Abstract
Background
There are limited population-wide trend data on kidney failure and kidney replacement therapy (KRT) in people with diabetes. We conducted a retrospective cohort study to report incidence trends of kidney failure and KRT and related mortality in people with diabetes in Hong Kong between 2002 and 2015.
Methods
We used territory-wide electronic medical records including laboratory, diagnostic and procedural data to identify people with kidney failure and KRT. We used Joinpoint regression models to estimate the average annual percent change (AAPC) of kidney failure and KRT incidence for entire study period, and annual percent change (APC) for each linear trend segment, along with 1-year and 5-year mortality rates.
Findings
During 4.9 million person-years of follow-up of 712,222 people with diabetes, 31,425 developed kidney failure, among whom 23.0% (n=7,233) received KRT. The incidence of kidney failure declined by 46.8% from 2002 to 2007 (APC: -11.6, 95% CI: -16.3, -6.7), then flattened from 2007 to 2015 (APC: -0.9, 95% CI: -3.1, 1.3). The incidence of KRT remained constant (AAPC: -1.6, 95% CI: -4.4, 1.2). The 1-year mortality rates declined statistically significantly after both kidney failure and KRT. The 5-year mortality rates declined after kidney failure but the decline was not statistically significant after KRT.
Interpretation
The findings of our study highlight the importance of developing new strategies to prevent a looming epidemic of kidney failure in people with diabetes in Hong Kong.
Funding
Asia Diabetes Foundation
Key words: diabetes, kidney failure, kidney replacement therapy, trend, incidence, mortality
Abbreviations: AAPC, Average annual percent change; APC, Annual percent change; EMR, Electronic medical record; HA, Hospital Authority; HD, Hemodialysis; HKDSD, Hong Kong Diabetes Surveillance Database; KRT, Kidney replacement therapy; PD, Peritoneal dialysis; RAAS, Renin-angiotensin-aldosterone system; RAMP-DM, Risk Assessment and Management Programme-Diabetes Mellitus
Research in context.
Evidence before this study
We searched PubMed and Google Scholar up to September 2020, for reviews and original research published in English using the search terms “trends” AND “diabetes” AND (“chronic kidney disease” OR “end-stage kidney disease” OR “end-stage renal disease” OR “kidney failure”). A review paper summarising global trends in complications of diabetes reported that there was a steep increase in incidence of diabetes-related kidney failure in the general population between 2002 and 2015. However, the trends in kidney failure in people with diabetes have not been well explored and population-based trend data from Asia are sparse. In addition, in the absence of serial laboratory data, previous nationwide studies only reported people with treated kidney failure who were receiving kidney replacement therapy (KRT).
Added value of this study
Using territory-wide data from the Hong Kong Hospital Authority, which provides about 95% of medical services throughout Hong Kong, we reported that the incidence rates of kidney failure in people with diabetes declined from 2002 to 2007, then levelled off from 2007 to 2015, whilst the incidence of KRT remained constant during the same surveillance period. The 1-year mortality rates declined statistically significantly after both kidney failure and KRT. The 5-year mortality rates declined after kidney failure but remained stable after KRT.
Implication of all the available evidence
After improvement from 2002 to 2007, the incidence rates of kidney failure in people with diabetes in Hong Kong plateaued. Given the rapidly increasing number of people living with diabetes, the burden of diabetes-related kidney failure is anticipated to grow. There is an urgent need to identify factors contributing to the unchanged kidney failure incidence and implement new strategies to prevent a looming epidemic of kidney failure in Hong Kong.
Alt-text: Unlabelled box
1. Introduction
Kidney failure is a major contributor to chronic disability, impaired quality of life and premature death in people with diabetes[1], [2], [3] . In recent estimates, between 40% and 50% of people newly initiating kidney replacement therapy (KRT) had underlying diabetes4,5 . In Hong Kong, the proportion of people entering KRT attributable to diabetes has increased from 26.2% in 1996 to 49.6% in 20136. The rapidly rising worldwide burden of diabetes in the past 20 years has contributed to increased number of people at high risk of kidney failure and related morbidity and mortality7,8. In the United States, population surveillance revealed a decline in the incidence of kidney failure with KRT in people with diabetes across a 20-year period between 1990 and 2010 which levelled off after 20109,10. In Germany, the incidence of KRT in people with diabetes was stable between 2002 and 201611, whilst that in Australia, increased between 2002 and 201312. Population-wide studies reporting trends in incidence of kidney failure among people with diabetes in Asia are sparse. Understanding the epidemiology of kidney failure in people with diabetes allows us to evaluate the quality of diabetes care, to develop approaches to primary and secondary prevention of kidney disease, and to plan health services to address unmet needs.
Using territory-wide data from Hong Kong, we conducted a 14-year retrospective cohort study to: 1) describe trends in incidence rates of kidney failure and KRT in people with diabetes from 2002 to 2015; 2) describe trends in 1-year and 5-year mortality rates after kidney failure and KRT in people with diabetes; and 3) compare all-cause and cause-specific years of life lost between diabetes population with and without kidney failure.
2. Methods
2.1. Study population
The Hong Kong Hospital Authority (HA) is a statutory government body established in 1990, responsible for managing all public hospitals and specialist/general out-patient clinics in Hong Kong with a population of 7.3 million. Because of the heavily subsidised public healthcare system, the HA provides about 95% of medical services throughout Hong Kong13. Since 2000, the HA electronic medical record (EMR) system captures clinical information of attendees of all public hospitals and clinics. The Hong Kong Diabetes Surveillance Database (HKDSD) is a territory-wide cohort of people with diabetes identified from the HA EMR system with data currently up to 31st December 2016. Details of the HKDSD have been described previously[14], [15], [16], [17]. Briefly, people with diabetes were diagnosed based on fasting plasma glucose ≥7.0 mmol/L, glycated haemoglobin (HbA1c) ≥48 mmol/mol (6.5%), prescription of insulin or non-insulin glucose lowering drugs, or diagnosis codes for diabetes by physicians. Women with gestational diabetes were not included in the HKDSD. This study was approved by the local clinical research ethics committee (CREC Ref. No 2017.298).
2.2. Ascertainment of incident kidney failure, KRT and mortality
We identified people with kidney failure using the following criteria: 1) a minimum of two estimated glomerular filtration rates (eGFR) <15 mL/min/1.73 m2 occurring at least three months apart but within one year based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation18. The requirement of two eGFR measurements at least three months apart concurs with the current definition and staging of chronic kidney disease and precluded inappropriate inclusion of acute kidney injury19,20. A total of 24,265,042 eGFR measurements were recorded in the HKDSD and 97.1% of the study population had at least two eGFR measurements. 2) KRT including maintenance peritoneal dialysis (PD) (ICD-9 procedure code: 54.98) or hemodialysis (HD) (ICD-9 procedure code: 39.95) for at least one month, or kidney transplant (ICD-9 procedure code: 55.6). People with KRT and concurrent acute kidney injury (ICD-9 diagnosis code: 584) were excluded; or 3) hospitalisation due to kidney failure (ICD-9 diagnosis code: 585.5, 585.6 or 586); or 4) mortality due to kidney failure (ICD-10 diagnosis code: N18.5, N18.6, N19).
We defined incident kidney failure as the first occurrence of any episode fulfilling the criteria of kidney failure between 1st January 2002 and 31st December 2015. A two-year kidney failure free observation period from 1st January 2000 to 31st December 2001 in the HA EMR system was used to exclude people with pre-existing kidney failure. We defined incident KRT as people who started KRT with PD, HD, or kidney transplant, whichever occurred first. Underlying cause of death retrieved from the Hong Kong Death Registry were grouped into 12 mutually exclusive categories using ICD-10 codes (Appendix Table 1).
2.3. Statistical analysis
We included all people with diabetes and without kidney failure aged ≥20 years between 2002 and 2015 in the HKDSD. The age of 20 years concurs with the cut-off used by the International Diabetes Federation for adults21 and few people developed diabetes and kidney failure who were younger than 20 years of age. We calculated the incidence rates of kidney failure/KRT as the number of incident cases divided by the person-years of follow-up in each calendar year. We calculated 1-year and 5-year cumulative mortality rates after incident kidney failure/KRT as the number of deaths within 1 year and 5 years after kidney failure/KRT divided by the number of people with incident kidney failure/KRT in each calendar year. All incidence rates of kidney failure/KRT were age-sex-standardised to the 2016 Hong Kong Census mid-year population16,22. All mortality rates after kidney failure/KRT were age-sex-standardised to the entire population with incident kidney failure in the HKDSD between 2002 and 2015. This approach avoids large changes in standardised versus crude mortality rates due to large differences in age structure between Hong Kong Census population and people with incident kidney failure. We also calculated the proportion of people living with kidney failure at the end of each year who were receiving KRT. However, because this is a cohort of incident cases of kidney failure, a large proportion of people living in the early part of the study period had newly developed kidney failure (Appendix Fig. 1). Therefore, we only made comparison from 2005 to 2015 to allow a generally stable ratio of incident cases to prevalent cases among people living with kidney failure across years.
To create comparable statistics with studies reporting trends in kidney failure in other countries9,10,12 and with studies reporting other diabetes-related complications in Hong Kong22,23, we used log-linear Joinpoint regression models to describe trends in incidence rates of kidney failure/KRT and 1-year mortality rates after kidney failure/KRT from 2002 to 2015, and trends in 5-year mortality rates after kidney failure/KRT from 2002 to 2011. The Joinpoint regression fits a piecewise linear regression to the natural logarithm of the standardised rates with the calendar year as the predictor variable and uses permutation tests to detect whether there are time points (joinpoints) at which trends in rates statistically significantly change in either direction or magnitude24. The Joinpoint regression compares models by starting with no joinpoint (no joinpoints corresponds a straight line) and then testing whether more joinpoints are needed to be added into the model to best fit the data. The best fitting model was selected to report the average annual percent change (AAPC) in rates for entire study period, and annual percent change (APC) for each linear trend segment detected. We conducted subgroup analyses by age (20-44, 45-64, 65-74, and ≥75 years) and sex. We used a wider age interval for younger than older age groups to increase statistical power in younger age groups and to make the age bands comparable with other studies6,10,25.
In sensitivity analyses, we excluded recipients of kidney transplant to reduce its potential effects on 1-year and 5-year mortality trends. We additionally compared key characteristics of people with kidney failure between those with and without incident KRT at the time of development of kidney failure, and described the trends in 1-year and 5-year mortality rates after kidney failure among these two groups.
We calculated excess life-years lost in people with kidney failure using a new method[26], [27], [28], [29]. We estimated the expected life expectancy for people after incident kidney failure and before a set upper age limit (95 years for this study) using age-specific mortality rates as the area under the survival curve. We chose 95 years as the upper age limit which is well above the life expectancy for the Hong Kong population with low survival probability. Next, life-years lost was calculated as the average number of years lost before the upper age limit (95 years minus the expected life expectancy). Finally, the number of excess life-years lost for all-cause and cause-specific mortality in people with versus without kidney failure was estimated as the differences in life-years lost between the two groups. Life-years lost due to cause-specific death was calculated through a competing risks model with mutually exclusive causes of death as competing events30. We performed all analyses using R (version 3.5.3, Vienna, Austria) or Joinpoint Regression Program (version 4.8.0.1; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute). There is no allowance for multiplicity. A two-tailed P value less than 0.05 was considered statistically significant.
2.4. Role of the funding source
The funding source of the study had no role in the study design, data collection, data analysis, data interpretation, or drafting of the manuscript. The corresponding authors had full access to all study data and are responsible for the decision to submit for publication.
3. Results
3.1. Characteristics of study population
The HKDSD included 712,222 people with diabetes and without pre-existing kidney failure, aged ≥20 years between 2002 and 2015 (Fig. 1). Over time, the mean age of people in the HKDSD increased, and the mean HbA1c and LDL-cholesterol levels improved (Table 1). The proportion of people who were using renin-angiotensin-aldosterone system (RAAS) inhibitors increased from 41.3% in 2002 to 47.2% in 2015, with the increase mainly observed between 2002 and 2008 (Appendix Table 2). During 4.9 million person-years of follow-up, 31,425 incident cases of kidney failure were recorded, among whom 23.0% (n=7,233) received KRT. Among all people with kidney failure, 96.2% of them were identified by eGFR and the proportion changed little over time (Appendix Fig. 2). Among people with KRT, 83.6% (n=6,044) had PD as the initial KRT modality and 1.6% (n=114) ever received a kidney transplant. The mean ages at development of kidney failure and initiation of KRT were 72.7 (standard deviation [SD]:12.1) years and 62.2 (SD: 12.1) years, respectively. The median time from development of kidney failure to KRT initiation was 12 (interquartile range [IQR]: 6-21) months at a median eGFR of 6.5 (IQR: 5-9) mL/min per 1.73m2.
Fig 1.
Flowchart of participants selection in the Hong Kong Diabetes Surveillance Database between 2002 and 2015.
Table 1.
Characteristics of people with incident kidney failure and KRT between 2002 and 2015 in the HKDSD.
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HKDSD | ||||||||||||||
| No. of people | 168,466 | 200,050 | 250,787 | 276,672 | 308,756 | 334,170 | 361,223 | 390,127 | 419,785 | 452,065 | 483,553 | 514,793 | 542,986 | 570,687 |
| % of men | 46.1 | 46.4 | 46.7 | 47.2 | 47.4 | 47.8 | 48.1 | 48.5 | 48.8 | 49.1 | 49.4 | 49.6 | 49.9 | 50.0 |
| Age, mean (SD), years | 64.9 (13.1) | 65.1 (13.1) | 65.2 (12.9) | 65.4 (12.9) | 65.5 (12.9) | 65.6 (12.9) | 65.8 (12.9) | 65.9 (13.0) | 66.1 (13.0) | 66.3 (13.0) | 66.5 (13.0) | 66.6 (12.9) | 66.8 (12.9) | 67.0 (12.9) |
| HbA1c, mean, mmol/mol (%) | 61.2 (7.8) | 61.5 (7.8) | 61.7 (7.8) | 61.9 (7.8) | 61.6 (7.8) | 61.6 (7.8) | 59.5 (7.6) | 59.1 (7.6) | 57.7 (7.4) | 58.0 (7.5) | 57.6 (7.4) | 57.3 (7.4) | 56.2 (7.3) | 56.3 (7.3) |
| LDL-cholesterol, mean (SD), mmol/L | 3.2 (0.9) | 3.1 (0.9) | 3.1 (1.0) | 3.0 (0.9) | 2.9 (1.0) | 2.9 (0.9) | 2.9 (0.9) | 2.9 (0.9) | 2.9 (0.9) | 2.8 (0.9) | 2.7 (0.8) | 2.5 (0.8) | 2.4 (0.8) | 2.3 (0.8) |
| Incident kidney failure | ||||||||||||||
| No. of incident kidney failure | 1,552 | 1,739 | 2,026 | 1,845 | 1,917 | 1,889 | 2,151 | 2,340 | 2,337 | 2,541 | 2,623 | 2,733 | 2,844 | 2,888 |
| % of men | 42.5 | 43.5 | 43.8 | 44.5 | 46.2 | 45.3 | 47.4 | 48.0 | 48.5 | 48.2 | 48.7 | 47.8 | 48.9 | 50.0 |
| Age at kidney failure, mean (SD), years | 70.3 (11.1) | 70.6 (11.5) | 71.5 (11.5) | 71.3 (11.5) | 72.1 (11.8) | 72.6 (11.5) | 72.6 (11.9) | 73.0 (12.0) | 73.1 (12.2) | 73.2 (12.4) | 73.2 (12.4) | 73.5 (12.8) | 74.2 (12.5) | 74.2 (12.8) |
| HbA1c, mean, mmol/mol (%) | 59.9 (7.6) | 57.3 (7.4) | 59.4 (7.6) | 60.7 (7.7) | 57.8 (7.5) | 57.9 (7.5) | 55.7 (7.2) | 58.2 (7.5) | 58.1 (7.5) | 58.9 (7.5) | 59.2 (7.6) | 58.3 (7.5) | 57.9 (7.5) | 57.3 (7.4) |
| LDL-cholesterol, mean (SD), mmol/L | 3.2 (1.4) | 3.0 (1.3) | 3.1 (1.3) | 2.9 (1.2) | 2.9 (1.2) | 2.9 (1.3) | 2.8 (1.2) | 2.9 (1.2) | 2.8 (1.2) | 2.7 (1.2) | 2.7 (1.1) | 2.6 (1.1) | 2.5 (1.1) | 2.5 (1.1) |
| eGFR, median (IQR), mL/min per 1.73 m2 | 12.6 (8-16) | 13.2 (9-18) | 13.3 (10-17) | 14.1 (11-18) | 14.1 (11-18) | 14.3 (11-19) | 14.1 (11-19) | 14.3 (11-19) | 14.5 (11-20) | 14.3 (11-20) | 14.4 (11-20) | 14.5 (11-20) | 14.7 (12-21) | 14.6 (12-21) |
| No. of 1-year mortality after kidney failure | 516 | 519 | 580 | 515 | 482 | 543 | 610 | 640 | 660 | 637 | 660 | 665 | 714 | 714 |
| No. of 5-year mortality after kidney failure | 1,239 | 1,370 | 1,581 | 1,429 | 1,474 | 1,465 | 1,671 | 1,777 | 1,784 | 1,893 | NA | NA | NA | NA |
| Incident KRT | ||||||||||||||
| No. of incident KRT | 197 | 332 | 386 | 403 | 429 | 531 | 511 | 524 | 562 | 628 | 644 | 710 | 696 | 680 |
| No. (%) of incident peritoneal dialysis* | 179 (90.9) | 301 (90.7) | 343 (88.9) | 359 (89.1) | 351 (81.8) | 442 (83.2) | 436 (85.3) | 441 (84.2) | 433 (77.0) | 503 (80.1) | 532 (82.6) | 589 (83.0) | 579 (83.2) | 556 (81.8) |
| No. (%) of incident hemodialysis* | 17 (8.6) | 30 (9.0) | 41 (10.6) | 44 (10.9) | 77 (17.9) | 88 (16.6) | 74 (14.5) | 81 (15.5) | 129 (23.0) | 124 (19.7) | 110 (17.1) | 120 (16.9) | 117 (16.8) | 124 (18.2) |
| No. (%) of incident kidney transplant* | 1 (0.5) | 1 (0.3) | 2 (0.5) | 0 (0) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 2 (0.4) | 0 (0) | 1 (0.2) | 2 (0.3) | 1 (0.1) | 0 (0) | 0 (0) |
| % of men | 54.8 | 59.6 | 58.8 | 57.8 | 63.2 | 62.3 | 61.6 | 62.8 | 68.3 | 65.3 | 64.0 | 64.6 | 64.1 | 67.5 |
| Age at KRT, mean (SD), years | 63.3 (10.9) | 61.0 (11.8) | 60.9 (11.7) | 62.7 (11.8) | 62.4 (11.2) | 62.5 (10.9) | 62.6 (10.8) | 62.4 (11.2) | 61.6 (11.8) | 62.6 (10.5) | 62.6 (11.0) | 61.6 (11.4) | 62.8 (10.3) | 62.4 (10.7) |
| Months** from kidney failure to KRT, median (IQR) | 8 (2-15) | 10 (4-19) | 13 (6-27) | 13 (6-25) | 13 (7-21) | 11 (6-19) | 13 (7-23) | 13 (7-22) | 12 (6-22) | 12 (6-22) | 12 (6-20) | NA | NA | NA |
| HbA1c, mean, mmol/mol (%) | 57.5 (7.4) | 54.6 (7.1) | 57.0 (7.4) | 56.8 (7.3) | 53.6 (7.1) | 55.9 (7.3) | 54.2 (7.1) | 54.0 (7.1) | 54.5 (7.1) | 55.5 (7.2) | 55.3 (7.2) | 55.1 (7.2) | 53.4 (7.0) | 53.7 (7.1) |
| LDL-cholesterol, mean (SD), mmol/L | 3.2 (1.2) | 2.9 (1.4) | 2.9 (1.4) | 2.7 (1.2) | 2.8 (1.2) | 2.7 (1.2) | 2.8 (1.2) | 2.8 (1.2) | 2.8 (1.3) | 2.7 (1.1) | 2.6 (1.2) | 2.6 (1.1) | 2.4 (1.0) | 2.5 (1.2) |
| eGFR, median (IQR), mL/min per 1.73 m2 | 7.0 (5-10) | 6.4 (5-9) | 6.7 (5-9) | 6.2 (5-9) | 6.3 (5-9) | 6.5 (5-9) | 6.8 (5-10) | 6.8 (5-9) | 6.6 (5-9) | 6.5 (5-9) | 6.6 (5-9) | 6.5 (5-9) | 6.3 (5-9) | 6.3 (5-9) |
| No. of 1-year mortality after KRT | 42 | 58 | 60 | 62 | 58 | 78 | 76 | 80 | 66 | 79 | 73 | 71 | 80 | 80 |
| No. of 5-year mortality after KRT | 130 | 216 | 264 | 275 | 257 | 344 | 344 | 325 | 363 | 396 | NA | NA | NA | NA |
*Number and proportion as the initial KRT modality.
**In the entire cohort, 96% of people who received KRT started KRT within 46 months after incident kidney failure. To reduce follow-up bias, four years (from 2013 to 2016) were used to remove people without complete follow-up from kidney failure to KRT. Thus, the median months from kidney failure to KRT were only reported from 2002 to 2012.
3.2. Trends in incidence rates of kidney failure
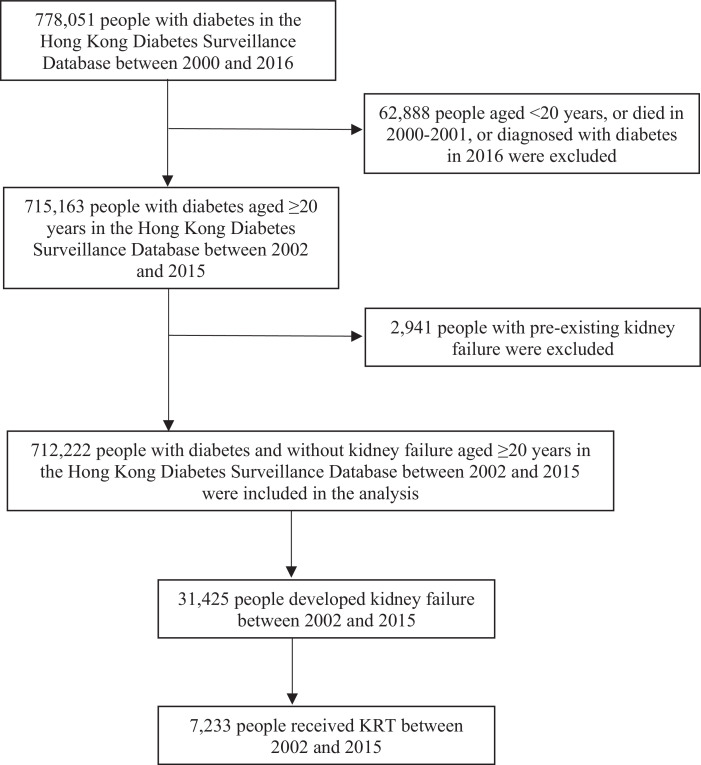
The number of incident cases of kidney failure increased from 1,552 in 2002 to 2,888 in 2015 (Table 1). The incidence rates of kidney failure declined by 46.8% from 62.9 (95% CI: 56.0, 69.8) per 10,000 person-years in 2002 to 33.4 (95% CI: 30.1, 36.7) per 10,000 person-years in 2007 (APC: -11.6, 95% CI: -16.3, -6.7), then flattened from 2007 to 2015 (APC: -0.9, 95% CI: -3.1, 1.3) with similar trends between sexes (Fig. 2 and Table 2). In age-stratified analysis, the changes in trends in rates of kidney failure after 2007 mainly occurred in 45-64 and 65-74 age groups. In people aged 20-44 and ≥75 years, the incidence rates of kidney failure decreased linearly during the whole study period. The cumulative incidence of kidney failure is shown in the Appendix Fig. 3.
Fig 2.
Trends in standardised incidence rates of kidney failure (a and b) and KRT (c and d) in people with diabetes in Hong Kong between 2002 and 2015, stratified by sex (a and c) and age (b and d). Dots are observed rates and solid lines are modelled rates from Joinpoint regression models.
Table 2.
Joinpoint analysis of trends in standardised incidence rates of kidney failure and KRT in people with diabetes in Hong Kong between 2002 and 2015.
| Rates per 10,000 person-years |
Period 1 |
Period 2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2002 | 2015 | AAPC (95% CI) | P | Period | APC (95% CI) | P | Period | APC (95% CI) | P | |
| Incident kidney failure | ||||||||||
| All | 62.9 | 37.3 | -5.2 (-7.2, -3.1) | <0.001 | 2002-2007 | -11.6 (-16.3, -6.7) | 0.001 | 2007-2015 | -0.9 (-3.1, 1.3) | 0.360 |
| Men | 71.0 | 45.3 | -5.1 (-7.5, -2.5) | <0.001 | 2002-2006 | -13.2 (-20.0, -5.8) | 0.004 | 2006-2015 | -1.2 (-3.6, 1.3) | 0.290 |
| Women | 54.9 | 29.3 | -5.6 (-7.9, -3.3) | <0.001 | 2002-2007 | -13.4 (-17.8, -8.6) | 0.001 | 2007-2015 | -0.5 (-3.6, 2.8) | 0.739 |
| 20-44 years | 32.0 | 32.5 | -3.8 (-7.3, -0.1) | 0.043 | 2002-2015 | -3.8 (-7.3, -0.1) | 0.043 | NA | ||
| 45-64 years | 62.4 | 27.0 | -6.3 (-8.1, -4.4) | <0.001 | 2002-2006 | -15.4 (-20.8, -10.1) | <0.001 | 2006-2015 | -1.8 (-3.4, -0.1) | 0.042 |
| 65-74 years | 114.4 | 40.1 | -8.0 (-9.7, -6.2) | <0.001 | 2002-2006 | -14.7 (-19.6, -9.6) | <0.001 | 2006-2015 | -4.8 (-6.4, -3.2) | <0.001 |
| ≥75 years | 158.8 | 101.7 | -3.3 (-4.2, -2.4) | <0.001 | 2002-2015 | -3.3 (-4.2, -2.4) | <0.001 | NA | ||
| Incident KRT | ||||||||||
| All | 11.2 | 13.8 | -1.6 (-4.4, 1.2) | 0.235 | 2002-2015 | -1.6 (-4.4, 1.2) | 0.235 | NA | ||
| Men | 12.5 | 19.7 | -1.3 (-4.6, 2.1) | 0.422 | 2002-2015 | -1.3 (-4.6, 2.1) | 0.422 | NA | ||
| Women | 9.8 | 8.0 | -2.2 (-4.8, 0.6) | 0.111 | 2002-2015 | -2.2 (-4.8, 0.6) | 0.111 | NA | ||
| 20-44 years | 7.5 | 12.9 | -3.3 (-9.3, 3.1) | 0.282 | 2002-2015 | -3.3 (-9.3, 3.1) | 0.282 | NA | ||
| 45-64 years | 15.1 | 16.4 | -1.1 (-2.5, 0.4) | 0.127 | 2002-2015 | -1.1 (-2.5, 0.4) | 0.127 | NA | ||
| 65-74 years | 15.9 | 16.0 | -1.6 (-3.0, -0.2) | 0.028 | 2002-2015 | -1.6 (-3.0, -0.2) | 0.028 | NA | ||
| ≥75 years | 6.8 | 5.1 | -2.8 (-5.3, -0.2) | 0.035 | 2002-2012 | -0.5 (-2.6, 1.8) | 0.650 | 2012-2015 | -10.1 (-19.3, 0.2) | 0.053 |
NA indicates no second trend identified
3.3. Trends in incidence rates of KRT
The number of people with incident KRT increased from 197 in 2002 to 680 in 2015 (Table 1). However, the incidence rates of KRT remained constant (AAPC: -1.6, 95% CI: -4.4, 1.2), which was 11.2 (95% CI: 8.7, 13.7) and 13.8 (95% CI: 10.9, 16.7) per 10,000 person-years in 2002 and 2015, respectively (Fig. 2 and Table 2). The trends were similar between sexes. Stratifying by age, the incidence rates of KRT declined slightly in the 65-74 (AAPC: -1.6, 95% CI: -3.0, -0.2) and ≥75 age groups (AAPC: -2.8, 95% CI: -5.3, -0.2). The cumulative incidence of KRT is shown in the Appendix Fig. 3. Among those living with kidney failure at the end of the year, proportion of people who were receiving KRT was 12.7% in 2002, and increased from 23.7% in 2005 to 31.3% in 2015, with the proportion consistently higher in men than women, and higher in younger than older ages (Appendix Fig. 4). The mean age (62.2 years [SD:12.1]) or median eGFR (6.5 mL/min per 1.73m2 [IQR: 5-9]) at KRT initiation and median time (12 months [IQR:6-21]) from incident kidney failure to KRT initiation were similar over time (Table 1).
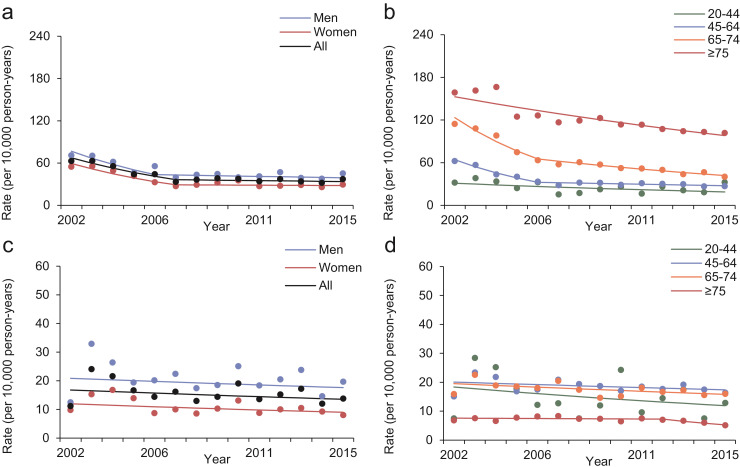
3.4. Trends in 1-year mortality rates after kidney failure and KRT
Between 2002 and 2015, the overall crude 1-year cumulative mortality rates after incident kidney failure and KRT initiation were 26.9% (95% CI: 26.4%, 27.4%) and 13.3% (95% CI: 12.5%, 14.1%), respectively. The 1-year mortality rates after kidney failure (AAPC: -2.7, 95% CI: -3.4, -1.9) and after KRT (AAPC: -3.8, 95% CI: -5.2, -2.4) both declined linearly (Fig. 3 and Table 3). The decline was observed for both sexes but varied by age. There was a non-statistically significant increasing trend in 1-year mortality rates after kidney failure in people aged 20-44 years (AAPC: 1.7, 95% CI: -6.2, 10.2). Sensitivity analyses excluding recipients of kidney transplant yielded similar declining trends in 1-year mortality after kidney failure and KRT (Appendix Fig. 5). Similar declining trends in 2-year (Appendix Table 3) and 3-year mortality rates (Appendix Table 4) after kidney failure and KRT were observed.
Fig 3.
Trends in standardised 1-year mortality rates after kidney failure (a and b) and KRT (c and d) in people with diabetes in Hong Kong between 2002 and 2015, stratified by sex (a and c) and age (b and d). Dots are observed rates and solid lines are modelled rates from Joinpoint regression models. Joinpoint regression analysis for 1-year mortality rates after KRT was not performed for people aged 20-44 years because no people aged 20-44 years died within one year after KRT in 2009.
Table 3.
Joinpoint analysis of trends in standardised 1-year and 5-year mortality rates after incident kidney failure and KRT in people with diabetes in Hong Kong between 2002 and 2015.
| Rates per 100 |
Period 1 |
||||||
|---|---|---|---|---|---|---|---|
| 1-year mortality rates | 2002 | 2015 | AAPC (95% CI) | P | Period | APC (95% CI) | P |
| After kidney failure | |||||||
| All | 35.9 | 23.3 | -2.7 (-3.4, -1.9) | <0.001 | 2002-2015 | -2.7 (-3.4, -1.9) | <0.001 |
| Men | 39.7 | 25.4 | -2.5 (-3.6, -1.4) | <0.001 | 2002-2015 | -2.5 (-3.6, -1.4) | <0.001 |
| Women | 32.1 | 21.1 | -3.0 (-4.0, -2.0) | <0.001 | 2002-2015 | -3.0 (-4.0, -2.0) | <0.001 |
| 20-44 years | 9.2 | 8.9 | 1.7 (-6.2, 10.2) | 0.666 | 2002-2015 | 1.7 (-6.2, 10.2) | 0.666 |
| 45-64 years | 16.7 | 13.0 | -2.7 (-4.3, -1.1) | 0.004 | 2002-2015 | -2.7 (-4.3, -1.1) | 0.004 |
| 65-74 years | 36.4 | 17.7 | -4.1 (-5.9, -2.2) | 0.001 | 2002-2015 | -4.1 (-5.9, -2.2) | 0.001 |
| ≥75 years | 44.6 | 30.9 | -2.3 (-3.1, -1.6) | <0.001 | 2002-2015 | -2.3 (-3.1, -1.6) | <0.001 |
| After KRT | |||||||
| All | 20.0 | 11.2 | -3.8 (-5.2, -2.4) | <0.001 | 2002-2015 | -3.8 (-5.2, -2.4) | <0.001 |
| Men | 17.8 | 13.0 | -3.1 (-4.3, -1.9) | <0.001 | 2002-2015 | -3.1 (-4.3, -1.9) | <0.001 |
| Women | 22.2 | 9.4 | -4.5 (-7.2, -1.8) | 0.004 | 2002-2015 | -4.5 (-7.2, -1.8) | 0.004 |
| 20-44 years* | 5.9 | 3.5 | / | / | / | / | / |
| 45-64 years | 15.1 | 9.5 | -2.9 (-5.9, 0.2) | 0.064 | 2002-2015 | -2.9 (-5.9, 0.2) | 0.064 |
| 65-74 years | 26.4 | 13.1 | -4.7 (-7.7, -1.7) | 0.005 | 2002-2015 | -4.7 (-7.7, -1.7) | 0.005 |
| ≥75 years | 29.6 | 16.6 | -4.6 (-7.7, -1.4) | 0.009 | 2002-2015 | -4.6 (-7.7, -1.4) | 0.009 |
| 5-year mortality rates | 2002 | 2011 | AAPC (95% CI) | P | Period | APC (95% CI) | P |
| After kidney failure | |||||||
| All | 81.8 | 73.6 | -1.0 (-1.2, -0.8) | <0.001 | 2002-2011 | -1.0 (-1.2, -0.8) | <0.001 |
| Men | 81.3 | 76.9 | -0.6 (-0.9, -0.2) | 0.007 | 2002-2011 | -0.6 (-0.9, -0.2) | 0.007 |
| Women | 82.3 | 70.4 | -1.4 (-1.8, -1.0) | <0.001 | 2002-2011 | -1.4 (-1.8, -1.0) | <0.001 |
| 20-44 years | 24.4 | 31.1 | 2.1 (-4.8, 9.4) | 0.520 | 2002-2011 | 2.1 (-4.8, 9.4) | 0.520 |
| 45-64 years | 60.8 | 48.4 | -2.5 (-3.4, -1.7) | <0.001 | 2002-2011 | -2.5 (-3.4, -1.7) | <0.001 |
| 65-74 years | 81.8 | 69.9 | -1.2 (-1.9, -0.5) | 0.003 | 2002-2011 | -1.2 (-1.9, -0.5) | 0.003 |
| ≥75 years | 92.9 | 87.7 | -0.5 (-0.9, -0.2) | 0.004 | 2002-2011 | -0.5 (-0.9, -0.2) | 0.004 |
| After KRT | |||||||
| All | 62.8 | 61.6 | -0.8 (-1.9, 0.3) | 0.115 | 2002-2011 | -0.8 (-1.9, 0.3) | 0.115 |
| Men | 66.7 | 62.0 | -0.2 (-1.6, 1.3) | 0.783 | 2002-2011 | -0.2 (-1.6, 1.3) | 0.783 |
| Women | 59.0 | 61.3 | -1.4 (-2.8, 0.1) | 0.058 | 2002-2011 | -1.4 (-2.8, 0.1) | 0.058 |
| 20-44 years | 20.4 | 44.3 | 5.3 (-0.5, 11.4) | 0.070 | 2002-2011 | 5.3 (-0.5, 11.4) | 0.070 |
| 45-64 years | 56.5 | 53.2 | -1.4 (-2.8, -0.1) | 0.043 | 2002-2011 | -1.4 (-2.8, -0.1) | 0.043 |
| 65-74 years | 75.0 | 72.7 | -0.9 (-1.9, 0.03) | 0.055 | 2002-2011 | -0.9 (-1.9, 0.03) | 0.055 |
| ≥75 years | 77.4 | 75.0 | -0.7 (-2.4, 1.2) | 0.427 | 2002-2011 | -0.7 (-2.4, 1.2) | 0.427 |
*Joinpoint regression analysis for 1-year mortality rates was not performed for people aged 20-44 years because no people aged 20-44 years died within one year after KRT in 2009.
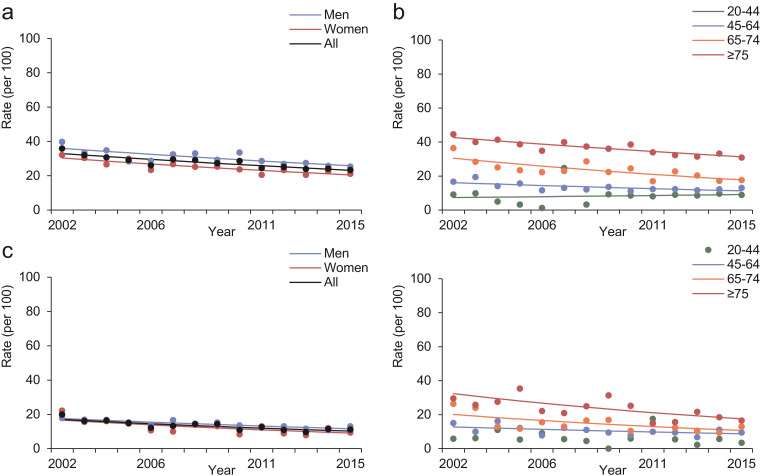
3.5. Trends in 5-year mortality rates after kidney failure and KRT
Between 2002 and 2011, the overall crude 5-year cumulative mortality rates after incident kidney failure and KRT were 77.1% (95% CI: 76.5%, 77.7%) and 64.7% (95% CI: 63.3%, 66.1%), respectively. During the study period, the 5-year mortality rates after kidney failure declined (AAPC: -1.0, 95% CI: -1.2, -0.8) with slightly less marked and not statistically significant decline after KRT (AAPC: -0.8, 95% CI: -1.9, 0.3) (Fig. 4 and Table 3). These trends were similar between sexes and across age subgroups, except for a non-statistically significant increasing trend in 5-year mortality rates after both kidney failure (AAPC: 2.1, 95% CI: -4.8, 9.4) and KRT (AAPC: 5.3, 95% CI: -0.5, 11.4) in people aged 20-44 years. Sensitivity analyses excluding recipients of kidney transplant yielded similar results (Appendix Fig. 6). The cumulative survival probability of people after kidney failure and KRT are shown in the Appendix Fig. 7. Among people who had received a kidney transplant, the overall crude 1-year and 5-year cumulative mortality rate after kidney transplant was 5.7% (95% CI: 1.3%, 10.3%) and 19.1% (95% CI: 7.9%, 30.4%), respectively.
Fig 4.
Trends in standardised 5-year mortality rates after kidney failure (a and b) and KRT (c and d) in people with diabetes in Hong Kong between 2002 and 2011, stratified by sex (a and c) and age (b and d). Dots are observed rates and solid lines are modelled rates from Joinpoint regression models.
3.6. Comparison between people with kidney failure with versus without incident KRT
Compared to people with KRT, those without KRT were more likely to be women (58.4% vs 36.6%), developed kidney failure at an older age (mean: 76.6 vs 61.1 years), had a higher eGFR (median: 14.6 vs 12.6 mL/min per 1.73 m2) at the time of development of kidney failure, and substantially higher crude 1-year (33.5% vs 4.7%) and 5-year (87.5% vs 48.3%) cumulative mortality rates after onset of kidney failure (Appendix Table 5). Kidney disease was the most common cause of death in people without KRT, whereas cardiovascular disease accounted for the highest proportion of deaths in people who received KRT. The declining trends in both 1-year and 5-year mortality rates after kidney failure were generally similar between people with and without incident KRT (Appendix Fig. 8 and Appendix Fig. 9).
3.7. Excess life-years lost in people with kidney failure
On average, the remaining life expectancy was 3.5 years for people with kidney failure after diagnosis. Kidney failure was associated with 12.5 excess life-years lost as compared to people without kidney failure (Appendix Table 6). The excess life-years lost decreased with increasing age of kidney failure onset. People diagnosed with kidney failure at the age of 45 years and 75 years had an estimated additional 31.3 and 9.3 years of life lost, respectively. Kidney disease, cardiovascular disease, and pneumonia were leading causes of excess life-years lost in people with kidney failure. People with kidney failure had a larger number of life-years lost than people without kidney failure for all specific causes of death, except for cancer.
4. Discussion
Using data from a territory-wide cohort of people with diabetes, we report four main findings. First, the initial downward trend between 2002 and 2007 was followed by subsequent stagnation in incidence rates of kidney failure in people with diabetes in Hong Kong. Second, the incidence rates of KRT remained constant during the surveillance period. Third, although the 1-year post-KRT mortality rates have decreased, survival at a longer term of 5-years post-KRT did not improve with a tendency of rising mortality among the 20-44 age group. Fourth, the occurrence of kidney failure shortened life expectancy by an average of 12.5 years which increased to 31 years if kidney failure developed at a younger age.
4.1. Lack of decline in rates of kidney failure in the last decade
The stagnated decline in rates of kidney failure in the last decade is concerning despite the 50-70% reduction in other diabetes-related complications during the same period22. There are several possible explanations. First, the reduction in morbidities has led to increased survival of people with diabetes in Hong Kong in the past 20 years16,22. From 2001 to 2016, the all-cause mortality rate in people with diabetes in Hong Kong has declined by about 50%16. This has given rise to an accumulating pool of older people living with long disease duration who were at high risk of developing kidney complications. Thus, the reduction in competing risk from death has allowed people with diabetes to live long enough to develop kidney failure, which has been supported by the increasing mean age at development of kidney failure over time in the HKDSD. Second, using the same dataset, we have reported a rising incidence of diabetes in people <45 years of age which could contribute to the increasing risk of kidney failure due to their long disease duration14. This is further compounded by the complex phenotypes in people with young-onset diabetes who have poor control of metabolic risk factors with both delayed and suboptimal management31. The stagnating rates of kidney failure was most evident in those aged 45-64 years, in keeping with the rising incidence of young-onset diabetes whose risk of kidney failure began to surge in middle age32. Third, the efforts to prevent kidney failure might have plateaued along with the lack of new treatment strategies. The initial decline in rates between 2002 and 2007 could be the combined effects from previous healthcare reform in Hong Kong16,22 and the introduction of RAAS inhibitors33. However, whilst the uptake of RAAS inhibitors has increased over time, albuminuria was present in up to one third of people with diabetes in Hong Kong in the most recent estimate32. Although glucagon-like peptide 1 receptor agonists and sodium-glucose cotransporter-2 inhibitor have kidney protective effects, in Hong Kong, these new drug classes were available in the public sector only in 2011 and 2015, respectively34. Fourth, the increasing number of people with kidney failure might reflect changes in screening practice. In 2009, a structured Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM) was expanded from hospital-based diabetes centres to all primary care clinics in Hong Kong to provide regular screening of complications for people with diabetes. Although data showed that RAMP-DM led to 12% decreased risk of microvascular complications compared to those who were not enrolled into the programme35, complication screening might also have increased the number of incident cases detected.
4.2. KRT in people with kidney failure
One in four people with kidney failure received KRT in our study. Although there are recognised indications for KRT, the decision to initiate KRT remains complex and is likely to be affected by multiple factors including the care practices of physicians, preferences of patients (KRT or comprehensive conservative care), and availability of resources36,37. In addition, due to a high competing risk of mortality, a large number of people with kidney failure may not survive to KRT. This is evidenced by the considerably higher 1-year mortality rates after onset of kidney failure among people without KRT than those who initiated KRT as reported in our study. We observed a slight increase in proportion of people with kidney failure receiving KRT from 2005 to 2015, although the incidence of KRT remained stable. The possible explanation is that the decrease in mortality after KRT has allowed more people with KRT to live in each year over time. Hong Kong has practiced PD-first policy for three decades and has the highest proportion of PD population in the world6. Additionally, organ donation rate is very low in Hong Kong as compared with other developed regions, and organ shortage has contributed to the small number of people receiving kidney transplant38.
4.3. Trends in mortality after kidney failure and KRT
The improvements in short- and long-term mortality rates after kidney failure are encouraging, but still, the absolute rates remained high at 24% in 1 year and 74% in 5 years. Short-term mortality rates after KRT have also declined, but the decline in rates at 5 years was not statistically significant. Nonetheless, these 5-year mortality rates after KRT ranging from 60% to 68%, are comparable to that of 62% reported in the United States in 200839 and 60% reported in the Europe in 2004-200840. The nominally increasing trends in mortality rates after both kidney failure and KRT in young people aged 20-44 years is concerning, with 31% and 44% of them having died within 5 years after development of kidney failure and KRT in the most recent estimates. These non-statistically significant trends may be related to the small sample size in this age group, but may also reflect delayed diagnosis and treatment among young people with diabetes16,41.
4.4. Excess life-years lost in people with kidney failure
Cardiovascular diseases accounted for more than 20% of the excess life-years lost in people with kidney failure. The contribution of cardiovascular diseases to loss of life-years increased with the younger age of kidney failure onset. Life expectancy was shortened by 9 years with onset of kidney failure at the age of 75 years versus 31 years with onset of kidney failure at 45 years of age. This underscores the importance of preventing and/or delaying the progression of diabetic kidney disease, and aggressive management of cardiovascular risk factors especially in young people. Given the high mortality among people without KRT, greater efforts are needed to broaden the availability of KRT to prevent premature deaths due to a lack of access to KRT treatment. Furthermore, kidney transplant is considered the most cost-effective form of KRT with the best outcomes among suitable candidates42. The extremely low organ donation rate in Hong Kong calls for new enhancement to existing organ transplantation policy and mass media promotion to improve public understanding and boost donor registration38.
4.5. Strengths and limitations
The major strengths of this study are the territory-wide coverage of the study population with minimal selection bias and a long period of follow-up. Moreover, compared to previous studies which only reported trends in KRT or used KRT as a proxy for kidney failure[9], [10], [11], [12], the large volume of eGFR measurements in the HA EMR system has enabled us to include the entire population of kidney failure regardless of treatment status. Our study has several limitations. First, despite our efforts to reduce misclassification bias, overestimation or underestimation of kidney failure and KRT was still possible, including using the CKD-EPI equation for Chinese ethnicity and people with diabetes43. However, misclassifications should be non-differential across study years and were unlikely to cause significant bias to the trend estimates. The ratio of incident PD to HD in our study was similar to that reported in the Hong Kong Renal Registry6, indicating a high degree of reliability. Second, we were unable to delineate non-diabetes causes that might contribute to kidney failure, such as hypertension, obstructive uropathy and primary glomerulonephritis. Third, the existing HA EMR system lacks information on people's sociodemographic and lifestyle factors. Therefore, we could not properly identify the drivers of the observed trends, and instead, our study was only designed to quantify the trends in incidence and mortality of kidney failure in people with diabetes in Hong Kong. Fourth, the effect of diabetes duration on excess life-years lost could not be assessed since age of diagnosis of diabetes was not documented in the EMR. Our results are interpreted as the average excess years of life lost in people with kidney failure versus their counterparts without kidney failure regardless of diabetes duration. Fifth, our data are subject to potential issue of validity as with all administrative data. Last, we were unable to differentiate between types of diabetes. However, using a validated algorithm, we have reported <1% of incident cases between 2002 and 2015 had type 1 diabetes in the HKDSD14. The influence of type 1 diabetes on our results is expected to be minimal.
In conclusion, we have provided territory-wide trends in incidence of kidney failure and KRT and related mortality in people with diabetes in Hong Kong. After improvement from 2002 to 2007, the incidence rates of kidney failure plateaued. With more people living with diabetes together with the rising number of people with young-onset diabetes, the burden of kidney failure related to diabetes is anticipated to escalate. There is an urgent need to identify factors contributing to the unchanged kidney failure incidence and implement new strategies to prevent a looming epidemic of kidney failure.
5. Contributors
HW contributed to conception of the article, statistical analysis, results interpretation, drafted the manuscript, revised the manuscript critically and approved the final version. AOYL and JCNC contributed to conception of the article, results interpretation, revised the manuscript critically and approved the final version. ESHL and AY contributed to conception of the article, results interpretation, revised the manuscript critically and approved the final version. SCC, RCWM, APSK, EC, and W-YS contributed to conception of the article, revised the manuscript critically and approved the final version. AOYL is the guarantor of this work, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of Competing Interest
Dr. Ma reports grants from AstraZeneca, Bayer, Pfizer, Novo Nordisk, Sanofi, personal fees from Worldwide Initiative for Diabetes Education (WorldWIDE Diabetes), Speaker honorarium from Boehringer Ingelheim, outside the submitted work; receives support from the Hong Kong Government Innovation and Technology Commission and its Technology Start-up Support Scheme for Universities (TSSSU), as co-founded a technology start-up which provides genetic testing for diabetes and diabetes complications. No income or renumeration received from the company. Dr. Chan reports grants from Astra Zeneca, Lilly, Hua Medicine, Lee Powder; grants and personal fees from Bayer, MSD, Merck, and Sanofi; act as CEO in Asia Diabetes Foundation and founding director in GemVCare, outside the submitted work; In addition, Dr. Chan has a patent Genetic markers for DKD issued. Dr. Luk reports non-financial support from Astra Zeneca, MSD, and Boehringer Inglheim; grants from Sanofi Hong Kong Limited and Amgan, acts as principal investigator of sponsored studies from MSD, Bayer, Roche, and Lee's Pharmaceutical, outside the submitted work.
Acknowledgments
Acknowledgements
We acknowledge the Hong Kong Hospital Authority for providing access to the electronic medical record. HW is partially supported by Asia Diabetes Foundation. The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data sharing statement
Data supporting the results of this study are hosted at the Hong Kong Hospital Authority. Details of data application and management procedures can be found on https://www3.ha.org.hk/data/Provision/Index/. More information is available on request to the corresponding author.
Editor note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100165.
Appendix. Supplementary materials
References
- 1.de Boer I.H., Caramori M.L., Chan J.C.N. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98(4):839–848. doi: 10.1016/j.kint.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Afkarian M., Sachs M.C., Kestenbaum B. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh H., Lin C., Li Y.R. Temporal trends of incident diabetes mellitus and subsequent outcomes in patients receiving kidney transplantation: a national cohort study in Taiwan. Diabetol Metab Syndr. 2020;12:34. doi: 10.1186/s13098-020-00541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Renal Data System. 2019 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2019.
- 5.ANZDATA Registry . Adelaide; Australia: 2019. Australia and New Zealand Dialysis and Transplant Registry. ANZDATA 42nd Annual Report 2019. [Google Scholar]
- 6.Leung C.B., Cheung W.L., Li P.K.T. Renal registry in Hong Kong—the first 20 years. Kidney Int Suppl. 2015;5(1):33–38. doi: 10.1038/kisup.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas M.C., Cooper M.E., Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12(2):73–81. doi: 10.1038/nrneph.2015.173. [DOI] [PubMed] [Google Scholar]
- 8.Harding J.L., Pavkov M.E., Magliano D.J., Shaw J.E., Gregg E.W. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 9.Gregg E.W., Hora I., Benoit S.R. Resurgence in diabetes-Related complications. JAMA. 2019;321(19):1867–1868. doi: 10.1001/jama.2019.3471. [DOI] [PubMed] [Google Scholar]
- 10.Burrows N.R., Li Y., Geiss L.S. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care. 2010;33(1):73–77. doi: 10.2337/dc09-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narres M., Claessen H., Kvitkina T. Incidence and relative risk of renal replacement therapy in people with and without diabetes between 2002 and 2016 in a German region. Diabetologia. 2020;63(3):648–658. doi: 10.1007/s00125-019-05067-6. [DOI] [PubMed] [Google Scholar]
- 12.Koye D.N., Magliano D.J., Reid C.M. Trends in incidence of ESKD in people with type 1 and type 2 diabetes in Australia. Am J Kidney Dis. 2019;73(3):300–308. doi: 10.1053/j.ajkd.2018.10.005. 2002-2013. [DOI] [PubMed] [Google Scholar]
- 13.Hong Kong Hospital Authority. Hospital Authority Annual Report 2018-2019. 2020. https://www.ha.org.hk/visitor/ha_visitor_index.asp?Content_ID=253826&Lang=ENG (accessed 19 November 2020).
- 14.Luk A.O.Y., Ke C., Lau E.S.H. Secular trends in incidence of type 1 and type 2 diabetes in Hong Kong: A retrospective cohort study. PLoS Med. 2020;17(2) doi: 10.1371/journal.pmed.1003052. e1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke C., Lau E., Shah B.R. Excess burden of mental illness and hospitalization in young-onset type 2 diabetes: a population-based cohort study. Ann Intern Med. 2019;170(3):145–154. doi: 10.7326/M18-1900. [DOI] [PubMed] [Google Scholar]
- 16.Wu H., Lau E.S.H., Ma R.C.W. Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001–2016: a retrospective cohort study. Diabetologia. 2020;63(4):757–766. doi: 10.1007/s00125-019-05074-7. [DOI] [PubMed] [Google Scholar]
- 17.Wu H., Yang A., Lau E.S.H. Secular trends in rates of hospitalisation for lower extremity amputation and 1 year mortality in people with diabetes in Hong Kong, 2001–2016: a retrospective cohort study. Diabetologia. 2020;63(12):2689–2698. doi: 10.1007/s00125-020-05278-2. [DOI] [PubMed] [Google Scholar]
- 18.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey A.S., Eckardt K.U., Dorman N.M. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97(6):1117–1129. doi: 10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Stevens P.E., Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 21.Saeedi P., Petersohn I., Salpea P. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. 9 (th) edition. [DOI] [PubMed] [Google Scholar]
- 22.Wu H., Lau E.S.H., Yang A. Trends in diabetes-related complications in Hong Kong, 2001–2016: a retrospective cohort study. Cardiovasc Diabetol. 2020;19(1):60. doi: 10.1186/s12933-020-01039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luk A.O.Y., Wu H., Lau E.S.H. Temporal trends in rates of infection-related hospitalisations in Hong Kong people with and without diabetes, 2001–2016: a retrospective study. Diabetologia. 2021;64(1):109–118. doi: 10.1007/s00125-020-05286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Gregg E.W., Li Y., Wang J. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 26.Andersen P.K. Life years lost among patients with a given disease. Stat Med. 2017;36(22):3573–3582. doi: 10.1002/sim.7357. [DOI] [PubMed] [Google Scholar]
- 27.Plana-Ripoll O., Pedersen C.B., Agerbo E. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. 2019;394(10211):1827–1835. doi: 10.1016/S0140-6736(19)32316-5. [DOI] [PubMed] [Google Scholar]
- 28.Erlangsen A., Andersen P.K., Toender A., Laursen T.M., Nordentoft M., Canudas-Romo V. Cause-specific life-years lost in people with mental disorders: a nationwide, register-based cohort study. Lancet Psychiatry. 2017;4(12):937–945. doi: 10.1016/S2215-0366(17)30429-7. [DOI] [PubMed] [Google Scholar]
- 29.Plana-Ripoll O., Canudas-Romo V., Weye N., Laursen T.M., McGrath J.J., Andersen P.K. lillies: An R package for the estimation of excess Life Years Lost among patients with a given disease or condition. Plos One. 2020;15(3):1–18. doi: 10.1371/journal.pone.0228073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen P.K. Decomposition of number of life years lost according to causes of death. Stat Med. 2013;32(30):5278–5285. doi: 10.1002/sim.5903. [DOI] [PubMed] [Google Scholar]
- 31.Luk A.O.Y., Lau E.S.H., Lim C. Diabetes-related complications and mortality in patients with young-onset latent autoimmune diabetes: a 14-year analysis of the prospective Hong Kong Diabetes Register. Diabetes Care. 2019;42(6):1042–1050. doi: 10.2337/dc18-1796. [DOI] [PubMed] [Google Scholar]
- 32.Luk A.O., Lau E.S., So W.-Y. Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care. 2014;37(1):149–157. doi: 10.2337/dc13-1336. [DOI] [PubMed] [Google Scholar]
- 33.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 34.Yang A., Wu H., Lau E.S.H. Trends in glucose-lowering drug use, glycemic control, and severe hypoglycemia in adults with diabetes in Hong Kong, 2002-2016. Diabetes Care. 2020;43(12):2967–2974. doi: 10.2337/dc20-0260. [DOI] [PubMed] [Google Scholar]
- 35.Wan E.Y.F., Fung C.S.C., Jiao F.F. Five-year effectiveness of the multidisciplinary Risk Assessment and Management Programme–Diabetes Mellitus (RAMP-DM) on diabetes-related complications and health service uses—a population-based and propensity-matched cohort study. Diabetes Care. 2018;41(1):49–59. doi: 10.2337/dc17-0426. [DOI] [PubMed] [Google Scholar]
- 36.Chan C.T., Blankestijn P.J., Dember L.M. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;96(1):37–47. doi: 10.1016/j.kint.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 38.International Registry in Organ Donation and Transplantation, 2020. https://www.irodat.org/?p=database&c=_H&year=2015#data (accessed 3 March 2021).
- 39.United States Renal Data System, 2013 USRDS annual data report, Atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013.
- 40.Kramer A., Pippias M., Stel V.S. Renal replacement therapy in Europe: a summary of the 2013 ERA-EDTA Registry Annual Report with a focus on diabetes mellitus. Clin Kidney J. 2016;9(3):457–469. doi: 10.1093/ckj/sfv151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeung R.O., Zhang Y., Luk A. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014;2(12):935–943. doi: 10.1016/S2213-8587(14)70137-8. [DOI] [PubMed] [Google Scholar]
- 42.Tonelli M., Wiebe N., Knoll G. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 43.Stevens L.A., Claybon M.A., Schmid C.H. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.