Abstract
Objective
National estimates of atherosclerotic cardiovascular disease (ASCVD) in the United States (US) are scarce, especially for patients grouped by cardiovascular risk, lipid-lowering therapy use, and low-density lipoprotein cholesterol (LDL-C) levels. The objective of this study was to estimate the size of the ASCVD population, including the subgroup at very high risk for recurrent events as defined by the 2018 Multi-Society Cholesterol Guidelines.
Methods
Patient-level data from the Truven MarketScan Research Database were used and extrapolated to approximate national figures based on known national demographic and ASCVD prevalence numbers. Demographic and clinical characteristics, including LDL-C levels and lipid-lowering therapy use, were captured.
Results
The extrapolated prevalence of ASCVD in 2014 was 18.3 million, of whom 690,524 had an acute coronary syndrome event in the past year. An estimated 41.4% of patients with ASCVD had diabetes, 44.9% had polyvascular disease, and 23.8% had multiple cardiovascular events. A third of those with ASCVD were estimated to be at very high risk for subsequent events per the 2018 Multi-Society Cholesterol Guidelines. Of those with ASCVD, 74.2% were estimated to have an LDL-C level of ≥70 md/dL, and more than half of these patients were neither on statins nor ezetimibe. Only 9.2% of patients with ASCVD and LDL-C ≥70 mg/dL were on a high-intensity statin.
Conclusions
The underutilization of lipid-lowering therapies in general, and in particular the relatively low usage of high-intensity statins among patients with uncontrolled LDL-C (including those at very high risk), suggests that eligible patients for proprotein convertase subtilisin/kexin type 9 inhibitor therapy may not be as numerous as previously estimated.
Keywords: Atherosclerotic cardiovascular disease, Lipid-lowering therapies, Low-density lipoprotein cholesterol
Highlights
-
•
The prevalence of atherosclerotic cardiovascular disease (ASCVD) amongst adults in the US is 18.3 million (8.0%).
-
•
690,524 adults had an acute coronary syndrome event last year, and over 6 million are at very high risk.
-
•
74% of ASCVD patients have low-density lipoprotein cholesterol (LDL-C) levels ≥70 mg/dL, including 67% at very high risk.
-
•
<50% with LDL-C ≥70 mg/dL are on statins; only 9.2% are on a high-intensity statin.
-
•
Only 24.4% of ASCVD patients with LDL-C ≥100 mg/dL receive statins.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) is a heterogeneous disease that puts patients at risk of cardiovascular events and is modulated by several risk factors, including low-density lipoprotein cholesterol (LDL-C) levels. Recently published US Multi-Society Cholesterol Guidelines recommend treatment to achieve an LDL-C reduction of ≥50% for patients with ASCVD using high-intensity statin or maximally tolerated statin doses, and state that very high-risk patients with ASCVD (those with a history of multiple major ASCVD events or a major event and multiple high-risk conditions) who are not able to reach an LDL-C threshold of <70 mg/dL may require additional non-statin therapy [1]. Similarly, the American Association of Clinical Endocrinologists 2017 Guidelines set LDL-C goals for patients based on risk level, with a goal of <70 mg/dL for those with ASCVD (termed “very high risk”) and a goal of <55 mg/dL for those at extreme risk (e.g., individuals with progressive ASCVD or those with clinical cardiovascular disease and diabetes) [2]. Guidelines in 2019 from the European Society of Cardiology/European Atherosclerosis Society recommend an LDL-C goal of <55 mg/dL for very high-risk patients, in which they include all patients with clinical ASCVD [3]. Statins are typically recommended prior to other agents, such as ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, for patients with ASCVD or familial hypercholesterolemia requiring additional LDL-C reduction. While there has been concern about the size of the appropriate patient population for treatment with PCSK9 inhibitors, limited national-level information from real-world data exists about the size of the ASCVD population overall, and especially the size of relevant disease and risk groups based on lipid-lowering therapy use and LDL-C levels.
While several database studies of the ASCVD population include detail on lipid-lowering therapy use and the clinical characteristics of patients, national estimates containing granular information are sparse [[4], [5], [6]]. The objective of the current study was to fill these gaps, aiming to provide a more complete picture of ASCVD and disease groups, including in terms of lipid-lowering therapy use and achieved LDL-C levels. Furthermore, we provide additional estimates for the clinical characteristics of the national population, including details on the prevalence of various risk factors.
2. Methods
This study used de-identified Health Insurance Portability and Accountability Act-compliant administrative healthcare claims from facilities, providers, and outpatient pharmacies from the Truven MarketScan Research Database, which contains healthcare data for more than 43.6 million covered lives (~13.4% of the US population [based on 2017 data]) [7,8]. The Truven MarketScan Research Database is a large US-managed care database representing patients covered by commercial and Medicare supplemental health plans. Data were linked to laboratory results where available. A previous study by Brookhart et al. [9] found little bias in common laboratory values, including LDL-C, when comparing patients with laboratory values in Truven Marketscan to nationally representative figures in NHANES, indicating no systematic difference between these two datasets.
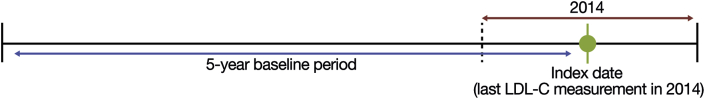
Enrollees who met all of the following criteria were included in the analytic cohort: at least 1 valid LDL-C measurement in 2014 (date of last measurement defined as index date), age ≥21 years as of the index date, continuous enrollment in the database for at least 5 years prior to the index date (baseline period was the 5 years prior to index for all patients; Fig. 1), a diagnosis of ASCVD or diabetes based on International Classification of Diseases, Ninth Revision (ICD-9) codes [10], or likely heterozygous familial hypercholesterolemia (based on claims-assessable Dutch Lipid Clinic Criteria [11]) during the baseline period (see Supplementary Table 1 for ICD-9 codes). The sizes of populations without ASCVD (including with diabetes or heterozygous familial hypercholesterolemia but without ASCVD) were also estimated. This was to control for any potential biases in the data based on age, gender, or diabetes status.
Fig. 1.
Study design.
LDL-C, low-density lipoprotein cholesterol.
To conduct the estimation, patients were stratified into mutually exclusive groups based on several demographic and clinical characteristics (described below). In doing so, the extrapolation accounted for differential weights by disease profiles and eliminated double-counting due to overlap between high-risk conditions. The sizes of patient groups were estimated using an optimization algorithm, according to which the cumulative prevalence of each demographic and clinical characteristic in the final extrapolated cohort aligned to national estimates (anchors). These anchors were set based on 2013 US census data for gender and age (21–64 years and ≥65 years) [12], and American Heart Association national statistics and published estimates for the prevalence of CHD [13], ischemic stroke [13], diabetes [13,14], PAD [15], and heterozygous familial hypercholesterolemia [16].
The weights per strata were estimated such that the squared error between the national prevalence and extrapolated prevalence was minimized across all anchor values (the objective function). A generalized reduced gradient non-linear optimization, via the Solver add-in for Microsoft Excel, was used to estimate the weights per strata. After that, an additional macro iterated through each weight, incrementally increasing and decreasing the weight to test whether it would decrease the value from the objective function. This additional optimization macro was run until the extrapolated values matched the national prevalence for all anchor values when rounded to the nearest integer.
This methodology ensured that the number of observations in the extrapolated dataset was in line with the adult US population and the national prevalence of CHD, ischemic stroke, diabetes, PAD, and heterozygous familial hypercholesterolemia. The final results ensured that each patient included in the analysis was extrapolated to the national level for the aforementioned variables, and also provided information on the following additional variables of interest: LDL-C value, history of acute coronary syndrome (ACS; including within 1 year or prior), evidence of multiple events, evidence of polyvascular disease, chronic kidney disease, and hypertension.
The primary analysis was conducted for 5 mutually exclusive cardiovascular risk groups, defined using the following hierarchy (patients were categorized into the first group they qualified for): (1) Recent ACS within ≤1 year (nonfatal myocardial infarction [MI] or unstable angina requiring hospitalization within the 12 months before the index date); (2) ACS >1 year, occurring more than 12 months before the index date; (3) ischemic stroke; (4) PAD (by non-coronary atherosclerotic disease, abdominal aortic aneurysm, or carotid artery stenosis); (5) other CHD (coronary revascularization [including coronary artery bypass graft and percutaneous coronary intervention], stable angina, or non-specific CHD diagnosis). Effectively, the hierarchical categorization amounted to first identifying patients with recent ACS ≤1 year, then patients without “recent ACS” who had ACS >1 year ago, then patients with ischemic stroke without any evidence of ACS, followed by patients with PAD without ACS or ischemic stroke, and finally patients with CHD other than any of the above.
A second analysis was conducted in which patients were classified into each category for which they qualified (“overlapping” disease groups). For example, a patient with evidence of both ischemic stroke and a prior PAD diagnosis would be categorized both into the ischemic stroke and PAD groups in the overlapping analysis (whereas they would only be categorized into the ischemic stroke group in the above hierarchical analysis).
Demographic and clinical characteristics, including comorbidities and risk factors of interest, such as polyvascular disease and recurrent events, were assessed for each disease group. Polyvascular disease was defined as disease in 2 or more vascular beds (coronary, cerebrovascular, peripheral); recurrent events were defined as 2 or more events (including the same type of event twice) of unstable angina with hospitalization, nonfatal ischemic stroke, nonfatal MI, or elective revascularization. A modified version (not including Ankle Brachial Index <0.85 and amputation in definition of symptomatic PAD due to data limitations) of ‘very high-risk’ patients, as defined in the 2018 Multi-Society Cholesterol Guidelines, was also assessed. ICD-9 codes were used to code all high-risk conditions and major ASCVD events aside from heterozygous familial hypercholesterolemia (defined using claims-assessable Dutch Lipid Clinic Criteria [11]).
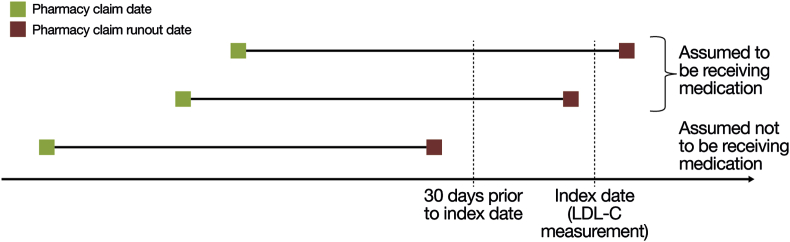
Treatment status and LDL-C were also assessed for each disease group. Treatment status was assessed according to evidence of a pharmacy claim for a statin and/or ezetimibe as of the index date; statin intensity was categorized according to previously published definitions [4]. A patient was assumed to be taking a medication at index if (a) the pharmacy claim date preceded the index date and the runout date was the index date or later, or (b) the pharmacy claim runout date was no later than 30 days prior to the index date (Fig. 2). Patients who had either no pharmacy claim or had a pharmacy claim with a runout date earlier than 30 days before index were not considered to be on the medication. Lipid-lowering therapy status and lipid values were assessed concurrently (i.e., lipid-lowering therapy pharmacy claim date within 30 days prior to LDL-C measurement), to ensure that lipid levels best reflected the impact of the current treatment regimen. Treatment status by disease group and LDL-C level (<55 mg/dL, <70 mg/dL, ≥70 mg/dL, and ≥100 mg/dL) as of the index date were also assessed.
Fig. 2.
Determination of treatment status at the index date (adapted from Steen et al. 20164).
LDL-C, low-density lipoprotein cholesterol.
3. Results
Patient counts for each hierarchical disease group are shown in Table 1; patient counts for overlapping disease groups are shown in Supplementary Table 2. The extrapolated prevalence of ASCVD was 18.3 million (8.0% of the adult population), of whom 690,524 had an ACS event in the past year.
Table 1.
Hierarchical ASCVD disease group count in the Truven Database and extrapolated population.
| Hierarchical disease group | Database, count | Database, % | Extrapolated US population size, count | Extrapolated US population size, % |
|---|---|---|---|---|
| Recent ACS ≤1 year | 4051 | 0.6 | 690,524 | 0.3 |
| ACS >1 year | 12,694 | 2.0 | 2,135,019 | 0.9 |
| Ischemic stroke | 11,396 | 1.8 | 4,912,555 | 2.1 |
| PAD | 42,999 | 6.9 | 3,588,654 | 1.6 |
| Other CHDa | 46,467 | 7.4 | 6,986,485 | 3.0 |
| No ASCVD | 509,647 | 81.3 | 211,121,795 | 92.0 |
| Total | 627,254 | 100 | 229,435,031 | 100 |
ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; PAD, peripheral arterial disease.
‘Other CHD’ includes coronary revascularization (including coronary artery bypass graft and percutaneous coronary intervention), stable angina, or another CHD diagnosis.
Demographic and clinical characteristics for each hierarchical disease group are shown in Table 2; characteristics for each overlapping disease group are shown in Supplementary Table 3. In the hierarchical analysis, polyvascular disease was estimated to be present in 44.6% of patients with ACS ≤1 year, 43.4% of patients with ACS >1 year, 89.8% of patients with ischemic stroke, and 71.9% of patients with PAD. The proportion of patients with recurrent cardiovascular events in the hierarchical analysis was estimated to be 59.5% in patients with ischemic stroke, 57.8% in patients with ACS ≤1 year, and 48.3% in patients with ACS >1 year. A total of 32.8% of patients with ASCVD were considered to be at very high risk according to the 2018 guideline. Hypertension was common across all disease groups, and Stage III chronic kidney disease was estimated to be present in 9–20% of patients in each disease group. Overall, an estimated 74.2% of patients with ASCVD had an LDL-C level of ≥70 mg/dL.
Table 2.
Demographic and clinical characteristics for hierarchical ASCVD disease groups of adults ≥21 years of age in the US.
| Recent ACS ≤1 year (N = 690,524) | ACS >1 year (N = 2,135,019) | Ischemic stroke (N = 4,912,555) | PAD (N = 3,588,654) | Other CHDa (N = 6,986,485) | Overall ASCVD (N = 18,313,236) | |
|---|---|---|---|---|---|---|
| Age, mean | 67.8 | 67.9 | 67.8 | 66.4 | 64.4 | 66.2 |
| Gender, % male | 61.9 | 63.2 | 50.1 | 50.5 | 59.1 | 55.6 |
| History of ACS, % | 100.0 | 100.0 | – | – | – | 15.4 |
| History of MI | 80.0 | 74.5 | – | – | – | 11.7 |
| History of UA with hospitalization | 43.0 | 41.8 | – | – | – | 6.5 |
| History of ischemic stroke, % | 30.6 | 28.9 | 100.0 | – | – | 31.4 |
| PAD, % | 34.8 | 33.7 | 45.8 | 100.0 | – | 37.1 |
| Elective revascularization, % | 10.4 | 11.4 | 0.7 | 0.8 | 1.0 | 2.4 |
| CKD Stage III, % | 19.5 | 16.9 | 14.0 | 11.1 | 8.7 | 12.0 |
| CKD Stage IV, % | 5.7 | 4.7 | 3.3 | 2.4 | 1.6 | 2.7 |
| CKD Stage V, % | 7.1 | 5.5 | 4.0 | 2.9 | 1.8 | 3.2 |
| Hypertension, % | 91.5 | 93.3 | 88.0 | 85.6 | 84.2 | 86.8 |
| Diabetes mellitus, % | 50.3 | 46.5 | 41.9 | 43.5 | 37.7 | 41.4 |
| Polyvascular disease, %b | 44.6 | 43.4 | 89.8 | 71.9 | 0.0 | 44.9 |
| Recurrent events, %c | 57.8 | 48.3 | 59.5 | 0.0 | 0.0 | 23.8 |
| Very high risk, %d | 90.0 | 70.4 | 76.2 | 3.8 | 0.0 | 32.8 |
| LDL-C, mean (mg/dL) | 83.8 | 88.5 | 94.0 | 99.5 | 95.4 | 94.6 |
| LDL-C <55 mg/dL, % | 19.1 | 14.1 | 11.0 | 7.8 | 8.8 | 10.2 |
| LDL-C <70 mg/dL, % | 40.5 | 33.6 | 26.5 | 20.9 | 23.9 | 25.8 |
| LDL-C ≥70 mg/dL, % | 59.5 | 66.4 | 73.5 | 79.1 | 76.1 | 74.2 |
| LDL-C ≥100 mg/dL, % | 25.3 | 29.0 | 37.6 | 42.8 | 38.6 | 37.5 |
ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; CKD, chronic kidney disease; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PAD, peripheral arterial disease; UA, unstable angina.
All data are percentages unless otherwise stated.
‘Other CHD’ includes coronary revascularization (including coronary artery bypass graft and percutaneous coronary intervention), stable angina, or another CHD diagnosis.
Defined as disease in ≥2 vascular beds (coronary, cerebrovascular, peripheral).
Defined as ≥2 of the following: UA with hospitalization, nonfatal ischemic stroke, MI, elective revascularization.
Defined using the 2018 Multi-Society Cholesterol Guidelines [1].
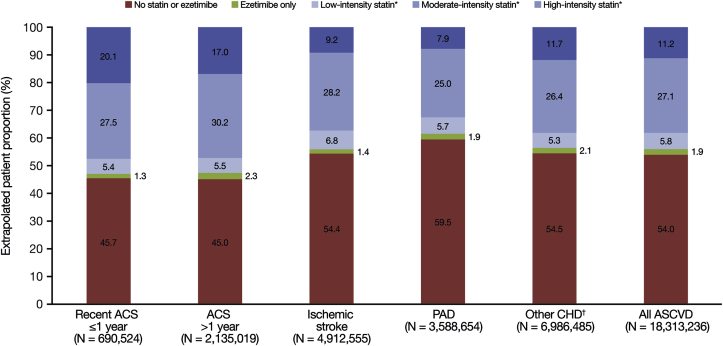
The proportion of patients with ASCVD without pharmacy claims for statin or ezetimibe was estimated to be 54.0% (ranging from 45.0% for ACS >1 year to 59.5% for PAD across the hierarchical disease groups; Fig. 3). In total, an estimated 44.1% of patients with ASCVD received a statin, 11.2% received high-intensity statin (ranging from 7.9% in the PAD group to 20.1% in the recent ACS ≤1 year group), 27.1% received moderate-intensity statin (ranging from 25.0% in the PAD group to 30.2% for ACS >1 year), and 5.8% received low-intensity statin (ranging from 5.3% for other CHD to 6.8% for ischemic stroke). Among patients with ASCVD, an estimated 2.4% of patients received ezetimibe, either as monotherapy or in combination with statins. Treatment characteristics by overlapping disease groups are shown in Supplementary Fig. 1.
Fig. 3.
Treatment status for hierarchical ASCVD disease groups of adults ≥21 years of age in the US.
ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; PAD, peripheral arterial disease.
∗Monotherapy or in combination with ezetimibe.
†‘Other CHD’ includes coronary revascularization (including coronary artery bypass graft and percutaneous coronary intervention), stable angina, or another CHD diagnosis.
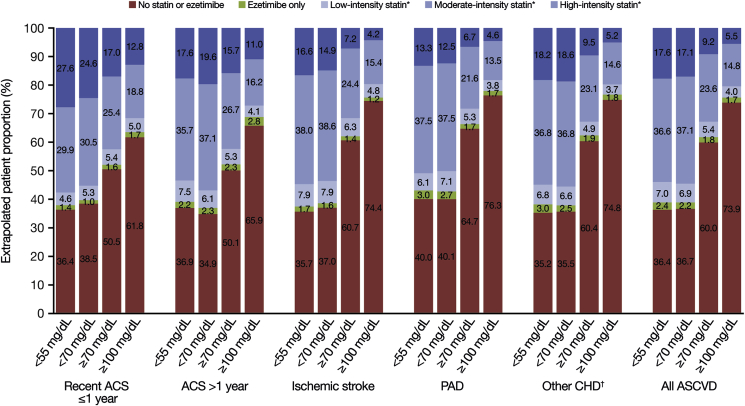
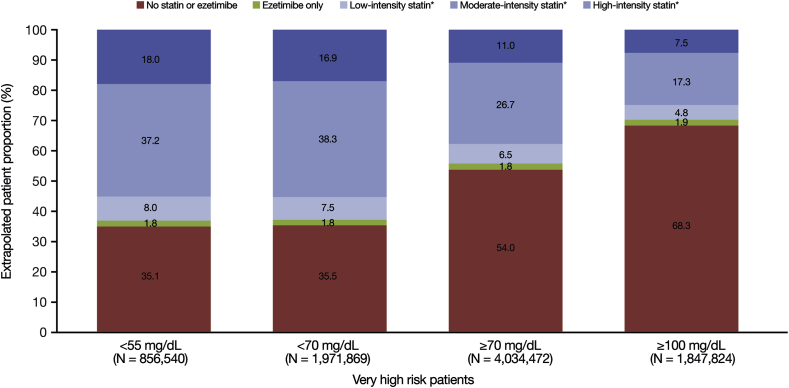
Among patients with ASCVD with an LDL-C level of <55 mg/dL, it was estimated that 61.2% received statins (Fig. 4). For patients with an LDL-C level of <70 mg/dL, this figure was 61.1%. By contrast, 38.2% and 24.4% of patients with LDL-C levels of ≥70 mg/dL and ≥100 mg/dL, respectively, were estimated to be on statins. The percentage of patients estimated to be on high-intensity statin was 17.6% for LDL-C <55 mg/dL, 17.1% for LDL-C <70 mg/dL, 9.2% for LDL ≥70 mg/dL, and 5.5% for LDL-C ≥100 mg/dL. In the lowest LDL-C group (<55 mg/dL), the estimated proportion of patients with no pharmacy claim for statin or ezetimibe across the hierarchical disease groups ranged from 35.2% in the other CHD group to 40.0% in the PAD group. In the highest LDL-C group (≥100 mg/dL), the proportion ranged from 61.8% in the recent ACS ≤1 year group to 76.3% in the PAD group. Treatment characteristics by overlapping disease groups and LDL-C level are shown in Supplementary Fig. 2. Figures were similar for the very high-risk ASCVD population compared with the overall population (Fig. 5).
Fig. 4.
Treatment characteristics by hierarchical ASCVD disease group and LDL-C level.
ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; LDL-C, low-density lipoprotein cholesterol; PAD, peripheral arterial disease.
∗Monotherapy or in combination with ezetimibe.
†‘Other CHD’ includes coronary revascularization (including coronary artery bypass graft and percutaneous coronary intervention), stable angina, or another CHD diagnosis.
Fig. 5.
Treatment characteristics and LDL-C level in very high-risk patients.
LDL-C, low-density lipoprotein cholesterol.
∗Monotherapy or in combination with ezetimibe. Very high-risk patients defined using the 2018 Multi-Society Cholesterol Guidelines [1].
4. Discussion
In the extrapolated population of 229 million adults in the US aged ≥21, the overall prevalence of ASCVD in 2014 was estimated to be 8.0%, representing more than 18.3 million people. These data are consistent with the previous estimate by Wong et al. of 17 million people, based on data from the NHANES database [17,18]. Interestingly, while the earlier estimate did take into account overlaps across disease groups, it did not include patients with PAD or coronary revascularization without an ACS or stroke event, as this information is not captured in the recent NHANES database.
In contrast to the estimated ASCVD population size, the point estimate for the proportion of US adults with a recent ACS event within 1 year was relatively small, corresponding to approximately 700,000 adults. This estimate appears consistent with data from the Healthcare Cost and Utilization Project, the largest collection of longitudinal hospital care data in the US, which show that there were 608,800 stays for acute MI in 2014 (although the figure may double-count patients with multiple MIs in the same year and does not include patients who have hospitalizations for unstable angina) [19]. In our study, 44.9% of patients with ASCVD were estimated to have polyvascular disease and 23.8% a history of recurrent cardiovascular events, further highlighting the burden of ASCVD in general, and in particular the high preponderance of patients not able to prevent a second event or progression to a second vascular bed. About one-third (32.8%) were estimated to be at ‘very high risk’ per the 2018 Multi-Society Cholesterol Guidelines [1], and 41.4% of patients with ASCVD were estimated to have diabetes mellitus, which would place them into an extreme risk category according to the American Association of Clinical Endocrinologists guidelines [2]. These particularly high-risk groups [18,[20], [21], [22]] may be an important area of public health focus and clinical impetus.
While the high burden of ASCVD in the US, as demonstrated in the literature [23] and in this study, is in itself concerning, we find the additional estimates of numbers of individuals untreated and undertreated alarming: more than 50% of the ASCVD population, corresponding to almost 9.9 million people, did not have a pharmacy claim for statins or for ezetimibe, although several guidelines recommend them unequivocally [1,2]. Even in patients considered to be at very high risk, nearly half of patients had no evidence of statin or ezetimibe. Although alternative lipid-lowering therapies are available, other studies suggest that the proportion of patients who receive these therapies without concomitant statin treatment is less than 5% [4,24]. These estimates are in line with similar proportions of patients with ASCVD in the US who have been reported as undertreated in other studies. Steen et al. found that 51% of adults aged ≥20 years with ASCVD in the US in 2014 were not taking a lipid-lowering therapy, based on data from the Optum Research database [4], and Wong et al. found that, of all statin-eligible patients in the US, only 63.7% of people with ASCVD received statins [18]. It is possible that the proportion of patients on statins may have been under-represented in the current analysis due to the Truven database not including prescriptions that were not billed for through insurance (e.g., those that may have been paid for by cash owing to the low cost of many generic statins). A past study by Wade et al. has shown that this underestimation is likely not too large but may have led to a deflated figure [25]. Nevertheless, it is clear that statins are significantly underutilized in adults with ASCVD in the US.
Treatment underutilization leads to a failure to achieve LDL-C goals, resulting in an increased risk of recurrent events [25]. In this study, in the group of adults with ASCVD who had an LDL-C level ≥70 mg/dL, an estimated 5.4% were receiving low-intensity statin and 60.0% had no pharmacy claim for either statins or ezetimibe (54% of those at very high risk had no pharmacy claim for statin or ezetimibe). Corresponding percentages for those with an LDL-C level ≥100 mg/dL were 4.0% and 73.9%. This highlights an opportunity to reduce LDL-C to below goal with higher-intensity statin in a large proportion of patients. However, some patients with ASCVD failed to reach LDL-C goals even with high-intensity statin therapy (9.2% with LDL-C ≥70 mg/dL and 5.5% with LDL-C ≥100 mg/dL, representing 1.25 million and ~400,000 patients, respectively); whereas 17.6% reached an LDL-C level of <55 mg/dL with high-intensity statin. Whether this failure to achieve LDL-C targets with high-intensity statin therapy is due to inadequate response or suboptimal adherence to the statin was not ascertained. Regardless, these patients may benefit from non-statin lipid-lowering therapy, such as PCSK9 inhibitors or ezetimibe. The relatively low percentage of patients who are not at goal despite high-intensity statin therapy also suggests that the number of eligible patients for PCSK9 inhibitors may not be as large as previously estimated, especially if patients are appropriately initiated and titrated, and adhere to their statin therapy [10,27]. In the very high-risk group, only 444,226 patients on high-intensity statin had an LDL-C level ≥70 mg/dL.
This analysis has several important limitations, pertaining to analytic methods and administrative claims data. Although the estimated weights accounted for key factors such as diagnoses of CHD, the analysis did not incorporate residual contributions from all known variables. The study cohort represents a subset of the US insured population, including those commercially insured in part by employers and those with Medicare supplement plans, which may limit generalizability to the uninsured, the Medicaid population, those using another commercial plan, or the US population in general. Furthermore, the data may underestimate the number of patients with ACS >1 year and subsequently overestimate the number with ‘other CHD’, as the baseline period was restricted to 5 years and there could be no surety that the ‘old MI’ ICD-9 code was used during that period in all patients with a history of MI. This may explain the gap between the 2.8 million patients with a history of ACS in this study and 7.1 million patients with a history of MI reported in Wong et al. [18]. Owing to the lack of Ankle Brachial Index and amputations in the data, as well as a cohort that was limited to ≥21 years of age, not all patients at very high risk per the 2018 Multi-Society Cholesterol Guidelines [1] may have been identified. Information on response to statins or potential intolerance to higher intensities of statin and race are also not available within these data.
5. Conclusion
Overall, we find that approximately 18 million US adults have ASCVD, with one-third of them being defined as “very high risk” according to the latest US Multi-Society Cholesterol Guidelines. This study provides some additional detail and insight into the size of different subsets of the ASCVD population in the US as well as the clinical and treatment characteristics therein. Furthermore, this analysis estimates the distribution of baseline treatments and corresponding LDL-C levels, highlighting a significant underutilization of statins, including in patients at very high risk according to the 2018 Multi-Society Cholesterol Guidelines. These data are of public health interest and may be an area of future therapeutic focus. The relatively low percentage of uncontrolled patients among those optimally treated with statins also suggests that the eligible population for PCSK9 inhibitors may not be as large as previously estimated.
Acknowledgments
The authors would like to thank Xue Song (Truven) for contributions to data acquisition.
Medical writing assistance and editorial support, under the direction of the authors, was provided by Rachel Wright, PhD, and Caroline Ayres, MPH, of Prime (Knutsford, UK) and was funded by Sanofi and Regeneron Pharmaceuticals, Inc., according to Good Publication Practice guidelines (https://annals.org/aim/fullarticle/2424869/good-publication-practice-communicating-company-sponsored-medical-research-gpp3).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajpc.2020.100010.
Funding
This study was funded by Sanofi and Regeneron Pharmaceuticals Inc.
Author contributions
Alexa Klimchak: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing original draft, writing review and editing.
Miraj Patel: conceptualization, methodology, validation, formal analysis, writing original draft, writing review and editing, supervision, project admin, funding acquisition.
Serban Iorga: conceptualization, methodology, validation, formal analysis, writing review and editing, supervision, project admin, funding acquisition.
Natasha Kulkarni: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing review and editing, visualization.
Nathan Wong: conceptualization, formal analysis, validation, writing review and editing, supervision.
Disclosures
The sponsors were involved in the study design, and collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. The authors had unrestricted access to study data, were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication. Alexa Klimchak was an employee of Axtria during the initial creation of the manuscript. Miraj Patel is an employee of and stockholder in Sanofi. Şerban Iorga was an employee of Regeneron Pharmaceuticals, Inc at the time of the study. Natasha Kulkarni is an employee of Axtria. Nathan Wong has received research support through his institution from Amgen, Amarin, Boehringer-Ingelheim, Novo Nordisk, and Regeneron Pharmaceuticals; Inc., has participated on advisory boards for Amarin and Esperion; and serves on the speaker bureaus for Amgen and Sanofi.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Jellinger P.S., Handelsman Y., Rosenblit P.D., Bloomgarden Z.T., Fonseca V.A., Garber A.J., Grunberger G., Guerin C.K., Bell D.S.H., Mechanick J.I., Pessah-Pollack R., Wyne K., Smith D., Brinton E.A., Fazio S., Davidson M. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1–87. doi: 10.4158/EP171764.APPGL. [DOI] [PubMed] [Google Scholar]
- 3.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 4.Steen D.L., Khan I., Becker L., Foody J.M., Gorcyca K., Sanchez R.J., Giugliano R.P. Patterns and predictors of lipid-lowering therapy in patients with atherosclerotic cardiovascular disease and/or diabetes mellitus in 2014: insights from a large US managed-care population. Clin Cardiol. 2017;40:155–162. doi: 10.1002/clc.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin I., Sung J., Sanchez R.J., Mallya U.G., Friedman M., Panaccio M., Koren A., Neumann P., Menzin J. Patterns of statin use in a real-world population of patients at high cardiovascular risk. J Manag Care Spec Pharm. 2016;22:685–698. doi: 10.18553/jmcp.2016.22.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Q., Grabner M., Sanchez R.J., Willey V.J., Cziraky M.J., Palli S.R., Power T.P. Clinical characteristics and unmet need among patients with atherosclerotic cardiovascular disease stratified by statin use. Am Health Drug Benefits. 2016;9:434–444. [PMC free article] [PubMed] [Google Scholar]
- 7.Truven Health Analytics . 2017. The Truven Health MarketScan Databases for life sciences researchers.https://truvenhealth.com/Portals/0/Assets/2017-MarketScan-Databases-Life-Sciences-Researchers-WP.pdf Available at: Accessed October 2018. [Google Scholar]
- 8.United States Census Bureau . 2017. Annual estimates of the resident population: April 1, 2010 to July 1, 2017.https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml Available at: Accessed October 2018. [Google Scholar]
- 9.Brookhart M.A., Todd J.V., Li X., Reams B.D., Pate V., Kshirsagar A.V. Estimation of biomarker distributions using laboratory data collected during routine delivery of medical care. Ann Epidemiol. 2014;24(10):754–761. doi: 10.1016/j.annepidem.2014.07.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon C.P., Khan I., Klimchak A.C., Reynolds M.R., Sanchez R.J., Sasiela W.J. Simulation of lipid-lowering therapy intensification in a population with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:959–966. doi: 10.1001/jamacardio.2017.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordestgaard B.G., Chapman M.J., Humphries S.E., Ginsberg H.N., Masana L., Descamps O.S., Wiklund O., Hegele R.A., Raal F.J., Defesche J.C., Wiegman A., Santos R.D., Watts G.F., Parhofer K.G., Hovingh G.K., Kovanen P.T., Boileau C., Averna M., Boren J., Bruckert E., Catapano A.L., Kuivenhoven J.A., Pajukanta P., Ray K., Stalenhoef A.F., Stroes E., Taskinen M.R., Tybjaerg-Hansen A. European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United States Census Bureau. 2013. Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States, Counties, and Puerto Rico Commonwealth and Municipios: April 1, 2010 to July 1, 2013.https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml Available at: Accessed August 2018. [Google Scholar]
- 13.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M., Das S.R., de Ferranti S., Despres J.P., Fullerton H.J., Howard V.J., Huffman M.D., Isasi C.R., Jimenez M.C., Judd S.E., Kissela B.M., Lichtman J.H., Lisabeth L.D., Liu S., Mackey R.H., Magid D.J., McGuire D.K., Mohler E.R., 3rd, Moy C.S., Muntner P., Mussolino M.E., Nasir K., Neumar R.W., Nichol G., Palaniappan L., Pandey D.K., Reeves M.J., Rodriguez C.J., Rosamond W., Sorlie P.D., Stein J., Towfighi A., Turan T.N., Virani S.S., Woo D., Yeh R.W., Turner M.B. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D., Benjamin E.J., Go A.S., Arnett D.K., Blaha M.J., Cushman M. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 15.Allison M.A., Ho E., Denenberg J.O., Langer R.D., Newman A.B., Fabsitz R.R., Criqui M.H. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg A.C., Hopkins P.N., Toth P.P., Ballantyne C.M., Rader D.J., Robinson J.G., Daniels S.R., Gidding S.S., de Ferranti S.D., Ito M.K., McGowan M.P., Moriarty P.M., Cromwell W.C., Ross J.L., Ziajka P.E. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1–S8. doi: 10.1016/j.jacl.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Stone N.J., Robinson J., Lichtenstein A.H., Bairey Merz C.N., Lloyd-Jones D.M., Blum C.B. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Wong N.D., Young D., Zhao Y., Nguyen H., Caballes J., Khan I., Sanchez R.J. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low-density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011–2012. J Clin Lipidol. 2016;10:1109–1118. doi: 10.1016/j.jacl.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Healthcare Cost and Utilization Project (HCUP) HCUP statistical brief #225: Trends in Hospital Inpatient Stays in the United States, 2005-2014. 2017. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb225-Inpatient-US-Stays-Trends.jsp?utm_source=ahrq&utm_medium=en1&utm_term=&utm_content=1&utm_campaign=ahrq_en7_5_2017 Available at: Accessed November 2018. [Google Scholar]
- 20.Suarez C., Zeymer U., Limbourg T., Baumgartner I., Cacoub P., Poldermans D., Rother J., Bhatt D.L., Steg P.G. Influence of polyvascular disease on cardiovascular event rates. Insights from the REACH Registry. Vasc Med. 2010;15:259–265. doi: 10.1177/1358863X10373299. [DOI] [PubMed] [Google Scholar]
- 21.van der Heijden A.A., Van’t Riet E., Bot S.D., Cannegieter S.C., Stehouwer C.D., Baan C.A., Dekker J.M., Nijpels G. Risk of a recurrent cardiovascular event in individuals with type 2 diabetes or intermediate hyperglycemia: the Hoorn Study. Diabetes Care. 2013;36:3498–3502. doi: 10.2337/dc12-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punekar R.S., Fox K.M., Richhariya A., Fisher M.D., Cziraky M., Gandra S.R., Toth P.P. Burden of first and recurrent cardiovascular events among patients with hyperlipidemia. Clin Cardiol. 2015;38:483–491. doi: 10.1002/clc.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 24.Bittner V., Deng L., Rosenson R.S., Taylor B., Glasser S.P., Kent S.T., Farkouh M.E., Muntner P. Trends in the use of nonstatin lipid-lowering therapy among patients with coronary heart disease: a retrospective cohort study in the Medicare population 2007 to 2011. J Am Coll Cardiol. 2015;66:1864–1872. doi: 10.1016/j.jacc.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 25.Wade R.L., Patel J.G., Hill J.W., De A.P., Harrison D.J. Estimation of missed statin prescription use in an administrative claims dataset. J Manag Care Spec Pharm. 2017;23:936–942. doi: 10.18553/jmcp.2017.23.9.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hess P.L., Kennedy K., Cowherd M., Virani S.S., Masoudi F.A., Navar A.M., Yeh R.W., Ho P.M., Maddox T.M. Implications of the FDA approval of PCSK9 inhibitors and FOURIER results for contemporary cardiovascular practice: an NCDR Research to Practice (R2P) project. Am Heart J. 2018;195:151–152. doi: 10.1016/j.ahj.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 26.Cholesterol Treatment Trialists Collaboration, Baigent C., Blackwell L., Emberson J., Holland L.E., Reith C., Bhala N., Peto R., Barnes E.H., Keech A., Simes J., Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.