Abstract
Objective
To determine the real-world use of pharmacotherapy with new evidence-based cardiovascular indications in an academic Preventive Cardiology Clinic.
Methods
A retrospective study of patients seen in our Center for Preventive Cardiology (CPC) and who received a new prescription, according to Food and Drug Administration (FDA) approved indications, for one of the following pharmacotherapies with new evidence-based cardiovascular indications from May 2019 to May 2020: proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i), eicosapentaenoic acid (EPA), sodium-glucose cotransporter 2 inhibitors (SGLT2i), and glucagon-like peptide-1 receptor agonists (GLP-1 RA). Treatment endpoints were prescription patterns, medication access, patient out-of-pocket expenses, medication tolerability, and clinical cardiovascular events while on these therapies.
Results
Of the 2390 patients seen in our CPC clinic over the observation period, 532 (22.3%) had already started and 291 (12.2%) were newly initiated on pharmacotherapy with new evidence-based cardiovascular indications with a median treatment duration of 9.1 months. Of these, 291 patients (for a total of 320 separate drug orders) – 93 (29.1%) were prescribed PCSK9i, 131 (40.9%) EPA, 46 (14.4%) SGLT2i, and 50 (15.6%) GLP-1 RA. Nearly 80% of cases required some form of provider intervention post-prescription (authorization, appeal, financial assistance, and/or side effect management). A total of 70% of adult patients with type 2 diabetes on metformin and with an HgbA1C >7% were treated with a SGLT2i and/or GLP-1 RA – either initiated prior to or during the study period. Median monthly drug cost for the total cohort was reduced from $595.00 pre-insurance approval to $70.50 post-insurance approval, to $7.00 post-financial assistance intervention. The medications were well tolerated with any side effect occurring in 28.3%, and discontinuation due to side effects in 5.8% of cases. Clinical cardiovascular events occurred in 2.7%, of which 1.9% was due to ASCVD and 0.8% to hospitalization for heart failure. Differences in medication access, cost, tolerability and clinical cardiovascular events varied widely between the medication classes.
Conclusions
Initiation and management of pharmacotherapy with new evidence-based cardiovascular indications in a real-world setting requires substantial provider intervention, a workflow amenable to a multi-disciplinary approach which allows for high rates of medication access and cost minimization, and low rates of medication side effects and clinical cardiovascular events.
Keywords: Cardiovascular disease, Prevention, Pharmacotherapy, Medication access
Highlights
-
•
Over 12 months, PCSK9i, EPA, SGLT2i, and GLP-1 RA where prescribed in 291 patients.
-
•
Nearly 80% of cases required some form of provider intervention post-prescription.
-
•
Multi-disciplinary efforts reduced median monthly patient cost from $595 to $7.
-
•
Cost was the leading factor for medication non-adherence.
-
•
Drugs with new CV indications were well tolerated with low rates of CV events.
1. Introduction
Cardiovascular disease (CVD) continues to lead the United States and World in morbidity and mortality [1,2]. The once substantial reductions in cardiovascular mortality over the past 50 years are no longer trending down and may even be reversing in some populations [2]. These changes may reflect missed opportunities at every step of the cardiovascular prevention and treatment continuum [2]. One of the tenets of cardiovascular preventive care is access to and willingness to incorporate new evidence-based pharmacotherapies with proven cardio-protective benefit into patient care.
Within the past five years, several Food and Drug Administration (FDA)-approved cardiovascular pharmacotherapy agents have been evaluated in large scale, randomized clinical trials and found to be effective at reducing major adverse cardiovascular events (MACE) when added to standards of care [[10], [11], [12], [13], [14], [15], [16], [3], [4], [5], [6], [7], [8], [9]]. In support of the positive outcomes data, all have received revised FDA approval for their respective cardiovascular indication, as well as endorsement by professional societies and guidelines to prevent or treat cardiovascular disease [[17], [18], [19], [20], [21], [22], [23], [24], [25]]. However, widespread adoption of these impactful therapies has been less than anticipated as a result of barriers to use, including often narrow FDA indications, clinical inertia, insurance denials, and prescription affordability that prevent or delay integration of these disease-modifying and life-improving therapies. The goal of this study was to systematically assess the real-world use of pharmacotherapies with new FDA approved cardiovascular indications in our academic Preventive Cardiology Clinic by evaluating prescription patterns, medication access, patient out-of-pocket expense, mediation tolerability, and clinical cardiovascular events.
2. Methods
This was a retrospective study of patients receiving medical care at the Center for Preventive Cardiology (CPC) at Oregon Health & Science University (OHSU). This study was approved by our Institutional Review Board (IRB #0021792). Adult patients from the CPC were included in the analysis if they received a new prescription for an agent from one of the following classes for the first time between May 2019 and May 2020: 1. Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i, alirocumab or evolocumab); 2. eicosapentaenoic acid (EPA, icosapent ethyl); 3. sodium-glucose cotransporter 2 inhibitors (SGLT2i, canagliflozin, dapagliflozin, empagliflozin, or ertugliflozin); and/or 4. glucagon-like peptide-1 receptor agonists (GLP-1 RA, dulaglutide, exenatide, liraglutide, lixisenatide, or semaglutide). Albiglutide use was not evaluated as this was removed from the market in May 2018. These medication classes were chosen for this analysis because all have randomized control trial data demonstrating cardiovascular benefits, as well as FDA approval for cardiovascular indications, guideline recommendations for their use in cardiovascular prevention, and often, insurance coverage for their use as cardio-protective agents.
Our care delivery model utilizes a multidisciplinary approach, relying on patient-centered management with coordinated contributions to care from physicians, advanced practice providers, a registered nurse, a clinical pharmacist, a dietitian, and medical assistants [26]. Patients prescribed PCSK9i in our CPC clinic agree to enter a structured protocol that consists of clinic visits prior to PCSK9i initiation and every 6 months while on therapy, with plasma samples (for lipid panel, lipoprotein (a) [Lp(a)], and PCSK9 levels) obtained at baseline and post-PCSK9i initiation, within 5 days after an injection, at 1, 6, and 12 months [26,27]. Patients prescribed EPA, SGLT2i and GLP-1 RA are managed according to our clinics standard of care, with follow up and laboratory assessments based on each patient’s needs and preferences.
Data was obtained through chart review of our electronic medical record (EMR). Baseline parameters included demographics, vitals, health status, laboratory parameters, and cardiovascular medications. Treatment endpoints included measures of medication access, cost, tolerability, and occurrence of clinical cardiovascular events (atherosclerotic cardiovascular disease [ASCVD], hospitalization for heart failure, and cardiovascular death) while on pharmacotherapy with new evidence-based cardiovascular indications. A separate endpoint was evaluated specifically for the SGLT2i and GLP-1 RA as a quality metric to gauge the CPC’s adherence to national guideline recommendations [18,19]. This metric consisted of the percent of adult patients with type 2 diabetes on metformin and with a hemoglobin A1C (HgbA1C) > 7% who were prescribed SGLT2i and/or GLP-1 RA. Data collection and follow up duration commenced in May 2019 and terminated September 2020.
3. Results
3.1. Baseline demographics
Over the course of 12 months, 2390 patients were seen in the CPC clinic, of whom 532 (22.3%) were treated with one or more of the pharmacotherapy with new evidence-based cardiovascular indications. Of these, 291 patients (for a total of 320 separate drug orders) met our inclusion criteria – 93 (29.1%) were prescribed PCSK9i, 131 (40.9%) EPA, 46 (14.4%) SGLT2i, and 50 (15.6%) GLP-1 RA (Table 1). Approximately 10% of our patients were initiated on more than one of these agents. Indications for prescribing the cardio-protective agents varied widely but followed FDA labeled indications which included: low-density lipoprotein cholesterol (LDL-C) lowering, triglyceride lowering, glucose lowering, weight loss, ASCVD prevention and treatment and/or heart failure prevention and treatment (Table 2). For the 258 cases in whom pharmacotherapy with new evidence-based cardiovascular indications was prescribed and initiated, the median treatment duration was 9.1 months. The average age in our cohort was 60.3 years, 45.3% of individuals were women, 76.3% had established ASCVD (mainly coronary artery disease) and 45.9% had diabetes (Table 1). Nearly all patients had some form of dyslipidemia (close to 25% had familial hypercholesterolemia), and 45–70% had hypertension, obesity, or family history of ASCVD. Rates of tobacco use (4.4%), heart failure (15%) and chronic kidney disease (13.4%) were low. Median blood pressure was 132/73 mmHg, weight 90.6 kg, and body mass index (BMI) 31.1 kg/m2 (Table 1). Baseline (prior to addition of study medication) LDL-C level was 71 mg/dL, Lp(a) 20 mg/dL, triglycerides 177 mg/dL, high-density lipoprotein cholesterol (HDL-C) 43 mg/dL, and HgbA1C 6.0% (Table 1). Baseline cardiovascular medication use included aspirin 66.6%, statins 61.6% (nearly all moderate-high intensity), ezetimibe 38.8%, metformin 32.2%, and antihypertensives 69.1% (with a mean of 2.1 agents used). In addition, 35% were already established on one or more pharmacotherapies with new evidence-based cardiovascular indications (mean of 1.1 agents used) prior to the addition of a new agent during the study period (Table 1).
Table 1.
Baseline characteristics.
| Variable | Combined Cohort | PCSK9i Cohort |
EPA Cohort | SGLT2i Cohort | GLP-1 RA Cohort |
|---|---|---|---|---|---|
| N | 320 | 93 | 131 | 46 | 50 |
| Age (mean ± SD) | 60.3 ± 12.9 | 60.3 ± 12.4 | 60.8 ± 12.2 | 60.5 ± 12.6 | 57 ± 14 |
| Female sex, N (%) | 145 (45.3) | 46 (49.5) | 50 (38.2) | 20 (43.5) | 29 (58) |
| ASCVD, N (%) | 244 (76.3) | 84 (90.3) | 112 (85.5) | 24 (52.2) | 24 (48) |
| CAD, N (%) | 224 (70) | 79 (84.9) | 102 (77.9) | 21 (45.7) | 22 (44) |
| CVD, N (%) | 22 (6.9) | 7 (7.5) | 10 (7.6) | 2 (4.3) | 3 (6) |
| PAD, N (%) | 63 (19.7) | 19 (20.4) | 33 (25.2) | 7 (15.2) | 4 (8) |
| Polyvascular, N (%) | 56 (17.5) | 17 (18.3) | 29 (22.1) | 6 (13) | 4 (8) |
| ASCVD risk factors, N (%) | |||||
| Hyperlipidemia | 317 (99) | 93 (100) | 131 (100) | 45 (97.8) | 48 (96) |
| FH | 77 (24) | 43 (46.2) | 29 (22.1) | 2 (4.3) | 3 (6) |
| Hypertension | 214 (66.9) | 46 (49.5) | 100 (76.3) | 35 (76.1) | 33 (66) |
| Diabetes | 147 (45.9) | 15 (16.1) | 48 (36.6) | 43 (93.5) | 41 (82) |
| Obesity | 193 (60.3) | 33 (35.5) | 82 (62.6) | 38 (82.6) | 40 (80) |
| Current tobacco use | 14 (4.4) | 4 (4.3) | 4 (3.1) | 4 (8.7) | 2 (4) |
| Family history ASCVD | 229 (71.6) | 79 (84.9) | 104 (79.4) | 22 (47.8) | 24 (48) |
| Heart failure | 48 (15) | 10 (10.8) | 16 (12.2) | 14 (30.4) | 8 (16) |
| Reduced EF | 20 (6.3) | 3 (3.2) | 7 (5.3) | 8 (17.4) | 2 (4) |
| Preserved EF | 28 (8.9) | 7 (7.5) | 9 (6.9) | 6 (13) | 6 (12) |
| Chronic kidney diseasea | 43 (13.4) | 11 (11.8) | 18 (13.7) | 11 (23.9) | 3 (6) |
| Vitals at baseline (median [IQR]) | |||||
| Systolic blood pressure (mmHg) | 132 (122–143) | 128 (117–136) | 135 (125–146) | 132 (123–144) | 134 (126–143) |
| Diastolic blood pressure (mmHg) | 73 (64–81) | 68 (63–79) | 74 (66–83.8) | 74 (67–80) | 72 (65–79) |
| Weight (kg) | 90.6 (79.9–106.8) | 81.4 (71.2–97.8) | 91.8 (81–103.2) | 93.4 (81.2–120.4) | 105.4 (87.7–123.9) |
| BMI (kg/m2) | 31.1 (27.2–35.9) | 27.8 (25–32.9) | 30.9 (27.5–33.9) | 32.9 (29–38.7) | 36.8 (31.8–42.3) |
| Laboratory parameters at baseline (median [IQR]) | |||||
| LDL-C (mg/dL) | 71 (43–101) | 101 (81–105) | 67.5 (42–101) | 72 (46–92) | 74 (51–101) |
| Lp(a) (mg/dL) | 20 (7–77) | 38 (12–90) | 17 (6–77.8) | 15.5 (8–33) | 10 (6–33) |
| Triglyceride (mg/dL) | 177 (112–265) | 112 (84–163) | 217 (159–306) | 170 (117–239) | 208 (126–308) |
| HDL-C (mg/dL) | 43 (37–53) | 50 (41–58) | 42 (36–52) | 41 (32–47) | 41 (35–47) |
| HgbA1C (%) | 6 (5.5–6.8) | 5.6 (5.3–6) | 5.8 (5.5–6.3) | 7 (6.4–8.5) | 6.8 (6.1–7.9) |
| Baseline cardiovascular therapies, N (%) | |||||
| Aspirin | 213 (66.6) | 62 (66.7) | 94 (71.8) | 33 (71.7) | 24 (48) |
| Statins | 197 (61.6) | 49 (52.7) | 78 (59.5) | 37 (80.4) | 33 (66) |
| High-intensity | 124 (38.8) | 31 (33.3) | 49 (37.4) | 24 (52.2) | 20 (40) |
| Moderate-intensity | 65 (20.3) | 12 (12.9) | 28 (21.4) | 12 (26.1) | 13 (26) |
| Low-intensity | 8 (2.5) | 6 (6.5) | 1 (0.8) | 1 (2.2) | 0 (0) |
| Ezetimibe | 124 (38.8) | 59 (63.4) | 51 (38.9) | 8 (17.4) | 6 (12) |
| Metformin | 103 (32.2) | 7 (7.5) | 35 (26.7) | 26 (56.5) | 35 (70) |
| Antihypertensive | 221 (69.1) | 53 (57) | 95 (72.5) | 38 (82.6) | 35 (70) |
| 0 | 99 (30.9) | 40 (43) | 36 (27.4) | 8 (17.4) | 15 (30) |
| 1 | 85 (26.6) | 24 (25.8) | 35 (26.7) | 10 (21.7) | 16 (32) |
| 2 | 61 (19.1) | 13 (14) | 26 (19.8) | 12 (26.1) | 10 (20) |
| 3 | 50 (15.6) | 13 (14) | 23 (17.6) | 9 (19.6) | 5 (10) |
| 4 | 17 (5.3) | 3 (3.2) | 7 (5.3) | 5 (10.9) | 2 (4) |
| 5 | 8 (2.5) | 0 (0) | 4 (3.1) | 2 (4.3) | 2 (4) |
| Pharmacotherapy with new evidence-based CV indications | 112 (35) | 7 (7.5) | 66 (50.4) | 19 (41.3) | 20 (40) |
| 0 | 208 (65) | 86 (92.5) | 65 (49.6) | 27 (58.7) | 30 (60) |
| 1 | 98 (30.6) | 6 (6.5) | 58 (44.3) | 17 (37) | 17 (34) |
| 2 | 14 (4.4) | 1 (1.1) | 8 (6.1) | 2 (4.3) | 3 (6) |
ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CAD, coronary artery disease; CV, cardiovascular disease; CVD, cerebrovascular disease; EF, ejection fraction; EPA, eicosapentaenoic acid; FH, familial hypercholesterolemia; GLP-1 RA, glucagon-like peptide-1 receptor agonists; HgbA1C, hemoglobin A1C; HDL-C, high-density lipoprotein cholesterol IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); N, number; PAD, peripheral artery disease; PCSK9i, protein convertase subtilisin/kexin type 9 inhibitors; SD, standard deviation; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
Stage 3-5 CKD, eGRF <60 ml/min.
Table 2.
Indications for Prescribing Pharmacotherapy with New Evidence-Based Cardiovascular Indications in our Center for Preventive Cardiology.
| Indication | PCSK9i | EPA | SGLT2i | GLP-1 RA |
|---|---|---|---|---|
| LDL-C lowering | ✓ | |||
| Triglyceride lowering | ✓ | |||
| Glucose lowering | ✓ | ✓ | ||
| Weight loss | ✓ | ✓ | ||
| ASCVD | ✓ | ✓ | ✓ | ✓ |
| HFrEF | ✓ |
ASCVD, atherosclerotic cardiovascular disease; HFrEF, heart failure with reduced ejection fraction; EPA, eicosapentaenoic acid; GLP-1 RA, glucagon-like peptide-1 receptor agonists; LDL-C, low-density lipoprotein cholesterol; PCSK9i, protein convertase subtilisin/kexin type 9 inhibitors; SGLT2i, sodium-glucose cotransporter 2 inhibitors.
A higher burden of atherosclerosis, lipid abnormalities, statin intolerance, and ezetimibe use at baseline was seen in individuals initiated on PCSK9i and/or EPA. Persons on SGLT2i and/or GLP-1 RA had higher rates of diabetes, higher HgbA1C levels, more metformin use, and higher rates of obesity. Persons on SGTL2i displayed the highest baseline rates of heart failure and chronic kidney disease. Persons on GLP-1 RA had the highest initial weight and BMI values. Persons initiated on treatment with EPA, SGLT2i, or GLP-1 RA were more likely to have been treated with a pharmacotherapy with new evidence-based cardiovascular indications at baseline (prior to the study period) compared to those who initiated treatment with a PCSK9i.
3.2. Medication access
For the total cohort, access to these medications required prior authorizations in 64.7% of cases and subsequent insurance appeals in 17.5% (Table 3). The median (IQR) time to approval was 2 (0–10.8) days and time to medication start was 12 days [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]]. Financial assistance in the form of copay cards and patient assistance programs were successfully utilized in 27.8% and 9.4% of patients, respectively. In nearly three quarters of cases, some form of provider intervention was required (prior authorization submission, appeal, and/or utilization of financial assistance resources) (Fig. 1). In total, 75.9% of cases were initiated and continued on the new cardiovascular pharmacotherapy, with the most common reason for non-adherence being cost (15.9%).
Table 3.
Medication access, tolerability, and outcomes.
| Variable | Combined Cohort | PCSK9i Cohort |
EPA Cohort | SGLT2i Cohort | GLP-1 RA Cohort |
|---|---|---|---|---|---|
| N | 320 | 93 | 131 | 46 | 50 |
| Taking the medication, N (%) | 243 (75.9) | 87 (93.5) | 87 (66.4) | 34 (73.9) | 35 (70) |
| Reason for not taking, N (%) | |||||
| Cost | 51 (15.9) | 2 (2.2) | 36 (27.5) | 2 (4.3) | 11 (22) |
| Side effects | 15 (4.7) | 2 (2.2) | 6 (4.6) | 4 (8.7) | 3 (6) |
| Patient preference | 9 (2.8) | 2 (2.2) | 2 (1.5) | 4 (8.7) | 1 (2) |
| Discontinued by provider | 2 (0.6) | 0 (0) | 0 (0) | 2 (4.3) | 0 (0) |
| Insurance type, N (%) | |||||
| Commercial | 154 (48.1) | 53 (57) | 64 (48.9) | 16 (34.8) | 21 (42) |
| Medicare | 113 (35.3) | 30 (32.3) | 48 (36.6) | 16 (34.8) | 19 (38) |
| Medicaid | 40 (12.5) | 7 (7.5) | 11 (8.4) | 12 (26.1) | 10 (20) |
| VA | 9 (2.8) | 1 (1.1) | 6 (4.6) | 2 (4.3) | 0 (0) |
| Uninsured | 4 (1.3) | 2 (2.2) | 2 (1.5) | 0 (0) | 0 (0) |
| Prior authorization, N (%) | 207 (64.7) | 90 (96.8) | 75 (57.3) | 20 (43.5) | 22 (44) |
| Appeals, N (%) | 56 (17.5) | 16 (17.2) | 26 (19.8) | 5 (10.9) | 9 (18) |
| Time to approval, days (median [IQR]) | 2 (0–10.8) | 5 (2–9.5) | 2 (0–14) | 1 (0–6) | 0 (0–14.5) |
| Medication cost, $ (median [IQR]) | |||||
| Drug cost | 595 (400–600) | 595 (595–595) | 400 (392–400) | 600 (600–619.3) | 980 (953–997) |
| Insurance copay | 70.5 (8–193.3) | 93 (30–180) | 88 (17.5–310) | 9.5 (0–86.8) | 41 (0–266.8) |
| Patient out-of-pocket cost | 7 (0–81) | 3.8 (0–5) | 9 (2.3–164) | 0 (0–28.9) | 25 (0–155) |
| Financial assistance, N (%) | |||||
| Copay card | 89 (27.8) | 41 (44.1) | 35 (26.7) | 5 (10.9) | 8 (16) |
| Patient assistance program | 30 (9.4) | 13 (14) | 14 (10.7) | 1 (2.2) | 2 (4) |
| Any insurance or cost intervention, N (%) | 236 (73.8) | 93 (100) | 93 (71) | 23 (50) | 27 (54) |
| Side effects, N (%)a | 73 (28.3) | 37 (41.6) | 12 (12.9) | 9 (23.7) | 15 (39.5) |
| Discontinued due to side effects, N (%)a | 15 (5.8) | 2 (2.2) | 6 (6.5) | 4 (10.5) | 3 (7.9) |
| Any insurance, cost, or side effect intervention, N (%) | 250 (78.1) | 93 (100) | 94 (71.8) | 28 (60.9) | 35 (70) |
| CV event on therapy, N (%)a | 7 (2.7) | 4 (4.5) | 0 (0) | 3 (7.9) | 0 (0) |
| ASCVD | 5 (1.9) | 4 (4.5) | 0 (0) | 1 (2.6) | 0 (0) |
| HHF | 2 (0.8) | 0 (0) | 0 (0) | 2 (5.3) | 0 (0) |
| CV death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
ASCVD, atherosclerotic cardiovascular disease; CV, cardiovascular; EPA, eicosapentaenoic acid; GLP-1 RA, glucagon-like peptide-1 receptor agonists; HHF, hospitalization for heart failure; IQR, interquartile range; PCSK9i, protein convertase subtilisin/kexin type 9 inhibitors; SGLT2i, sodium-glucose cotransporter 2 inhibitors; VA, veterans administration.
To account for patients who initiated therapy, N is out of 258, 89, 93, 38, and 38 for combined, PCSK9i, EPA, SGLT2i, and GLP-1 RA cohorts respectively.
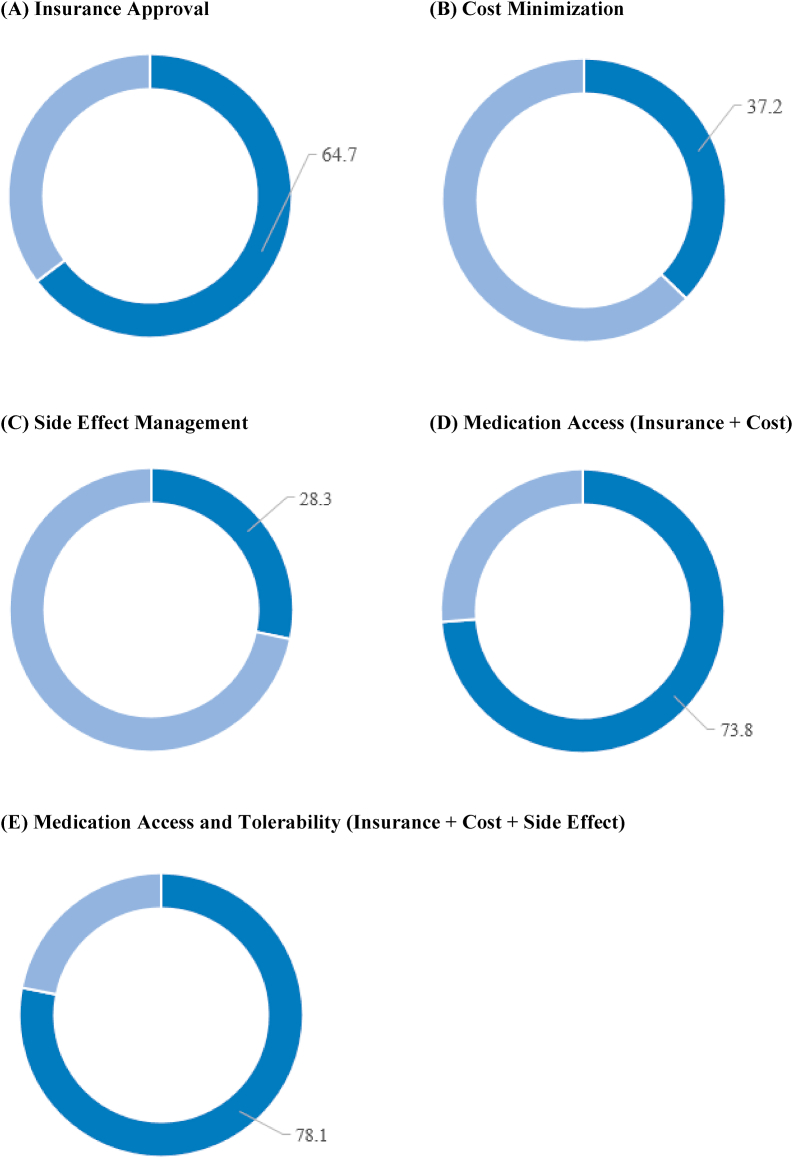
Fig. 1.
Percent of Patients Requiring Provider Intervention for Management of Pharmacotherapies with New Evidence-Based Cardiovascular Indications.
Figures A-E demonstrate the percent of patients requiring provider interaction to obtain or tolerate pharmacotherapy with new evidence-based cardiovascular indications. Dark blue = patients requiring provider intervention; light blue = patients not requiring intervention. Figure A – 64.7% of patients required insurance approval to obtain the medication. Figure B – 37.2% of patients required cost minimization strategies to obtain afford the medication. Figure C – 28.3% of patients required side effect management. Figure D – 73.8% of patients required an intervention for medication access (insurance approval or cost minimization). Figure E − 78.1% of patients required an intervention for medication access and tolerability (insurance approval, cost minimization, or side effect management).
Medication accessibility and adherence varied among the cardio-protective medication classes. Individuals treated with PCSK9i had the highest adherence to therapy (93.5%), with low rates of discontinuation due to cost (2.2%) or side effects (2.2%). The PCSK9i cohort also had the longest time to approval (5 days) and required the most resources, with nearly a 2-fold higher requirement for insurance authorizations compared to the other agents (96.8% vs 44–57%), and the highest need for provider intervention (100%). The EPA cohort had the highest rate of treatment non-adherence (33.6%), followed by GLP-1 RA (30%) and SGLT2i (26.1%), mostly due to the cost factors described below. The EPA cohort required moderate resource utilization, with prior authorizations in 57.3% and any provider intervention in 71% of patients. Both the SGLT2i and GLP-1 RA cohort had a lower utilization of resources, with prior authorizations required in 43.5% and 44% and any provider intervention in 50% and 54% respectively. Finally, a total of 70% of individuals with type 2 diabetes on metformin and HgbA1C >7% were prescribed SGLT2i and/or GLP-1 RA.
3.3. Medication cost
Median monthly drug cost for the total cohort was reduced from $595 pre-insurance approval to $70 post-insurance approval to $7 post-financial assistance intervention (Table 3). Wide variations in medication costs were seen between drug classes. EPA was the least expensive drug therapy ($400 per month prior to insurance authorization) yet had the lowest rate of treatment adherence (66.4%) and highest rate of non-adherence due to cost, accounting for 70% of all non-adherent cases due to cost. The SGLT2i cohort had the lowest insurance copay ($9.5 per month) and patient out-of-pocket cost (zero dollars per month), largely owing to a preponderance of Medicaid recipients (26.1%). The GLP-1 RA was the most expensive drug category at $980 per month without insurance authorization, and $41 per month after insurance approval, with a wide distribution (IQR: $0-$267 per month). The lower copay was likely due the large contribution of Medicaid insurance in our cohort. However, non-adherence due to cost was still elevated at 22%.
3.4. Medication tolerability
These cardiovascular pharmacotherapies were well tolerated with side effects occurring in just over a quarter of the total cohort, and medication discontinuation a result of side effects occurring in only 5.8% of cases (Table 3). EPA therapy was the best tolerated, with side effects occurring in 12.9%. Parenteral therapies (PCSK9i and GLP-1 RA) pooled had higher rates of side effects compared with the other medications, 40.9% versus 16.4%, respectively. Despite the higher rate of side effects with parenteral agents, there were no discernible differences in discontinuation rates among parenteral versus oral therapies, implying that most side-effects were mild and/or transient. Accounting for side effect management, 78.1% of patients required some form of provider intervention for medication access and/or tolerability (authorization, appeal, financial assistance, and/or side effect management) (Fig. 1).
3.5. Clinical cardiovascular events
Clinical cardiovascular events were rare, occurring in only 2.7% of patients over a median treatment duration of 9.1 months, consisting of 1.9% ASCVD, 0.8% hospitalization for heart failure, and zero cardiovascular deaths (Table 3). This event rate corresponds to an annualized event rate of approximately 3.6%. Persons on either EPA or GLP-1 RA therapies experienced no clinical cardiovascular events over the follow up period. The SGLT2i cohort had the most frequent occurrence of clinical cardiovascular events (7.9%), largely driven by hospitalizations for heart failure (5.3%), which reflected the higher rate of heart failure at baseline in this cohort. Of the 88 patients successfully initiated on more than one pharmacotherapy with new evidence-based cardiovascular indications, only one experienced a clinical cardiovascular event (1.1%) as compared to six events in the group of 170 patients initiating only one newer agent.
4. Discussion
We described a one-year experience with standardized use of cardio-protective medications in patients with recent FDA indications to receive such therapies who were seen in our academic Center for Preventive Cardiology. The real-world initiation of new evidence-based and guideline-endorsed cardiovascular pharmacotherapy was substantial, well tolerated, and overall affordable to patients, but required expert and protocol-based intervention from our clinical care team and support staff. Nearly 25% of patients were initiated on these cardio-protective medications, and 75% of these effectively started, continued, and tolerated therapy. The rapid adoption and high utilization of these therapies is one of several areas of opportunity listed by the recent American Heart Association Presidential Advisory for a call to action to address urgent challenges in cardiovascular disease [2]. Our study results demonstrate a substantial improvement in this metric when evaluating prescribing patterns of the novel antidiabetic agents, SGLT2i and/or GLP-1 RA. In a report by Vaduganathan et al., in 2017, they highlighted low utilization of SGTL2i use despite clinical trial evidence suggesting cardiovascular disease benefit [28]. They also identified cardiologists as the least likely prescriber, accounting for only 5% of SGLT2i and 4.5% of GLP- RA prescriptions [28]. In contrast, our cardiology-based CPC that includes endocrinologists has integrated the use of SGLT2i and GLP-1 RA agents into standard-of-care practices for our patient population, thus far coopting 70% of eligible patients. Though substantial work is still needed to optimize use of cardio-protective antidiabetic agents in at risk populations, our data suggest that steps are being made towards achieving this goal.
The enhanced emphasis on use of pharmacotherapy with new evidence-based cardiovascular indications in our CPC was associated with increased workload for providers and support staff, which went above and beyond standard duties in the outpatient setting. Nearly 80% of cases involving these agents required some provider intervention (prior authorization, appeal, cost savings initiative, or side effect management) for medication initiation and maintenance of therapy, with the vast majority of interventions (73.8%) tied to medication access. This is a vital point, one which highlights the important notion that the providers duties do not cease with signing the prescription. This workflow is most amenable to a multi-disciplinary team approach, leveraging the assistance and expertise of the various healthcare team members which in our case consist of physicians, advanced practice providers, a registered nurse, a clinical pharmacist, a dietitian, medical assistants, and clerical staff [29,30].
As with any branded medication, cost considerations are critical for optimal utilization of these cardio-protective therapies. Concerns over barriers to medication access and cost emerged in contemporary cardiovascular disease management with the use of PCSK9i therapy [[31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. Our group’s previous analyses in this area delineated how a multi-disciplinary team approach that leveraged a formalized and structured protocol for PCSK9i initiation significantly reduced patient cost as a barrier to appropriate patient management [26,27,41]. In the current study, cost to the patient was reduced nearly 80-fold, from a median price of $595 per month to $7 per month with insurance approval and application of financial assistance resources. It has been documented that medication non-adherence significantly rises when patient out-of-pocket expense is >$50 per month [[42], [43], [44]]. However, cost was still the leading cause for patients not adhering to our recommendations, occurring in 15.9% of cases though varying substantially by drug class. PCSK9i had the lowest rates of cost-associated medication non-adherence (2.2%) and EPA was the highest (27.5%). Explanations for this difference are related to 1) internal protocols for drug prescribing in our clinic, 2) insurance approval criteria, and 3) access to financial assistance programs. First, only PCSK9i initiation followed a structured protocol in our clinic during the study period. The differences in clinic structure and resources devoted to patients on the structured protocol for PCSK9i compared to other drugs likely contributed to differences in cost containment. Second, insurance approval criteria were most stringent for EPA as the therapy did not obtain FDA cardiovascular indications until December 2019, a full 7 months into our study. Prior to FDA approval, insurance approval for cardiovascular risk reduction was challenging. In addition, many insurance companies inappropriately required patients to use an elusive “over-the-counter equivalent” in place of the prescription-grade and evidence-based EPA therapy. This scenario is not uncommon and requires many levels of appeals and a lapse of weeks to months to get the appropriate EPA therapy approved. These challenges with obtaining approval for EPA still persist despite the new FDA indication for ASCVD [45]. Third, many insurers “cover” the pharmacotherapy with new evidence-based cardiovascular indications, but classify them as “specialty medications” or non-formulary medications, placing them on high-tiered copay structures. Many insurers require a co-insurance payment instead of a copay, which shifts the high medication cost burden to the patient, an act that is particularly problematic for Medicare patients as they, by law, have less access to financial assistance resources (i.e., copay or discount cards). Though all pharmacotherapies with new evidence-based cardiovascular indications provide financial assistance programs (i.e., copay cards and/or patient assistance programs), there are differences in income requirements and the need for patients to have spent a certain amount of out-of-pocket dollars to qualify. These programs are most accommodating for PCSK9i and less so for the others. As cost remains a significant barrier for the cardiovascular patient, accounting for non-adherence in 1 of 8 Americans [46], workflows and best practices aimed at minimizing cost are vital for patients to realize the full therapeutic benefit of these risk reducing therapies.
Pharmacotherapies with new evidence-based cardiovascular indications were well tolerated in our cohort, with adverse drug events reported in less than a quarter of patients, most of which were mild and self-limiting. Adverse drug events were 2 to 3-fold higher with the parenteral agents (PCSK9i and GLP-1 RA), primarily consisting of local injection site reactions (i.e., pain, rash) or cold/flu-like syndrome (i.e, muscle aches, malaise, nasopharyngitis) primarily with PCSK9i and gastrointestinal symptoms with GLP-1 RA. Medication discontinuation due to adverse drug events was low at 5.8%.
Although our study does not have sufficient numbers of patients or duration of follow-up to assess changes in rates of CV events, our patients on cardio-protective pharmacotherapy displayed a low occurrence of MACE, overall only 2.7% over 9.1 months of median follow up for an annualized event rate of approximately 3.6%. Not surprisingly, the highest rate of MACE was seen in the PCSK9i cohort, due to pre-existing ASCVD (90.3%), familial hypercholesterolemia (46.2%), and statin intolerance (47.3%). Heart failure events occurred most commonly in the SGLT2i cohort (5.3%), which was expected given this group of patients had 2–3 fold higher prevalence of heart failure (30%), and chronic kidney disease (23.9%) comorbidities at baseline. To our knowledge, this is the first study to compare MACE outcomes in a real-world setting across classes of pharmacotherapy with new evidence-based cardiovascular indications. Our extrapolated annual event rate of 3.6%, is equal to or lower than the annualized event rates observed in cardiovascular outcome trials for PCSK9i (3.4–4.5% on treatment versus 4–5.1% on placebo) [7,10], EPA (3.5% on treatment versus 4.5% on placebo) [12], SGL2Ti in diabetes population (2.1–3.4% on treatment versus 2.2–4% on placebo) [4,8,13], SGLT2i in a heart failure population (6.6–9.9% on treatment versus 13.7–14% on placebo) [15,16], and GLP-1 RA (2.2–6.4% on treatment versus 2.5–6.3% on placebo) [5,6,11,14,47,48].
Strengths of this study include a large cohort of well-characterized patients initiated on pharmacotherapy with new evidence-based cardiovascular indications, including the PCSK9i group within an IRB-approved structured protocol. The validity of our data is driven by the systematic collection and documentation of clinical events, laboratory measures and vitals, both at baseline and those accrued during follow up, as well as medication access, cost, and tolerability evaluated routinely and longitudinally for patients seen within our practice. This study documenting our experience is also the first demonstration of how to integrate the quickly expanding set of cardiovascular agents into clinical practice – applying data from large cardiovascular outcome trials, traversing the issue of medication access in today’s healthcare landscape and applying it to high-risk cardiovascular patients in practice and closely monitoring for medication tolerability and clinical outcomes. The overarching message of this study is that it is possible to overcome the barriers to implementing pharmacotherapy with new evidence-based cardiovascular indications. Three core components are necessary to improve attainment of this goal, which include: 1) overcoming clinical inertia, 2) having a structured, protocol-based approach to pharmacotherapy screening, initiation, and follow up, and 3) utilizing a multi-disciplinary care team to deliver on these actionable items.
Limitations of this study are inherent to its single-center, retrospective nature, and real-world data design. Additionally, our selection of cardiovascular pharmacotherapies was not all inclusive, as we did not examine use of newer antithrombotic and anticoagulation agents such as ticagrelor and rivaroxaban. Another limitation pertains to event rate capture, since our patient population comes from across the region and the country, tabulation of MACE outcomes is challenging. There is the possibility of failing to account for MACE outcomes due to patients not divulging events during follow up or not being treated within our healthcare system network of interconnected EMRs. Though we captured MACE outcomes in those initiating newer pharmacotherapies (N = 291), we did not evaluate such endpoints in those who did not initiate newer pharmacotherapies (N = 2099), precluding any determination regarding event rate reduction with use of newer agents. Finally, we did not assess initiation or intensification of background cardiovascular pharmacotherapies (i.e., statins, antihypertensive, etc) once the study commenced. Although changes in background cardiovascular pharmacotherapies may have occurred during the observation period, it is unlikely as this task is generally optimized prior to initiation of newer pharmacotherapies.
5. Conclusions
We describe the real-world use of pharmacotherapy with new evidence-based cardiovascular indications among a widely diverse group of patients with established ASCVD or very high risk for developing cardiovascular disease. Incorporating in practice the routine prescribing of these agents in line with guideline recommendations requires a multi-disciplinary, patient-centered, structured approach. In the majority of these patients, a prescription for these cardiovascular pharmacotherapy agents necessitated additional healthcare team involvement to obtain insurance authorization and cost minimization for the patient. Despite best efforts by our team, a quarter of patients were unable to adhere to the medications long term, mainly due to excessive medication costs. These medications were well tolerated with most side effects mild and self-limiting, with very few discontinuing due to adverse events. Similar to results from cardiovascular outcome trials, few clinical cardiovascular events occurred when patients were established on these pharmacotherapies with new evidence-based cardiovascular indications. The clinic model and experience we have described herein may help guide increased use of these agents in other clinical settings.
Disclosures
BAW reports institutional grant from Akcea.
PBD reports advisory work for Esperion, Regeneron, RegenxBio, and Sanofi. Institutional grants from Regeneron, RegenxBio, and Retrophin.
JQP reports clinical trial work with Amgen, Novartis, and Akcea.
SF reports advisory work for Kowa, Amgen, Novo Nordisk, Astra Zeneca and Amarin.
The other authors have no disclosures to report.
Authors’ contributions
BAW and SF conceived the study, BAW designed the study, performed the data collection and drafted the manuscript. All authors contributed to patient care procedures and to the writing and/or editing of the manuscript and have read and approved the final version of the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Acknowledgments
Gray Winkler for his assistance with data acquisition.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Shay C.M., Spartano N.L., Stokes A., Tirschwell D.L., VanWagner L.B., Tsao C.W. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.McClellan M., Brown N., Califf R.M., Warner J.J. Call to action: urgent challenges in cardiovascular disease: a presidential advisory from the American heart association. Circulation. 2019;139:e44–e54. doi: 10.1161/CIR.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 3.Bonaca M.P., Bhatt D.L., Cohen M., Steg P.G., Storey R.F., Jensen E.C., Magnani G., Bansilal S., Fish M.P., Im K., Bengtsson O., Oude Ophuis T., Budaj A., Theroux P., Ruda M., Hamm C., Goto S., Spinar J., Nicolau J.C., Kiss R.G., Murphy S.A., Wiviott S.D., Held P., Braunwald E., Sabatine M.S. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 4.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., Broedl U.C., Inzucchi S.E. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 5.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A., Nissen S.E., Pocock S., Poulter N.R., Ravn L.S., Steinberg W.M., Stockner M., Zinman B., Bergenstal R.M., Buse J.B. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jódar E., Leiter L.A., Lingvay I., Rosenstock J., Seufert J., Warren M.L., Woo V., Hansen O., Holst A.G., Pettersson J., Vilsbøll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A., Kuder J.F., Wang H., Liu T., Wasserman S.M., Sever P.S., Pedersen T.R. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 8.Neal B., Perkovic V., Mahaffey K.W., de Zeeuw D., Fulcher G., Erondu N., Shaw W., Law G., Desai M., Matthews D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 9.Eikelboom J.W., Connolly S.J., Bosch J., Dagenais G.R., Hart R.G., Shestakovska O., Diaz R., Alings M., Lonn E.M., Anand S.S., Widimsky P., Hori M., Avezum A., Piegas L.S., Branch K.R.H., Probstfield J., Bhatt D.L., Zhu J., Liang Y., Maggioni A.P., Lopez-Jaramillo P., O’Donnell M., Kakkar A.K., Fox K.A.A., Parkhomenko A.N., Ertl G., Störk S., Keltai M., Ryden L., Pogosova N., Dans A.L., Lanas F., Commerford P.J., Torp-Pedersen C., Guzik T.J., Verhamme P.B., Vinereanu D., Kim J.H., Tonkin A.M., Lewis B.S., Felix C., Yusoff K., Steg P.G., Metsarinne K.P., Cook Bruns N., Misselwitz F., Chen E., Leong D., Yusuf S. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz G.G., Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Diaz R., Edelberg J.M., Goodman S.G., Hanotin C., Harrington R.A., Jukema J.W., Lecorps G., Mahaffey K.W., Moryusef A., Pordy R., Quintero K., Roe M.T., Sasiela W.J., Tamby J.F., Tricoci P., White H.D., Zeiher A.M. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez A.F., Green J.B., Janmohamed S., D’Agostino R.B., Sr., Granger C.B., Jones N.P., Leiter L.A., Rosenberg A.E., Sigmon K.N., Somerville M.C., Thorpe K.M., McMurray J.J.V., Del Prato S. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Jr., Juliano R.A., Jiao L., Granowitz C., Tardif J.C., Ballantyne C.M. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 13.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A., Silverman M.G., Zelniker T.A., Kuder J.F., Murphy S.A., Bhatt D.L., Leiter L.A., McGuire D.K., Wilding J.P.H., Ruff C.T., Gause-Nilsson I.A.M., Fredriksson M., Johansson P.A., Langkilde A.M., Sabatine M.S. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 14.Gerstein H.C., Colhoun H.M., Dagenais G.R., Diaz R., Lakshmanan M., Pais P., Probstfield J., Riesmeyer J.S., Riddle M.C., Rydén L., Xavier D., Atisso C.M., Dyal L., Hall S., Rao-Melacini P., Wong G., Avezum A., Basile J., Chung N., Conget I., Cushman W.C., Franek E., Hancu N., Hanefeld M., Holt S., Jansky P., Keltai M., Lanas F., Leiter L.A., Lopez-Jaramillo P., Cardona Munoz E.G., Pirags V., Pogosova N., Raubenheimer P.J., Shaw J.E., Sheu W.H., Temelkova-Kurktschiev T. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 15.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Køber L., Kosiborod M.N., Martinez F.A., Ponikowski P., Sabatine M.S., Anand I.S., Bělohlávek J., Böhm M., Chiang C.E., Chopra V.K., de Boer R.A., Desai A.S., Diez M., Drozdz J., Dukát A., Ge J., Howlett J.G., Katova T., Kitakaze M., Ljungman C.E.A., Merkely B., Nicolau J.C., O’Meara E., Petrie M.C., Vinh P.N., Schou M., Tereshchenko S., Verma S., Held C., DeMets D.L., Docherty K.F., Jhund P.S., Bengtsson O., Sjöstrand M., Langkilde A.M. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 16.Packer M., Anker S.D., Butler J., Filippatos G., Pocock S.J., Carson P. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 17.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Davies M.J., D’Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G., Rossing P., Tsapas A., Wexler D.J., Buse J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetologia. 2018;61:2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 19.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., Michos E.D., Miedema M.D., Muñoz D., Smith S.C., Jr., Virani S.S., Williams K.A., Sr., Yeboah J., Ziaeian B. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74:e177–e232. doi: 10.1016/j.jacc.2019.03.010. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orringer C.E., Jacobson T.A., Maki K.C. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J Clin Lipidol. 2019;13:860–872. doi: 10.1016/j.jacl.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S111–s134. doi: 10.2337/dc20-S010. 10. [DOI] [PubMed] [Google Scholar]
- 22.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., Huikuri H.V., Johansson I., Jüni P., Lettino M., Marx N., Mellbin L.G., Östgren C.J., Rocca B., Roffi M., Sattar N., Seferović P.M., Sousa-Uva M., Valensi P., Wheeler D.C. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019;41:255–323. doi: 10.1093/eurheartj/ehz486. 2020. [DOI] [PubMed] [Google Scholar]
- 23.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., Graham I.M., Halliday A., Landmesser U., Mihaylova B., Pedersen T.R., Riccardi G., Richter D.J., Sabatine M.S., Taskinen M.R., Tokgozoglu L., Wiklund O. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455. 2020. [DOI] [PubMed] [Google Scholar]
- 24.O’Meara E., McDonald M., Chan M., Ducharme A., Ezekowitz J.A., Giannetti N., Grzeslo A., Heckman G.A., Howlett J.G., Koshman S.L., Lepage S., Mielniczuk L.M., Moe G.W., Swiggum E., Toma M., Virani S.A., Zieroth S., De S., Matteau S., Parent M.C., Asgar A.W., Cohen G., Fine N., Davis M., Verma S., Cherney D., Abrams H., Al-Hesayen A., Cohen-Solal A., D’Astous M., Delgado D.H., Desplantie O., Estrella-Holder E., Green L., Haddad H., Harkness K., Hernandez A.F., Kouz S., LeBlanc M.H., Lee D., Masoudi F.A., McKelvie R.S., Rajda M., Ross H.J., Sussex B. CCS/CHFS heart failure guidelines: clinical trial update on functional Mitral regurgitation, SGLT2 inhibitors, ARNI in HFpEF, and tafamidis in amyloidosis. Can J Cardiol. 2020;36:159–169. doi: 10.1016/j.cjca.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Das S.R., Everett B.M., Birtcher K.K., Brown J.M., Januzzi J.L., Jr., Kalyani R.R., Kosiborod M., Magwire M., Morris P.B., Neumiller J.J., Sperling L.S. Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2020;76:1117–1145. doi: 10.1016/j.jacc.2020.05.037. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman T.M., Duell P.B., Purnell J.Q., Wojcik C., Fazio S., Shapiro M.D. Application of PCSK9 inhibitors in practice: challenges and opportunities. Circ Res. 2017;121:499–501. doi: 10.1161/CIRCRESAHA.117.311532. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman T.M., Warden B.A., Minnier J., Miles J.R., Duell P.B., Purnell J.Q., Wojcik C., Fazio S., Shapiro M.D. Application of PCSK9 inhibitors in practice. Circ Res. 2019;124:32–37. doi: 10.1161/CIRCRESAHA.118.314191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vardeny O., Vaduganathan M. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Failure. 2019;7:169–172. doi: 10.1016/j.jchf.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro M.D., Fazio S. Setting the agenda for preventive cardiology. Circ Res. 2017;121:211–213. doi: 10.1161/CIRCRESAHA.117.311390. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro M.D., Maron D.J., Morris P.B., Kosiborod M., Sandesara P.B., Virani S.S., Khera A., Ballantyne C.M., Baum S.J., Sperling L.S., Bhatt D.L., Fazio S. Preventive cardiology as a subspecialty of cardiovascular medicine: JACC council perspectives. J Am Coll Cardiol. 2019;74:1926–1942. doi: 10.1016/j.jacc.2019.08.1016. [DOI] [PubMed] [Google Scholar]
- 31.Kazi D.S., Moran A.E., Coxson P.G., Penko J., Ollendorf D.A., Pearson S.D., Tice J.A., Guzman D., Bibbins-Domingo K. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. Jama. 2016;316:743–753. doi: 10.1001/jama.2016.11004. [DOI] [PubMed] [Google Scholar]
- 32.Baum S.J., Toth P.P., Underberg J.A., Jellinger P., Ross J., Wilemon K. PCSK9 inhibitor access barriers-issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40:243–254. doi: 10.1002/clc.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navar A.M., Taylor B., Mulder H., Fievitz E., Monda K.L., Fievitz A., Maya J.F., Lopez J.A.G., Peterson E.D. Association of prior authorization and out-of-pocket costs with patient Access to PCSK9 inhibitor therapy. JAMA Cardiol. 2017;2:1217–1225. doi: 10.1001/jamacardio.2017.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowles J.W., Howard W.B., Karayan L., Baum S.J., Wilemon K.A., Ballantyne C.M., Myers K.D. Access to nonstatin lipid-lowering therapies in patients at high risk of atherosclerotic cardiovascular disease. Circulation. 2017;135:2204–2206. doi: 10.1161/CIRCULATIONAHA.117.027705. [DOI] [PubMed] [Google Scholar]
- 35.Hess G.P., Natarajan P., Faridi K.F., Fievitz A., Valsdottir L., Yeh R.W. Proprotein convertase subtilisin/kexin type 9 inhibitor therapy: payer approvals and rejections, and patient characteristics for successful prescribing. Circulation. 2017;136:2210–2219. doi: 10.1161/CIRCULATIONAHA.117.028430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazi D.S., Penko J., Coxson P.G., Moran A.E., Ollendorf D.A., Tice J.A., Bibbins-Domingo K. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the FOURIER trial. Jama. 2017;318:748–750. doi: 10.1001/jama.2017.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hlatky M.A., Kazi D.S. PCSK9 inhibitors: economics and policy. J Am Coll Cardiol. 2017;70:2677–2687. doi: 10.1016/j.jacc.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Virani S.S., Akeroyd J.M., Nambi V., Heidenreich P.A., Morris P.B., Nasir K., Michos E.D., Bittner V.A., Petersen L.A., Ballantyne C.M. Estimation of eligibility for proprotein convertase subtilisin/kexin type 9 inhibitors and associated costs based on the FOURIER trial (further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk): insights from the department of veterans affairs. Circulation. 2017;135:2572–2574. doi: 10.1161/CIRCULATIONAHA.117.028503. [DOI] [PubMed] [Google Scholar]
- 39.Mark D.B., Schulman K.A. PCSK9 inhibitors and the choice between innovation, efficiency, and affordability. Jama. 2017;318:711–712. doi: 10.1001/jama.2017.8907. [DOI] [PubMed] [Google Scholar]
- 40.Arrieta A., Hong J.C., Khera R., Virani S.S., Krumholz H.M., Nasir K. Updated cost-effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the FOURIER trial. JAMA Cardiol. 2017;2:1369–1374. doi: 10.1001/jamacardio.2017.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warden B.A., Shapiro M.D., Fazio S. The role of the clinical pharmacist in a preventive cardiology practice. Ann Pharmacother. 2019;53:1214–1219. doi: 10.1177/1060028019864669. 1060028019864669. [DOI] [PubMed] [Google Scholar]
- 42.Bibeau W.S., Fu H., Taylor A.D., Kwan A.Y. Impact of out-of-pocket pharmacy costs on branded medication adherence among patients with type 2 diabetes. J Managed Care Specialty Pharm. 2016;22:1338–1347. doi: 10.18553/jmcp.2016.22.11.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawaskar M.D., Xu L., Tang Y., Puckrein G.A., Rajpathak S.N., Stuart B. Effect of medication copayment on adherence and discontinuation in Medicare beneficiaries with type 2 diabetes: a retrospective administrative claims database analysis. Diabetes Ther: Resear, Treat Educ Diabetes Related Disorders. 2018;9:1979–1993. doi: 10.1007/s13300-018-0489-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eaddy M.T., Cook C.L., O’Day K., Burch S.P., Cantrell C.R. How patient cost-sharing trends affect adherence and outcomes: a literature review. P T : Peer-Rev J Formulary Manag. 2012;37:45–55. [PMC free article] [PubMed] [Google Scholar]
- 45.Boden W.E., Baum S., Toth P.P., Fazio S., Bhatt D.L. Impact of expanded FDA indication for icosapent ethyl on enhanced cardiovascular residual risk reduction. Future Cardiol. 2020;17:155–174. doi: 10.2217/fca-2020-0106. [DOI] [PubMed] [Google Scholar]
- 46.Khera R., Valero-Elizondo J., Das S.R., Virani S.S., Kash B.A., de Lemos J.A., Krumholz H.M., Nasir K. Cost-related medication nonadherence in adults with atherosclerotic cardiovascular disease in the United States, 2013 to 2017. Circulation. 2019;140:2067–2075. doi: 10.1161/CIRCULATIONAHA.119.041974. [DOI] [PubMed] [Google Scholar]
- 47.Pfeffer M.A., Claggett B., Diaz R., Dickstein K., Gerstein H.C., Køber L.V., Lawson F.C., Ping L., Wei X., Lewis E.F., Maggioni A.P., McMurray J.J., Probstfield J.L., Riddle M.C., Solomon S.D., Tardif J.C. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 48.Holman R.R., Bethel M.A., Mentz R.J., Thompson V.P., Lokhnygina Y., Buse J.B., Chan J.C., Choi J., Gustavson S.M., Iqbal N., Maggioni A.P., Marso S.P., Öhman P., Pagidipati N.J., Poulter N., Ramachandran A., Zinman B., Hernandez A.F. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]