Abstract
The extraordinary expansion of cardiometabolic risk factors, the impact they generate in the development of hypertension and its specific phenotypes, and its implications in cardiovascular risk and therapeutic decision-making deserve an extensive and careful reflection. The aim of this review is to analyze the available evidence and gaps in the relationship between cardiometabolic risk factors and hypertension phenotypes. Overweight or obese patients, dyslipidemic, carbohydrate intolerant and type 2 diabetic patients have a significantly higher probability of suffering from high blood pressure than subjects without metabolic disorders. Masked hypertension should be systematically suspected in subjects with type 2 diabetes or metabolic disorders and borderline hypertension independently of the debate on the reproducibility of blood pressure phenotypes diagnosis. Some minor difficulties emerge to understand the phenotypes of hypertension in diabetic individuals, since clinical practice guidelines are not homogeneous in their postulates regarding the blood pressure targets at office and ambulatory and home blood pressure monitoring. The small number of diabetic hypertensive patients included in epidemiological studies, and the presence of confounding factors, such as the duration of diabetes, the quantity and type of drugs indicated for the treatment of both hypertension and diabetes, or the level of diabetes control, undermine the possibilities to draw conclusions of value for the clinical practice.
Keywords: Hypertension phenotypes, Metabolic disorders, Target organ damage, Cardiovascular prognosis
The effects of cardiometabolic risk factors in blood pressure profile behavior are not fully understood. Several studies have investigated specific aspects of this relationship in a context of an extraordinary worldwide expansion of cardiometabolic risk factors. The impact they generate in the development of hypertension and its specific phenotypes, and its implications in cardiovascular risk and therapeutic decision-making deserve an extensive and careful reflection.
Hypertension is a biological parameter with intrinsic and cause-specific variability, demonstrable both in normotensive and hypertensive subjects, this may seem to be exaggerated in some conditions and scenarios. At the same time, the frequent disparity between office and daily-life blood pressure are well-known. The aim of this review is to analyze the available evidence and gaps in the knowledge about the relationship between cardiometabolic risk factors and hypertension phenotypes.
1. Growth of cardiometabolic risk factors
The interaction of environmental factors such as low socio-economic level, limited access to health services, a low level of education and culture, high consumption of alcoholic beverages, rural and urban habits of life, air and sonic pollution, or the poor quality of drinking water have created an enabling environment to the growth of cardiometabolic risk factors.
1.1. Worldwide
The prospective cohort study PURE (Prospective Urban Rural Epidemiology) quantified and compared the association and the attributable population fraction of 14 modifiable risk factors to cardiovascular disease and mortality in individuals without prior cardiovascular disease, stratified according to countries high, medium or low income level. Risk factors were grouped into behavioral, socio-economic, psychosocial, and metabolic factors. Within the latter, high blood pressure or a history of hypertension, dysglycemia or a history of diabetes, non-HDL cholesterol, and abdominal obesity were considered. Regardless of the income category of the countries, the cardiometabolic risk factors were the ones with greatest contribution to the development of cardiovascular disease. In the global sample, the highest contribution to the population risk attributable to cardiovascular disease was high blood pressure with 22.3%, followed by high levels of non-HDL cholesterol with 8.1% [1].
1.2. In the Americas
Many epidemiological cohort studies have shown overwhelming evidence of the worldwide increase in cardiometabolic risk factors frequency. The prevalence of obesity increased about 2.77–4.71 times in men and 2.2 to 2.5 times in women from 1980 to 2014 in an analysis of 389 population studies from 37 American countries. At the same time, the frequency of diabetes rose 1.5 to a little more than 2 times both in men and female. In contrast, the prevalence of high blood pressure decreased by around 40% in North America, in both men and women, and between 20 and 30% in the rest of America. This study, beyond the evident disparities between countries, exposes the high prevalence of cardiometabolic risk factors that crosses the whole continent, and therefore, the need for a comprehensive approach to cardiovascular prevention [2].
Overweight or obese patients, dyslipidemic subjects, carbohydrate intolerant and diabetic individuals have a significantly higher probability of suffering from high blood pressure with specific phenotypes which are discussed in this manuscript.
2. Hypertension phenotypes
The terms white coat hypertension (WCH), office high blood pressure and ambulatory normal blood pressure, and masked hypertension (MH), office normal blood pressure and ambulatory hypertension were initially coined for patients not receiving antihypertensive treatment in the diagnostic stage of the disease. Subsequently, their use was extended to individuals receiving treatment for hypertension, taking a different meaning and context from their first use. Calling them isolated office hypertension or white-coat uncontrolled hypertension (WUCH) implies that blood pressure is adequately controlled on an outpatient basis and patients are responders to the therapy. Masked uncontrolled hypertension (MUCH) refers to blood pressure not meeting therapeutic goals on an outpatient basis despite appearing controlled in the office. At the same time, non-responders treated patients mean that they did not achieve blood pressure treatment targets both at office and ambulatory and are named sustained uncontrolled hypertension (SUCH) [3].
2.1. Hypertension phenotypes prevalence
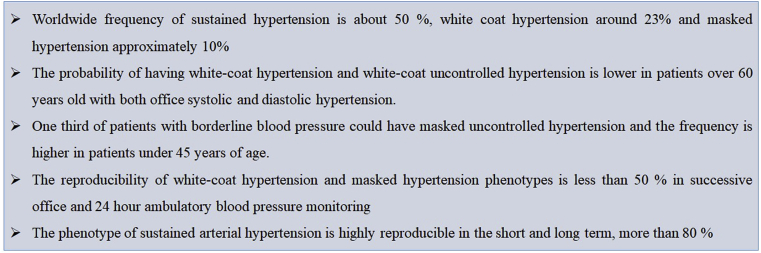
The ARTEMIS registry (international Ambulatory blood pressure Registry: TEleMonitoring of hypertension and cardiovascular rISk project) included patients from Europe, Africa, America, Asia and Australia. Sustained hypertension was detected in 49% of the sample, white coat hypertension in 23% of the cases with no difference between untreated and treated subjects, and masked hypertension in 10% of the cases (11% in untreated and 9% in treated). The most important determinants of the presence of white coat hypertension or white-coat uncontrolled hypertension were age and obesity; while for masked hypertension and masked uncontrolled hypertension were diabetic men, smokers, and people living in Asia [4].
A home blood pressure monitoring registry was carried out in two independent populations in Brazil. Masked hypertension was present in 10% of patients under 40 years of age, 13% of patients of ages between 40 and 60 years, and 20% of patients over 60; while masked uncontrolled hypertension was present in 14%, 15% and 21% of patients in the same age strata, respectively. The presence of white coat hypertension and white-coat uncontrolled hypertension was higher in isolated systolic hypertension than in isolated diastolic hypertension. Patients with both systolic and diastolic hypertension presented lower presence of white coat hypertension and white-coat uncontrolled hypertension, which means that these patients were more likely truly sustained hypertensive. At the same time, subjects with ages over 60 years showed a significantly lower presence of white coat hypertension and white-coat uncontrolled hypertension than individuals younger than 60 years. This means that older patients with office hypertension have a highly significant probability of being truly sustained hypertensive [5].
Out of 625 individuals included in the Strong Heart Study without cardiovascular disease, 38% developed hypertension in a four-year follow-up period. The probability of developing new hypertension increased by 10% for every 10 cm the waist circumference increases, and by 273% with the presence of Diabetes Mellitus. The evolution to sustained hypertension could be powerfully predicted by the detection of Diabetes Mellitus, associated with the basal values of systolic blood pressure, and the left ventricular mass index and the stroke volume measured by echocardiography [6,7].
3. Borderline blood pressure, masked blood pressure and cardiovascular risk
According to meta-analysis of twenty prospective cohort studies, the risk of having a fatal cardiovascular event increased by 28% in individuals with systolic blood pressure between 130 and 139 mm Hg and diastolic blood pressure between 85 and 89 mm Hg, compared to subjects with blood pressure lower than 120–80 mm Hg [8]. The Strong Heart Study showed individuals in these pressure ranges are at greater risk of developing hypertension, and the study by Huang et al. evidenced their higher cardiovascular risk. The Registry on Ambulatory Blood Pressure Monitoring of the Spanish Society of Hypertension helps to understand why. A sub-analysis of the registry included 14,840 hypertensive treated patients who achieved target office blood pressure, but, 31.1% of them had masked uncontrolled hypertension. In 60% of the cases, masked uncontrolled hypertension happened during daytime and nighttime, but the proportion of nighttime masked uncontrolled hypertension was twice of that of daytime. The findings were evaluated by repeating the ambulatory monitoring in a small sample of the registry on average one month after the first study, and in 87.4% of the cases the diagnosis of masked uncontrolled hypertension was reconfirmed. Although the presence of this phenotype was detected in all blood pressure levels, including those with optimal blood pressure, patients with systolic blood pressure between 130 and 139 mm Hg and diastolic blood pressure 80–89 mm Hg had the most frequency of masked uncontrolled hypertension. Independently of the amount of drugs the patients received or their cardiovascular risk, this situation was seen in 36.7% of the cases, and it was significantly higher in patients under 45 years of age. The probability of suffering masked uncontrolled hypertension, being associated with male sex and smoking as predictors of this type of blood pressure behavior, was significantly increased by the presence of obesity in 20% of the cases, and by Diabetes in 25% [9].
4. The problem of blood pressure phenotypes reproducibility
The reproducibility of different blood pressure phenotypes was addressed by de la Sierra et al. in a group of subjects naïve of treatment by repeating measurements of office and 24 h ambulatory blood pressure monitoring separated on average by three months. In the first evaluation, 17% of the sample was normotensive, 24% had white coat hypertension, 9% evidenced mask hypertension, and 50% had sustained hypertension; while in the second evaluation it changes to 6%, 21%, 10% and 53%, respectively. Thirty two and a half percent of the subjects had discordant results between the two measurements. The diagnosis of hypertension had a reproducibility of 82.2%, while of normal blood pressure was 52.5%, white coat hypertension 55.6%, and masked hypertension 47.4%. Some patients were re-categorized as sustained hypertensive as follows: 19.1% of normotensive patients, 25.9% of white coat hypertensive subjects and 33.3% of masked hypertensive. The only parameter associated to the reproducibility of the results was the time lapse between the two ambulatory blood pressure monitoring. When the evaluations were done less than one month apart, 80% of the results were the same, while if the tests were performed after a month there was only a 30% of similarity. As a conclusion the phenotype of sustained arterial hypertension is highly reproducible in the short and long term, but the phenotypes of white coat hypertension and masked hypertension only have good reproducibility in the short term (Fig. 1) [10].
Fig. 1.
Key points in hypertension phenotypes.
5. Hypertension phenotypes in patients with metabolic disorders
One of the aims of this manuscript is to thoroughly review the available evidence and gaps in the relationship between cardiometabolic risk factors and hypertension phenotypes.
5.1. Blood pressure behavior in diabetic subjects
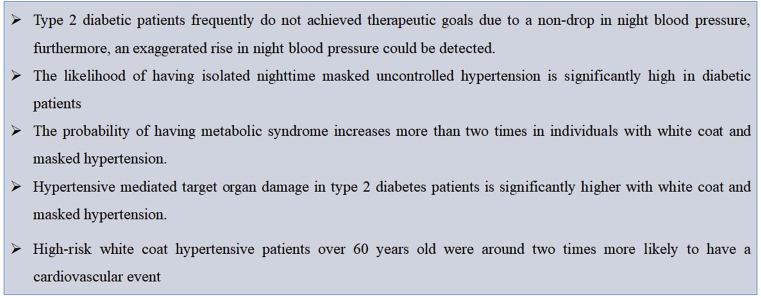
The Registry on Ambulatory Blood Pressure Monitoring of the Spanish Society of Hypertension included more than twelve thousand diabetic hypertensive subjects. Even though these patients received a significantly greater amount of antihypertensive drugs than non-diabetics subjects, the mean value of systolic blood pressure in the active daytime period and the night test period were significantly higher. There was a greater number of patients not reaching therapeutic goals, particularly during the night period, due to a higher frequency of non-drop in blood pressure (non-dipper pattern) detected in more than 60% of cases. Furthermore, an exaggerated rise in night blood pressure (riser pattern) was detected in 25% of the subjects, with no dependency of the body mass index and the history of cardiovascular disease. Thus, data takes to the conclusion that the night-time behavior of blood pressure in diabetic patients is expressed with a specific and characteristic phenotype [11].
5.2. Diabetes and hypertension phenotypes
The association between diabetes and ambulatory blood pressure phenotypes was evaluated in a cross-sectional analysis of subjects from the Jackson Heart Study receiving drug treatment for high blood pressure. The average body mass index was significantly higher while the mean value of HDL-C and office diastolic blood pressure were significantly lower in diabetic patients. This last data allows to infer that the pulse pressure is higher in diabetic subjects when compared to non-diabetics, and in general terms, the stiffness of large central arteries is increased. Despite receiving a greater number of antihypertensive drugs, diabetic individuals presented significantly higher systolic blood pressure values in ambulatory monitoring than non-diabetics ones, both in the 24-h period as well as in the day and nighttime. After multivariate adjustment, diabetic patients were shown to be 32% more likely to have daytime hypertension, a 46% increase in the risk of having masked uncontrolled hypertension, and a 39% increased likelihood of suffering isolated nighttime masked uncontrolled hypertension. However, if the systolic blood pressure in the daytime period is added to the adjustment, the association of the other phenotypes of hypertension with diabetes is reduced [2].
5.3. Phenotypes frequency in metabolic patients
According to the Registry on Ambulatory Blood Pressure Monitoring of the Spanish Society of Hypertension, by applying blood pressure thresholds of 140–90 mm Hg at office and daytime ambulatory blood pressure of 135–85 mm Hg, the frequency of white coat hypertension was reduced from 33% to 15.4% when cut-off points of 130–80 mm Hg were used at office and 125-75 mm Hg for daytime ambulatory blood pressure. Diabetic and non-diabetic patient information was not compared, and the therapeutic status of the subjects was not reported [11]. The Jackson Heart Study did report this data and the frequency of white-coat uncontrolled hypertension was significantly lower in diabetic patients (25.5%) than in non-diabetic individuals (35.5%) [12], [13]. According to the PAMELA study (Pressione Arteriose Monitorate E Loro Associazioni), 8.5% of the sample presented white coat hypertension. These subjects had values of total cholesterol, serum triglycerides and body mass index significantly higher than normotensive individuals, and at the same time, HDL-cholesterol was significantly lower and similar to the average levels of sustained hypertensive patients. The frequency of diabetes and carbohydrate intolerance, the average plasma glucose values, as well as the prevalence of metabolic syndrome were also significantly higher in individuals with white coat hypertension [14].
A sample of diabetic subjects evaluated for high blood pressure in Japan was 42% less likely to have white coat hypertension than non-diabetics patients. The frequency of white coat hypertension in diabetic patients was 22.2% in risers, 16.1% in non-dippers and 12.2% in dippers, without statistically significant differences with non-diabetic subjects, which was 38%; 26.9% and 19.5%, respectively [15].
The previous studies described some specific considerations that must be taken into account when assisting hypertensive patients with metabolic disorders. First, overweight or obese patients, dyslipidemic individuals, carbohydrate intolerant and type 2 diabetic subjects have a significantly higher probability of suffering from high blood pressure than subjects without these metabolic disorders. However, even in the lower stages of hypertension, an office high blood pressure subject with metabolic disorders must be suspected of having sustained hypertension rather than white coat hypertension, an opposite situation to what happens in the general population. Finally, in individuals with borderline hypertension who have type 2 diabetes, the presence of masked hypertension should be systematically suspected, and therefore, there is a precise indication to evaluate the blood pressure behavior outside the doctor’s office. In this context, however, understanding the phenotype of hypertension in diabetic individuals derive in some minor difficulties, since clinical practice guidelines are not homogeneous in their postulates regarding the blood pressure values at office to diagnose and treat high blood pressure. There are not established guidelines whether the cut-off values for ambulatory and home blood pressure monitoring should follow the practices of the general population or should be specific for diabetic subjects [16,17]. The possibilities to draw conclusions of value for the clinical practice are undermined by the small number of diabetic hypertensive patients included in epidemiological studies and the presence of confounding factors, such as the duration of diabetes, the quantity and type of drugs indicated for the treatment of both hypertension and diabetes, or the level of diabetes control.
6. Phenotypes of hypertension, metabolic disorders and hypertensive mediated target organ damage
Epidemiological studies have shown a close relationship between metabolic disturbances and the different phenotypes of blood pressure, at the same time, this interaction could have impact on the development of target organ damage, these issues will be addressed in the following paragraphs.
6.1. Metabolic risk and hypertension phenotypes
One of the key challenges to be solved is the impact of the typical phenotypes previously described of hypertension on metabolic disorders and target organ damage [18]. The PAMELA study assessed whether people with white coat hypertension and masked hypertension had also an increased risk of having type 2 diabetes. After 10 years of follow-up, patients with white coat hypertension by ambulatory blood pressure monitoring had a significant increase in risk of having type 2 diabetes (2.88 times), in comparison with those with masked hypertension (2.71 times), without significant differences to sustained hypertension. White coat hypertension at home blood pressure monitoring increased the risk by 2.99 times. In the multivariate analysis, the most important independent predictor of new diabetes or glucose intolerance development was the baseline blood glucose level. Body mass index and 24-h and home diastolic blood pressure were other independent predictors. This could imply that both white coat hypertension and masked hypertension lose statistical significance to the risk of developing new disorders of carbohydrate metabolism when adjusted for other metabolic variables. Therefore, part of the increased cardiovascular risk of these two phenotypes of hypertension could be better linked to the level of blood pressure than to the alterations of the metabolism of carbohydrates [19].
After doing another sub-analysis of the PAMELA study, it can be said that 16.2% of the selected patients presented metabolic syndrome according to the criteria of ATP III (Adult Treatment Panel III) of the National Program Report of Cholesterol Education (NCEP). The basal frequency of metabolic syndrome was 6.5% in true normotensive patients, 12.4% in white coat hypertensive patients, 15.1% in masked hypertensive patients, and 14.2% in sustained hypertensive patients. After 10 years of follow-up, it is noticed that 8.8% of the subjects developed a new metabolic syndrome. In the multivariate analysis, triglyceridemia, female gender, waist circumference, HDL-C levels, 24-h mean systolic pressure, and blood glucose were independently associated with the probability of developing metabolic syndrome. Interestly enough, only ambulatory blood pressure monitoring showed an association with the risk of developing metabolic syndrome, without significant evidence with home blood pressure monitoring. After carrying out an analysis adjusted by age and sex, the probability of suffering from metabolic syndrome increased significantly when the criteria derived from ambulatory blood pressure monitoring were applied. The increase was of 2.03 times in individuals with white coat hypertension; 2.55 times in patients with masked hypertension and 2.28 in sustained hypertension compared to normotensive patients. And only white coat hypertension, with an increase in risk of 2.16 times, reached statistical significance when home blood pressure monitoring was used to compare with the normotensive patients [20].
6.2. Hypertension phenotypes and target organ damage
The meta-analysis of 25 epidemiological studies showed that the average left ventricular mass index was 88.1 ± 1.8 grs/m2 in normotensive, 95.7 ± 1.8 grs/m2 in white coat hypertensive people, and 109.2 ± 2.5 grs/m2 in sustained hypertensive patients, with statistically significant differences between white coat hypertensive and normotensive patients, and between sustained hypertensive and white coat hypertensive patients. Thus, concluding that white coat hypertension is not a benign blood pressure phenotype [21].
In a multi-ethnic probabilistic sample of the Dallas Heart Study, the presence of hypertensive mediated target organ damage was evaluated according to the blood pressure phenotypes. After a multivariate analysis, untreated subjects with white coat hypertension and sustained hypertension had a significantly higher aortic pulse wave velocity when compared to normotensive patients. Among patients receiving treatment, those with white-coat uncontrolled hypertension, masked uncontrolled hypertension and sustained hypertension had higher aortic pulse wave velocity values than normotensive subjects. White coat hypertension, masked hypertension and sustained hypertension phenotypes had higher albuminuria values compared to normotension, regardless of their therapeutic status [22].
A study was carried out on 304 diabetic on-hypertension treatment patients of more than 10 years of evolution and with an average glycosylated hemoglobin of 7.9%. Half of the patients were obese and a fourth of the individuals had a history of major cardiovascular events. The findings show that the frequency of diabetic nephropathy was 21.3% in controlled hypertension, 37.2% in white-coat uncontrolled hypertension, 25.5% in masked uncontrolled hypertension and 44.7% in sustained hypertension, reaching statistically significant differences just between sustained and controlled hypertensive patients. No statistically significant differences were detected between the four groups in terms of indexed left ventricular mass, indexed left atrial volume, or diastolic function parameters [23].
7. Prognosis of hypertension phenotypes in patients with metabolic disorders
Independently of the previous discussion, one of the main knowledge gaps is the relationship between hypertension phenotypes in metabolic patients and hard end-points. Few epidemiological studies and meta-analysis have delved in this topic.
A meta-analysis with an average follow-up of 8 years uncovered that mortality from cardiovascular disease was 1.2% in normotensive subjects, 4% in patients with white coat hypertension, and 6.6% in untreated sustained hypertensive patients. This implies an increase in cardiovascular risk of 270% in individuals with white coat hypertension compared to normotensive patients, and a 53% lower risk in relation to untreated sustained hypertensive patients. Patients with white coat hypertension had 64% less risk of a stroke than sustained untreated hypertensive patients and a similar risk compared to normotensive patients. No attempt was made in this study to identify the variables that could lead to the development of sustained hypertension or increased cardiovascular risk; therefore, more exhaustive reviews are needed [24].
During a population study that considered eleven of the twelve IDACO (International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes) cohorts, untreated subjects with white coat hypertension and normotensive controls matched by age from the total sample were stratified into five levels of cardiovascular risk according to the Hypertension European Guidelines. The presence of up to 2 cardiovascular risk factors was considered low risk, and subjects with 3–5 cardiovascular risk factors, diabetes, or a history of cardiovascular disease were considered high risk. After a little more than ten years of follow-up, high-risk white coat hypertensive patients were 2.06 times more likely to suffer a cardiovascular event (cardiovascular mortality, sudden death, non-fatal stroke, non-fatal myocardial infarction, coronary revascularization, non-fatal heart failure). Individuals older than 60 years had 2.19 times more risk, and younger subjects did not show significant differences. High cardiovascular risk subjects over 60 years of age with white coat hypertension represent approximately one over six subjects with this hypertensive phenotype [25].
In a more recent meta-analysis, in order to determine the potential cardiovascular risk associated to white coat hypertension, Huang et al. considered individuals without antihypertensive treatment with an average follow-up of 9.6 years, and subjects receiving antihypertensive drugs with an average follow-up of 5.3 years [26,27]. White coat hypertension was associated with a 38% increased risk of cardiovascular disease in cohorts of untreated individuals, but no increased risk was observed in patients who had achieved therapeutic goals with drug therapy. Likewise, hypertensive patients with white coat phenotype who did not receive antihypertensive treatment had a significant increase in all-cause mortality by 20% when compared to those with normal blood pressure. It is recognized that other metabolic risk factors such as carbohydrate intolerance, overweight and obesity, or atherogenic dyslipidemia are significantly more frequent in individuals with white coat hypertension than in normotensive patients. However, adjustments for co-variables carried out in these cohorts greatly reduced confounding factors that may influence the association between white coat hypertension and cardiovascular morbidity and mortality [28]. Furthermore, small differences in ambulatory pressures can only partially explain the magnitude of the impact of this hypertensive phenotype on the cardiovascular prognosis of untreated subjects (Fig. 2).
Fig. 2.
Key findings in hypertension phenotypes and metabolic disorders.
8. Implications for preventive cardiology practice
The potential disparity between office and out-of-office hypertension diagnosis is well-known since early in the 21st. Patients with metabolic disorders have shown the greatest discordances in blood pressure values. White coat hypertension and white-coat uncontrolled hypertension are more frequent in isolated systolic or diastolic hypertension and in patients younger than 60 years old, but, patients with cardiometabolic diseases are less-likely to have these hypertension phenotypes. Diabetic and obese patients with borderline blood pressure had significantly higher probability of having masked hypertension or masked uncontrolled hypertension [29]. Hypertensive subjects with metabolic disorders have a significant higher frequency of hypertensive mediated organ damaged and cardiovascular events, even white coat hypertensive diabetic subjects are at high cardiovascular risk. These epidemiological data support the recommendation of measuring blood pressure on daily-life by ambulatory or home blood pressure monitoring to fit hypertension diagnosis and management [16,17]. Metabolic disorders are a platform in which hypertensive phenotypes are expressed in a more extreme way and with a greater impact on medical decision-making.
9. Conclusions
The profile of cardiovascular risk factors has changed dramatically in recent decades. The inadequate lifestyle habits have a great impact on the increase of overweight, obesity, and therefore type 2 diabetes mellitus and atherogenic dyslipidemia. This epidemiological transition is reflected in the phenotypes in which high blood pressure is expressed. Despite debatable reproducibility in the diagnosis of these disease models, a higher frequency of masked hypertension and uncontrolled masked hypertension appears to be apparent in metabolic patients. At the same time, hypertensive patients have shown a greater predisposition to develop cardiometabolic disorders. Because of this, there is a greater risk of cardiovascular events, and therefore, a specific approach to high blood pressure and metabolic risk factors management is required.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Yusuf S., Joseph P., Rangarajan S. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395:795–808. doi: 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC)—Americas Working Group Trends in cardiometabolic risk factors in the Americas between 1980 and 2014: a pooled analysis of population-based surveys. Lancet Glob Health. 2020;8:e123–e133. doi: 10.1016/S2214-109X(19)30484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien E., Parati G., Stergiou G. European society of hypertension working group on blood pressure M. European society of hypertension position paper on ambulatory blood pressure monitoring. J. Hypertens. 2013;31:1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 4.Omboni S., Aristizabal D., De la sierra A. On behalf of ARTEMIS (international Ambulatory blood pressure Registry: TEleMonitoring of hypertension and cardiovascular rISK project) Investigators. Hypertension types defined by clinic and ambulatory blood pressure in 14143 patients referred to hypertension clinics worldwide. Data from the ARTEMIS study. J. Hypertens. 2016;34:2187–2198. doi: 10.1097/HJH.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 5.Feitosa A.D.M., Mota-Gomes M.A., Barroso W.S. Relationship between office isolated systolic or diastolic hypertension and white-coat hypertension across the age spectrum: a home blood pressure study. J. Hypertens. 2020;38:663–670. doi: 10.1097/HJH.0000000000002320. [DOI] [PubMed] [Google Scholar]
- 6.De Marco M., de Simone G., Roman M.J. Cardiovascular and metabolic predictors of progression of prehypertension into hypertension. The Strong heart study. Hypertension. 2009;54:974–980. doi: 10.1161/HYPERTENSIONAHA.109.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parati G., Stergiou G., O’Brien E. European society of hypertension working group on blood pressure monitoring and cardiovascular variability. European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014;32:1359–1366. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y., Su L., Cai X. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. Am. Heart J. 2014;167:160–168. doi: 10.1016/j.ahj.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Banegas J.R., Ruilope L.M., de la Sierra A. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur. Heart J. 2014;35:3304–3312. doi: 10.1093/eurheartj/ehu016. [DOI] [PubMed] [Google Scholar]
- 10.de la Sierra A., Vinyoles E., Banegas J.R. Short-term and long-term reproducibility of hypertension phenotypes obtained by office and ambulatory blood pressure measurements. J. Clin. Hypertens. 2016;18:927–933. doi: 10.1111/jch.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorostidi M., de la Sierra A., Gonzàlez-Albarraìn O. On behalf of the Spanish Society of Hypertension ABPM Registry investigators. Abnormalities in ambulatory blood pressure monitoring in hypertensive patients with diabetes. Hypertens. Res. 2011;34:1185–1189. doi: 10.1038/hr.2011.100. [DOI] [PubMed] [Google Scholar]
- 12.Bromfield S.G., Shimbo D., Bertoni A.G., Sims W., Carson A.P., Muntner P. Ambulatory blood pressure monitoring phenotypes among individuals with and without diabetes taking antihypertensive medication: the Jackson Heart Study. J. Hum. Hypertens. 2016;30:731–736. doi: 10.1038/jhh.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas SJ, Booth 3rd JN, Bromfield SG. Clinic and Ambulatory Blood Pressure in a Population-Based Sample of African Americans: The Jackson Heart Study. J Am Soc Hypertens. 2017;11:204–212. doi: 10.1016/j.jash.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sega R., Trocino G., Lanzarotti A. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study) Circulation. 2001;104:1385–1392. doi: 10.1161/hc3701.096100. [DOI] [PubMed] [Google Scholar]
- 15.Eguchi K., Pickering T.G., Hoshide S. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am. J. Hypertens. 2008;21:443–450. doi: 10.1038/ajh.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whelton P.K., Carey R.M., Aronow W.S. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary. A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2017;2018(71):2199–2269. [Google Scholar]
- 17.Williams B., Mancia G., Spiering W. ESC/ESH guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European society of cardiology (ESC) and the European society of hypertension (ESH) Eur. Heart J. 2018;2018(39):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 18.Franklin S.S., Wong N. The complexity of masked hypertension: diagnostic and management challenges. Curr. Hypertens. Rep. 2014;16:474. doi: 10.1007/s11906-014-0474-4. [DOI] [PubMed] [Google Scholar]
- 19.Mancia G., Bombelli M., Facchetti R. Increased long-term risk of new-onset diabetes mellitus in white-coat and masked hypertension. J. Hypertens. 2009;27:1672–1678. doi: 10.1097/HJH.0b013e32832be5f9. [DOI] [PubMed] [Google Scholar]
- 20.Cuspidi C., Facchetti R., Bombelli M. Risk of new-onset metabolic syndrome associated with white-coat and masked hypertension: data from a general population. J. Hypertens. 2018;36:1833–1839. doi: 10.1097/HJH.0000000000001767. [DOI] [PubMed] [Google Scholar]
- 21.Cuspidi C., Rescaldani M., Tadic M., Sala C., Grassi G., Mancia G. White-coat hypertension, as defined by ambulatory blood pressure monitoring, and subclinical cardiac organ damage: a meta-analysis. J. Hypertens. 2015;33:24–32. doi: 10.1097/HJH.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 22.Tientcheu D., Ayers C., Das S.R. Target organ complications and cardiovascular events associated with masked hypertension and white-coat hypertension. Analysis from the Dallas heart study. J. Am. Coll. Cardiol. 2015;66:2159–2169. doi: 10.1016/j.jacc.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiria L.F., Severo M.D., Ledur P.S. White coat effect and masked uncontrolled hypertension in treated hypertensive-diabetic patients: prevalence and target organ damage. J. Diabetes. 2015;7:699–707. doi: 10.1111/1753-0407.12231. [DOI] [PubMed] [Google Scholar]
- 24.Briasoulis A., Androulakis E., Palla M., Papageorgiou N., Tousoulis D. White-coat hypertension and cardiovascular events: a meta-analysis. J. Hypertens. 2016;34:593–599. doi: 10.1097/HJH.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 25.Franklin S.S., Thijs L., Asayama K. IDACO investigators. The cardiovascular risk of white-coat hypertension. J. Am. Coll. Cardiol. 2016;68:2033–2043. doi: 10.1016/j.jacc.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y., Huang W., Mai W. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J. Hypertens. 2017;35:677–688. doi: 10.1097/HJH.0000000000001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancia G. White-coat hypertension: growing evidence in favour of its adverse prognostic significance. J. Hypertens. 2017;35:710–712. doi: 10.1097/HJH.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 28.Mancia G., Facchetti R., Bombelli M., Grassi G., Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–853. doi: 10.1161/01.HYP.0000215363.69793.bb. [DOI] [PubMed] [Google Scholar]
- 29.Frankin S.S., O`Brien E., Staessen J.A. Masked hypertension: understanding its complexity. Eur. Heart J. 2017;38:1112–1118. doi: 10.1093/eurheartj/ehw502. [DOI] [PubMed] [Google Scholar]