Abstract
Background
The Dutch cascade screening model for FH was the most successful of such programs in the world. It remains unclear whether aspects of the Dutch model (i.e. direct engagement with FH probands and relatives outside usual healthcare settings) are feasible in the US. This is especially important since prior attempts at cascade screening in the US have had very low screening rates (<10% of families screened).
Methods
We conducted a multi-site single-arm proof-of-concept study in which the US-based FH Foundation (a 501c3 research and advocacy organization) directly engaged with FH probands and relatives similar to the approach taken by the Dutch “Foundation for Tracing FH.”
Results
Eleven unrelated probands with genetically confirmed FH were enrolled. Mean age was 43 years; 82% were women, and 82% were of European ancestry. Prior to enrolling into the study, only 2 families (18% screening rate) were screened for FH with both lipid measurements and genetic testing. Two probands declined cascade screening due to fear over genetic discrimination. Nine total relatives engaged with the FH Foundation. Mean age was 43 years and 44% were women. Seven of those relatives (from 6 families; 55% screening rate) consented to be screened for FH with lipid measurement and genetic testing. The two additional relatives – men ages 39 and 49 – agreed to lipid measurements but not genetic testing, each noting he would like to think more about genetic testing.
Conclusions
Our proof-of-concept study demonstrates the feasibility of the FH Foundation engaging FH probands and their relatives outside the usual healthcare settings for cascade screening, similar to the Dutch model. We found only 18% of families had already been screened, and after engaging with the FH Foundation, 55% of families were willing to participate in cascade screening. These findings suggest the methods described here may improve cascade screening rates in the US.
Keywords: Familial hypercholesterolemia, Cascade screening, Cholesterol, LDLR, Family screening, Dutch lipid clinic network, Hyperlipidemia, Hypercholesterolemia dyslipidemia, Severe hypercholesterolemia
Graphical abstract
1. Introduction
Familial Hypercholesterolemia (FH) is a common genetic disorder (estimated prevalence: 1 in 250 individuals) characterized by markedly elevated low-density lipoprotein cholesterol (LDL-C) from birth onward [1], [2], [3], [4], resulting in significantly increased risk for early atherosclerotic cardiovascular disease (ASCVD). One in 10 premature myocardial infarctions (MIs) is caused by FH. Yet 90% of the 1.3 million United States (US) residents living with FH remain undiagnosed [1], suggesting a missed opportunity for ASCVD prevention.
As an autosomal dominant genetic disorder, first degree relatives of a person with FH have a 50% chance of also having FH [1]. Cascade screening – the process of screening relatives of an proband (i.e. index case) for FH – has the potential to identify up to 8 additional FH cases per proband [5]. Indeed, because of its potential for a positive impact on public health, cascade screening for FH has been designated a “Tier 1 Genomics Application” by the Centers for Disease Control and Prevention's (CDC) Office of Genomics and Precision Public Health [6].
Unfortunately, in the US, few efforts have been made to develop cascade screening models, and few published reports exist of successful cascade screening programs. Furthermore, in clinical context, the standand of care (i.e. usual care) remains for healthcare providers to counsel FH patients on the importance of their relatives being screened [6,7]. No active efforts are made to ensure relatives are actually screened.
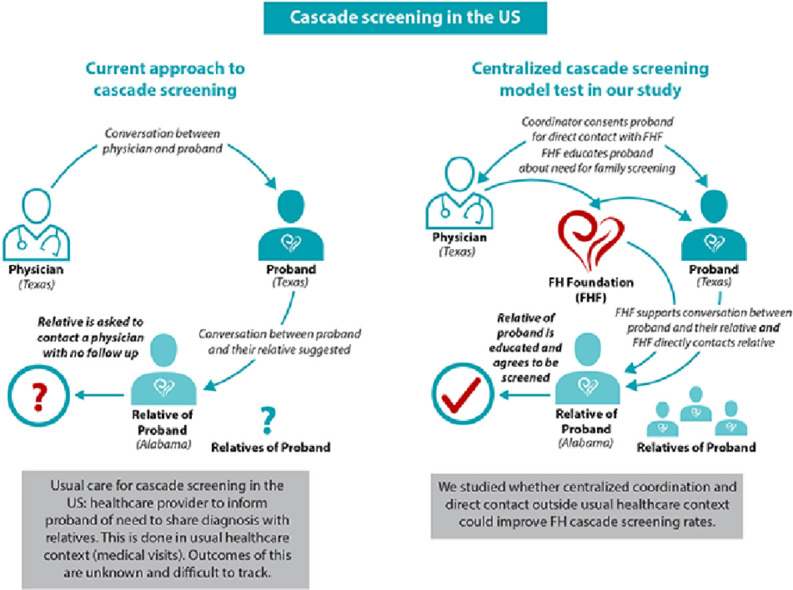
In contrast, several other countries have implemented efforts to ensure relatives get screened [8], [9], [10], [11], [12], [13]. Of these, the Netherlands is widely recognized as having had the most successful national cascade screening program, with over 70% of all individuals with FH identified nationwide [10,11]. In the Dutch model, healthcare providers identified FH probands and, once genetically confirmed to have FH, forwarded their contact information to a centralized coordinating office funded by the government, called the “Foundation for Tracing FH.” The probands, and subsequently their family members, were contacted, family history gathered, and screening visits scheduled. Of note, 90% of FH probands had family members screened for FH. The success of this approach has been linked to several key aspects: engagement with probands and families outside of usual healthcare settings by the “Foundation for Tracing FH,” direct contact with relatives, and in-home visits for sample collection (for laboratory and genetic testing).
To begin assessing whether key aspects of the Dutch model are feasible in the US, we conducted a multi-site single-arm proof-of-concept study. In our study, the US-based FH Foundation directly engaged with FH probands and relatives outside of usual healthcare settings – similar to the “Foundation for Tracing FH” in the Netherlands – to facilitate cascade screening.
2. Methods
All patients gave informed consent with the approval of the Institutional Review Board at the University of Texas Southwestern (UTSW) Medical Center or University of Pennsylvania (UPENN). Inclusion criteria for probands was age ≥ 18 and a diagnosis of FH confirmed by genetic testing. Exclusion criteria included 1) majority of relatives living outside of US, 2) less than two first-degree relatives living, 3) inability to give informed consent. The study ran for 8 weeks.
Study investigators identified and consented FH probands from specialty lipid clinics (UTSW, UPENN) or from individuals actively involved as volunteers with the FH Foundation. Study coordinators from UTSW or UPENN then arranged for telephone or video visits with the proband and the Chief Medical Officer at the FH Foundation. The FH Foundation (Winter Park, Florida), a 501c3 research and advocacy organization, engaged with FH probands during these telephone and video visits, similar to the approach taken by the Dutch “Foundation for Tracing FH.” Specifically, the FH Foundation counseled probands on the importance of family screening for individuals with FH, and a semi-structured set of questions regarding family history (modeled after the Dutch protocol) was used to collect family information (see Supplementary material).
To assess whether our model improved cascade screening in the families enrolled, we asked probands detailed questions regarding which relatives had already been screened for FH. A relative was considered to be screened if they had previously had both lipid measurement and genetic testing. Based on the Dutch experience, both lipid measurement and genetic testing are needed for effective cascade screening as approximately 15% of relatives will harbor pathogenic variants but have normal LDL-C levels [11,14].
Probands were asked by the FH Foundation to contact their first degree relatives and inform them of the study and the need to be screened for FH. Concomitantly, contact information for first-degree relatives was collected by study coordinators to arrange for telephone or video visits with the FH Foundation.
During the calls between relatives and the FH Foundation, discussions focused on the relatives’ risk of having FH, risk of ASCVD, benefits of cholesterol lowering if they were found to be affected, and the benefits and risks of genetic testing. Risks of genetic testing included a description of the US Genetic Information Nondiscrimination Act as well as the exceptions to this act (e.g. life insurance). If the relative agreed, informed consent was taken to enroll in the study for FH screening with both genetic testing and lipid measurements.
Originally, we planned to have local study teams arrange collection of blood or sputum samples from relatives via home visits, site clinic appointments, or mailed blood or sputum kits (for relatives who live away from study sites). Our main outcome variable was the number of families screened. Because of COVID-19 limitations on human research activities, we reduced the scope of our original study design by changing the main outcome from families “screened” to “consented” as blood or saliva samples could not be collected due to restrictions on in-person research visits.
3. Results
3.1. Probands
In June and July 2020, we identified 11 unrelated probands with genetically confirmed FH and all 11 agreed to participate (Table 1). Mean age was 43 years; 82% were women, and 82% were of European ancestry.
Table 1.
Characteristics of participants in proof-of-concept study of centralized cascade screening.
| Characteristic of probands | |
| Probands, n | 11 |
| Age, probands, years | 45 (30–79) |
| Women, n | 9 (82%) |
| Ethnicity/Race, n | |
| European Ancestry | 9 (82%) |
| African American | 1 (9%) |
| Hispanic | 0 (0%) |
| Asian | 1 (9%) |
| Preferred method(s) of communication | |
| Phone | 45% |
| Text | 27% |
| 64% | |
| Number of living 1st degree relatives per family | 4 (2–8) |
| 1st degree relatives already screened for FH per family* | 0 (0–2) |
| Characteristics of relatives | |
| Total relatives contacted, n | 9 |
| # families with any relatives consented, n | 6 (55%) |
| # famlies with at least 2 relatives consented | 3 |
| Age, years | 46 (6–75) |
| Women, n | 5 (56%) |
| Relationship, n | |
| Son | 2 |
| Daughter | 1 |
| Sister | 4 |
| Brother | 1 |
Both lipids and genetic testing done.
Data shown as median (range) unless otherwise specified.
These 11 probands reported having a total of 49 first degree relatives, of which 35 were reported to have had at least a lipid measurement previously. Prior to enrolling in the study, only 3 relatives from 2 families (18%) had been screened for FH with both lipid measurements and genetic testing. At the conclusion of our study, 55% of the families had at least one relative consented for FH screening for both lipid measurements and genetic testing.
3.2. Relatives
Nine total relatives engaged with the FH Foundation. Mean age was 43 years and 44% were women. Six families had at least one relative who consented to be screened for FH with lipid measurement and genetic testing. Among those six families, seven relatives total consented. The time between the proband being consented and the relative being consented was median (range) 12 (0–29) days. Two additional relatives – men ages 39 and 49 – agreed to lipid measurements but not genetic testing, each noting he would like to think more about genetic testing.
3.3. Interviews
Two subjects expressed concern over genetic discrimination.
-
•
One proband whose relatives had already been screened expressed regret regarding having had two of her three young children tested genetically. She stated that if she could do it over, she would not have them screened genetically due to fear they would not be able to get life insurance in the future.
-
•
Another proband had proactively decided not to genetically test her son, also due to concern regarding his ability to obtain life insurance in the future.
One of the probands and one relative of a different proband were not on lipid lowering therapy because neither could afford a medical office visit or lab testing due to lack of health insurance coverage.
One proband, who had already lost one of her three children to an MI, with seven siblings said her siblings “don't believe in preventive care,” and her two living children were “too busy with work to be screened.”
4. Discussion
Despite the limitations imposed by the COVID-19 pandemic, our proof-of-concept study demonstrates the feasibility of the FH Foundation with regards to engagement of FH probands and their relatives outside usual healthcare settings for the purposes of improving cascade screening rates, similar to the Dutch model. Of note, only 18% of probands had relatives previously screened for FH with lipid measurements and genetic testing. By the end of the study, 55% of probands had relatives willing to be screened with lipid measurements and genetic testing, suggesting the direct engagement of FH probands and relatives by the FH Foundation has potential to improve cascade screening results.
Previously, the FH Foundation conducted a nationwide web-based study (“Patient Acceptance of Genetic Testing,” PAGENT) offering free genetic testing to individuals who met clinical criteria for FH (n = 57) [15]. Subsequently, the individuals with pathogenic or likely-pathogenic mutations were asked to discuss genetic testing – at no cost – with their relatives. Of note, no direct interaction between the FH Foundation and those relatives occurred, and only 9% of families participated. Our results build on this prior experience and suggest interaction outside of routine healthcare settings may improve cascade screening rates.
Because of several unique characteristics of the US healthcare setting (e.g., nearly 1000 different healthcare payers with varying coverage policies), cascade screening efforts within the US have lacked a centralized approach as established in the Netherlands. Prior research protocols for cascade screening in the US have not utilized aspects of the Dutch approach. The MyCode Genomic Screening and counseling (GSC) Program at Geisinger has reported the return of genetic testing results to 125 individuals found to have FH pathogenic variants [3]. Although these individuals were invited to have their blood relatives screened, only 4% of families were screened. The West Virginia University Coronary Artery Risk Detection In Appalachian Communities (CARDIAC) Project screened lipids in 5th-grade students throughout rural Appalachian West Virginia (n = 637) [16]. These children were then seen in pediatric lipid clinics and relatives recruited for screening, but only 5% of families were screened. Overall, several factors have likely affected the reach of cascade screening among US FH families: lack of centralized coordination, little direct contact with probands and relatives outside of usual healthcare settings, a system that relies on family members to travel to an appointment to be evaluated in a healthcare setting, and possibly inconsistent use of genetic testing. To maximize detection, multiple complementary approaches to FH diagnosis may be beneficial in the US, including centralized cascade screening as explored in this study as well as child-parent cascade screening as has been advocated by others [17].
During our interactions with probands and relatives, several expressed hesitancy about genetic discrimination, and prior studies have noted 25% of people decline to participate in genomic-sequencing research in the US due to fear of discrimination by life insurance companies [18]. In the Netherlands – where genetic discrimination laws are stricter than US – fewer people (10%) declined genetic testing due to fear of social consequences (such as testing having a negative impact on employment and insurance) [10,11]. While the U.S. Genetic Information Nondiscrimination Act (GINA) of 2008 bars the use of genetic information in making decisions related to health insurance or employment, there are no provisions baring discrimination in obtaining life or longterm care insurance [19]. As noted by others, GINA has failed to alleviate anxieties about medical genetic testing, despite the fact that as of 2019, only 48 unique court cases were resolved involving GINA [19]. Efforts are underway to remove the exceptions from GINA, and in the state of Florida, life insurance companies are prohibited from using genetic information in underwriting unless the information is accompanied by a diagnosis of a medical condition [20]. Nonetheless, until this issue is further addressed in the US, this may remain a barrier to widespread acceptance of genetic testing in FH.
A few limitations of our proof-of-concept study require further efforts to address. Although the FH Foundation served a central role in this proof-of-concept study in terms of direct contact and obtaining social and health information from both the proband and relatives, the Foundation did not directly reach out to schedule the proband or family members, as this was coordinated by study coordinators at UTSW and UPENN. Our timeframe was short and our sample size was small. On average, we recruited only 1.5 relatives per proband who had family willing to be screened, which may be below a previously published threshold for cost-effectiveness [21]. Before broad implementation, the Dutch cascade screening model requires further adaptation to account for differences between the Dutch and US health-care systems, especially regarding barriers experienced by US FH patients such as insurance gaps with a job change or loss and suspicion related to genetic testing. Other issues that must be considered are screening and diagnosis of underrepresented populations (i.e., racial/ethnic minorities and rural populations) and the diverse healthcare system of the US.
Heretofore, home visits from a centralized site would be nearly impossible in a country as large as the US. Our study was performed early in the COVID-19 pandemic before widespread acceptance of telehealth. Ironically, now more than 10 months into the pandemic, the use and acceptance of telehealth has grown exponentially. Telehealth allows a centralized cascade testing approach to overcome the issue of “in person” direct contact, permitting initial contact with the proband and subsequent virtual home visits with both the proband and relatives anywhere in the US. Additionally, several commercial laboratories offer in-home lab kits, enabling individuals to provide a blood or saliva sample by mail (https://www.letsgetchecked.com, https://www.invitae.com/).
In summary, our proof-of-concept study demonstrated the FH Foundation can serve in a role similar to the “Foundation for Tracing FH” in the Netherlands, directly engaging with FH probands and relatives outside usual healthcare settings to improve the uptake of cascade screening.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Acknowledgments
Funding statement
REDCap (CTSA NIH Grant UL1-RR024982) was utilized for data collection.
Author contributions
MPM: Conceptualization, Data curation, Investigation, Methodology, Writing - original draft; Writing - review & editing.
MC: Conceptualization, Investigation, Methodology, Writing - original draft; Writing - review & editing.
CDA: Conceptualization, Writing - review & editing.
AK: Writing - review & editing.
WSW: Writing - review & editing.
KW: Conceptualization, Funding acquisition, Writing - review & editing.
ZA: Conceptualization, Data curation, Formal analysis, Supervision, Writing - original draft; Writing - review & editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2021.100170.
Appendix. Supplementary materials
References
- 1.McGowan M.P., Hosseini Dehkordi S.H., Moriarty P.M., Duell P.B. Diagnosis and treatment of heterozygous familial hypercholesterolemia. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjouke B., Hovingh G.K., Kastelein J.J., Stefanutti C. Homozygous autosomal dominant hypercholesterolaemia: prevalence, diagnosis, and current and future treatment perspectives. Curr Opin Lipidol. 2015;26:200–209. doi: 10.1097/MOL.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 3.Abul-Husn N.S., Manickam K., Jones L.K. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science. 2016:354. doi: 10.1126/science.aaf7000. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y.V., Damrauer S.M., Hui Q. Effects of genetic variants associated with familial hypercholesterolemia on low-density lipoprotein-cholesterol levels and cardiovascular outcomes in the million veteran program. Circ Genom Precis Med. 2018;11 doi: 10.1161/CIRCGEN.118.002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowles J.W., Rader D.J., Khoury M.J. Cascade screening for familial hypercholesterolemia and the use of genetic testing. JAMA. 2017;318:381–382. doi: 10.1001/jama.2017.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen M.S., Kolor K., Dotson W.D., Ned R.M., Khoury M.J. Public health action in genomics is now needed beyond newborn screening. Publ Health Genom. 2012;15:327–334. doi: 10.1159/000341889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation. 2018 CIR0000000000000625. [Google Scholar]
- 8.Bhatnagar D., Morgan J., Siddiq S., Mackness M.I., Miller J.P., Durrington P.N. Outcome of case finding among relatives of patients with known heterozygous familial hypercholesterolaemia. BMJ. 2000;321:1497–1500. doi: 10.1136/bmj.321.7275.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts GF, Sullivan DR, Poplawski N et al. Familial hypercholesterolaemia: A model of care for Australasia. Atheroscler Suppl. [DOI] [PubMed]
- 10.Nordestgaard B.G., Chapman M.J., Humphries S.E. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478. doi: 10.1093/eurheartj/eht273. -90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umans-Eckenhausen M.A., Defesche J.C., Sijbrands E.J., Scheerder R.L., Kastelein J.J. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet. 2001;357:165–168. doi: 10.1016/S0140-6736(00)03587-X. [DOI] [PubMed] [Google Scholar]
- 12.Lazaro P., Perez de Isla L., Watts G.F. Cost-effectiveness of a cascade screening program for the early detection of familial hypercholesterolemia. J Clin Lipidol. 2017;11:260–271. doi: 10.1016/j.jacl.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Rubio-Marin P., Michan-Dona A., Maraver-Delgado J. Cascade screening program for familial hypercholesterolemia. Endocrinol Diabetes Nutr. 2018;65:280–286. doi: 10.1016/j.endinu.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Wiegman A., Rodenburg J., de Jongh S. Family history and cardiovascular risk in familial hypercholesterolemia: data in more than 1000 children. Circulation. 2003;107:1473–1478. doi: 10.1161/01.cir.0000058166.99182.54. [DOI] [PubMed] [Google Scholar]
- 15.Gidding S.S., Sheldon A., Neben C.L. Patient acceptance of genetic testing for familial hypercholesterolemia in the CASCADE FH Registry. J Clin Lipidol. 2020;14:218–223. doi: 10.1016/j.jacl.2020.02.001. e2. [DOI] [PubMed] [Google Scholar]
- 16.Elliott E., Lilly C., Murphy E., Pyles L.A., Cottrell L., Neal W.A. The coronary artery risk detection in appalachian communities (CARDIAC) project: an 18 year review. Curr Pediatr Rev. 2017;13:265–276. doi: 10.2174/1573400514666180117093652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wald D.S., Bestwick J.P., Morris J.K., Whyte K., Jenkins L., Wald N.J. Child-parent familial hypercholesterolemia screening in primary care. N Engl J Med. 2016;375:1628–1637. doi: 10.1056/NEJMoa1602777. [DOI] [PubMed] [Google Scholar]
- 18.Green R.C., Lautenbach D., McGuire A.L. GINA, genetic discrimination, and genomic medicine. N Engl J Med. 2015;372:397–399. doi: 10.1056/NEJMp1404776. [DOI] [PubMed] [Google Scholar]
- 19.Areheart B., Roberts J. GINA, big data, and the future of employee privacy. Yale Law J. 2019:710. [Google Scholar]
- 20.Rothstein M.A., Brothers K.B. Banning genetic discrimination in life insurance – time to follow florida's lead. N Engl J Med. 2020;383:2099–2101. doi: 10.1056/NEJMp2024123. [DOI] [PubMed] [Google Scholar]
- 21.Kerr M., Pears R., Miedzybrodzka Z. Cost effectiveness of cascade testing for familial hypercholesterolaemia, based on data from familial hypercholesterolaemia services in the UK. Eur Heart J. 2017;38:1832–1839. doi: 10.1093/eurheartj/ehx111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


