disease Control
- FDA
Food and Drug Administration
- USA
United States of America
- US
United States
- EVALI
e-cigarette or Vaping Associated Lung Injury
REFERENCES
- 1.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. The Lancet. 2016; 20: 761–775. [DOI] [PubMed] [Google Scholar]
- 2.Park TH, Ko Y, Lee SJ, Lee KB, Lee J, Han MK, et al. Identifying target risk factors using population attributable risks of ischemic stroke by age and sex. J Stroke. 2015; 17: 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aigner A, Grittner U, Rolfs A, Norrving B, Siegerink B, Busch MA. Contribution of established stroke risk factors to the burden of stroke in young adults. Stroke. 2017; 48: 1744–1751. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011; 123: el8–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Stroke Association. Awareness and Knowledge of Stroke Prevention: A Study of Adults, 50 Years of Age and Over. Englewood, Colo: National Stroke Association; 1996. [Google Scholar]
- 6.Pancioli AM, Broderick J, Kothari R, Brott T, Tuchfarber A, Miller R, et al. Public perception of stroke warning signs and knowledge of potential risk factors. JAMA. 1998; 279: 1288–1292. [DOI] [PubMed] [Google Scholar]
- 7.Stroke Symptoms. 2021. [Google Scholar]
- 8.DiGiovanni BF, Sundem LT, Southgate RD, Lambert DR. Musculoskeletal medicine is underrepresented in the American medical school clinical curriculum. Clin Orthop Relat Res. 2016; 474: 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feroze R, Prabhu AV, Agarwal N. Stroke Education Is Underrepresented in US Undergraduate Clinical Curricula. Acad Med. 2017; 92: 435. [DOI] [PubMed] [Google Scholar]
- 10.Yi X, Luo H, Zhou J, Yu M, Chen X, Tan L, et al. Prevalence of stroke and stroke related risk factors: a population based cross sectional survey in southwestern China. BMC Neurol. 2020; 20: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonita R, Duncan J, Truelsen T, Jackson RT, Beaglehole R. Passive smoking as well as active smoking increases the risk of acute stroke. Tobacco Control. 1999; 8: 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension Prevalence and Control Among Adults: United States, 2015-2016. NCHS Data Brief. 2017; 289: 1–8. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services. 2020. [Google Scholar]
- 14.Carroll MD, Fryar CD, Nguyen DT. Total and high-density lipoprotein cholesterol in adults: United States, 2015-2016. NCHS Data Brief. 2017; 290: 1–8. [PubMed] [Google Scholar]
- 15.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. 2020. [PubMed] [Google Scholar]
- 16.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994; 25: 40–43. [DOI] [PubMed] [Google Scholar]
- 17.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010; 67: 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen CL, Bayraktutan U. Risk factors for ischaemic stroke. Int J Stroke. 2008; 3:105–116. [DOI] [PubMed] [Google Scholar]
- 19.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008; 7: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arrick DM, Sharpe GM, Sun H, Mayhan WG. nNOS-dependent reactivity of cerebral arterioles in Type 1 diabetes. Brain Res. 2007; 1184: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009; 40: S40–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007; 38: 2136–2141. [DOI] [PubMed] [Google Scholar]
- 23.Zou MH. Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004; 11: 89–97. [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010; 67:181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol. 2002; 441: 1–14. [DOI] [PubMed] [Google Scholar]
- 26.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014; 276: 618–632. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen VG, Hafner DT, Steinbrenner EB. Tobacco smoke-induced hypercoagulation in human plasma: role of carbon monoxide. Blood Coagul Fibrinolysis. 2013; 24: 405–410. [DOI] [PubMed] [Google Scholar]
- 28.Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, et al. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm. 2011; 8: 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, et al. Tobacco product use and cessation indicators among adults-United States, 2018. Morbidity and Mortality Weekly Report. 2019; 68: 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [PubMed] [Google Scholar]
- 31.Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ. 1989; 298: 789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colditz GA, Bonita R, Stampfer MJ, Willett WC, Rosner B, Speizer FE, et al. Cigarette smoking and risk of stroke in middle-aged women. N Engl J Med. 1988; 318: 937–941. [DOI] [PubMed] [Google Scholar]
- 33.Markidan J, Cole JW, Cronin CA, Merino JG, Phipps MS, Wozniak MA, et al. Smoking and risk of ischemic stroke in young men. Stroke. 2018; 49: 1276–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan B, Jin X, Jun L, Qiu S, Zheng Q, Pan M. The relationship between smoking and stroke: A meta-analysis. Medicine. 2019; 98: e14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults-United States, 2005-2015. Morbidity and Mortality Weekly Report. 2016; 65: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 36.Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, et al. Tobacco product use and cessation indicators among adults-United States, 2018. Morbidity and Mortality Weekly Report. 2019; 68:1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adults with Mental Illness or Substance Use Disorder Account for 40 Percent of All Cigarettes Smoked. 2014. [Google Scholar]
- 38.Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans-United States, 2010–2015. Morbidity and Mortality Weekly Report. 2018; 67: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haddock CK, Hyder ML, Poston WS, Jahnke SA, Williams LN, Lando H. A longitudinal analysis of cigarette prices in military retail outlets. Am J Public Health. 2014; 104: e82–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith EA, Jahnke SA, Poston WS, Malone RE, Haddock CK. Tobacco pricing in military stores: views of military policy leaders. Nicotine Tob Res. 2016; 18: 2041–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Federal Trade Commission. Federal trade commission cigarette report for 2018. 2019. [Google Scholar]
- 42.US Federal Trade Commission. Federal trade commission smokeless tobacco for 2018. 2019. [Google Scholar]
- 43.McAfee presentation (June 11, 2012); Campaign for Tobacco-Free Kids (2012b,d); CDC; (2007c). [Google Scholar]
- 44.Campaign for Tobacco-Free Kids. Spending vs. Tobacco Company marketing. 2012b. [Google Scholar]
- 45.Perks SN, Armour B, Agaku IT. Cigarette Brand Preference and Pro-Tobacco Advertising Among Middle and High School Students-United States, 2012-2016. Morbidity and Mortality Weekly Report. 2018; 67: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.US Department of Health and Human Services. Women and smoking: A report of the Surgeon General. Washington, DC: Public Health Service, Office of the Surgeon General. Executive summary. MMWR Recomm Rep. 2002; 51:1–13. [PubMed] [Google Scholar]
- 47.National Cancer Institute. The role of the media in promoting and reducing tobacco use. Tobacco Control Monograph. No.19. 2008. [Google Scholar]
- 48.US Department of Health and Human Services. Tobacco use among US racial/ethnic minority groups-African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A report of the surgeon general. National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 1998; 90. [Google Scholar]
- 49.Jha P, Chaloupka FJ. The economics of global tobacco control. BMJ. 2000; 321: 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jha P, Chaloupka FJ. Tobacco control in developing countries. Oxford University Press; 2000. [Google Scholar]
- 51.Orzechowski W, Walker R. The Tax Burden on Tobacco, Historical Compilation, 1970-2018. Arlington, VA: Orzechowski& Walker Consulting. 2018. [Google Scholar]
- 52.King BA, Pechacek TF, Mariolis P. Best practices for comprehensive tobacco control programs. 2014. [Google Scholar]
- 53.Broken Promises to Our Children. Campaign for Tobacco-Free Kids. 2020. [Google Scholar]
- 54.Congressional Budget Office. Historical Tables of the Budget of the US government Washington, DC: Congressional Budget Office. 2020; 41–50. [Google Scholar]
- 55.Benowitz NL. Nicotine addiction. New England Journal of Medicine. 2010; 362: 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends in Pharmacological Sciences. 2004; 25: 317–324. [DOI] [PubMed] [Google Scholar]
- 57.Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997; 20: 92–98. [DOI] [PubMed] [Google Scholar]
- 58.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005; 8:1445–1449. [DOI] [PubMed] [Google Scholar]
- 59.McEntee WJ, Crook TH. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology. 1993; 111: 391–401. [DOI] [PubMed] [Google Scholar]
- 60.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000; 27: 349–357. [DOI] [PubMed] [Google Scholar]
- 61.Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiatry. 2003; 64: 21–27. [PubMed] [Google Scholar]
- 62.Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009; 78: 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, et al. Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006; 63: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007; 16: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cigarette Smoking Among U.S. Adults Lowest Ever Recorded: 14% in 2017 CDC Newsroom. US Department of Health and Human Services. 2018. [Google Scholar]
- 66.Smoking is down, but almost 38 million American adults still smoke [Internet]. CDC Newsroom. US Department of Health and Human Services. 2018. [Google Scholar]
- 67.Ghosh S, Drummond MB. Electronic cigarettes as smoking cessation tool: are we there? Current Opinion in Pulmonary Medicine. 2017; 23: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Surgeon General’s Advisory on E-cigarette Use among Youth. E Cigarettes Surgeon General. Department of Health and Human Services; 2018. [Google Scholar]
- 69.Wang TW, Gentzke A, Sharapova S, Cullen KA, Ambrose BK, Jamal A. Tobacco product use among middle and high school students-United States, 2011-2017. MMWR Morb Mortal Wkly Rep. 2018;67:629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang TW, Neff LJ, Park-Lee E, Ren C, Cullen KA, King BA. E-cigarette use among middle and high school students-United States, 2020. Morbidity and Mortality Weekly Report. 2020; 69: 1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang TW, Gentzke AS, Creamer MR, Cullen KA, Holder-Hayes E, Sawdey MD, et al. Tobacco product use and associated factors among middle and high school students-United States, 2019. MMWR Surveill Summ. 2019; 68: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.US Food and Drug Administration. Enforcement priorities for electronic nicotine delivery systems (“ENDS”) and other deemed products on the market without premarket authorization. 2020. [Google Scholar]
- 73.Ali FR, Diaz MC, Vallone D, Tynan MA, Cordova J, Seaman EL, et al. E-cigarette Unit Sales, by Product and Flavor Type-United States, 2014–2020. Morbidity and Mortality Weekly Report. 2020; 69: 1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, et al. E-cigarette use among youth in the United States, 2019. JAMA. 2019; 322: 2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. [Google Scholar]
- 76.Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, et al. Tobacco product use among adults-United States, 2017. Morbidity and Mortality Weekly Report. 2018; 67: 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014-2018. JAMA. 2019; 322:1824–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.National Center for Health Statistics. National Health Interview Survey 2018 Data Release internal calculation. DC: Washington; 2018. [Google Scholar]
- 79.Hu SS, Homa DM, Wang T, Gomez Y, Walton K, Lu H, et al. Peer Reviewed: State-Specific Patterns of Cigarette Smoking, Smokeless Tobacco Use, and E-Cigarette Use Among Adults-United States, 2016. Preventing Chronic Disease. 2019; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.National Academies of Sciences, Engineering, and Medicine 2018. Public Health Consequences of E-Cigarettes. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 81.Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students-United States, 2011-2018. Morbidity and Mortality Weekly Report. 2018; 67: 1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jackler RK, Chau C, Getachew BD, Whitcomb MM, Lee-Heidenreich J, Bhatt AM, et al. JUUL advertising over its first three years on the market. SRITA White Paper. 2019: 1–48. [Google Scholar]
- 83.Haardörfer R, Cahn Z, Lewis M, Kothari S, Sarmah R, Getachew B, Berg CJ. The advertising strategies of early e-cigarette brand leaders in the United States. Tob Regul Sci. 2017; 3: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eaton DL, Alberg AJ, Goniewicz MA, Leventhal A, Manautou JE, McGrath-Morrow SH, Mendez D, et al. Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public health consequences of e-cigarettes. Consensus study report for the US Food and Drug Administration. Washington (DC): US National Academies of Science, Medicine and Engineering. 2018. [Google Scholar]
- 85.What Do We Know About E-cigarettes? American Cancer Society. [Google Scholar]
- 86.Electronic Cigarettes -An Overview. German Cancer Research Center (Ed.) Heidelberg. 2013; 19: 1–39. [Google Scholar]
- 87.Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S, Wehrli FW. Acute effects of electronic cigarette aerosol inhalation on vascular function detected at quantitative MRI. Radiology. 2019; 293: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parekh T, Pemmasani S, Desai R. Risk of stroke with E-cigarette and combustible cigarette use in young adults. Am J Prev Med. 2020; 58: 446–452. [DOI] [PubMed] [Google Scholar]
- 89.Farsalinos K, Abrams D, Niaura R. Can the Association Between Electronic-Cigarette Use and Stroke Be Interpreted as Risk of Stroke?. Am J Prev Med. 2020; 58: 895–896. [DOI] [PubMed] [Google Scholar]
- 90.Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020; 382: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhat TA, Kalathil SG, Bogner PN, Blount BC, Goniewicz ML, Thanavala YM. An animal model of inhaled vitamin E acetate and EVALI-like lung injury. N Engl J Med. 2020; 382: 1175–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. 2020. [Google Scholar]
- 93.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, Navon L, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin. N Engl J Med. 2020; 382: 903–916. [DOI] [PubMed] [Google Scholar]
- 94.Gaiha SM, Cheng J, Halpern-Felsher B. Association between youth smoking, electronic cigarette use, and COVID-19. J Adolesc Health. 2020; 67: 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang H, Rostami MR, Leopold PL, Mezey JG, O’Beirne SL, Strulovici-Barel Y, et al. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am J Respir Crit Care Med. 2020; 202: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharma P, Zeki AA. Does vaping increase susceptibility to COVID-19? Am J Respir Crit Care Med. 2020; 202: 1055–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaur G, Lungarella G, Rahman I. SARS-CoV-2 COVID-19 susceptibility and lung inflammatory storm by smoking and vaping. J Inflamm (Lond). 2020; 17: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.FDA - Deeming Regulations for E-Cigarettes, Cigars, and All Other Tobacco Products. [Internet]. U.S. Food and Drug Administration. FDA. 2020. [Google Scholar]
- 99.US Department of Health and Human Services. Cigarette smoking among adults, United States, 1998. MMWR. 2000; 48: 881–884. [PubMed] [Google Scholar]
- 100.Cohen S, Lichtenstein E, Prochaska JO, Rossi JS, Gritz ER, Carr CR, et al. Debunking myths about self-quitting: Evidence from 10 prospective studies of persons who attempt to quit smoking by themselves. Am Psychol. 1989; 44: 1355–1365. [DOI] [PubMed] [Google Scholar]
- 101.Hughes JR. Motivating and helping smokers to stop smoking. J Gen Intern Med. 2003; 18: 1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services. 2008. [Google Scholar]
- 103.Emmons KM, Rollnick S. Motivational interviewing in health care settings: opportunities and limitations. Am J Prev Med. 2001; 20: 68–74. [DOI] [PubMed] [Google Scholar]
- 104.Prochaska JO, Goldstein MG. Process of smoking cessation. Implications for clinicians. Clin Chest Med. 1991; 12: 727–735. [PubMed] [Google Scholar]
- 105.Stead LF, Bergson G, Lancaster T, Preciado N, Sanchez G, Hartmann-Boyce J. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013; 2013: CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev. 2017; 3: CD001007. [DOI] [PMC free article] [PubMed] [Google Scholar]


