Abstract
Background
Epilepsy affects more than 50 million people worldwide, 80% of whom live in low- and middle-income countries (LMICs). In Southeast Asia, the prevalence is moderate (6‰), and the main public health challenge is reducing the treatment gap, which reaches more than 90% in rural areas.
Methods
This 12-month comparative study (intervention vs. control areas) assessed the community effectiveness of two different strategies for the identification and home follow-up of people with epilepsy by Domestic Health Visitors for epilepsy (DHVes). In Lao PDR, DHVes were health center staff covering several villages via monthly visits; in Cambodia, DHVes were health volunteers living in the villages.
Findings
At baseline, the treatment gap was >95% in Lao PDR and 100% in Cambodia. After 12 months, the treatment gap in Lao PDR decreased by 5·5% (range: 4·0–12·2) in the intervention area and 0·5% (range: 0·4–0·8) in the control area (p<0·0001). In Cambodia, the treatment gap decreased by 34·9% (range: 29·0–44·1) in the intervention area and 8·1% (range: 6·7–10·2) in the control area (p<0·0001). Among the PWEs followed at home by the DHVes, the proportion adhering to drug treatment was 85·2% in Lao PDR and 78·1% in Cambodia. The cost associated with strategy implemented in Cambodia, compared with the control area, was lower than the cost associated with strategy implemented in Lao PDR.”
Interpretation
The treatment gap was significantly reduced with both intervention strategies, but the effect was larger in Cambodia. The results of this cost analysis pave the way for scaling-up in rural areas of Lao PDR and Cambodia, and experimental adaptation in other LMICs.
Funding
The study was funded by the Global Health Department of Sanofi and Grand Challenges Canada (grant number 0325–04).
Research in context.
Evidence before this study
Epilepsy affects more than 50 million people worldwide, 80% of whom live in low- and middle-income countries. The treatment gap, defined as the proportion of people with epilepsy who are not adequately treated, is higher than 80% in many countries. In Asia, the treatment gap ranges from 30 to 98% and is higher in rural areas than in urban areas. Reasons include beliefs, lack of trained personnel, availability, cost and quality of antiepileptic drugs, and distance from the point of care. Demonstration projects have showed that it is feasible to reduce the treatment gap through a pack of interventions (provision of cost-free drugs, adherence reinforcement, education, community awareness) but no study tried to assess each action separately.
Added value of this study
This 12-month comparative study (intervention vs. control areas) assessed the effectiveness of community strategies involving Domestic Health Visitors for epilepsy (DHVes) for the identification and home follow-up of people with epilepsy, whilst all other determinants remained constant. In Lao PDR, DHVes were health center staff covering several villages via monthly visits; in Cambodia, DHVes were volunteers living in the villages. At baseline, the treatment gap was >95% in Lao PDR and 100% in Cambodia. After 12 months, the treatment gap in Lao PDR decreased by 5·5% in the intervention area and 0·5% in the control area (p<0·0001). In Cambodia, the treatment gap decreased by 34·9% in the intervention area and 8·1% in the control area (p<0·0001).
Implications of all the available evidence
The treatment gap has been substantially and significantly reduced relying on health volunteers living in the villages. These results pave the way for scaling-up this strategy in rural areas of other low- and middle-income countries.
Alt-text: Unlabelled box
1. Introduction
Epilepsy is a ubiquitous disease affecting more than 50 million people worldwide, 80% of whom live in low- and middle-income countries (LMICs) [1]. LMICs in tropical regions bear a significant share of the global burden of epilepsy, with high incidence and mortality [2,3]. The epidemiology of epilepsy in these countries is complicated by cultural beliefs, lack of reliable medical records, limited diagnostic expertise, and a shortage of investigative resources for examining risk factors and causes [4]. Thus, the medical management of epilepsy is often sub-optimal, and access to care for people with epilepsy is limited.
The treatment gap (TG), defined as the proportion of people with epilepsy (PWEs) who are not appropriately treated [5], is >80% in many countries. In Asian countries, the TG ranges from 30 to 98% and is higher in rural areas than in urban areas [6,7]. Reasons for the TG include beliefs, lack of trained personnel, availability, cost and quality of antiepileptic drugs (AEDs), and distance from the point of care [8]. Efforts should be made to use a valid methodology [9,10] in these countries to evaluate community-based intervention strategies for the management of epilepsy that are in line with available resources and involve local healthcare systems and authorities.
The present study evaluated two intervention strategies in rural settings in two Southeast Asian countries: Lao PDR and Cambodia.
2. Methods
2.1. Program overview
This study aimed to test the community effectiveness of the identification and home follow-up of PWEs by Domestic Health Visitors for epilepsy (DHVes) in reducing the TG. Two strategies were tested. In Lao PDR, DHVes were health center staff covering several villages. In Cambodia, DHVes were village health volunteers covering their residential village. This was a 12-month comparative study of intervention and control areas in each of the two countries (from November 2014 to October 2015 in Lao PDR and from July 2016 to June 2017 in Cambodia).
2.1.1. Primary endpoint
The primary endpoint was the difference of the TG (before and after the intervention) in the intervention area compared to the control area. The expected numbers of PWEs in the studied areas were estimated using the prevalence reported by population-based studies in the concerned countries [11,12].
2.1.2. Secondary endpoints
The secondary endpoints were adherence to treatment, stigma, and cost of the intervention. These indicators showed positive changes between the first visit, 1 month after starting treatment, and the last visit. Morisky scale [13] was used to assess adherence to treatment. The score is based on the answers to four questions, with each answer scored 0 or 1. A PWE was adherent with a score of 0 and non-adherent with a score >0. Jacoby scale [14,15] was used to assess stigma. The score is based on the answers to three questions, with each answer scored 0 to 1. A PWE experienced stigma when his/her score was >0. The cost analysis is conducted from a governmental perspective. It estimates the difference in costs between intervention and control areas, divided by the difference in the outcome of interest (number of cases under treatment and cases adhering treatment) [16,17]. The calculation was made using only direct costs.
2.2. Study areas
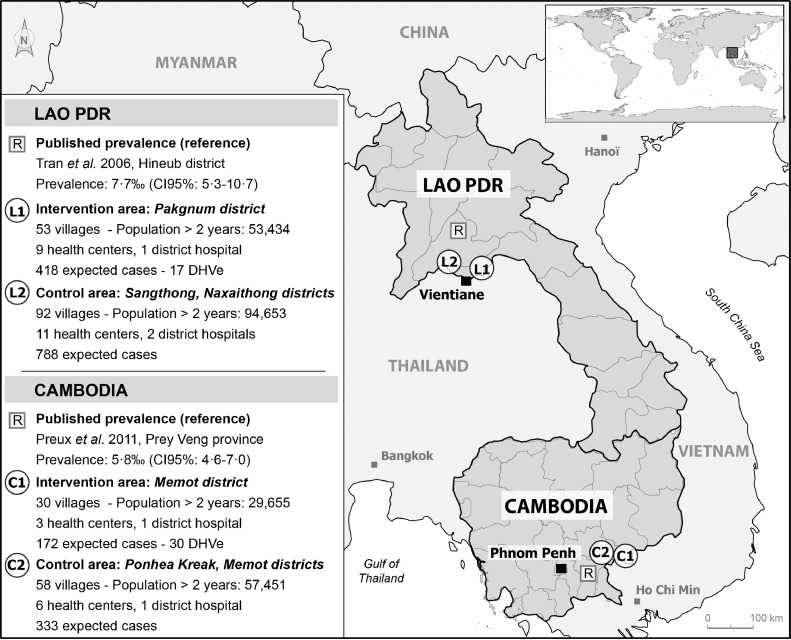
A map of the study area is shown in Fig. 1. There were no sociodemographic differences in the general population between intervention and control areas in each country at baseline (Appendix 4).
Fig. 1.
Study areas in Lao PDR and Cambodia.
2.2.1. Lao PDR strategy
In Lao PDR, 418 PWEs (95% CI 283–572) were expected in the intervention area and 788 PWEs (95% CI 501–1013) in the control area. The expected number of PWEs was based on a door-to-door survey conducted by Tran and colleagues [11] suggesting a 7·7‰ prevalence (95% CI 5·3–10·7). To cover the 53 villages of the intervention area (53,434 inhabitants), 17 DHVes (distributed in the 9 primary health centers) covered 3 or 4 villages each, with their monthly visits. The 92 villages in the control area (94,653 inhabitants) were serviced by staff of 2 district hospitals and 11 primary health centers.
2.2.2. Cambodia strategy
In Cambodia, 172 PWEs (95% CI 136–207) were expected in the intervention area and 333 PWEs (95% CI 264–402) in the control area. The expected number of PWEs was based on a local door-to-door survey, conducted by Preux et al. [12], suggesting a 5·8‰ prevalence (95% CI 4·6–7·07). To cover the 30 villages of the intervention area (29,655 inhabitants), each of the 30 DHVes covered the village in which they lived. The 58 villages of the control area (57,451 inhabitants) were serviced by 1 district hospital and 3 primary health centers.
2.3. Study oversight
The study was approved by the Ethics Committee of Lao PDR MoH and of Cambodia MoH, and by the committee for the protection of persons in Nouvelle Aquitaine region (France) (Appendix 2). All of the authors vouch for the completeness and accuracy of the data and analyses, and for compliance with the study protocol. All included subjects provided their written informed consent.
2.4. Implementation
2.4.1. Preparation phase
In both the intervention and control areas, health services were involved equally except concerning DHVes. AEDs and IEC materials were made available in district hospitals and primary health centers. Questionnaires (Knowledge, Attitudes, and Practices [KAP] surveys) and monitoring forms were produced. All questionnaires were translated, pre-tested, and completed by a trained person speaking the local language. KAP surveys for health personnel, general population, and PWEs were carried out (Appendix 3). Patient registries from district hospitals and non-governmental organizations were assessed at baseline. Training of health personnel (physicians, pharmacists, primary health care personnel, and DHVes) was carried out by neurologists and public health physicians. Referral physicians in the district hospitals (2 per district hospital) carried out diagnoses and prescriptions. The primary health center staff (2 per primary health center in the study area) ensured logistical follow-up (see study protocol).
2.4.2. Intervention areas
DHVes organized public information and awareness meetings on epilepsy and disseminated IEC materials to the general population (e.g., comic strips, quizzes, first aid cards) (Appendix 5: Table S.4). In the villages, they actively searched for PWEs, interviewing key informants. DHVes were responsible for identifying suspected cases using a validated screening questionnaire [18,19]. In Lao PDR, 17 DHVes were selected from the nine primary health center staff working in the intervention area. In Cambodia, 30 DHVes were recruited, one in each village in the intervention area. Suspected cases were referred to district hospitals, where a trained physician confirmed the diagnosis. For confirmed PWEs, DHVes provided home visits for drug delivery and follow-up. The DHVes periodically reported their activities to primary health center managers and district hospital physicians.
2.4.3. Control areas
Identification of PWEs only occurred through routine consultations in primary health centers or district hospitals. No public information and awareness meetings were organized, no IEC materials were proactively disseminated in the villages (but they were available in the health centers and district hospitals), and no village screening was carried out, as there were no DHVes.
2.4.4. Cost of treatment of PWEs
Suspected cases paid for the consultation at the district hospital (diagnosis and AED treatment for the first month). Subsequently, drug treatment was available free of charge via monthly home delivery in the intervention area and at the district hospital or health center in the control area.
2.4.5. Quality control
Diagnosis confirmation and follow-up of PWEs were assessed by a neurologist to ensure that patient management was appropriate. Data were collected monthly by the study team.
2.5. Patients
Confirmed cases were diagnosed by district hospital physicians. Epilepsy was diagnosed according to the ILAE epidemiological definition: two or more unprovoked seizures at least 24 h apart [20]. Children under 2 years of age were not included. The calculation of number of subjects required is presented in the study protocol.
2.6. Statistical analysis
Categorical variables were described using absolute numbers and percentages, and quantitative variables using means and standard deviations. Estimated expected cases and TG ranges were estimated using the 95% confidence intervals (95% CI) of the published prevalence. The primary and secondary endpoints were compared between intervention and control areas using chi-square tests. Sociodemographic variables were compared using the chi-square test or Fisher exact test and t-test. All statistical comparisons used a significance level of 5% (see statistical plan for details).
2.7. Role of the funding source
Funders had no role in the study design, data collection, data analysis, interpretation and writing of the report.
3. Results
3.1. Identification and confirmation of PWEs
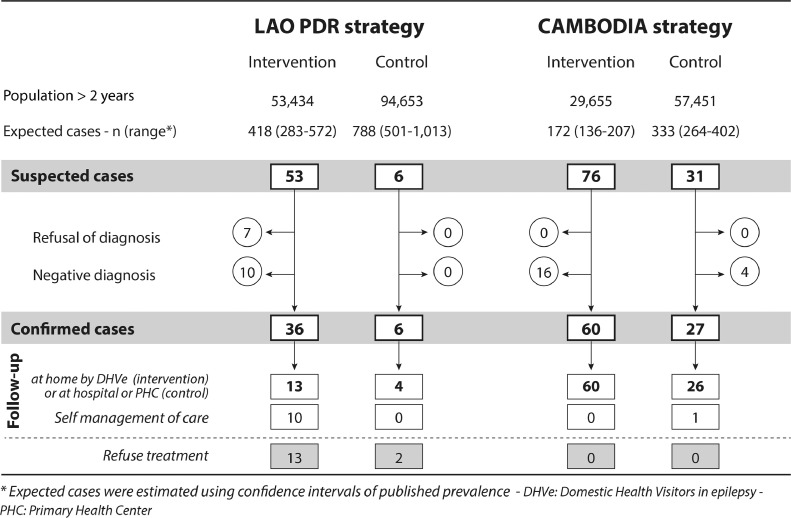
In the Lao PDR intervention area, 21 PWEs were identified at baseline and reconfirmed (Appendix 6). During the 12-month period, 9779 inhabitants (18·3% of the population) attended community information meetings. Out of the 53 suspected cases identified by DHVes, 36 cases were confirmed. In the control area, 24 PWEs were identified at baseline (and reconfirmed), and all 6 suspected cases identified through routine consultation were confirmed (Fig. 2).
Fig. 2.
Recruitment of persons with epilepsy during a 12-month period in Lao PDR (2014–2015) and Cambodia (2016–2017).
In the entire study area in Cambodia, no PWE was known at baseline. During the 12-month period, 7471 inhabitants (25·2% of the population) in the intervention area attended community information meetings. Out of the 76 suspected cases identified by DHVes, 60 were confirmed. In the control area, out of the 31 suspected cases identified through routine consultation, 27 cases were confirmed.
3.2. Characteristics of PWEs confirmed during the 12-month period
Few significant differences were found in the sociodemographic and clinical data of the PWEs identified in the intervention and control areas (Table 1). There were however statistically significantly more men in the intervention than control area in Lao PDR, and a trend towards older age and more generalized epilepsy. The sociodemographic and clinical characteristics of PWEs were similar to those usually described in the literature [3].
Table 1.
Sociodemographic and clinical data for people with epilepsy identified during a 12-month period in Lao PDR (2014–2015) and Cambodia (2016–2017).
|
LAO PDR strategy |
CAMBODIA strategy |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total | Intervention | Control | p | Total | Intervention | Control | p | |
| Confirmed cases | 42 | 36 | 6 | – | 87 | 60 | 27 | – |
| Mean age,years (SD) | 30·2 (18·1) | 32·3 (17·0) | 18·0 (19·7) | 0·073 | 27·4 (13·8) | 28·7 (14·8) | 27·4 (10·9) | 0·633 |
| Men | 20 (47·6) | 18 (50·0) | 2 (33·3) | 0·002 | 50 (57·4) | 36 (60·0) | 14 (48·1) | 0·477 |
| Status Available data | 25 | 21 | 4 | – | 83 | 57 | 26 | – |
| Single | 9 (36·0) | 8 (38·1) | 1 (25·0) | 0·843 | 39 (47·0) | 29 (50·9) | 10 (38·5) | 0·568 |
| Married | 11 (44·0) | 9 (42·8) | 2 (50·0) | 37 (44·6) | 23 (40·4) | 14 (53·8) | ||
| Divorced | 2 (8·0) | 2 (9·5) | 0 (0·0) | 2 (2·4) | 1 (1·8) | 1 (3·8) | ||
| Widowed | 3 (12·0) | 2 (9·5) | 1 (25·0) | 5 (6·0) | 4 (7·0) | 1 (3·8) | ||
| Education Available data | 25 | 21 | 4 | – | 86 | 60 | 26 | |
| Not educated | 6 (24·0) | 5 (23·8) | 1 (25·0) | 1·000 | 38 (44·2) | 24 (40·0) | 14 (53·8) | 0·426 |
| Primary | 11 (44·0) | 9 (42·6) | 2 (50·0) | 33 (38·4) | 23 (38·3) | 10 (38·5) | ||
| Secondary | 7 (28·0) | 6 (28·6) | 1 (25·0) | 14 (16·3) | 12 (20·0) | 2 (7·7) | ||
| Professional training | 1 (4·0) | 1 (4·8) | 0 (0·0) | 1 (1·1) | 1 (1·7) | 0 (0·0) | ||
| Activity Available data | 21 | 17 | 4 | – | 81 | 54 | 27 | – |
| Unemployed | 7 (33·3) | 6 (35·3) | 1 (25·0) | 0·673 | 8 | 7 (13·0) | 1 (3·7) | 0·699 |
| Student | 1 (4·8) | 1 (5·9) | 0 (0·0) | 14 | 9 (16·7) | 5 (18·5) | ||
| Worker/employee | 6 (28·6) | 4 (23·5) | 2 (50·0) | 3 | 2 (3·7) | 1 (3·7) | ||
| Farmer | 7 (33·3) | 6 (35·3) | 1 (25·0) | 56 | 36 (66·6) | 20 (74·1) | ||
| Age at first seizure | 35 | 30 | 5 | – | 87 | 60 | 27 | – |
| Mean, years (SD) | 15·9 (13·6) | 17·4 (13·8) | 7·0 (5·1) | 0·114 | 11·5 (9·2) | 13·0 (9·7) | 11·2 (7·3) | 0·460 |
| Seizure frequency | 42 | 36 | 6 | – | 87 | 60 | 27 | – |
| ≤ 4 /month | 32 (76·2) | 27 (76·5) | 5 (83·3) | 1·000 | 52 (59·8) | 37 (61·7) | 15 (55·6) | 0·591 |
| > 4 /month | 10 (23·8) | 9 (23·5) | 1 (16·7) | 35 (40·2) | 23 (38·3) | 12 (44·5) | ||
| Type Available data | 42 | 36 | 6 | – | 87 | 60 | 27 | – |
| Focal | 13 (31·0) | 9 (25·0) | 4 (66·7) | 0·063 | 3 (3·4) | 2 (3·3) | 1 (3·7) | 0·584 |
| Generalized | 29 (69·0) | 27 (75·0) | 2 (33·3) | 84 (96·6) | 58 (96·7) | 26 (96·3) | ||
| Under treatment | ||||||||
| Available data | 27 | 23 | 4 | – | 87 | 60 | 27 | – |
| Phenobarbitone | 18 (66·7) | 14 (60·9) | 4 (100·0) | 0·768 | 77 (88·5) | 52 (86·7) | 25 (92·6) | 0·802 |
| Phenytoin | 5 (18·5) | 5 (21·7) | 0 (0·0) | 1 (1·2) | 1 (1·7) | 0 (0·0) | ||
| Valproate | 2 (7·4) | 2 (8·7) | 0 (0·0) | 9 (10·3) | 7 (11·6) | 2 (7·4) | ||
| Other | 2 (7·4) | 2 (8·7) | 0 (0·0) | – | – | |||
Data are given as n or n (%) unless otherwise noted.
3.3. Primary endpoint analysis
During the 12-month period, 86·8% of suspected cases in Lao PDR and 100·0% in Cambodia agreed to the physician visit to confirm the diagnosis. In Lao PDR, 36·1% of PWEs agreed to home-based care by DHVes, whereas 27·8% took care of AEDs themselves and 36·1% refused treatment. In Cambodia, 100·0% of PWEs signed up for home-based care by DHVes (Fig. 1).
The TG significantly decreased in the intervention vs. control areas in both countries (Table 2). The same significant results, though with a slightly greater effect, were observed for generalized epilepsy. The kinetics of the intervention were not linear. Out of the total number of PWEs identified, 98·3% of the PWEs were identified during the first 6 months in Cambodia and 67·8%in Lao PDR (Appendix 9).
Table 2.
Epilepsy treatment gap in the intervention and control areas during a 12-month period in Lao PDR (2014–2015) and Cambodia (2016–2017).
| Area | LAO PDR strategy Prevalence: 7·7‰ (95% CI 5·3–10·7) Generalized seizures: 63·6% (95% CI 45·1–82·2) |
CAMBODIA strategy Prevalence: 5·8‰ (95% CI 4·6–7·0) Generalized seizures: 90·6% (95% CI 80·1–100·0) |
||||
|---|---|---|---|---|---|---|
| Intervention | Control | p | Intervention | Control | p | |
| Population > 2 years old | 53,434 | 94,653 | – | 29,655 | 57,451 | |
| OVERALL | ||||||
| Expected cases, n (range*) | 418 (283–572) | 788 (501–1013) | – | 172 (136–207) | 333 (264–402) | – |
| Cases under treatment at baseline, n | 21 | 24 | – | 0 | 0 | – |
| Total cases at endline, n | 57 | 30 | – | 60 | 27 | – |
| Cases under treatment at endline, n | 44 | 28 | 60 | 27 | ||
| Treatment gap at baseline, % (range) | 95·0 (92·6–96·3) | 96·9 (95·2–97·6) | 0·063 | 100·0 | 100·0 | – |
| Treatment gap at endline, % (range) | 89·5 (84·4–92·3) | 96·4 (94·4–97·2) | < 0·0001 | 65·1 (55·8–71·0) | 91·9 (89·8–93·3) | < 0·0001 |
| Treatment gap reduction, % (range) | 5·5 (4·0–12·2) | 0·5 (0·4–0·8) | < 0·0001 | 34·9 (29·0–44·1) | 8·1 (6·7–10·2) | < 0·0001 |
| GENERAL SEIZURES | ||||||
| Expected cases, n (range*) | 266 (188–344) | 501 (355–648) | – | 156 (138–187) | 302 (267–333) | – |
| Cases under treatment at baseline, n | 9 | 16 | – | 0 | 0 | – |
| Total cases at endline, n | 36 | 18 | 58 | 26 | ||
| Cases under treatment at endline, n | 36 | 18 | – | 58 | 26 | – |
| Treatment gap at baseline, % (range) | 96·6 (95·2–97·4) | 96·8 (95·5–97·5) | 0·888 | 100·0 | 100·0 | – |
| Treatment gap at endline, % (range) | 86·5 (80·6–89·5) | 96·4 (94·2–97·2) | < 0·0001 | 62·8 (58·0–69·0) | 91·4 (90·3–92·2) | < 0·0001 |
| Treatment gap reduction, % (range) | 10·1 (7·1–16·0) | 0·4 (0·4–2·6) | < 0·0001 | 37·2 (31·0–42·0) | 8·6 (7·8–9·7) | < 0·0001 |
Expected cases were estimated using the value and confidence intervals of the published prevalence
3.4. Secondary endpoints (Table 3)
Table 3.
Secondary endpoints for PWEs identified during a 12-month period and under treatment, in Lao PDR (2014–2015) and Cambodia (2016–2017).
| LAO PDR strategy |
CAMBODIA strategy |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention area (n=23) |
Control area (n=4) |
*p | Intervention area (n=60) |
Control area (n=27) |
*p | |||||||||
| First visit | Last visit | p | First visit | Last visit | p | First visit | Last visit | p | First visit | Last visit | p | |||
| Adherence to Anti-Epileptics Drugs | ||||||||||||||
| Adherent | 13 (56·5) | 20 (87·0) | 0·023a | 2 (50·0) | 3 (75·0) | 1·000a | 1·000c | 45 (75·0) | 47 (78·3) | 0·480a | 22 (81·5) | 21 (77·8) | 1·000 a | 1·000 c |
| Non adherent | 10 (43·5) | 3 (13·0) | 2 (50·0) | 1 (25·0) | 15 (25·0) | 13 (21·7) | 5 (18·5) | 6 (22·2) | ||||||
| Evolution of seizures number | ||||||||||||||
| Decreased | 17 (73·9) | 5 (21·7) | <0·0001b | 3 (75·0) | 1 (25·0) | 0·480a | 1·000 c | 38 (63·3) | 5 (8·3) | <0·0001b | 16 (59·3) | 2 (7·4) | <0·0001b | 0·785 c |
| Stable + Increased | 6 (26·1) | 18 (78·3) | 1 (25·0) | 3 (75·0) | 22 (36·7) | 55 (91·7) | 11 (40·7) | 25 (92·6) | ||||||
| increased | 1 (4·3) | 2 (8·7) | – | 0 | 0 | – | – | 2 (3·3) | 3 (5·0) | – | 1 (3·7) | 1 (3·7) | – | – |
| Stigma | ||||||||||||||
| Reporting no stigma | 9 (39·1) | 13 (56·5) | 0·134 a | 1 (25·0) | 1 (25·0) | 1·000 a | 1·000 c | 35 (58·3) | 39 (65·0) | 0·134 a | 11 (40·7) | 12 (44·4) | 1.000 a | 1.000 c |
| Reporting stigma | 14 (60.9) | 10 (43.5) | 3 (75.0) | 3 (75.0) | 25 (41.7) | 21 (35.0) | 16 (59.3) | 15 (55.6) | ||||||
| Cost-effectiveness | Intervention area (n=23) | Control area (n=4) | Intervention area (n=60) | Control area (n=27) | ||||||||||
| Directs costs (total) | 15,415.80 | 2777.60 | 13,868 | 2807.60 | ||||||||||
| per patient for 1 month | 55·85 | 57·86 | 19.26 | 8·66 | ||||||||||
| per 10,000 person·year | 2885.02 | 293.45 | 4676.45 | 488.69 | ||||||||||
| Cost per case under treatment | 665.17 | ref | 335.16 | ref | ||||||||||
| Cost per case adhering to treatment | 742.99 | ref | 425.83 | ref | ||||||||||
First visit: follow-up at home or at PHC for first replenishment of AEDs (one month after confirmation); Last visit: during endline survey
difference between evolutions in intervention and control areas
Mac Nemar test with Yates correction
Mac Nemar test
Fisher's exact test; ICER: Incremental cost-effectiveness ratio
In both countries, the PWEs who received drug treatment, were adhering to it (>75%), and showed a decrease in seizure frequency, and then stabilization. Overall, more than 40% of PWEs reported stigma. In the intervention areas, these three indicators showed positive changes between the first visit (1 month after starting treatment) and the last visit. The changes in stigma were not statistically significant (Table 3).
The costs associated with the strategy implemented in Lao PDR, compared with the control area, is 665 USD per case under treatment and 742 USD per case adhering to treatment. The costs associated with the strategy implemented in Cambodia, compared with control area, is 335 USD per case under treatment and 425 USD per case adhering to treatment. The cost analysis for the first 6 months showed similar results (Appendix 10).
The medical competencies of health personnel were improved in both countries. Neurologists from the central hospitals validated all diagnoses of epilepsy and globally their types (generalized or focal) (Appendix 11). Knowledge levels improved for physicians, primary health center staff, and DHVes. Although beliefs that epilepsy is a form of insanity was still frequent in the general population and among the PWEs at the end of the intervention, knowledge about treatment had improved for all (Appendix 12).
4. Discussion
In most low- and middle-income countries, the epilepsy TG is a major public health concern as more than 90% of PWEs are not receiving appropriate treatment. Low identification and diagnosis rates and poor adherence to treatment are the main obstacles to reducing the TG. We have performed two community intervention studies in which DHV identified suspected PWE, facilitated the confirmation of their diagnosis and assisted with their ongoing treatment. The proposed strategy in Cambodia was more effective than that of Lao PDR, resulting in a significant reduction in the TG of 34·0%. The percentage of PWEs adherent to treatment increased, and more than half of PWEs reported reduced seizure frequency and reduced stigma.
This study used a quasi-experimental evaluation methodology, which is rather innovative in intervention studies in epilepsy; it aims to specifically assess the contribution of DHVes to the identification and home follow-up of PWEs by keeping other important determinants constant (i.e., IEC, availability of medicine, staff training). The official definition of community health workers in the International Labor Organization International Standard Classification of Occupations (ISCO) refers to community health workers as a distinct occupational group within the associate health professionals category: community health workers provide health education and referrals for a wide range of services, and provide support and assistance to communities, families and individuals with preventive health measures and gaining access to appropriate curative health and social services. They create a bridge between providers of health, social and community services and communities that may have difficulty in accessing these services [21]. But as WHO (2018) highlights also, unclear nomenclature and classification complicate the policy discourse on CHWs: the term “community health workers” is often used in a non-specific way, referring to a diverse typology of lay and educated, formal and informal, paid and unpaid health workers. For this reason, although we recognize that DHVes are fully integrated into the broad category of CHWs, we have preferred to continue using the term DHVes, which was chosen at the initiation of this project, which began before the publication of the WHO guidelines. We underline that home visiting and active identification were major tasks of these workers in the project.
In most published studies, the intervention consisted of several components, which does not provide information on the effectiveness of each component. A single difference between the two countries makes it possible to evaluate the two strategies. The audit carried out by external neurologists showed the accuracy of the diagnoses made by physicians, reflecting the high level of skills acquired during training. The study includes a health-economics evaluation, which is quite novel in this type of study in LMICs, though based only on direct costs, as indirect costs are extremely difficult to collect and price in this context. A recent review of the models of community-based primary care for epilepsy in LMICs found only 24 reports the majority addressing only active convulsive epilepsy only and without evaluating impact assessment at the local level [22].
Our study has several limitations. The study areas were selected on the basis of feasibility and of the representativeness of the countries’ rural areas, in agreement with government authorities, who also provided significant support to the project. This is not an experimental study at an individual level, which would be yet more valid but more complex to conduct. We have chosen close areas to limit the potential differences. There were two different strategies and time periods for evaluation and personnel types doing the test of the community effectiveness of the identification and home follow-up of PWEs. It was not possible in this design to apply multivariate technique or a propensity analysis considering the possible influence of differences between areas or periods. The primary study endpoint is based on the TG estimated from the results of door-to-door studies carried out in the general population of the countries, though a few years earlier and not in the same areas.
However, these prevalence data are close to the median prevalence found in Asia in systematic reviews [6,7] and are likely to be similar in the study areas. Given that the TG is estimated based on an extrapolated denominator of expected cases, caution should be exercised interpreting TG and other clinico-epidemiological measures. These studies used a 2-year age cut-off rather than the 6-year age cut-off typically used in similar studies; we used the same 2-year age cut-off in our study, that means that some children theoretically at risk of infantile spasms/febrile seizures may have been included. It is unlikely that this had a major biasing effect on the findings.
Health interventions involving DHVes and community health workers in LMICs are not new. Their effectiveness has already been shown in several other disease areas, but mainly for communicable diseases (e.g., HIV, tuberculosis, malaria [21]). The results presented here show that this concept can be applied to chronic non-communicable diseases [23]. Recently, a study showed that proactive home visits by trained government community health workers who were linked with existing public health care infra- structure led to a greater reduction in blood pressure than usual care among adults with hypertension [24].
The reduction in the TG is significant for both strategies but much larger in Cambodia (34·9%) than in Laos (5·5%).
In Lao PDR, the results of the intervention, though significant, were limited. In this country, the DHVes were health center staff and had to travel to each village once a month. This did not allow for sufficient identification of suspected epilepsy cases. In addition, as they did not live in these villages, they did not know the inhabitants well, leading to some reluctance within the village community. Furthermore, In Cambodia the DHVes had about 988 subjects to cover (mean population of the villages) compared to about 3143 in Lao PDR. Surprisingly, the proportion of PWEs refusing care was high despite offering home delivery of medicine [25]. In Cambodia, the DHVes lived in the villages. These people were already trained in other health interventions, such as vaccination. They were able to easily include additional activities after a short training period, without disrupting the existing system.
Community-based epilepsy care is recommended by the WHO, which has implemented several projects in LMICs over the last two decades. Community-based intervention programs have been conducted in China [26], Vietnam, Myanmar, Mozambique, and Ghana [27]. However, the results of these community-based interventions have not yet been published except for Ghana, where the "epilepsy contact coverage" (i.e., the proportion of the target population in contact with services) has improved from 14·5% to 38·3% in 5 years [28], but without confirmation that patients were actually taking their medications. In Asia, the TG has been documented in many countries [6], but little evidence has been published regarding the impact of community-based screening and care projects. We are aware of the Global Campaign Against Epilepsy demonstration project in rural China, which contributed to a decrease in the TG from 62·6% to 49·8% in a population of 51,644 people [26].
In Cambodia, nearly all cases were identified and confirmed within the first 6 months of the intervention, which brings into question the need for a longer intervention. However, the vast majority of confirmed cases were generalized epilepsy, which is well known by the population and more easily identifiable. This form is also the most serious and must be treated as a priority. Some epidemiological studies now focus only on active and convulsive forms [29], [30], [31]. The value of additional training on focal epilepsy before or at 6 months could be a further improvement of our current intervention and should be evaluated. Furthermore, regular reminders to identify incident cases of epilepsy could also improve the intervention.
The secondary end-points apply to only small numbers of patients in intervention and control areas. There is therefore very limited statistical power and any inferences should be made with great caution.
The health-economics analysis shows that it is possible to increase the number of cases treated at a relatively low additional cost. It would be possible to further reduce costs by including, within the scope of using DHVes, other chronic non-communicable diseases that are easily identifiable and can be managed in the community (e.g., hypertension).
Implementation of the intervention will require allocating additional resources to epilepsy, which could be estimated based on this study. There is still a need to produce more data of this type from the patients’ perspective, integrating indirect costs and benefits (e.g., the patient's productivity back at work), and evaluating if increasing the workload of community health workers could divert them from other tasks and affect the quality of service. These studies could help raise awareness among governments of the value of investing in the management of epilepsy and prioritizing it over other actions.
Contributors
All authors contributed significantly to this paper and participated in several brainstorming sessions to optimize and adjust the analysis and interpretation. Farid Boumediene and Pierre-Marie Preux established the objectives and the study design. All other authors contributed to case recruitment and data acquisition. Farid Boumediene and Pierre-Marie Preux performed the datamanagement, validation of the data and statistical analyses. All authors contributed to interpretation of the results. Clémence Thèbaut supervised the medio-analysis interpretation. Farid Boumediene and Pierre-Marie Preux wrote the first manuscript version. All authors contributed to the final manuscript.
Declaration of Competing Interest
Dr. Boumediene, Dr. Preux, Dr. Hun, Dr. Thebaut, Dr. Chhour, Dr. Chum, Dr. Ros, and Dr. Samleng report grants from Sanofi Global Health Programs, during the conduct of the study; Dr. Boumediene and Dr. Preux report grants from Sanofi Global Health Programs, outside the submitted work; Dr. Chivorakoun, Dr. Souvong, Dr. Bounlu, and Dr. Vorachit report grants and non-financial support from Sanofi Global Health Programs and grants from Grand Challenges Canada during the conduct of the study.
Acknowledgments
Acknowledgements
We thank the two Ministries of Health for their involvement and motivation in involving health actors from district hospitals and health centers. We thank the members of two associations that facilitated this fieldwork: ACLE (Association Cambodgienne de Lutte contre l'Epilepsie) in Cambodia and APE (Association for Patients with Epilepsy) in Lao PDR.
Data sharing statement
Study protocol including statistical analysis plan is available at the following URL: https://www.unilim.fr/ient/wp-content/uploads/ECIR/LANCET_Boumediene_Protocol
Data collected for the study (deidentified participant data) could be accessed on demand to the corresponding author after signature of a data signature agreement and upon submission of a protocol summary.
Footnotes
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2020.100042.
Appendix. Supplementary materials
References
- 1.Ngugi A.K., Bottomley C., Kleinschmidt I., Sander J.W., Newton C.R. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding D., Wang W., Wu J. Premature mortality in people with epilepsy in rural China: a prospective study. Lancet Neurol. 2006;5:823–827. doi: 10.1016/S1474-4422(06)70528-2. [DOI] [PubMed] [Google Scholar]
- 3.Newton C.R., Preux P.-M. Epilepsy. In: Academic P, editor. Neuroepidemiology in tropical health. San Diego (USA): Pierre-Marie Preux. Michel Dumas; 2017. p. 340. [Google Scholar]
- 4.Yemadje L.P., Houinato D., Quet F., Druet-Cabanac M., Preux P.M. Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia. 2011;52:1376–1381. doi: 10.1111/j.1528-1167.2011.03099.x. [DOI] [PubMed] [Google Scholar]
- 5.Meinardi H., Scott R.A., Reis R., Sander J.W. World ICotD. The treatment gap in epilepsy: the current situation and ways forward. Epilepsia. 2001;42:136–149. doi: 10.1046/j.1528-1157.2001.32800.x. [DOI] [PubMed] [Google Scholar]
- 6.Trinka E., Kwan P., Lee B., Dash A. Epilepsy in Asia: Disease burden, management barriers, and challenges. Epilepsia. 2018 doi: 10.1111/epi.14458. [DOI] [PubMed] [Google Scholar]
- 7.Mac T.L., Tran D.S., Quet F., Odermatt P., Preux P.M., Tan C.T. Epidemiology, aetiology, and clinical management of epilepsy in Asia: a systematic review. Lancet Neurol. 2007;6:533–543. doi: 10.1016/S1474-4422(07)70127-8. [DOI] [PubMed] [Google Scholar]
- 8.Mbuba C.K., Ngugi A.K., Newton C.R., Carter J.A. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49:1491–1503. doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurman D.J., Beghi E., Begley C.E. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(Suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 10.Boumédiène F., Marin B., Preux P.-M. Methodological challenges of neuroepidemiological studies in low- and middle-income countries. In: Preux P.M., Dumas M., editors. Neuroepidemiology in tropical health. Elsevier; 2017. pp. 3–12. [Google Scholar]
- 11.Tran D.S., Odermatt P., Le T.O. Prevalence of epilepsy in a rural district of central Lao PDR. Neuroepidemiology. 2006;26:199–206. doi: 10.1159/000092407. [DOI] [PubMed] [Google Scholar]
- 12.Preux P.M., Chea K., Chamroeun H. First-ever, door-to-door cross-sectional representative study in Prey Veng province (Cambodia) Epilepsia. 2011;52:1382–1387. doi: 10.1111/j.1528-1167.2011.03102.x. [DOI] [PubMed] [Google Scholar]
- 13.Morisky D.E., Green L.W., Levine D.M. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Jacoby A., Snape D., Baker G.A. Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol. 2005;4:171–178. doi: 10.1016/S1474-4422(05)01014-8. [DOI] [PubMed] [Google Scholar]
- 15.Taylor J., Baker G.A., Jacoby A. Levels of epilepsy stigma in an incident population and associated factors. Epilepsy Behav. 2011;21:255–260. doi: 10.1016/j.yebeh.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Drummond M., Claxton K., Stoddart G., Torrence G. 4th ed. Oxford University Press; UK: 2015. Methods for the economic evaluation of health care programmes. [Google Scholar]
- 17.Marseille E., Larson B., Kazi D.S., Kahn J.G., Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preux P.M. Questionnaire in a study of epilepsy in tropical countries. Bull Soc Pathol Exot. 2000;93:276–278. [PubMed] [Google Scholar]
- 19.Diagana M., Preux P.M., Tuillas M., Ould Hamady A., Druet-Cabanac M. Depistage de l'epilepsie en zones tropicales: validation d'un questionnaire en Mauritanie. Bull Soc Pathol Exot. 2006;99:103–107. [PubMed] [Google Scholar]
- 20.International League Against Epilepsy Guidelines for epidemiologic studies on epilepsy. Commiss Epidemiol Prognosis. 1993;34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x. Epilepsia. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO; 2018. WHO guideline on health policy and system support to optimize community health worker programmes. Geneva. [PubMed] [Google Scholar]
- 22.Singh G., Sharma M., Krishnan A. Models of community-based primary care for epilepsy in low- and middle-income countries. Neurology. 2020;94:165–175. doi: 10.1212/WNL.0000000000008839. [DOI] [PubMed] [Google Scholar]
- 23.Ezzati M., Pearson-Stuttard J., Bennett J.E., Mathers C.D. Acting on non-communicable diseases in low- and middle-income tropical countries. Nature. 2018;559:507–516. doi: 10.1038/s41586-018-0306-9. [DOI] [PubMed] [Google Scholar]
- 24.Jafar T.H., Gandhi M., de Silva H.A. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717–726. doi: 10.1056/NEJMoa1911965. [DOI] [PubMed] [Google Scholar]
- 25.Bounlu M., Auditeau E., Vorachit S. Explanatory factors of adherence to community-based management of epilepsy in Lao PDR. Epilepsy Behav. 2018;88:74–80. doi: 10.1016/j.yebeh.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Wang W., Wu J., Dai X. Global campaign against epilepsy: assessment of a demonstration project in rural China. Bull World Health Organ. 2008;86:964–969. doi: 10.2471/BLT.07.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Information Kit on Epilepsy - What you can do? WHO Programs on reducing the epilepsy treatment2015. Accessed on 05/03/2019 athttps://www.who.int/mental_health/neurology/epilepsy/epilepsy_global_toolkit.pdf.)
- 28.World Health Organization. "Fight against epilepsy” initiative in Ghana. WHO Programme on reducing the epilepsy treatment gap 2012–2016. ISBN: 978-9988-2-8267-72018.
- 29.Kariuki S.M., Matuja W., Akpalu A. Clinical features, proximate causes, and consequences of active convulsive epilepsy in Africa. Epilepsia. 2014;55:76–85. doi: 10.1111/epi.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngugi A.K., Bottomley C., Kleinschmidt I. Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol. 2013;12:253–263. doi: 10.1016/S1474-4422(13)70003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngugi A.K., Bottomley C., Scott J.A. Incidence of convulsive epilepsy in a rural area in Kenya. Epilepsia. 2013;54:1352–1359. doi: 10.1111/epi.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.