Abstract
Cardiovascular disease remains a major contributor to morbidity and mortality in the US and elsewhere, and stroke is a leading cause of disability worldwide. Despite recent success in diminishing stroke incidence in the general US population, in parallel there is now a concerning propensity for strokes to happen at younger ages. Specifically, the incidence of stroke for US adults 20–44 years of age increased from 17 per 100,000 US adults in 1993 to 28 per 100,000 in 2015. Occurrence of strokes in young adults is particularly problematic as these patients are often affected by physical disability, depression, cognitive impairment and loss of productivity, all of which have vast personal, social and economic implications. These concerning trends among young adults are likely due to increasing trends in the prevalence of modifiable risk factors amongst this population including hypertension, hyperlipidemia, obesity and diabetes, highlighting the importance of early detection and aggressive prevention strategies in the general population at early ages. In parallel and compounding to the issue, troublesome trends are evident regarding increasing rates of substance abuse among young adults. Higher rates of strokes have been noted particularly among young African Americans, indicating the need for tailored prevention and social efforts targeting this and other vulnerable groups, including the primordial prevention of risk factors in the first place, reducing stroke rates in the presence of prevalent risk factors such as hypertension, and improving outcomes through enhanced healthcare access. In this narrative review we aim to emphasize the importance of stroke in young adults as a growing public health issue and increase awareness among clinicians and the public health sector. For this purpose, we summarize the available data on stroke in young adults and discuss the underlying epidemiology, etiology, risk factors, prognosis and opportunities for timely prevention of stroke specifically at young ages. Furthermore, this review highlights the gaps in knowledge and proposes future directions moving forward.
Keywords: Review, Stroke, Young adults
Abbreviations and acronyms
- ACC
American College of Cardiology
- AF
atrial fibrillation
- AHA
American Heart Association
- ASCVD
atherosclerotic cardiovascular disease
- CVD
cardiovascular disease
- ICH
intracerebral hemorrhage
- LDL-C
low-density lipoprotein cholesterol
- SAH
subarachnoid hemorrhage
- SSS
Stop Stroke Study
- TOAST
Trial of ORG 10172 in Acute Stroke Treatment
1. Introduction
Stroke is the fifth leading cause of death in the United States (US) and a major cause of mortality and disability worldwide [1]. While traditionally stroke was considered a disease of middle-aged and elderly patients, an emerging trend has recently been noticed of increasing strokes in younger adults [[2], [3], [4]]. For example, the National Inpatient Sample (NIS) reported increasing rates of hospitalizations for acute ischemic stroke patients aged 18–44 years in 2012 [4]. More recently, similar data form the NIS still indicate increasing rates for young adults in 2017 [5].
These concerning trends, which have also paralleled the increase in myocardial infarction among young individuals, not only have direct health consequences, but also important implications for productivity, societies, economies, and for the sustainability of healthcare systems [[2], [3], [4],6]. However, despite those implications most of the current understanding about stroke is from the older populations. Consequently, as of 2020 tailored prevention interventions and early detection efforts targeting younger adults are lacking compared to their older counterparts.
Given the current lack of insight about characteristics and patterns of stroke in younger adults, in this narrative review we sought to summarize the most up-to-date evidence on ischemic and hemorrhagic stroke burden, etiology, prognosis, opportunities for enhanced prevention in young adults, and future directions in this field. While inconsistencies exist in the literature regarding the age cut-off to define “young adults”, at least partly driven by increasing longevity [4,7] for this review we use the most consistent definition of 18–45 years old, although relevant studies comprising populations up to 55 years of age are also described when appropriate.
2. Epidemiology of stroke in young adults
2.1. Burden, stroke subtypes and trends
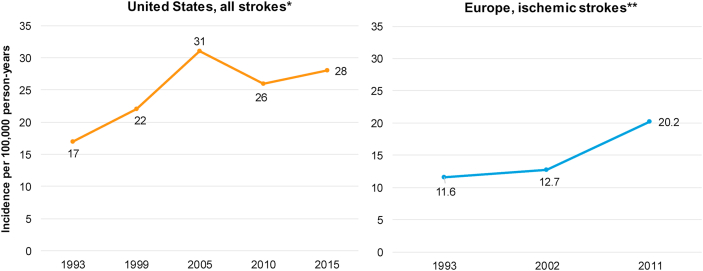
In the US, the prevalence of stroke in young adults represents about 10–15% of all strokes [8,9]. The incidence of stroke for adults aged 20–44 was shown to increase from 17 per 100,000 US adults in 1993 to 28 per 100,000 US adults in 2015 (Fig. 1) [10]. In Europe, for adults younger than 55 years the incidence for ischemic strokes increased from 10.7 per 100,000 adults in 1994–2002 to 18.1 per 100,000 adults in 2003–2011 [11]. Data are scarce for Asia, however in India a population-based study estimated that in the period of 2003–2005 the average annual incidence of stroke was only 4 per 100,000 in patients <40 years, and exceptionally higher at 41 per 100,000 for the 40–44 age group [12]. In a review specifically focused on ethnic and worldwide geographic differences in the burden of stroke in young adults, Yesilot et al. noted incidence rates of ischemic stroke among young adults ranging from as low as 5.8/100,000 in central Italy to as high as 97.7/100,000 in China. Of note, the review included studies conducted during different time periods and including individuals of varying age ranges [13].
Fig. 1.
Incidence of stroke in young adults per 100,000 in the US and Europe. ∗All stroke in patients aged 20–44 Years old. ∗∗ Ischemic stroke in patients aged <55 Years old. Adapted from Ref. [10] Madsen TE, Khoury JC, Leppert M, Alwell K, Moomaw CJ, Sucharew H, Woo D, Ferioli S, Martini S, Adeoye O, Khatri P. Temporal Trends in Stroke Incidence Over Time by Sex and Age in the GCNKSS. Stroke. 2020 Apr; 51 (4):1070–6. [11]; Béjot Y, Daubail B, Jacquin A, Durier J, Osseby GV, Rouaud O, Giroud M. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: the Dijon Stroke Registry. Journal of Neurology, Neurosurgery & Psychiatry. 2014 May 1; 85 (5):509–13.
The prevalence of stroke subtypes has specific considerations in young adults [14]. A population-based stroke epidemiologic study in the Greater Cincinnati/Northern Kentucky region found that ischemic strokes represented about 65% of all strokes in young adults aged 20–44 years, while 17% were diagnosed with intracerebral hemorrhage (ICH) and 16% with subarachnoid hemorrhage (SAH) [15]. George et al. have demonstrated that the national rate of hospitalization in the US for ischemic stroke in young adults aged between 18 and 44 years old has doubled between 2003 and 2012 while it remained stable for ICH and SAH [4].
2.2. Etiology
Etiology of ischemic stroke in young adults is diverse and varies according to age, sex and geographical location. Table 1 illustrates the etiology of ischemic stroke according to Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria and their prevalence in young adults. Despite recent healthcare advancements including improved diagnosis and management, undetermined etiology remains the leading cause in this age group [[16], [17], [18], [19], [20]]. Importantly, the main drawback of the TOAST classification is a high proportion of undetermined etiology due to lack of detailed investigation. To minimize this issue, the updated Stop Stroke Study TOAST (SSS-TOAST) system divides each of the original TOAST subtypes into three subcategories as “evident,” “probable,” or “possible” [21]. An automated version of SSS-TOAST, the Causative Classification System (CCS), was devised to improve its usefulness and accuracy for stroke subtyping through a computerized algorithm [22]. In addition, ASCO (Atherosclerosis, Small vessel disease, Cardiac source, Other cause) was proposed as a new classification for stroke subtyping in 2009 [23]. The application of the causative classification system and ASCO classifications in young stroke patients may reduce the number of strokes classified as undetermined etiology, however, these still represent a relatively large proportion [24].
Table 1.
TOAST classification of ischemic stroke.
| TOAST classification | Proportion rangea |
|---|---|
| Large-artery atherosclerosis | 6–15% |
| Cardioembolism | 10–24% |
| Small-vessel occlusion | 12–26% |
| Stroke of other determined etiology | 9–26% |
| Stroke of undetermined etiology | 24–53% |
Abbreviations: TOAST: Trial of ORG 10172 in Acute Stroke Treatment.
Estimates based on published studies.
Cardioembolism is another important cause of ischemic stroke in young adults. While electrocardiogram monitoring is used to detect atrial dysrhythmias such as paroxysmal atrial fibrillation (AF) [25], transesophageal and transthoracic echocardiograms are considered as important tools in diagnosing cardioembolic stroke [26]. Patent foramen ovale is present in up to 45% of young patients with cryptogenic stroke (strokes with no clear identifiable etiology after proper neurologic work up) [27]. Meta-analyses have shown that patent foramen ovale closure results in a significant reduction in the recurrence of ischemic stroke when compared to aspirin therapy alone, while its superiority compared to anticoagulation is unclear [28,29].
Large artery atherosclerosis and small vessels diseases are a more common cause of stroke after the age of 35–40 [20]. Of note, with the increased prevalence of traditional cardiovascular risk factors among young adults [4], these causes are expected to increase in proportion to other causes among patients with a stroke. However, the current data reporting increased prevalence of ischemic strokes lacks in terms of classifying the underlying mechanisms involved. Additionally, traditional risk factors also have a role as underlying causes of thromboembolic ischemic strokes, for example hypertension, which causes left ventricle overload, left atrial enlargement and subsequent AF at relatively young ages [30].
Arterial dissection also significantly contributes to the stroke burden in young adults [16]. Cervical artery dissection accounts for 10–25% of all strokes in young adults, as opposed to 2% of all ischemic strokes in all age groups [31]. Most arterial dissections occur spontaneously, however, rarely, some are associated with trauma and several genetic and connective tissue disorders such as Ehlers-Danlos syndrome, Marfan syndrome, and fibromuscular dysplasia. They occur more commonly in the internal carotid artery than the vertebral artery [32]. Several conditions that increase the risk of ischemic stroke include migraine, infection, inflammatory vasculopathy, coagulation disorders, immunological and rheumatological disorders, and oral contraceptive drugs [[33], [34], [35]]. Table 2 summarizes common and uncommon causes of ischemic stroke in young [[16], [17], [18], [19]].(36).
Table 2.
Common and uncommon causes of strokes in young adults.
| Common causes | Uncommon causes |
|---|---|
| Large-artery atherosclerosis | Large-artery atherosclerosis |
| Middle cerebral artery, internal carotid artery and vertebrobasilar artery | Posterior cerebral artery and anterior cerebral artery |
| Small-vessel occlusion | |
| Atherothrombotic vasculopathy | |
| Cardioembolism | Cardioembolism |
| Patent foramen ovale, dilated cardiomyopathy, and atrial fibrillation | Infective endocarditis, congenital cardiac malformation, mechanical aortic valve, left ventricular thrombus, hypokinetic left ventricular segment, akinetic left ventricular segment, atrial myxoma and nonbacterial thrombotic endocarditis |
| Stroke of other determined etiology | Stroke of other determined etiology |
| Dissection of cervical artery and vasculitis | Systemic lupus erythematosus, Hereditary and acquired coagulation disorder, active malignancy and radiation vasculopathy, infective vasculitis, Inflammatory vasculopathy, Hereditary diseases, antiphospholipid antibodies, reversible cerebral vasoconstriction syndrome |
| Stroke of undetermined etiology |
Hemorrhagic strokes include subarachnoid and intracerebral types. Hypertensive microangiopathy is a major cause of hemorrhagic strokes. The likelihood of having hypertensive microangiopathy as the etiology of ICH increases with advancing age, nonetheless it is still a relevant cause in young adults [37]. Drug abuse is another important cause of hemorrhagic stroke in young adults [38]. The increased use of substances such as cocaine and amphetamine have led to an increase in cardiovascular disease (CVD) in the young [39,40], and urine toxicology is an important consideration in these patients. Other causes of hemorrhagic stroke in young adults are listed in Table 3 [37]. [41].
Table 3.
Causes of ICH in young adults.
Hypertensive microangiopathy
|
Structural lesions
|
Medication/drug abuse
|
Systemic disease
|
| Undetermined cause |
2.3. Demographic characteristics
Racial, ethnic and sex disparities in the incidence of stroke have been reported in a number of studies. Considering the overall incidence across all age groups, strokes (of both types) are more common among men, but women are more severely affected and have a higher case-fatality rate, which may be explained by higher prevalence of embolic strokes among women [10,42]. In the young, gender disparities in the incidence of stroke are inconsistent across studies. In a population-based study in France investigating the incidence of all strokes in different age groups of men and women, the latter had a higher incidence of stroke below the age of 35, the women/men incidence rate ratio being 1.89 (95% confidence interval 1.27–2.80). However, the incidence in the ages 35–44 was not significantly different between the two groups. Men had a significantly higher incidence in the age group 45–84. In the Netherlands, a nationwide cohort demonstrated an increased risk of stroke for women aged 18–44 than men [43]. Pregnancy, puerperium, and exposure of young women to estrogenic oral contraception have been proposed as potential explanations for a higher risk of stroke among young women compared to men [44]. In contrast, in the US, the Greater Cincinnati/Northern Kentucky Stroke Study showed that men had a higher but non-significant difference in the incidence for 20–44 age group. Of note, while the incidence for young men has increased over time in the US, it has remained relatively stable for young women [10].
The burden of stroke also varies by race and ethnicity. The Northern Manhattan study documented that the incidence of stroke in Black and Hispanic adults was higher in all age groups compared with Whites [45]. The incidence of all stroke in young adults (aged 20–45 years) per 100,000 for Blacks was 25, in Hispanics 26, and in Whites 10 [46,47]. However, the increased incidence for Hispanics compared to non-Hispanic Whites is not consistent throughout all reports. For example, from the national stroke inpatient registry in the US, George et al. showed that Hispanics did not have increased risk compared to non-Hispanic whites for patients younger than 55 years [4]. This inconsistency is believed to be mainly due to marked sub-ethnic heterogenicity across Hispanic subgroups, which are exposed to varying levels of both cardiovascular-protective and cardiovascular-harmful socioeconomic features [[48], [49], [50]]. On the other hand, the higher risk of stroke among Black men and women is more consistent across US studies, and is likely explained by a higher prevalence of stroke-specific risk factors at younger ages, particularly hypertension; more adverse average socioeconomic conditions, and healthcare system challenges such as lack of access to medical care, insufficient health coverage, and lower quality of preventive and acute care [46,48].
The increasing role of traditional risk factors in young adults with stroke.
Initially, it was shown that the risk factor profile in young stroke patients was unique when compared with the risk profile of elderly individuals: strokes in young adults were infrequent, and attributed to characteristics such as hematologic diseases, vasculitis, malignancies, illicit drug use, pregnancy, thrombophilia, patent foramen ovale, and oral contraception use [4,51]. Nonetheless, recently there has been a shift in the risk factor profile of younger patients towards an increasing importance of traditional cardiovascular risk factors including hypertension, dyslipidemia, diabetes, smoking and others [15,52,53]. As a consequence, currently those traditional cerebrovascular risk factors identified as relevant in the genesis of strokes in older populations also account for a large proportion of strokes in younger adults [54].
For instance, George et al. explored the prevalence of cardiovascular risk factors among patients hospitalized for stroke in the US, noting that the increase in young adult strokes were predominantly ischemic. The authors also compared the prevalence of risk factors in 2003–2004 versus 2011–2012 and found almost universal increase in hypertension, hyperlipidemia, diabetes, smoking, and obesity across the following age groups with ischemic stroke: ([18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]), ([35], [36], [37], [38], [39], [40], [41], [42], [43], [44]), ([45], [46], [47], [48], [49], [50], [51], [52], [53], [54]) years old. In parallel, there was a decrease in patients without risk factors, and an upward trend for patients with 1–2 and 3–5 risk factors [4]. Table 4 shows a summary of risk factor prevalence in ischemic stroke patients from the analysis by George et al. ICH patients had comparable risk profile with increased prevalence of hypertension especially in the ([35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]) age group, and a lower prevalence hyperlipidemia, diabetes, and smoking than in young ischemic patients [4].
Table 4.
Prevalence of risk factors among patients hospitalized with acute ischemic stroke by age and sex.
| HTN | HLD | Diabetes | Tobacco | Obesity | AF | IHD | No RFs∗∗ | 1-2 RFs | 3-5 RFs | |
|---|---|---|---|---|---|---|---|---|---|---|
| Males | ||||||||||
| 18–34 YO | ||||||||||
| 2003–04 | 34.0% | 14.6% | 15.3% | 23.1% | 6.8% | 2.1% | 6.4% | 42.0% | 48.9% | 9.1% |
| 2011–12 | 41.1%∗ | 29.1%∗ | 15.2% | 35.7%∗ | 13.3%∗ | 2.9% | 5.5% | 27.9%∗ | 55.9%∗ | 16.2%∗ |
| 35–44 YO | ||||||||||
| 2003–04 | 54.% | 29.0% | 24.3% | 31.3% | 7.7% | 3.2% | 11.1% | 22.8% | 58.6% | 18.6% |
| 2011–12 | 65.9%∗ | 47.8%∗ | 30.3%∗ | 41.7%∗ | 15.2%∗ | 4.3% | 11.2% | 12.1%∗ | 52.9%∗ | 35.0%∗ |
| Females | ||||||||||
| 18–34 YO | ||||||||||
| 2003–04 | 26.1% | 9.6% | 11.8% | 21.1% | 9.1% | 1.7% | 2.1% | 48.6% | 45.8% | 5.6% |
| 2011–12 | 30.7%∗ | 21.7%∗ | 15.5%∗ | 26.5%∗ | 15.7%∗ | 1.8% | 3.9% | 38.5% | 48.0% | 13.5%∗ |
| 35–44 YO | ||||||||||
| 2003–04 | 50.1% | 20.8% | 24.2% | 26.9% | 10.9% | 1.2% | 7.3% | 28.1% | 56.5% | 15.4% |
| 2011–12 | 57.3%∗ | 37.8%∗ | 31.4%∗ | 35.8%∗ | 21.0%∗ | 2.3%∗ | 7.2%∗ | 18.6%∗ | 49.9%∗ | 31.6% |
Abbreviations: AF, atrial fibrillation; HLD, lipid disorder; HTN, hypertension; IHD, ischemic heart disease; RF, risk factors; YO, years old. Adapted from Ref. [4] George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA neurology. 2017 Jun 1; 74(6):695–703.
∗Clinically significant change.
∗∗Risk factors include; (HTN, Diabetes, HLD, Obesity, and Tobacco Use).
In a German study, somewhat different risk factors among 2125 young stroke patients (ischemic and hemorrhagic) (18–55 years old) were examined. Population-attributable risks were: low physical activity (accounting for 59.7% of strokes), hypertension (27.1%), heavy alcohol use (17.5%), and smoking (12.8%). Other significant risk factors in this study were obesity and diabetes [54]. In contrast, coronary artery disease and hyperlipidemia were not significant risk factors in this young stroke population. In another study in China of stroke patients (ischemic and hemorrhagic) aged 35–45, the authors concluded that in their population, the key risk factors in the order of importance were hypertension, smoking, alcohol use, previous stroke, heart disease, diabetes, and hyperlipidemia [55].
2.4. Prognosis of stroke in young adults
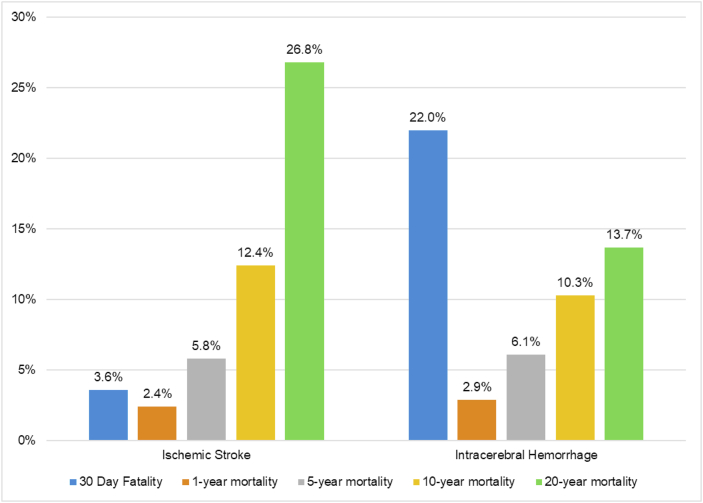
The prognosis of young stroke is not as favorable as previously thought, with respect either to mortality or physical/psychosocial consequences [8]. Of note, while stroke mortality has decreased overall in recent years, this decline has been slower in young adults compared with older patients [2]. A key reason underlying this phenomenon is stroke etiology, which has substantial impact on mortality and morbidity: hemorrhagic strokes represent a higher proportion of strokes among young adults, and while the case-fatality for ischemic stroke is estimated to be 3.6%, this goes up to 22% for ICH. Nonetheless, it must be noted that the long-term mortality for ischemic stroke among young patients is also high, with a cumulative mortality of 12.4% after 10 years and 26.8% after 20 years. Around half of the deaths were found to be related to a vascular cause, indicating that the underlying cause of stroke at a young age continues to be active throughout life (Fig. 2) [56].
Fig. 2.
30-day fatality and cumulative mortality in 30-day survivors of stroke in young adults∗ ∗ Data for ≥ 1-year mortality presented as cumulative incidence of mortality among survivors of first 30 days post-stroke. Adapted from Ref. [54] Rutten-Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, de Leeuw FE. Long-term mortality after stroke among adults aged 18–50 years. Jama. 2013 Mar 20; 309 (11):1136–44.
In addition, the survivors’ road to recovery is filled with obstacles that may prevent the patients from fully returning to their normal lives. This is particularly relevant among young, working-age stroke survivors, as well as among those with dependents such as children or elderly parents. The complications of strokes can be categorized into physical and psychosocial consequences and are summarized in Table 5. It is important to note that there is scarcity of data with regards to the long-term consequences of stroke specifically in young adults, and further research is needed to better characterize the implications of this growing phenomenon.
Table 5.
Long-term consequences of strokes in young adults.
| Consequence | Description |
|---|---|
| Physical | |
| Functional disability |
|
| Pain Syndromes |
|
| Epilepsy |
|
| Psychosocial | |
| Cognitive impairment |
|
| Depression | |
| Anxiety | |
| Fatigue |
|
| Sexual Dysfunction |
|
| Return to work |
|
2.5. Prevention
Globally, 90% of stroke burden is attributable to modifiable risk factors, and it has been proposed that control of behavioral and metabolic risk factors could avert about three-quarters of the global stroke burden [57]. With the recent increase in traditional cardiovascular risk factors among young adults, together with the parallel increase in myocardial infarction among young adults, aggressive preventive measures targeting the young become more important than ever. Although several recommendations on the management and prevention of stroke have been published, these strategies are not universally adopted [58].
2.6. Primordial prevention
Primordial prevention strategies aim at preventing the emergence of risk factors. Healthy lifestyle changes such as smoking cessation, managing body weight, regular physical activity, and a healthy diet lower CVD morbidity and mortality, including that related to stroke by mitigating the development of key traditional risk factors such as hypertension, diabetes, hyperlipidemia, obesity and exposure to tobacco products [59].
Tobacco smoking is associated with hypercoagulable state, inflammation, oxidative stress, insulin resistance, dyslipidemia, and increased blood pressure [60]. Smoking shows a strong dose–response relationship between the number of cigarettes smoked daily and ischemic stroke among young men [61]. Moreover, George et al. demonstrated a trend of increased smoking prevalence in young adults in the period 2003–2012 in the US [4]. Additionally, although complete smoking cessation is the goal, smoking fewer cigarettes may reduce the risk of ischemic stroke in young adults [62]. The use of electronic cigarettes has been exponentially increasing in the past decade, especially among youth and younger adults. In a wide cross-sectional study in the US, the use of electronic cigarettes have been associated with higher odds of stroke, myocardial infarction, and coronary artery disease when compared to non-users [1]. [63].
Diet can influence a range of cardiovascular risk factors and is, therefore, a primary target of primordial prevention strategies. In observational data, limiting the intake of salt, alcohol, refined sugars and saturated fats has been shown to be associated with a lower prevalence of cardiovascular risk factors [64]. In addition, Iacoviello et al. provided evidence supporting a favorable role of dietary models characterized by a relatively high consumption of plant foods (fruits, vegetables, unrefined cereals, legumes, nuts), use of extra virgin olive oil as main source of fat, moderate consumption of fish, milk and dairy products, and a lower consumption of meat (particularly of red and processed meats) [65].
The role of physical inactivity has also been well described. In the US, the prevalence of physical inactivity among adults ≥18 years of age, has decreased from 1998 to 2017, with the largest drop occurring in the past decade, from 40.2% to 25.9% between 2005 and 2017, respectively [1]. Physical inactivity has been associated with greater risk of CVD [66]. The mechanism by which exercise has been shown to protect from CVD includes a lower risk of hypertension, a lowering of blood sugar, and an increase in high-density lipoprotein cholesterol, as shown in meta-analysis by Wewege et al. [67].
Weight loss has beneficial effects on cardiovascular risk factors and these changes would be expected to decrease later cardiovascular events including strokes. Weight loss significantly reduced systolic and diastolic blood pressure, low-density lipoprotein cholesterol (LDL-C), triglycerides, fasting plasma glucose, and hemoglobin A1c [68].
With increased rates of substance abuse (including smoking, alcohol, and illegal drug use), emphasis should be placed on the dangers associated with such habits; the mounting evidence of their association with CVD should prompt nationwide programs to curb their use and harmful consequences [69]. In a recent notionally representative cross-sectional study, Parekh et al. demonstrated that young (18–44 years old) marijuana users were at an increased risk of stroke, with even higher risk in frequent users (>10 times/day) and concurrent smokers. Despite recent trends of legalizing marijuana use, the nation should move with caution and healthcare workers should spread awareness regarding its possible risks [70].
Previous studies have shown that targeted counselling regarding primordial prevention amongst asymptomatic healthy adults, taking into consideration their cultural background, home environments and literacy leads to better outcomes at least in the short-term [71]. Given the fact that risk factors and strokes are on the rise in young adults, there is a need to stress on the importance of and explore community-based strategies including awareness programs at educational institutions and workplaces. In addition, there is a need to further enhance preventive medicine programs with better clinic-community linkage and active engagement of the young adult population to better implement these prevention strategies which can prevent and delay the development of risk factors. Further, it is imperative for clinicians to consider that even their younger patients are at a risk for strokes and understand the positive impact of effective counselling; including but not limited to simple strategies like taking stairs at work and exploring the opportunity for physical work-outs at work places.
2.7. Primary prevention
Primary stroke prevention encompasses actions aimed at stroke prevention in at-risk asymptomatic population. It entails identifying and controlling known risk factors such as hypertension, hyperlipidemia, diabetes and smoking through promoting lifestyle modification including smoking cessation, adoption of healthy diets, moderation of alcohol consumption and adequate physical activity.
Hypertension is an important risk factor across all age groups, accounting for half the deaths from stroke globally [72]. Interestingly, there has been a decline in stroke mortality over the past several decades, mostly in older adults, which is strongly believed to be attributable, in part, to improved blood pressure control [73,74]. The most recent 2017 American College of Cardiology (ACC) hypertension guidelines have also recommended more aggressive blood pressure control with a target blood pressure of <130/80 mmHg as compared to <140/90 mmHg previously, for patients with a 10-year atherosclerotic CVD (ASCVD) risk ≥10% [75]. However, it is of note that this risk score is heavily influenced by age, and thus most at-risk young individuals will not meet this criteria for more aggressive blood pressure control [76]. These guidelines now categorize previously considered normal blood pressure (systolic 120–129 mm Hg, diastolic 80–89 mm Hg) as ‘elevated blood pressure’ and recommend starting with at least style modifications in such individuals with close follow up [75].
In terms of hyperlipidemia, the recent American Heart Association (AHA)/ACC/Multi-society Cholesterol guidelines establish that the decision to start a statin depends on the risk factor profile and the 10-year ASCVD risk. Unfortunately, this 10-year risk calculator only applies to individuals 40–79 years of age, and likely underestimates risk in young individuals [76]. For young adults (20–39 years old) there are lifetime risk calculators available, although their use to guide statin therapy allocation is currently limited. Overall, in these individuals, efforts should be directed towards optimizing the risk profile. Statin therapy is indicated in young adults with an LDL-C levels ≥190 mg/dL and those with persistent, moderate hypercholesterolemia (LDL-C 160–189 mg/dL) might benefit from statin therapy depending on their risk profile [77]. Of note, and despite initial concerns, meta-analyses of randomized controlled trials have shown that statins do not increase the risk of ICH as previously thought [78]. However, this misconception continues to be observed in the population.
There are other potential therapies that may have a selected role in primary prevention for the reduction of stroke, including therapies to lower lipoprotein(a), which is associated with an increased risk of CVD including ischemic strokes, that are currently under development [79]. Low-dose aspirin also has a guideline-endorsed role in the primary prevention of CVD events, including strokes. However, the ACC/AHA recommends restricting consideration of aspirin therapy to adults <70 years who are at higher ASCVD risk but not at high risk of bleeding, and recent analysis by Cainzos-Achirica et al. suggested that the coronary artery calcium score might help identify the most optimal candidates for therapy [80]. It is important to stress, however, that these considerations only apply to individuals free of AF. For persons with it, anticoagulants should be prioritized over aspirin. In summary, both statins and aspirin may play a role in primary prevention for specific individuals. Clinical risk scores to inform primary prevention in young adults are currently lacking, however, some of these therapies may be indicated in patients with a high burden of traditional risk factors (e.g., statins in patients with severe hypercholesterolemia), as well as in individuals with a high burden of established subclinical disease (such as a high burden or coronary atherosclerosis, as detected in cardiac computed tomography testing).
Although intensive glycemic control has not shown to reduce macrovascular complications of diabetes, but a holistic approach consisting of life-style modifications, controlling hyperglycemia, and treating cardiovascular risk factors associated with diabetes is beneficial to the cardiovascular risk profile of those patients [[81], [82], [83]].
2.8. Vulnerable groups
As previously mentioned, in the US young Black individuals have a significantly higher incidence of stroke than Whites [84]. Targeted interventions are needed to address this public health issue, through both policies and interventions aimed at enhancing the primordial and primary prevention of CVD and its risk factors among young Black adults [84,85] In addition, the early detection of risk factors and their management once present (e.g., aggressive management of hypertension) are of vital importance. For these improvements to happen, structural and social interventions tackling social determinants of health (i.e. socioeconomic stability, health access and literacy, physical environment, education) are crucial, as these are likely to be key factors underpinning the excess burden of stroke compared to other racial/ethnic groups in the US.
Although observations for premature stroke in other minority groups such as Hispanics/Latinos are currently not as consistent as in Blacks, the concerning trends reported in some studies [46,47] require close follow-up, research efforts aimed at the identification of highly vulnerable subgroups, and implementation of appropriate, effective health and social interventions to reduce this burden.
2.9. Future directions
In combination with the need to continue conducting population-level research on the rising stroke incidence and prevalence experienced by young adults, it is crucial to increase general awareness of the growing burden of relevant stroke risk factors along with contemporary stroke trends and their consequences including delayed diagnoses and/or inadequate therapies. Although recent studies have yielded a better understanding of the burden, features and opportunities for prevention of stroke in younger adults, further research is needed. Future studies should continue to describe contemporary stroke rates and outcomes in young adults, so that these can be used to inform timely, effective public health, prevention and management interventions.
Furthermore, more data is needed on the long-term physical and psychosocial consequences faced by young stroke patients to enable clinicians to identify and treat such long-term consequences — with special attention to managing the psychological impact to the patient and their family. These complications may directly affect the outcome, quality of life, and perceived life satisfaction and could hold the key for improved longitudinal outcomes in stroke survivors. On the same note, health expenditure, health care resource utilization and comorbidity burden need to be further described in young adults, along with their impact on the clinical outcomes of these patients, to align health managers and administrators with clinicians and public health experts.
In addition, efforts should also concentrate on implementing strategies for enhancing stroke prevention and reducing the prevalence of traditional cardiovascular and cerebrovascular risk factors at early ages and identifying the at-risk population. Most primordial interventions to prevent stroke are also beneficial for the prevention of coronary heart disease, diabetes, and multiple types of cancers, among other diseases. The increasing rates of stroke among younger adults provide thus further rationale to implement such interventions, with special attention to primordial prevention. Also, the associated risk of stroke could be used by cardiovascular prevention professionals to further motivate patients for healthy lifestyle change. We hope that the considerations included in this review can be used to inform effective interventions targeting both the young adult population at large, and specifically demographic and other subgroups at increased risk.
Besides primordial prevention actions to be implemented mostly at a population level, in primary prevention development of additional, accurate risk assessment strategies and tools will be needed to better identify apparently healthy individuals at highest risk of stroke during young adulthood. For instance, the risk prediction models including those primarily meant for stroke risk estimation such as Framingham, Atherosclerosis Risk in Communities and CHS stroke scores were developed based on relatively older population. With the increasing burden of strokes in younger population and more people meeting the criteria for risk factors with newer guidelines, there is a need to update existing models and develop new risk calculation tools specifically for this younger population. Optimal, timely identification of those individuals will be crucial to implement the most aggressive individual-level preventive interventions.
3. Conclusion
In the last decade stroke in young adults has emerged as a growing public health problem in many countries such as the US. The recent trend of increasing strokes in young adults seems to be primarily driven by increase in ischemic strokes, in part it is mainly attributed to higher prevalence of modifiable risk factors, such as hypertension, hyperlipidemia, obesity, and smoking. Prevention remains the most important strategy to impact long-term clinical and economic consequences. The high mortality rates, the striking impact of recurrent stroke on the risk of death, and the devastating long-term consequences should lead to development of more robust primordial, primary and secondary prevention strategies for young adults.
Declaration of competing interest
Dr. Virani reports grant support: Department of Veterans Affairs, World Heart Federation, Tahir and Jooma Family Honorarium: American College of Cardiology (Associate Editor for Innovations, acc.org) Steering Committee member: Patient and Provider Assessment of Lipid Management (PAM) registry at the Duke Clinical Research Institute (no financial remuneration). Dr. Ron Blankstein reports research support from Amgen Inc and Astellas Inc. The other authors have no conflicts of interest relevant to the content of this manuscript.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation. 2020:E139–E596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Krishnamurthi R.V., Moran A.E., Feigin V.L., Barker-Collo S., Norrving B., Mensah G.A., Taylor S., Naghavi M., Forouzanfar M.H., Nguyen G., Johnson C.O. Stroke prevalence, mortality and disability-adjusted life years in adults aged 20-64 years in 1990-2013: Data from the global burden of disease 2013 study. Neuroepidemiology. 2015;45(3):190–202. doi: 10.1159/000441098. [DOI] [PubMed] [Google Scholar]
- 3.Ekker M.S., Boot E.M., Singhal A.B., Tan K.S., Debette S., Tuladhar A.M., de Leeuw F.E. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018 Sep 1;17(9):790–801. doi: 10.1016/S1474-4422(18)30233-3. [DOI] [PubMed] [Google Scholar]
- 4.George M.G., Tong X., Bowman B.A. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA neurology. 2017 Jun 1;74(6):695–703. doi: 10.1001/jamaneurol.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan S.U. Midlife and Older; 2020. Clinical and economical burden of stroke among young. [Google Scholar]
- 6.Yang J., Biery D.W., Singh A., Divakaran S., DeFilippis E.M., Wu W.Y., Klein J., Hainer J., Ramsis M., Natarajan P., Januzzi J.L. Risk factors and outcomes of very young adults who experience myocardial infarction: the Partners YOUNG-MI registry. Am J Med. 2020 May 1;133(5):605–612. doi: 10.1016/j.amjmed.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivioja R., Pietilä A., Martinez-Majander N., Gordin D., Havulinna A.S., Salomaa V., Aarnio K., Curtze S., Leiviskä J., Rodríguez-Pardo J., Surakka I. Risk factors for early-onset ischemic stroke: A case-control study. Journal of the American Heart Association. 2018 Nov 6;7(21) doi: 10.1161/JAHA.118.009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maaijwee N.A., Rutten-Jacobs L.C., Schaapsmeerders P., Van Dijk E.J., de Leeuw F.E. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol. 2014 Jun;10(6):315. doi: 10.1038/nrneurol.2014.72. [DOI] [PubMed] [Google Scholar]
- 9.Ji R., Schwamm L.H., Pervez M.A., Singhal A.B. Ischemic stroke and transient ischemic attack in young adults: Risk factors, diagnostic yield, neuroimaging, and thrombolysis. JAMA neurology. 2013 Jan 1;70(1):51–57. doi: 10.1001/jamaneurol.2013.575. [DOI] [PubMed] [Google Scholar]
- 10.Madsen T.E., Khoury J.C., Leppert M., Alwell K., Moomaw C.J., Sucharew H., Woo D., Ferioli S., Martini S., Adeoye O., Khatri P. Temporal trends in stroke incidence over time by sex and age in the GCNKSS. Stroke. 2020 Apr;51(4):1070–1076. doi: 10.1161/STROKEAHA.120.028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Béjot Y., Daubail B., Jacquin A., Durier J., Osseby G.V., Rouaud O., Giroud M. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: The Dijon Stroke Registry. J Neurol Neurosurg Psychiatr. 2014 May 1;85(5):509–513. doi: 10.1136/jnnp-2013-306203. [DOI] [PubMed] [Google Scholar]
- 12.Das S.K., Banerjee T.K., Biswas A., Roy T., Raut D.K., Mukherjee C.S., Chaudhuri A., Hazra A., Roy J. A prospective community-based study of stroke in Kolkata, India. Stroke. 2007 Mar 1;38(3):906–910. doi: 10.1161/01.STR.0000258111.00319.58. [DOI] [PubMed] [Google Scholar]
- 13.Yesilot N., Putaala J., Bahar S.Z., Tatlısumak T. Ethnic and geographical differences in ischaemic stroke among young adults. Curr Vasc Pharmacol. 2017;15(5):416–429. doi: 10.2174/1570161115666170202161719. [DOI] [PubMed] [Google Scholar]
- 14.Marini C., Russo T., Felzani G. Incidence of stroke in young adults: a review. Stroke Res Treat. 2010 Dec 19:2011. doi: 10.4061/2011/535672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissela B.M., Khoury J.C., Alwell K., Moomaw C.J., Woo D., Adeoye O., Flaherty M.L., Khatri P., Ferioli S., La Rosa F.D., Broderick J.P. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012 Oct 23;79(17):1781–1787. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung J.W., Park S.H., Kim N., Kim W.J., Park J.H., Ko Y., Yang M.H., Jang M.S., Han M.K., Jung C., Kim J.H. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification and vascular territory of ischemic stroke lesions diagnosed by diffusion-weighted imaging. Journal of the American Heart Association. 2014 Aug 11;3(4) doi: 10.1161/JAHA.114.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smajlović D., Salihović D., Ibrahimagić O.Ć., Sinanović O. Characteristics of stroke in young adults in Tuzla Canton, Bosnia and Herzegovina. Coll Antropol. 2013 Jul 1;37(2):515–519. [PubMed] [Google Scholar]
- 18.Putaala J., Metso A.J., Metso T.M., Konkola N., Kraemer Y., Haapaniemi E., Kaste M., Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009 Apr 1;40(4):1195–1203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 19.Wu T.Y., Kumar A., Wong E.H. Young ischaemic stroke in South Auckland: a hospital-based study. NZ Med J. 2012 Oct 26;(1364):125. [PubMed] [Google Scholar]
- 20.Cerrato P., Grasso M., Imperiale D., Priano L., Baima C., Giraudo M., Rizzuto A., Azzaro C., Lentini A., Bergamasco B. Stroke in young patients: etiopathogenesis and risk factors in different age classes. Cerebrovasc Dis. 2004;18(2):154–159. doi: 10.1159/000079735. [DOI] [PubMed] [Google Scholar]
- 21.Ay H., Furie K.L., Singhal A., Smith W.S., Sorensen A.G., Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Official Journal of the American Neurological Association and the Child Neurology Society. 2005 Nov;58(5):688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 22.Ay H., Furie K.L., Singhal A., Smith W.S., Sorensen A.G., Koroshetz W.J. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol: Official Journal of the American Neurological Association and the Child Neurology Society. 2005 Nov;58(5):688–697. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 23.Amarenco P., Bogousslavsky J., Caplan L.R., Donnan G.A., Hennerici M.G. New approach to stroke subtyping: The ASCO (phenotypic) classification of stroke. Cerebrovasc Dis. 2009;27(5):502–508. doi: 10.1159/000210433. [DOI] [PubMed] [Google Scholar]
- 24.Gökçal E., Niftaliyev E., Asil T. Etiological classification of ischemic stroke in young patients: A comparative study of TOAST, CCS, and ASCO. Acta Neurol Belg. 2017 Sep 1;117(3):643–648. doi: 10.1007/s13760-017-0813-8. [DOI] [PubMed] [Google Scholar]
- 25.Jabaudon D., Sztajzel J., Sievert K., Landis T., Sztajzel R. Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke. 2004 Jul 1;35(7):1647–1651. doi: 10.1161/01.STR.0000131269.69502.d9. [DOI] [PubMed] [Google Scholar]
- 26.Lerakis S., Nicholson W.J., Stouffer G.A., Lenihan D., Lerakis S., Sheahan R.G. Part I: Use of echocardiography in the evaluation of patients with suspected cardioembolic stroke. Am J Med Sci. 2005 Jun 1;329(6):310–316. doi: 10.1097/00000441-200506000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Handke M., Harloff A., Olschewski M., Hetzel A., Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007 Nov 29;357(22):2262–2268. doi: 10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]
- 28.Abo-salem E., Chaitman B., Helmy T., Boakye E.A., Alkhawam H., Lim M. Patent foramen ovale closure versus medical therapy in cases with cryptogenic stroke, meta-analysis of randomized controlled trials. J Neurol. 2018 Mar 1;265(3):578–585. doi: 10.1007/s00415-018-8750-x. [DOI] [PubMed] [Google Scholar]
- 29.De Rosa S., Sievert H., Sabatino J., Polimeni A., Sorrentino S., Indolfi C. Percutaneous closure versus medical treatment in stroke patients with patent foramen ovale: A systematic review and meta-analysis. Ann Intern Med. 2018 Mar 6;168(5):343–350. doi: 10.7326/M17-3033. [DOI] [PubMed] [Google Scholar]
- 30.Renna R., Pilato F., Profice P., Della Marca G., Broccolini A., Morosetti R., Frisullo G., Rossi E., De Stefano V., Di Lazzaro V. Risk factor and etiology analysis of ischemic stroke in young adult patients. J Stroke Cerebrovasc Dis. 2014 Mar 1;23(3):e221–e227. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Hathidara M.Y., Saini V., Malik A.M. Stroke in the young: A global update. Curr Neurol Neurosci Rep. 2019 Nov 1;19(11):91. doi: 10.1007/s11910-019-1004-1. [DOI] [PubMed] [Google Scholar]
- 32.Debette S., Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009 Jul 1;8(7):668–678. doi: 10.1016/S1474-4422(09)70084-5. [DOI] [PubMed] [Google Scholar]
- 33.Jillella D.V., Wisco D.R. Infectious causes of stroke. Curr Opin Infect Dis. 2019 Jun 1;32(3):285–292. doi: 10.1097/QCO.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 34.Brey R.L. Antiphospholipid antibodies in young adults with stroke. J Thromb Thrombolysis. 2005 Oct 1;20(2):105–112. doi: 10.1007/s11239-005-3204-6. [DOI] [PubMed] [Google Scholar]
- 35.Schürks M., Rist P.M., Bigal M.E., Buring J.E., Lipton R.B., Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. Bmj. 2009 Oct 27:339. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012 Oct 1;11(10):906–917. doi: 10.1016/S1474-4422(12)70135-7. [DOI] [PubMed] [Google Scholar]
- 37.Tatlisumak T., Cucchiara B., Kuroda S., Kasner S.E., Putaala J. Nontraumatic intracerebral haemorrhage in young adults. Nat Rev Neurol. 2018 Apr;14(4):237. doi: 10.1038/nrneurol.2018.17. [DOI] [PubMed] [Google Scholar]
- 38.Westover A.N., McBride S., Haley R.W. Stroke in young adults who abuse amphetamines or cocaine: A population-based study of hospitalized patients. Arch Gen Psychiatr. 2007 Apr 1;64(4):495–502. doi: 10.1001/archpsyc.64.4.495. [DOI] [PubMed] [Google Scholar]
- 39.DeFilippis E.M., Bajaj N.S., Singh A., Malloy R., Givertz M.M., Blankstein R., Bhatt D.L., Vaduganathan M. Marijuana use in patients with cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. 2020 Jan 28;75(3):320–332. doi: 10.1016/j.jacc.2019.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeFilippis E.M., Singh A., Divakaran S., Gupta A., Collins B.L., Biery D., Qamar A., Fatima A., Ramsis M., Pipilas D., Rajabi R. Cocaine and marijuana use among young adults with myocardial infarction. J Am Coll Cardiol. 2018 Jun 5;71(22):2540–2551. doi: 10.1016/j.jacc.2018.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Renjen P.N. Stroke in young. Apollo Medicine. 2013 Dec 1;10(4):265–269. [Google Scholar]
- 42.Appelros P., Stegmayr B., Terént A. Sex differences in stroke epidemiology: A systematic review. Stroke. 2009 Apr 1;40(4):1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 43.Ekker M.S., Verhoeven J.I., Vaartjes I., Van Nieuwenhuizen K.M., Klijn C.J., de Leeuw F.E. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology. 2019 May 21;92(21):e2444–e2454. doi: 10.1212/WNL.0000000000007533. [DOI] [PubMed] [Google Scholar]
- 44.Giroud M., Delpont B., Daubail B., Blanc C., Durier J., Giroud M., Béjot Y. Temporal trends in sex differences with regard to stroke incidence: the Dijon stroke registry (1987–2012) Stroke. 2017 Apr;48(4):846–849. doi: 10.1161/STROKEAHA.116.015913. [DOI] [PubMed] [Google Scholar]
- 45.Gardener H., Sacco R.L., Rundek T., Battistella V., Cheung Y.K., Elkind M.S. Race and ethnic disparities in stroke incidence in the northern manhattan study. Stroke. 2020 Apr;51(4):1064–1069. doi: 10.1161/STROKEAHA.119.028806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong J.Y., Sacco R.L. Epidemiology of stroke in young adults: Race/ethnic differences. J Thromb Thrombolysis. 2005 Oct 1;20(2):77–83. doi: 10.1007/s11239-005-3201-9. [DOI] [PubMed] [Google Scholar]
- 47.Pandey D.K., Gorelick P.B. Epidemiology of stroke in african Americans and hispanic Americans. Medical Clinics. 2005 Jul 1;89(4):739–752. doi: 10.1016/j.mcna.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Trimble B., Morgenstern L.B. Stroke in minorities. Neurol Clin. 2008 Nov 1;26(4):1177–1190. doi: 10.1016/j.ncl.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgenstern L.B., Smith M.A., Sanchez B.N., Brown D.L., Zahuranec D.B., Garcia N., Kerber K.A., Skolarus L.E., Meurer W.J., Burke J.F., Adelman E.E. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann Neurol. 2013 Dec;74(6):778–785. doi: 10.1002/ana.23972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgenstern L.B., Smith M.A., Lisabeth L.D., Risser J.M., Uchino K., Garcia N., Longwell P.J., McFarling D.A., Akuwumi O., Al-Wabil A., Al-Senani F. Excess stroke in Mexican Americans compared with non-Hispanic whites: The brain attack surveillance in Corpus Christi project. Am J Epidemiol. 2004 Aug 15;160(4):376–383. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singhal A.B., Biller J., Elkind M.S., Fullerton H.J., Jauch E.C., Kittner S.J., Levine D.A., Levine S.R. Recognition and management of stroke in young adults and adolescents. Neurology. 2013 Sep 17;81(12):1089–1097. doi: 10.1212/WNL.0b013e3182a4a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleindorfer D., Khoury J., Alwell K., Moomaw C.J., Woo D., Flaherty M.L., Adeoye O., Ferioli S., Khatri P., Kissela B.M. The impact of Magnetic Resonance Imaging (MRI) on ischemic stroke detection and incidence: minimal impact within a population-based study. BMC Neurol. 2015 Dec;15(1):1–6. doi: 10.1186/s12883-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George Mary G. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol. 2011;70(5):713–721. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 54.Aigner A., Grittner U., Rolfs A., Norrving B., Siegerink B., Busch M.A. Contribution of established stroke risk factors to the burden of stroke in young adults. Stroke. 2017 Jul;48(7):1744–1751. doi: 10.1161/STROKEAHA.117.016599. [DOI] [PubMed] [Google Scholar]
- 55.Bi Q., Wang L., Li X., Song Z. Risk factors and treatment of stroke in Chinese young adults. Neurol Res. 2010 May 1;32(4):366–370. doi: 10.1179/016164110X12656393665288. [DOI] [PubMed] [Google Scholar]
- 56.Rutten-Jacobs L.C., Arntz R.M., Maaijwee N.A., Schoonderwaldt H.C., Dorresteijn L.D., van Dijk E.J., de Leeuw F.E. Long-term mortality after stroke among adults aged 18 to 50 years. Jama. 2013 Mar 20;309(11):1136–1144. doi: 10.1001/jama.2013.842. [DOI] [PubMed] [Google Scholar]
- 57.Feigin V.L., Roth G.A., Naghavi M., Parmar P., Krishnamurthi R., Chugh S., Mensah G.A., Norrving B., Shiue I., Ng M., Estep K. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016 Aug 1;15(9):913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 58.Krishnamurthi R., Hale L., Barker-Collo S., Theadom A., Bhattacharjee R., George A., Arroll B., Ranta A., Waters D., Wilson D., Sandiford P. Mobile technology for primary stroke prevention: A proof-of-concept pilot randomized controlled trial. Stroke. 2019 Jan;50(1):196–198. doi: 10.1161/STROKEAHA.118.023058. [DOI] [PubMed] [Google Scholar]
- 59.Foraker R.E., Olivo-Marston S.E., Allen N.B. Lifestyle and primordial prevention of cardiovascular disease: challenges and opportunities. Current Cardiovascular Risk Reports. 2012 Dec 1;6(6):520–527. [Google Scholar]
- 60.Salahuddin S., Prabhakaran D., Roy A. Pathophysiological mechanisms of tobacco-related CVD. Global heart. 2012 Jul 1;7(2):113–120. doi: 10.1016/j.gheart.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Markidan J., Cole J.W., Cronin C.A., Merino J.G., Phipps M.S., Wozniak M.A., Kittner S.J. Smoking and risk of ischemic stroke in young men. Stroke. 2018 May;49(5):1276–1278. doi: 10.1161/STROKEAHA.117.018859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jamal A., Phillips E., Gentzke A.S., Homa D.M., Babb S.D., King B.A., Neff L.J. Current cigarette smoking among adults—United States, 2016. Morbidity and Mortality Weekly Report. 2018 Jan 19;67(2):53. doi: 10.15585/mmwr.mm6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ndunda P.M., Muutu T.M. Electronic cigarette use is associated with a higher risk of stroke. Stroke. 2019 Feb;50 (Suppl_1):A9- [Google Scholar]
- 64.Claas S.A., Arnett D.K. The role of healthy lifestyle in the primordial prevention of cardiovascular disease. Curr Cardiol Rep. 2016 Jun 1;18(6):56. doi: 10.1007/s11886-016-0728-7. [DOI] [PubMed] [Google Scholar]
- 65.Iacoviello L., Bonaccio M., Cairella G., Catani M.V., Costanzo S., D’Elia L., Giacco R., Rendina D., Sabino P., Savini I., Strazzullo P. Diet and primary prevention of stroke: systematic review and dietary recommendations by the ad hoc Working Group of the Italian Society of Human Nutrition. Nutr Metabol Cardiovasc Dis. 2018 Apr 1;28(4):309–334. doi: 10.1016/j.numecd.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Pandey A., Salahuddin U., Garg S., Ayers C., Kulinski J., Anand V., Mayo H., Kumbhani D.J., de Lemos J., Berry J.D. Continuous dose-response association between sedentary time and risk for cardiovascular disease: a meta-analysis. JAMA cardiology. 2016 Aug 1;1(5):575–583. doi: 10.1001/jamacardio.2016.1567. [DOI] [PubMed] [Google Scholar]
- 67.Wewege M.A., Thom J.M., Rye K.A., Parmenter B.J. Aerobic, resistance or combined training: a systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. 2018 Jul 1;274:162–171. doi: 10.1016/j.atherosclerosis.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Zomer E., Gurusamy K., Leach R., Trimmer C., Lobstein T., Morris S., James W.P., Finer N. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes Rev. 2016 Oct;17(10):1001–1011. doi: 10.1111/obr.12433. [DOI] [PubMed] [Google Scholar]
- 69.de los Ríos F., Kleindorfer D.O., Khoury J., Broderick J.P., Moomaw C.J., Adeoye O. Trends in substance abuse preceding stroke among young adults: a population-based study. Stroke. 2012;43(12):3179–3183. doi: 10.1161/STROKEAHA.112.667808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parekh T., Pemmasani S., Desai R. Marijuana use among young adults (18–44 Years of age) and risk of stroke: a behavioral risk factor surveillance system survey analysis. Stroke. 2020;51(1):308–310. doi: 10.1161/STROKEAHA.119.027828. [DOI] [PubMed] [Google Scholar]
- 71.Richards A., Cheng E.M. Stroke risk calculators in the era of electronic health records linked to administrative databases. Stroke. 2013;44(2):564–569. doi: 10.1161/STROKEAHA.111.649798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lackland D.T., Weber M.A. Global burden of cardiovascular disease and stroke: hypertension at the core. Can J Cardiol. 2015 May 1;31(5):569–571. doi: 10.1016/j.cjca.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Lackland D.T., Roccella E.J., Deutsch A.F., Fornage M., George M.G., Howard G., Kissela B.M., Kittner S.J., Lichtman J.H., Lisabeth L.D., Schwamm L.H. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014 Jan;45(1):315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lackland D.T., Carey R.M., Conforto A.B., Rosendorff C., Whelton P.K., Gorelick P.B. Implications of recent clinical trials and hypertension guidelines on stroke and future cerebrovascular research. Stroke. 2018 Mar;49(3):772–779. doi: 10.1161/STROKEAHA.117.019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Himmelfarb C.D., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., MacLaughlin E.J. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018 May 7;71(19) doi: 10.1016/j.jacc.2017.11.006. e127-248. [DOI] [PubMed] [Google Scholar]
- 76.Singh A., Collins B.L., Gupta A., Fatima A., Qamar A., Biery D., Baez J., Cawley M., Klein J., Hainer J., Plutzky J. Cardiovascular risk and statin eligibility of young adults after an MI: partners YOUNG-MI Registry. J Am Coll Cardiol. 2018 Jan 15;71(3):292–302. doi: 10.1016/j.jacc.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., Goldberg R. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019 Jun 17;73(24) doi: 10.1016/j.jacc.2018.11.003. e285-350. [DOI] [PubMed] [Google Scholar]
- 78.McKinney J.S., Kostis W.J. Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke. 2012 Aug;43(8):2149–2156. doi: 10.1161/STROKEAHA.112.655894. [DOI] [PubMed] [Google Scholar]
- 79.Berman A.N., Blankstein R. Current and future role of lipoprotein (a) in preventive cardiology. Curr Opin Cardiol. 2019 Sep 1;34(5):514–518. doi: 10.1097/HCO.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 80.Cainzos-Achirica M., Miedema M.D., McEvoy J.W., Al Rifai M., Greenland P., Dardari Z., Budoff M., Blumenthal R.S., Yeboah J., Duprez D.A., Mortensen M.B. Coronary artery calcium for personalized allocation of aspirin in primary prevention of cardiovascular disease in 2019: The MESA study (Multi-Ethnic study of atherosclerosis) Circulation. 2020 May 12;141(19):1541–1553. doi: 10.1161/CIRCULATIONAHA.119.045010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Action to control cardiovascular risk in diabetes study group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Terry T., Raravikar K., Chokrungvaranon N., Reaven P.D. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes?: insights from ACCORD, ADVANCE, and VADT. Curr Cardiol Rep. 2012 Feb 1;14(1):79–88. doi: 10.1007/s11886-011-0238-6. [DOI] [PubMed] [Google Scholar]
- 83.Huang D., Refaat M., Mohammedi K., Jayyousi A., Al Suwaidi J., Abi Khalil C. Macrovascular complications in patients with diabetes and prediabetes. BioMed Res Int. 2017 Oct;2017 doi: 10.1155/2017/7839101. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hozawa A., Folsom A.R., Sharrett A.R., Chambless L.E. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects—atherosclerosis Risk in Communities Study. Arch Intern Med. 2007 Mar 26;167(6):573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 85.Clark L.T., El-Atat F. Metabolic syndrome in African Americans: Implications for preventing coronary heart disease. Clin Cardiol. 2007 Apr;30(4):161–164. doi: 10.1002/clc.20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varona J.F., Bermejo F., Guerra J.M., Molina J.A. Long-term prognosis of ischemic stroke in young adults. J Neurol. 2004 Dec 1;251(12):1507–1514. doi: 10.1007/s00415-004-0583-0. [DOI] [PubMed] [Google Scholar]
- 87.Treister A.K., Hatch M.N., Cramer S.C., Chang E.Y. Demystifying poststroke pain: from etiology to treatment. PM&R. 2017 Jan 1;9(1):63–75. doi: 10.1016/j.pmrj.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harno H., Haapaniemi E., Putaala J., Haanpää M., Mäkelä J.P., Kalso E. Central poststroke pain in young ischemic stroke survivors in the Helsinki Young Stroke Registry. Neurology. 2014;83(13):1147–1154. doi: 10.1212/WNL.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 89.Arntz R.M., Maaijwee N.A., Rutten-Jacobs L.C., Schoonderwaldt H.C., Dorresteijn L.D., van Dijk E.J., de Leeuw F.E. Epilepsy after TIA or stroke in young patients impairs long-term functional outcome: the FUTURE Study. Neurology. 2013 Nov 26;81(22):1907–1913. doi: 10.1212/01.wnl.0000436619.25532.f3. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y., Yang S., Jia J. Factors related to long-term post-stroke cognitive impairment in young adult ischemic stroke. Med Sci Mon Int Med J Exp Clin Res: international medical journal of experimental and clinical research. 2015;21:654. doi: 10.12659/MSM.892554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waje-Andreassen U., Thomassen L., Jusufovic M., Power K.N., Eide G.E., Vedeler C.A., Naess H. Ischaemic stroke at a young age is a serious event–final results of a population-based long-term follow-up in Western Norway. Eur J Neurol. 2013 May;20(5):818–823. doi: 10.1111/ene.12073. [DOI] [PubMed] [Google Scholar]
- 92.Maaijwee N.A., Tendolkar I., Rutten-Jacobs L.C., Arntz R.M., Schaapsmeerders P., Dorresteijn L.D., Schoonderwaldt H.C., van Dijk E.J., de Leeuw F.E. Long-term depressive symptoms and anxiety after transient ischaemic attack or ischaemic stroke in young adults. Eur J Neurol. 2016 Aug;23(8):1262–1268. doi: 10.1111/ene.13009. [DOI] [PubMed] [Google Scholar]
- 93.Pompili M., Venturini P., Lamis D.A., Giordano G., Serafini G., Murri M.B., Amore M., Girardi P. Suicide in stroke survivors: Epidemiology and prevention. Drugs Aging. 2015 Jan;32(1):21–29. doi: 10.1007/s40266-014-0233-x. [DOI] [PubMed] [Google Scholar]
- 94.Maaijwee N.A., Arntz R.M., Rutten-Jacobs L.C., Schaapsmeerders P., Schoonderwaldt H.C., van Dijk E.J., de Leeuw F.E. Post-stroke fatigue and its association with poor functional outcome after stroke in young adults. J Neurol Neurosurg Psychiatr. 2015 Oct 1;86(10):1120–1126. doi: 10.1136/jnnp-2014-308784. [DOI] [PubMed] [Google Scholar]
- 95.Bugnicourt J.M., Hamy O., Canaple S., Lamy C., Legrand C. Impaired sexual activity in young ischaemic stroke patients: an observational study. Eur J Neurol. 2014 Jan;21(1):140–146. doi: 10.1111/ene.12277. [DOI] [PubMed] [Google Scholar]
- 96.Aarnio K., Rodríguez-Pardo J., Siegerink B., Hardt J., Broman J., Tulkki L. Return to work after ischemic stroke in young adults: A registry-based follow-up study. Neurology. 2018;91(20):e1909–e1917. doi: 10.1212/WNL.0000000000006510. [DOI] [PMC free article] [PubMed] [Google Scholar]