Abstract
Objective
To identify the prevalence, treatment, and low-density lipoprotein cholesterol (LDL-C) control of individuals with LDL-C ≥190 mg/dL in contemporary clinical practice.
Methods
We included adults (age ≥18 years) with LDL-C ≥190 mg/dL, at least one LDL-C level drawn from 255 health systems participating in Cerner HealthFacts database (2000–2017, n = 4,623,851), and a detailed examination within Duke University Health System (DUHS, 2015–2017, n = 267,710). Factors associated with LDL-C control were evaluated using multivariable logistic regression modeling.
Results
The cross-sectional prevalence of LDL-C ≥190 mg/dL was 3.0% in Cerner (n = 139,539/4,623,851) and 2.9% at DUHS (n = 7728/267,710); among these, rates of repeat LDL-C measurement within 13 months were low: 27.9% (n = 38,960) in Cerner, 54.5% (n = 4211) at DUHS. Of patients with follow-up LDL-C levels, 23.6% in Cerner had a 50% of greater reduction in LDL-C, 18.3% achieved an LDL-C <100 mg/dL and 2.7% < 70 mg/dL. At DUHS, 28.4% had a 50% or greater reduction in LDL-C, 28.4% achieved an LDL-C ≤100 mg/dL and 4.4% achieved <70 mg/dL. Within DUHS, 71.6% with LDL-C ≥190 mg/dL were on any statin during follow-up, but only 28.5% were on a high-intensity statin. In multivariable modeling, seeing a cardiologist (Cerner odds ratio [OR] 1.56, confidence interval [CI] 1.33–1.83; DUHS OR 1.89, 95% CI 1.18–3.01) and having diabetes (Cerner OR 1.34 CI 1.23–1.46; DUHS OR 2.07, CI 1.62–2.65) increased odds of LDL-C control, defined as a ≥50% reduction in LDL-C (at Cerner) or initiation of high intensity statin (at DUHS). Prior atherosclerotic cardiovascular disease (OR 1.19, CI 1.07–1.33), hypertension (OR 1.10, CI 1.03–1.18), African American race (OR 0.79, CI 0.71–0.89), and government (vs. private) insurance (OR 0.90, CI 0.83–0.98) were associated with LDL-C control at Cerner. Female sex was associated with lower odds of appropriate therapy (OR 0.69, CI 0.59–0.81) at DUHS.
Conclusions
Approximately 3% of United States adults have LDL-C ≥190 mg/dL. Among those with very high LDL-C, rates of repeat measurement within one year were low; of those retested, only about one-fourth met guideline-recommended LDL-C treatment goals.
Keywords: LDL-C, Hyperlipidemia, Statin therapy
Highlights
-
•Large numbers of U.S. adults with extremely high LDL-C.
-
oCan be identified using available EHR data
-
oOften have no follow-up lipid measurement
-
oAre not treated with recommended lipid-lowering therapies
-
oDo not achieve guideline-recommended LDL-C reduction goals
-
o
1. Introduction
Low-density lipoprotein cholesterol (LDL-C) represents a key modifiable risk factor for the prevention of atherosclerotic cardiovascular disease (ASCVD) [[1], [2], [3]]. Adults with extremely high LDL-C (≥190 mg/dL) are at particularly high risk for future cardiovascular events. Compared with individuals with LDL-C <130 mg/dL, those with LDL-C ≥190 mg/dL have an accelerated risk for cardiovascular disease (CVD) by 10–20 years in men and 20–30 years in women, and more than four times the hazard of ASCVD (hazard ratio [HR] 4.1, 95% confidence interval [CI] 1.2–13.4) [4]. The risk conferred by these elevations in LDL-C is largely modifiable [3,[5], [6], [7], [8], [9], [10]], with statins representing the gold-standard treatment [11]. For this reason, multiple guidelines for the treatment of blood cholesterol consistently recommend high-intensity statin treatment in adults 40–75 years old with LDL-C ≥190 mg/dL, with a goal of at least 50% LDL-C reduction [3,12]. The prevalence of adults with extremely high LDL-C is estimated to be up to 7% in national survey data [13,14], yet characteristics of these adults and their consequent treatment patterns, remain understudied.
Given the uncertainty surrounding this high-risk population, we set out to assess the prevalence and characteristics of adults with severe hyperlipidemia (LDL-C ≥190 mg/dL) from two separate populations: 1) the Cerner HealthFacts database, which is a large dataset of patients seen across 255 United States (U.S.) health systems; and 2) a single large health system, Duke University Health System (DUHS), which has detailed clinical and treatment data. We subsequently used these two complementary data sources to: 1) describe characteristics of adults with extremely high LDL-C; 2) understand patterns of care for these adults, including the proportion who achieve guideline-recommended LDL-C reduction; and 3) factors associated with achieving guideline-recommended care for severe hyperlipidemia.
2. Methods
2.1. Cerner HealthFacts data
We retrospectively evaluated the prevalence of adults with a single LDL-C measurement ≥190 mg/dL in two complementary data sources: the Cerner HealthFacts database and the Duke University Health System (DUHS). Cerner HealthFacts is a de-identified database that includes 172,920,586 patients seen across 428 health systems across the United States that use the Cerner electronic health record (EHR) and have opted in for participation. The time period of data available for this analysis was between 2000 and 2017. During this time period, Cerner HealthFacts lacked accurate outpatient medication data. Since health systems could change EHRs used, or stop participating in HealthFacts, some health systems did not have data through 2017. Therefore, in order to ensure sufficient time to capture follow-up lipid measurements, we required the lipid measurement to occur at least 13 months prior to the end of each health system’s participation in HealthFacts, or the end of data collection in HealthFacts. In sensitivity analysis, we restricted the population to those in the Cerner HealthFacts database between 2013 and 2015 with follow up through 2017 to understand if more contemporary data yielded similar results.
For this study, we included adults 18 years of age and older with at least 1 draw of LDL-C. For our analysis of severe hyperlipidemia, we identified adults with an LDL-C ≥190 mg/dL at any point, using the first instance of LDL-C ≥190 mg/dL as the baseline, including patients with multiple values that exceeded the threshold. In order to identify adults on whom clinical data would be available in the EHR, we further required at least one outpatient or clinic encounter with primary care, cardiology, obstetrics and gynecology, nephrology, or endocrinology within 30 days (before or after) the baseline LDL-C lab draw. Patient comorbidities were collected using International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) codes associated with the baseline visit, or the closest outpatient encounter, for the following diagnoses: hypertension, diabetes (type 1 and type 2), ASCVD, heart failure, peripheral arterial disease, coronary heart disease, cerebrovascular disease, hypothyroidism, hyperthyroidism, and nephrotic syndrome [[15], [16], [17], [18], [19]]. eTable 1 shows ICD-9 and ICD-10 codes used to capture baseline comorbidities.
2.2. DUHS data
DUHS is a large academic medical center with both inpatient and outpatient primary care and specialty clinics that uses a single non-Cerner system EHR. Unlike HealthFacts, the DUHS data also allowed for analysis of outpatient medications. The inclusion criteria for this analysis were: 1) age 18 and older; 2) at least one LDL-C measurement between January 1, 2015 and 11/30/2017; and 3) at least one follow-up visit within 2 years of the baseline draw. The requirement for a follow-up visit was put into place to identify a cohort of patients followed longitudinally at DUHS. Of eligible adults, we identified the first LDL-C measurement ≥190 mg/dL within the evaluation period, and identified the closest outpatient encounter to that laboratory draw as the index encounter. From that index encounter, we obtained the following information from the EHR: patient age, sex, race, body mass index, insurance type, allergies, laboratory data including LDL-C, high-density lipoprotein cholesterol (HDL-C), triglycerides, blood pressure, ordering provider information, and medications at the time of the visit. Where information was missing, data from the most recent prior outpatient encounter were used. Data on patient comorbidities were assessed by identifying ICD-9 or ICD-10 codes for the following conditions either listed as a billing diagnosis or on the patient problem list at any point between January 7, 2014 (the date of initiation of a new EHR at DUHS) and the index visit: hypertension, diabetes (type 1 and type 2), ASCVD, heart failure, peripheral artery disease, coronary heart disease, cerebrovascular disease, hypothyroidism, hyperthyroidism, hyperlipidemia, and nephrotic syndrome (codes listed in eTable 1). Patient medication lists at the index visit and future outpatient visits were used to identify medications used at baseline and initiated follow-up. Statin intensity was defined as: high-intensity (rosuvastatin ≥20 mg per day or atorvastatin ≥40 mg/day), moderate-intensity (rosuvastatin 5–<20 mg, atorvastatin 10–<40, simvastatin ≥20 mg, pravastatin ≥40 mg, lovastatin ≥40 mg, fluvastatin 80 mg, pitavastatin ≥2 mg), and low-intensity (any other statin dose).
Table 1.
Patient characteristics of patients with very high LDL-C in DUHS and cerner.a.
| Characteristic | Duke Health (N = 7728) | Cerner (N = 139,539) |
|---|---|---|
| Age | 57 (48, 66) | 56 (48–66) |
| Sex (female) | 63.9% (4938) | 64.6% (82508) |
| Race | ||
| Caucasian | 67.7% (5230) | 84.6% (98112) |
| African American | 24.9% (1928) | 8.6% (10021) |
| Other | 2.5% (190) | 3.6% (4153) |
| Hispanic | 1.8% (136) | 2.2% (2551) |
| Asian | 3.0% (234) | 1.0% (1185) |
| Insurance | ||
| Commercial | 65.3% (4921) | 53.2% (40392) |
| Government | 34.2% (2577) | 41.4% (31438) |
| No insurance | 2.5% (191) | 4.8% (3671) |
| Other | 0.5% (39) | 0.6% (455) |
| SBP, mmHg | 124 (116, 138) | |
| Baseline LDL-C (mg/dL) | 203 (195, 217) | 204 (196–219) |
| Comorbidities | ||
| Hypertension | 27.2% (2105) | 19.1% (26636) |
| Diabetes | ||
| Type 1 | 0.3% (21) | 0.7% (934) |
| Type 2 | 9.3% (720) | 8.0% (11119) |
| ASCVD | 6.1% (473) | 4.6% (6447) |
| Heart failure | 0.9% (73) | 0.9% (1315) |
| PAD (PAD + AAA) | 0.8% (59) | 0.4% (598) |
| Coronary heart disease | 4.0% (307) | 3.6 (5014) |
| Cerebrovascular disease (CVD + strokes) | 2.0% (153) | 1.1% (1478) |
| Hypothyroidism | 10.1% (784) | 7.4% (10370) |
| Hyperthyroidism | 0.5% (41) | 0.5% (686) |
| Nephrotic syndrome | 0.0% (0) | 0.1% (148) |
| BMI | 29 (26, 33) | |
| Family history of MI | 15.9% (1228) | |
| Current smoker | 11.0% (838) | |
| Meeting guideline recommendation without LDL-C considered | 50.7% (3916) | |
| No ASCVD and calculated risk ≤7.5% | 50.5% (2337) | |
| ASCVD 10-year risk | 7.5 (3.8, 14.3) | |
| Provider type | ||
| Cardiology | 2.8% (217) | 2.5% (3467) |
| Endocrinology | 2.4% (186) | 0.3% (413) |
| Primary care: internal | 55.5% (4285) | 12.1% (16914) |
| Primary care: family | 31.3% (2419) | 23.5% (32742) |
| Other provider type or not otherwise specified | 8.0% (621) | 61.6% (86,003) |
| Facility type | ||
| Urban | 87.7% (122423) | |
| Rural | 12.3% (17116) | |
| Facility region | ||
| Midwest | 10.7% (14886) | |
| Northeast | 55.6% (77644) | |
| South | 15.6% (21744) | |
| West | 18.1% (25265) | |
| Baseline treatment | ||
| None | 86.8% (6707) | |
| Non-statin only | 1.9% (148) | |
| Low-intensity statin | 2.2% (171) | |
| Moderate-intensity statin | 5.4% (418) | |
| High-intensity statin | 3.7% (284) | |
Abbreviations: AAA, abdominal aortic aneurysm; ASCVD, atherosclerotic cardiovascular disease; CVD, cardiovascular disease; DUHS, Duke University Health System; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PAD, peripheral artery disease. Where cells are blank, data unavilable for that data source.
Data presented as median (25th, 75th percentile) or percentage (N). All calculations are made as the percentage of non-missing values.
2.3. Lipid control definitions
In order to evaluate the trajectory of LDL-C in patients with severe hyperlipidemia, we evaluated all subsequent LDL-C measurements between 30 days and 13 months after the index LDL-C higher than 190 mg/dL. A 30-day window was used after the initial draw to screen out repeat lipid panels drawn as confirmation and to identify follow-up lipid panels that may have changed in response to treatments. The lowest LDL-C achieved at follow-up is described by data sources by binning into the following categories: <70, 70–99, 100–129, 130–159, 160–189, ≥190 mg/dL. In the Cerner HealthFacts data, lipid control was defined as a 50% or more reduction in LDL-C, using the lowest value in the 30-day to 13-month window. Since outpatient medications are not available in HealthFacts, we did not evaluate medication use in this population. At DUHS, outpatient medications were available, so we defined appropriate therapy as one of the following: 1) initiation or up-titration to a high-intensity statin within 13 months; or 2) LDL-C reduction by at least 50% over the subsequent 13 months, regardless of baseline or follow-up medication treatment. This definition was based on the most recent guideline recommendations for patients with LDL-C ≥190 mg/dL that emphasize initiation of high-intensity statin therapy and target LDL-C reduction by 50% or more [3,12]. Additionally, for the small proportion of subjects who were already on a high-intensity statin at baseline (<4% overall), we considered a reduction of at least 30% within 13 months of meeting criteria for appropriate therapy. For the evaluation of lipid control at DUHS, we excluded individuals for whom no follow-up medication data were available (n = 1353).
2.4. Statistical analysis
Baseline characteristics were presented descriptively as medians (25th, 75th percentile) for continuous variables or N (percentage) for categorical variables. Percentages were calculated as the percent of non-missing values. Multivariable logistic regression models were created to evaluate the relationship between individual variables of interest and the primary outcomes of: 1) LDL-C reduction of ≥50% in the Cerner population; and 2) appropriate therapy in the DUHS population as defined above. The following variables were pre-specified for inclusion in the Cerner model: age, sex, race, insurance status, hypertension, ASCVD, hypertension, diabetes, provider specialty, and facility location (rural vs. urban). In the DUHS model, similar variables were included with the exception of facility location (as this included only 1 facility), and the addition of body mass index and family history of myocardial infarction, which were not available in the Cerner database. Results were presented as odds ratios (ORs) with 95% CIs. This study was approved by the Duke University Institutional Review Board (Pro00089790 and Pro00087975), and all analyses were performed using SAS version 9.4 (Cary, NC).
3. Results
3.1. Cerner HealthFacts cohort
Overall, we studied 255 U S. health systems that contributed data to the Cerner HealthFacts database and met inclusion criteria. In these systems, a total of 4,623,851 unique individuals had at least one LDL-C level available. Out of that population, 139,539 (3.0%) patients were identified as having at least one LDL-C ≥190 mg/dL at any point (Table 1). The median percentage of patients with LDL-C ≥190 mg/dL across the 255 health systems was 2.4% (interquartile range [IQR] 1.6–3.3%). The median number of patients per hospital was 1842 patients (IQR 105–12,498).
Of the 4,285,400 patients included and with known sex, 3.5% of female patients (N = 82,508/2,391,506) and 2.4% of male patients (N = 45,305/1,893,894) had LDL-C ≥190 mg/dL. Of those with extremely high cholesterol, the median age was 56 years (IQR 48–66), with 11% younger than 40 years, 84.6% were Caucasian, 8.6% were African American, and 64.6% were female. The prevalence of CVD and CVD risk factors was low: 4.6% had ASCVD, 19.1% had hypertension, and 8.7% had diabetes. The median baseline LDL-C level was 204 mg/dL (IQR 196–219).
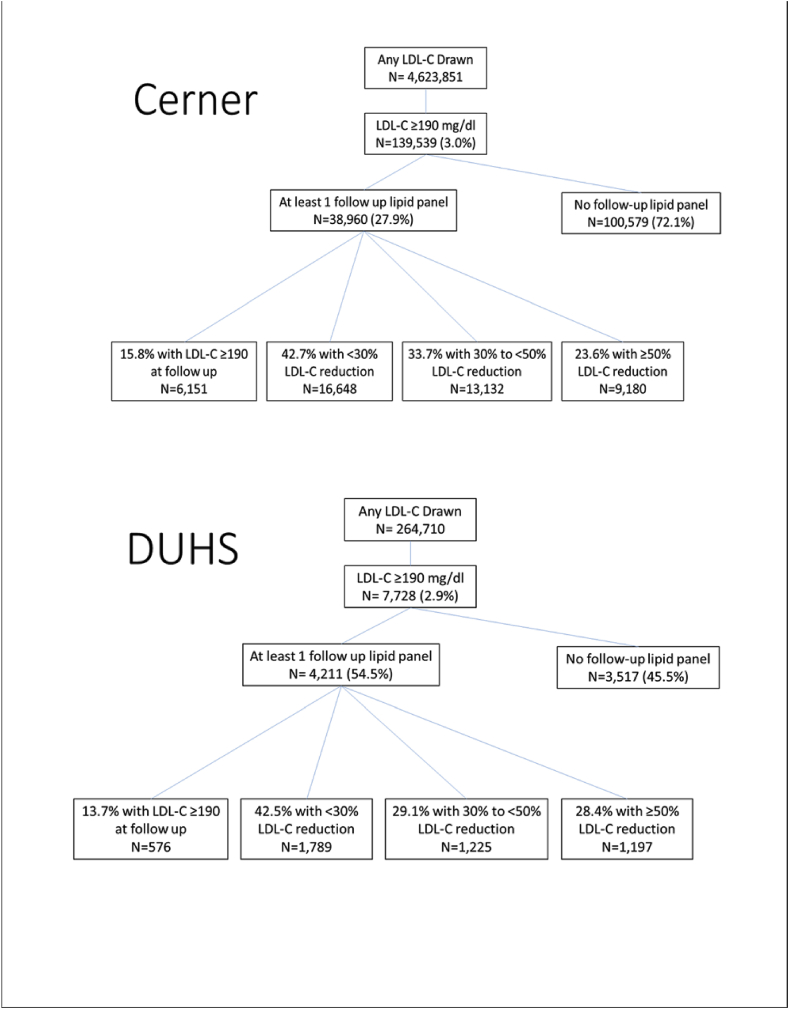
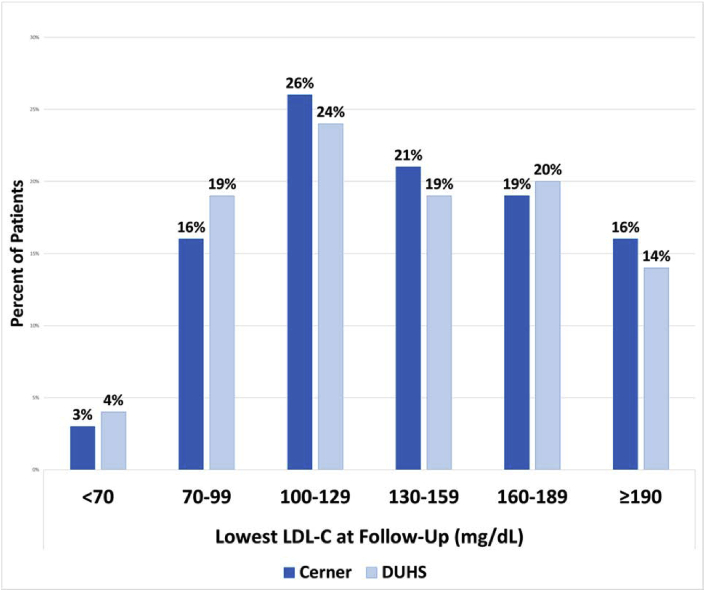
Among 139,539 patients with extremely high LDL-C, n = 38,960 (27.9%) had a follow-up LDL-C measured in the subsequent year (between 1 and 13 months, Fig. 1). The median number of days between index and first follow-up LDL-C lab was 117 (IQR 78, 192). Among those who had at least one additional LDL-C drawn at follow-up, 23.6% achieved a 50% or greater reduction in LDL-C, only 18.3% achieved an LDL-C <100 mg/dL, and 2.7% achieved LDL-C <70 mg/dL (Fig. 2). Many patients (15.8%) had a follow-up LDL-C that remained higher than 190 mg/d.
Fig. 1.
Treatment of DUHS patients with very high LDL-C (≥90) Fig. 1 demonstrates a flow diagram of the patients seen at Cerner and DUHS, respectively, and having an LDL-C drawn, follow-up LDL-C drawn, and strata of LDL-C reduction.
Abbreviations: DUHS, Duke University Health System; LDL-C, low-density lipoprotein cholesterol.
Fig. 2.
Distribution of lowest LDL-C achieved in Cerner and DUHS databases
Fig. 2 shows the lowest achieved LDL-C among those with at least one additional LDL-C drawn at follow-up (n = 49,002) at Cerner and DUHS, respectively.
Abbreviations: DUHS, Duke University Health System; LDL-C, low-density lipoprotein cholesterol.
In sensitivity analysis restricting to data in 2013 and after, similar patterns were seen. Among 1,011,696 patients with any LDL-C drawn, 30,625 (3.0%) had an LDL-C ≥190 mg/dL. Of those with LDL-C ≥190 mg/dL, 21.6% had a follow up LDL-C measurement. In this population, 20.1% had a 50% or greater reduction in LDL-C, and 20.0% had an LDL-C ≥190 mg/dL at follow up.
3.2. DUHS cohort
Within DUHS, we identified 264,710 adults with an LDL-C drawn at any point during the study period (2015–2017) and had an eligible follow-up visit. Of those, 2.9% (n = 7728) had an LDL-C higher than 190 mg/dL. The median LDL-C among these patients was 203 mg/dL (IQR 195–217). Clinical characteristics of these adults were similar to those in the Cerner system (Table 1), and included mostly younger adults (median age 57, IQR 48–66), more female patients (63.9% female), and 67.7% were Caucasian. Similar to the Cerner cohort, the prevalence of CVD and CVD risk factors in the DUHS cohort was low: 6.1% had established ASCVD, 27.2% had hypertension, and 9.6% had diabetes. Among those without ASCVD, the median ASCVD 10-year risk score was 7.5% (IQR 3.8–14.3).
Follow-up lipid testing was more common in the DUHS cohort than the Cerner cohort (Fig. 1). Of those with LDL-C ≥190 mg/dL, 54.5% (n = 4211) had an eligible follow-up LDL-C measurement (median time-to-measurement 175 days; IQR 98, 282). Nevertheless, among those with follow up LDL-C measurements, only 28.4% achieved a 50% or greater reduction in LDL-C, 22.9% achieved an LDL-C <100 mg/dL, and 4.4% achieved LDL-C <70 mg/dL (Fig. 2). Of those with follow-up LDL-C levels, 13.7% continued to have an LDL-C higher than 190 mg/dL.
At baseline, 86.8% of those in DUHS with an LDL-C ≥190 mg/dL were not on a lipid-lowering therapy drug, and only 3.7% were on a high-intensity statin (Table 2). Even fewer patients were on both a statin and a non-statin lipid-lowering medication at baseline (0.6%). By one year, 71.6% were on some form of statin therapy, yet only 28.5% were on a high-intensity statin. Patients who saw cardiologists for their baseline visit were more likely to be placed on a high-intensity statin compared with those seen by primary care (51.0 vs. 27.8%, p < 0.001). Among those with LDL-C ≥190 mg/dL, 2.8% saw a cardiologist in follow-up. Those seeing a cardiologist were more likely to be initiated on a high-intensity statin in follow-up than those seeing only primary care (40.7% vs. 24.5%, p < 0.001). Initiation of non-statin therapy (ezetimibe, niacin, fibrate, bile acid sequestrant, omega 3 fatty acid, or a PCSK9 inhibitor) in subjects not on lipid-lowering therapy at baseline was rare overall (4.4% of patients), but slightly more common among those seen by a cardiologist than a primary care physician (11.3% vs. 3.9%, p < 0.001). Of the 284 patients at DUHS who were on a high-intensity statin at baseline, 156 had a follow-up lipid test. Among these, 15 (9.6%) had an LDL-C <70 mg/dL and 52 had an LDL-C < 100 mg/dL.
Table 2.
Baseline and follow-up treatment for very high LDL-C at DUHS.
| Provider Type |
p-values |
|||||
|---|---|---|---|---|---|---|
| ∖ | Overall | Cardiology | Primary Care | Other | Primary care vs. Cardiology | Primary care vs. Other |
| Treatment status at baselinea | <0.001 | 0.068 | ||||
| None | 85.49% (5450) | 78.13% (150) | 85.83% (4753) | 84.81% (547) | ||
| Non-statin only | 2.13% (136) | 1.04% (2) | 1.99% (110) | 3.72% (24) | ||
| Low-intensity statin | 2.51% (160) | 3.65% (7) | 2.46% (136) | 2.64% (17) | ||
| Moderate-intensity statin | 5.91% (377) | 5.73% (11) | 5.96% (330) | 5.58% (36) | ||
| High-intensity statin | 3.95% (252) | 11.46% (22) | 3.77% (209) | 3.26% (21) | ||
| Treatment status at first follow-up visit | <.001 | <.001 | ||||
| None | 23.80% (1517) | 10.94% (21) | 24.30% (1346) | 23.26% (150) | ||
| Non-statin only | 7.04% (449) | 16.67% (32) | 6.23% (345) | 11.16% (72) | ||
| Low-intensity statin | 6.57% (419) | 4.69% (9) | 6.59% (365) | 6.98% (45) | ||
| Moderate-intensity statin | 40.77% (2599) | 25.52% (49) | 41.51% (2299) | 38.91% (251) | ||
| High-intensity statin | 21.82% (1391) | 42.19% (81) | 21.36% (1183) | 19.69% (127) | ||
| Most intense treatment status during follow-up | <.001 | 0.013 | ||||
| None | 23.80% (1517) | 10.94% (21) | 24.30% (1346) | 23.26% (150) | ||
| Non-statin only | 4.61% (294) | 9.38% (18) | 4.15% (230) | 7.13% (46) | ||
| Low-intensity statin | 4.58% (292) | 3.65% (7) | 4.68% (259) | 4.03% (26) | ||
| Moderate-intensity statin | 38.48% (2453) | 25.00% (48) | 39.08% (2164) | 37.36% (241) | ||
| High-intensity statin | 28.53% (1819) | 51.04% (98) | 27.79% (1539) | 28.22% (182) | ||
Abbreviations: DUHS, Duke University Health System; LDL-C, low-density lipoprotein cholesterol.
For the follow-up evaluation of lipid-lowering treatment at DUHS, we excluded individuals for whom no follow-up medication data were available (n = 1353).
3.3. Multivariable modeling
In the Cerner cohort, factors associated with increased likelihood of adequate LDL-C control in follow-up included: seeing a cardiologist (OR 1.56, CI 1.33–1.83), having a history of ASCVD (OR 1.19, CI 1.07–1.33), hypertension (OR 1.10, CI 1.03–1.18), and diabetes (OR 1.34 CI 1.23–1.46); Table 3. In contrast, those who were African American (OR 0.79, CI 0.71–0.89) or had government-issued insurance (OR 0.90, CI 0.83–0.98) were significantly less likely than their peers to achieve LDL-C target reductions. eTable 2 shows the proportion of adults by subgroup who achieved LDL-C control.
Table 3.
Multivariable associations with reaching LDL-C target reductiona in cerner healthfacts.
|
Characteristic |
LDL-C Controlled N = 9180 |
LDL-C Uncontrolled N = 29,780 |
Adjusted OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Ageb | 58 (50, 68) | 57 (49, 66) | 1.06 | (1.03, 1.08) | <.001 |
| Sex (female) | 63.9% (5102) | 64.1% (16791) | 0.96 | (0.91, 1.02) | 0.219 |
| Race | <.001 | ||||
| Caucasianc | 88.4% (6478) | 86.5% (20640) | 1.00 | (ref) | |
| African American | 6.2% (453) | 7.3% (1750) | 0.79 | (0.71, 0.89) | |
| Other | 5.4% (396) | 6.2% (1469) | 0.90 | (0.80, 1.01) | |
| Insurance | <.001 | ||||
| Commercialc | 27.3% (2505) | 28.9% (8599) | 1.00 | (ref) | |
| Government | 22.5% (2069) | 22.6% (6715) | 0.90 | (0.83, 0.98) | |
| Other/no insurance/not specified | 50.2% (4606) | 48.6% (14466) | 1.12 | (1.05, 1.20) | |
| ASCVD | 7.8% (717) | 5.7% (1706) | 1.19 | (1.07, 1.33) | 0.001 |
| Hypertension | 26.1% (2394) | 23.0% (6859) | 1.10 | (1.03, 1.18) | 0.004 |
| Diabetes | 13.1% (1205) | 9.9% (2933) | 1.34 | (1.23, 1.46) | <.001 |
| Provider | <.001 | ||||
| Cardiology | 3.7% (335) | 2.2% (640) | 1.56 | (1.33, 1.83) | |
| Primary carec | 37.1% (3401) | 37.3% (11113) | 1.00 | (ref) | |
| Other/not specified | 59.3% (5444) | 60.5% (18027) | 1.01 | (0.96, 1.07) | |
| Facility type | 0.012 | ||||
| Ruralc | 9.5% (876) | 10.6% (3163) | 1.00 | (ref) | |
| Urban | 90.5% (8304) | 89.4% (26617) | 1.12 | (1.02, 1.23) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; ref: reference category.
Results from multivariable logistic regression model adjusting for all factors in the table.
The following multivariable model results present odds ratios of the association between the clinical variables of interest and the outcome of LDL-C control defined as ≥50% lowering within 1 year.
OR for age is calculated per 10-year increase.
Indicates the reference category. There were 31185/38960 observations used in the adjusted model due to 7775 missing values of race and/or gender.
Similarly, in the DUHS cohort, those seeing a cardiologist (OR 1.89 CI 1.18–3.01) and with diabetes (OR 2.07, CI 1.62–2.65) were more likely to achieve LDL-C target reductions, while female sex was associated with lower odds of reaching target reductions (OR 0.69, CI 0.59–0.81). In DUHS, African Americans and Caucasians had similar odds of appropriate therapy (OR 0.93, CI 0.78–1.11); Table 4. Supplement Table 1 shows the percent of adults who achieved LDL-C goals or received appropriate therapy by subgroup.
Table 4.
Multivariable associations reaching LDL-C target reduction or on appropriate therapya in DUHS.
| Characteristic | Appropriate therapy N = 1753 |
Inappropriate therapy N = 2458 |
Adjusted OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Ageb | 59 (50, 67) | 59 (49, 68) | 1.01 | (0.94, 1.10) | 0.713 |
| Sex (female) | 60.6% (1063) | 66.2% (1627) | 0.69 | (0.59, 0.81) | <.001 |
| Race | 0.033 | ||||
| Caucasianc | 66.6% (1158) | 72.4% (1761) | 1.07 | (0.90, 1.28) | |
| African American | 26.4% (458) | 22.6% (549) | 1.00 | (ref) | |
| Other | 7.0% (122) | 5.1% (124) | 1.50 | (1.10, 2.03) | |
| Insurance | 0.050 | ||||
| Commercialc | 59.0% (1034) | 61.3% (1507) | 1.00 | (ref) | |
| Government | 38.4% (673) | 37.2% (914) | 1.08 | (0.89, 1.32) | |
| Other/no insurance/not specified | 2.6% (46) | 1.5% (37) | 1.89 | (1.11, 3.19) | |
| ASCVD | 10.2% (178) | 5.9% (146) | 1.22 | (0.91, 1.66) | 0.188 |
| Hypertension | 36.6% (642) | 30.4% (746) | 1.04 | (0.87, 1.24) | 0.671 |
| Diabetes | 15.9% (279) | 8.1% (198) | 2.07 | (1.62, 2.65) | <.001 |
| Provider | 0.010 | ||||
| Cardiology | 4.5% (79) | 2.3% (57) | 1.89 | (1.18, 3.01) | |
| Primary carec | 85.2% (1494) | 87.1% (2142) | 0.85 | (0.65, 1.10) | |
| Other/not specified | 10.3% (180) | 10.5% (259) | 1.00 | (ref) | |
| BMI | 29 (26, 33) | 29 (25, 33) | 1.01 | (1.00, 1.02) | 0.040 |
| Family history of MI | 17.8% (312) | 16.6% (408) | 0.99 | (0.81, 1.21) | 0.916 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CI, confidence interval; DUHS, Duke University Health System; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; OR, odds ratio; ref, reference category.
Results from multivariable logistic regression model adjusting for all factors in the table.
We defined “appropriate therapy” as meeting any of the following criteria: 1) New initiation or intensification to a high-intensity statin within 13 months, 2) LDL-C reduction by at least 50% within 13 months, or 3) LDL-C reduction of at least 30% within 13 months in subjects already on a high-intensity statin at baseline.
Odds ratio for age is calculated per 10 year increase.
Indicates the reference category There were 3035/4211 observations used in the adjusted model due to 1176 missing values of race and/or BMI.
4. Discussion
Adults with extremely high LDL-C levels are at high lifetime risk for developing ASCVD. In two separate analyses of U.S. health systems, we found that around 3.0% of adults had a cross-sectional prevalence of LDL-C level higher than 190 mg/dL. Despite very high LDL-C levels, many did not have follow-up LDL-C testing and, of those who did have follow-up, only a quarter achieved guideline-recommended LDL-C treatment reduction goals. Additionally, only a minority of patients were treated with high-intensity statins, and very few were on a statin in combination with a non-statin LDL-C lowering agent.
While the prevalence of familial hypercholesterolemia (FH) has been estimated at approximately 1:250 in the U.S adult population [20,21], the prevalence of severe hypercholesterolemia has been estimated at 5–7% [4,13,14]. A number of patients excluded from our study may have had an LDL-C ≥190 mg/dL at some point, but were already started on statin therapy, which helped to lower their LDL-C. Consequently, our data represent the remaining prevalent reserve of real-world patients with extremely high LDL-C (3.0%) who are seen in the healthcare system. A few prior studies have included some patients on statin therapy, or applied correction factors that estimate pre-treatment LDL-C for patients on statin therapy, which likely impacted the estimated prevalence of severe hypercholesterolemia [4,13,14].
Across both datasets, adults with extremely high LDL-C (≥190 mg/dL) were generally young (median age 56–57 years) and otherwise healthy, with low rates of hypertension, diabetes, and prevalent CVD. Those with extremely high LDL-C were more likely to be female than male. Given their young age and low prevalence of ASCVD, many of these patients would have had no other indication for statin therapy other than their elevated LDL-C level. This reinforces the importance of broad routine cholesterol screening, even in patients who may be masquerading as “low-risk,” and highlights the importance of recognizing extremely high LDL-C levels as an indication for treatment [22].
Identifying patients with extremely high LDL-C is an important step in the detection of familial hypercholesterolemia. Although relatively few adults with LDL-C higher than 190 mg/dL (<2.0%) carry a mutation for familial hypercholesterolemia, those that do are at particularly high risk for CVD [13]. FH patients are known to suffer from delayed diagnosis and inadequate LDL-C control [23]. Even among FH patients on maximal lipid-lowering therapy in the Spanish Familial Hypercholesterolemia Cohort Study (SAFEHEART), only 11.2% of patients reached an LDL-C treatment target of <100 mg/dL [24]. Such evidence emphasizes the importance of screening for FH in adult patients with LDL-C ≥190 mg/dL, as well as the need for vigilance in pursuing aggressive treatment for these individuals and their families.
Individuals with LDL-C ≥190 mg/dL with and without familial hypercholesterolemia are at high long-term risk of ASCVD, which can be addressed with LDL-C lowering [4,13,22]. Our study identified several gaps in achievement of guideline-recommended reductions in LDL-C levels. The first gap identified was in follow-up lipid testing: the majority of patients with LDL-C ≥190 mg/dL did not have a follow-up lipid profile. While some of these patients may have transferred to a different health system, had their LDL-C measured at out-of-system laboratories, or were lost to follow-up, we attempted to account for these possibilities by requiring at least one follow-up visit within the same healthcare system in the year following their baseline LDL-C lab draw. Even among those who did have longitudinal LDL-C measurement, the vast majority did not achieve guideline-recommended LDL-C reduction. While approximately half of patients achieved a 30% or more LDL-C reduction at one year (57.3% in Cerner, 57.5% in DUHS), far fewer achieved a guideline-recommended 50% or greater reduction (23.6% in Cerner, 28.4% in DUHS). Only around one-fifth of patients achieved a repeat LDL-C goal of <100 mg/dL (18.3% in Cerner, 22.9% at DUHS). Almost one in six patients in both populations had a second repeat LDL-C level higher than 190 mg/dL at follow-up (15.8% in Cerner, 13.6% in DUHS). Given the 2-fold higher observed risk of death at 20 years from major cardiovascular events when compared to individuals with LDL-C ≥190 mg/dL [22], these results demonstrate a population that needs more aggressive targeting for CVD risk reduction.
While complete medication data were unavailable in the Cerner dataset, we were able to evaluate treatment patterns for adults at DUHS, where only 1 in 4 of patients were started on an American College of Cardiology/American Heart Association guideline-recommended high-intensity statin at follow-up [3,12]. This finding parallels prior studies demonstrating poor evidence-based utilization of lipid-lowering therapies among individuals with LDL-C ≥190 mg/dL and significant practice-level variation in the care of these patients [25]. In one study, only half of patients seen at the Veterans Affairs Health System with LDL-C ≥190 mg/dL were treated with a statin and <10% were on high-intensity statin therapy [26]. Gaps in knowledge regarding lipid treatment among providers may partially drive the observed undertreatment. While evidence-based utilization of lipid-lowering therapies was low across provider types, in our study, patients seen by cardiologists were significantly more likely to receive a high-intensity statin compared with those seen in primary care. A survey performed on health care professionals demonstrated that only half of respondents could identify the four treatment categories for high-intensity statin therapy [27]. While this study used a 50% reduction in LDL-C as a target, this may actually overestimate the degree of control achieved in this high-risk population. Even with a 50% or greater reduction in LDL-C, many patients, particularly those with established CVD, will fail to reach treatment goals with statin therapy alone. Achieving LDL-C control in adults with severe hypercholesterolemia should start with high-intensity statin, but providers should be vigilant to follow up lipid panels and consider additional lipid lowering therapy as needed.Despite the high frequency of undertreatment, some patients in both cohorts with LDL-C ≥190 mg/dL did receive appropriate statin treatment and consequent LDL-C control. Positive predictors of LDL-C lowering included the presence of comorbid conditions, including hypertension and diabetes, as well as seeing a cardiologist. In the SAFEHEART study, treatment goal attainment was similarly associated with the presence of diabetes [24]. Individuals with greater comorbidities are more likely to trigger their providers to recognize their high-risk status compared to patients with relatively few comorbidities. Provider recognition of the ASCVD risk phenotype promotes more aggressive treatment, as does cardiovascular specialist referral, yet given the overall low burden of comorbidities in the population overall, these findings should be considered with caution. Female sex (in DUHS) and African American race (in Cerner) both predicted lower rates of appropriate treatment/LDL-control. Racial and sex differences in cardiovascular risk, statin treatment, and lipid control have been well-described in literature [[28], [29], [30], [31], [32], [33]]. A recent study from our group identified the forces underlying racial differences in statin use and potential future targets for improvement, discerning significant differences in clinical characteristics, socioeconomic factors, patient beliefs, risk perception, and clinician trust [31]. More studies are necessary to investigate the potential reasons underlying health disparities in statin treatment, and integration of creative interventions is needed to adequately address these treatment gaps.
Our study demonstrates the power of utilizing EHR to capture a high-risk cohort of patients with severe hypercholesterolemia, whose treatment can be optimized with affordable and low-risk medications in order to prevent future adverse outcomes. The potential implications for providing a pragmatic approach to identify both patient-specific and system-wide care gaps are tremendous [[34], [35], [36], [37], [38], [39]]. With an enhanced ability to pinpoint these patients, we will also have opportunities to develop and capitalize on novel implementation strategies to actually improve patient care. For example, clinical support tools via EHR have demonstrated in randomized trial settings to significantly improve blood glucose and blood pressure control in outpatient diabetics [34,39]. While the last few decades have brought a wealth of efficacious lipid-lowering therapies, improving the uptake of these therapies will rely on the intersection of technology and implementation science. Given the ease of identifying patients with severe hyperlipidemia, future EHR-based interventions should be studied that can be used to close the gaps in follow up testing and treatment. Through the EHR, providers can be alerted to the presence of severe hyperlipidemia, repeat lipid testing can be automatically ordered, patient educational materials can be distributed, referrals offered, and care pathways suggested to providers. In addition, the patient- and provider-facing aspects of the lipid report, often distributed in the EHR, can be used to improve awareness about the risks of severe hyperlipidemia and suggested pathways of care.
4.1. Limitations
Our study had several limitations. First, Cerner HealthFacts data did not have any medication information, preventing us from analyzing the link between LDL-C trends and pharmacologic management. Second, the low overall rate of follow-up LDL-C measurement may have been influenced by patients being lost to follow-up, switching health care systems, or having LDL-C measurement at out-of-system laboratories, but this was partially attenuated by requiring at least one follow-up encounter within that system. Third, as mentioned above, our population does not represent true incident cases of LDL-C ≥190 mg/dL, but rather, embodies the prevalent reserve of those with very high LDL-C in the real world. Fourth, patients with a previous LDL-C ≥190 mg/dL who were already on treatment and had their LDL-C levels lowered prior to our cross-section, would not have met our inclusion criteria. Fifth, while our data are relatively recent (including patients through 2017), utilization of non-statin therapies may have increased in recent years; however, market data indicate that the uptake of non-statin lipid-lowering therapy continues to be sluggish. Sixth, comorbidity data were assessed using ICD-9 and -10 codes based on the prior literature [[15], [16], [17], [18], [19]]; however, billing codes lack complete accuracy, which may lead to imprecise estimation of comorbidities. In addition, we are unable to evaluate whether other factors such as a consultation with a nutritionist or dietary counseling was performed and whether this impacted LDL-C changes over time. Finally, despite the large number of health systems evaluated in our study, the prevalence estimate is only applicable to those with an LDL-C value who have sought care and received lipid screening.
5. Conclusions
Large numbers of U.S. adults with extremely high LDL-C can be identified using available EHR data. These adults are often first identified when they are relatively young and without CVD or other comorbidities used by providers to identify high-risk patients. Unfortunately, many of those with extremely high LDL-C do not have a follow-up lipid measurement, are not treated with recommended LDL-reduction therapies, and do not achieve guideline-recommended LDL-C reduction goals. This study highlights opportunities to use EHRs to more effectively find, track, and ultimately ensure appropriate treatment and follow-up of individuals with extremely high LDL-C.
Sources of funding
Dr. Navar is funded by NIH K01HL133416-01. Dr. Nanna is supported by NIH training grant T-32-HL069749-15.
Declaration of competing interest
ME Gold: No relationship(s) to disclose.
MG Nanna: Dr. Nanna is supported by NIH training grant T-32-HL069749-15.
SM Doerfler: No relationship(s) to disclose.
T Schibler: No relationship(s) to disclose.
D Wojdyla: No relationship(s) to disclose.
ED Peterson: Research Grant: Significant; Amgen, Sanofi, Astrazeneca, Merck. Consultant/Advisory Board; Modest; Amgen. Consultant/Advisory Board: Significant; Cerner, AstraZeneca, Merck, and Sanofi Aventis.
AM Navar: Research Grant: Significant; Amarin, Janssen, Amgen, Sanofi, and Regeneron Pharmaceuticals. Consultant/Advisory Board: Significant; Cerner, Amarin, Amgen, Esperion, 89 Bio, New Amsterdam, Pfizer, Novartis, Novonordisk, AstraZeneca, Sanofi and Regeneron.
Acknowledgments
We thank Erin Campbell, MS, for her editorial contributions to this manuscript. Ms. Campbell did not receive compensation for her contributions, apart from her employment at the institution where this study was conducted.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajpc.2020.100079.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pokharel Y., Tang Y., Bhardwaj B. Association of low-density lipoprotein pattern with mortality after myocardial infarction: insights from the TRIUMPH study. J Clin Lipidol. 2017;11:1458–1470.e4. doi: 10.1016/j.jacl.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf S., Hawken S., Ounpuu S. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 3.Stone N.J., Robinson J.G., Lichtenstein A.H. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. 2014. [DOI] [PubMed] [Google Scholar]
- 4.Perak A.M., Ning H., de Ferranti S.D., Gooding H.C., Wilkins J.T., Lloyd-Jones D.M. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation. 2016;134:9–19. doi: 10.1161/CIRCULATIONAHA.116.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor F., Ward K., Moore T.H. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011:CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cholesterol Treatment Trialists’ (CTT) Collaborators, Mihaylova B., Emberson J. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholesterol Treatment Trialists’ (CTT) Collaboration, Fulcher J., O’Connell R. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz G.G., Steg P.G., Szarek M. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 9.Sabatine M.S., Giugliano R.P., Keech A.C. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 10.Cannon C.P., Blazing M.A., Giugliano R.P. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 11.Cannon C.P., Steinberg B.A., Murphy S.A., Mega J.L., Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 12.Grundy S.M., Stone N.J., Bailey A.L. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Khera A.V., Won H.H., Peloso G.M. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–2589. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bucholz E.M., Rodday A.M., Kolor K., Khoury M.J., de Ferranti S.D. Prevalence and predictors of cholesterol screening, awareness, and statin treatment among US adults with familial hypercholesterolemia or other forms of severe dyslipidemia (1999–2014) Circulation. 2018;137:2218–2230. doi: 10.1161/CIRCULATIONAHA.117.032321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barchielli A., Balzi D., Naldoni P. Hospital discharge data for assessing myocardial infarction events and trends, and effects of diagnosis validation according to MONICA and AHA criteria. J Epidemiol Community Health. 2012;66:462–467. doi: 10.1136/jech.2010.110908. [DOI] [PubMed] [Google Scholar]
- 16.Birman-Deych E., Waterman A.D., Yan Y., Nilasena D.S., Radford M.J., Gage B.F. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 17.McCormick N., Bhole V., Lacaille D., Avina-Zubieta J.A. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PloS One. 2015;10 doi: 10.1371/journal.pone.0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan H., Sundararajan V., Halfon P. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Saczynski J.S., Andrade S.E., Harrold L.R. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abul-Husn N.S., Manickam K., Jones L.K. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science. 2016;354 doi: 10.1126/science.aaf7000. [DOI] [PubMed] [Google Scholar]
- 21.de Ferranti S.D., Rodday A.M., Mendelson M.M., Wong J.B., Leslie L.K., Sheldrick R.C. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States national health and nutrition examination surveys (NHANES) Circulation. 2016;133:1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791. [DOI] [PubMed] [Google Scholar]
- 22.Vallejo-Vaz A.J., Robertson M., Catapano A.L. Low-density lipoprotein cholesterol lowering for the primary prevention of cardiovascular disease among men with primary elevations of low-density lipoprotein cholesterol levels of 190 mg/dL or above: analyses from the WOSCOPS (West of Scotland Coronary Prevention Study) 5-year randomized trial and 20-year observational follow-up. Circulation. 2017;136:1878–1891. doi: 10.1161/CIRCULATIONAHA.117.027966. [DOI] [PubMed] [Google Scholar]
- 23.deGoma E.M., Ahmad Z.S., O’Brien E.C. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: data from the CASCADE-FH Registry. Circ Cardiovasc Genet. 2016;9:240–249. doi: 10.1161/CIRCGENETICS.116.001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez de Isla L., Alonso R., Watts G.F. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART Registry follow-up. J Am Coll Cardiol. 2016;67:1278–1285. doi: 10.1016/j.jacc.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Virani S.S., Kennedy K.F., Akeroyd J.M. Variation in lipid-lowering therapy use in patients with low-density lipoprotein cholesterol >/=190 mg/dL: insights from the National Cardiovascular Data Registry-Practice Innovation and Clinical Excellence Registry. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez F., Knowles J.W., Maron D.J., Virani S.S., Heidenreich P.A. Frequency of statin use in patients with low-density lipoprotein cholesterol >/=190 mg/dl from the Veterans Affairs Health System. Am J Cardiol. 2018;122:756–761. doi: 10.1016/j.amjcard.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virani S.S. The 2013 ACC/AHA Cholesterol Management Guideline: clearing the confusion from noncontroversial components. Tex Heart Inst J. 2016;43:313–314. doi: 10.14503/THIJ-16-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bostean G., Roberts C.K., Crespi C.M. Cardiovascular health: associations with race-ethnicity, nativity, and education in a diverse, population-based sample of Californians. Ann Epidemiol. 2013;23:388–394. doi: 10.1016/j.annepidem.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Y., Xu Q. Health disparities and delayed health care among older adults in California: a perspective from race, ethnicity, and immigration. Publ Health Nurs. 2016;33:383–394. doi: 10.1111/phn.12260. [DOI] [PubMed] [Google Scholar]
- 30.Kanchi R., Perlman S.E., Chernov C. Gender and race disparities in cardiovascular disease risk factors among New York City adults: New York City Health and Nutrition Examination Survey (NYC HANES) 2013–2014. J Urban Health. 2018;95:801–812. doi: 10.1007/s11524-018-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanna M.G., Navar A.M., Zakroysky P. Association of patient perceptions of cardiovascular risk and beliefs on statin drugs with racial differences in statin use: insights from the Patient and Provider Assessment of Lipid Management Registry. JAMA Cardiol. 2018;3:739–748. doi: 10.1001/jamacardio.2018.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamboa C.M., Colantonio L.D., Brown T.M., Carson A.P., Safford M.M. Race-sex differences in statin use and low-density lipoprotein cholesterol control among people with diabetes mellitus in the Reasons for Geographic and Racial Differences in Stroke Study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters S.A.E., Colantonio L.D., Zhao H. Sex differences in high-intensity statin use following myocardial infarction in the United States. J Am Coll Cardiol. 2018;71:1729–1737. doi: 10.1016/j.jacc.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor P.J., Sperl-Hillen J.M., Rush W.A. Impact of electronic health record clinical decision support on diabetes care: a randomized trial. Ann Fam Med. 2011;9:12–21. doi: 10.1370/afm.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geboy A.G., Nichols W.L., Fernandez S.J., Desale S., Basch P., Fishbein D.A. Leveraging the electronic health record to eliminate hepatitis C: screening in a large integrated healthcare system. PloS One. 2019;14 doi: 10.1371/journal.pone.0216459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin H., Tang X., Shen P. Using big data to improve cardiovascular care and outcomes in China: a protocol for the Chinese Electronic health Records Research in Yinzhou (CHERRY) Study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu J.V., Chu A., Donovan L.R. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. doi: 10.1161/CIRCOUTCOMES.114.001416. [DOI] [PubMed] [Google Scholar]
- 38.Lucas J.E., Bazemore T.C., Alo C., Monahan P.B., Voora D. An electronic health record based model predicts statin adherence, LDL cholesterol, and cardiovascular disease in the United States Military Health System. PloS One. 2017;12 doi: 10.1371/journal.pone.0187809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali M.K., Singh K., Kondal D. Effectiveness of a multicomponent quality improvement strategy to improve achievement of diabetes care goals: a randomized, controlled trial. Ann Intern Med. 2016;165:399–408. doi: 10.7326/M15-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.