Abstract
Background
There is heterogeneity in the clinical manifestations and responses to drugs in RA patients due to variety of factors such as genes and environment. Despite advances in the treatment of rheumatoid arthritis (RA), approximately 40% of RA patients still do not achieve primary clinical outcomes in randomized trials, and its low remission rate and high economic consumption remain unresolved, especially in developing countries. Iguratimod (IGU) is a new disease-modifying anti-rheumatic drug (DMARD) with a low price that has demonstrated good efficacy and safety in clinical trials and was approved for active RA in China and Japan. As the most populous country in the Western Pacific region, it is warranted to conduct a study with a large scale of patients in a real-life setting. Our study confirms the new option for RA patients, which is potentially benificial for public health in developing countries.
Methods
This was a nationwide, prospective real-world study of IGU. Eligible subjects were active adult RA patients who aged 18 to 85 with or without multiple comorbidities such as hypertension and diabetes with DMARDs at a stable dosage for at least 12 weeks, or without ongoing DMARDs. A two-stage design was used for this study. In the first stage (the first 12 weeks), IGU 25 mg bid was added as monotherapy or to the background therapy, and in the second stage (the latter 12 weeks), adjustment of RA medicines other than IGU was allowed according to the participants’ disease activity. The primary endpoints were American College of Rheumatology 20% response (ACR20) 24 weeks and adverse events during 24 weeks. The secondary endpoints were ACR50 and ACR70 over 24 weeks, the changes of DAS28 and Health Assessment Questionnaire (HAQ) at week 12 and week 24 from baseline. The trial was registered with ClinicalTrials.gov, number NCT01554917.
Findings
Between March 2012 and January 2015, 1759 participants were enrolled, of whom 81•5% (1433/1759) completed the study. Notably, 1597 patients in the full analysis set were assessed for the effectiveness and 1751 patients were in the safety analysis set; 71•9% (1148/1597) of the patients achieved the primary endpoint of ACR20 response at week 24, and 51•7% (906/1751) patients had at least 1 adverse event (AE). The incidence of the clinical significant AE (grade≥3) of special interest was 3•4% (54 patients for grade 3 and 6 patients for grade 4), and 0•7% (13/1751) of patients developed SAEs associated with IGU. The most common clinical significant AEs were infection in 0•6% (10/1751) of the patients, abdominal discomfort in 0•5% (9/1751) of the patients including 0•2% (3/1751) gastric ulcer, fracture in 0•4% (7/1751), and increased alanine aminotransferase (ALT) in 0•2% (3/1751) of the patients. The secondary endpoint of ACR50 and ACR70 response rates at week 24 were 47•4% (757/1597) and 24•0% (384/1597). DAS28 was 4•11±1•27 and 3•75±1•32 at week 12 and 24, which was significantly decreased -1•40±1•10 and -1•75±1•26 compared with baseline (P<0•001) respectively. Changes in HAQ at week 12 and 24 from baseline were -7•4 ± 9•18 and -8•5 ± 9•97, respectively (all P<0•001). Stratified analysis results showed that the patients with shorter disease duration, male gender had better response to IGU. There was no significant difference in ACR20/50/70 responses between elderly patients(≥65 years) and younger patients(<65 years), IGU monotherapy or combined with other DMARDs. However, more fractures (1•1% vs 0•5%; P = 0•64) and infections (8•7% vs 7•9%; P = 0•69) were observed in elderly patients in our study.
Interpretation
Our results confirmed the effectiveness and safety of IGU as a new DMARD for active patients with RA as monotherapy or combination therapy.
Funding
This study was supported by “the 11th Five-Year-Plan for Science and Technology Support Program (2012ZX09104-103-01)”.
1. Introduction
Rheumatoid arthritis (RA) is a chronic disease leading to disability with serious heterogeneity, which makes it difficult to control and seriously affects the life and health of the public. Nowadays, disease-modifying anti-rheumatic drugs (DMARDs), including conventional synthetic DMARDs, biological DMARDs, and target synthetic DMARDs, are widely used for RA treatment. However, the remission rate of patients with RA remains low [1], [2], [3]. Furthermore, the economic burden of RA in developing countries is heavy. In 2017, the average cost was $1917•21±2559•06 per patient with RA per year and was approximately $13•9–22•4 billion in China, mainly drug expense [4]. New, affordable, oral and tolerable DMARDs are necessary to help patients, especially those in developing countries, achieve treatment targets.
Iguratimod (IGU) is a methane sulfonanilide chemically composed of (N-[7-[(methanesulfonyle) amino]−4-oxo-6-phenoxy-4H-1-benzopyran-3-yl]-formamide) [5]. It was found to have an inhibitory effect on the expression of interleukin (IL)−1 and IL-6 in monocytes, and selectively inhibited cyclooxygenase (COX)−2 activity and reduced the expression of COX-2 mRNA [6,7]. Further investigations have revealed that IGU down-regulated NF-κB activation, inhibited the expression of IgM and IgG in B cells, and reduced serum levels of TNF-α, IL-1, IL-6, and IL-17 in collagen-induced arthritis mice models [8], [9], [10]. It was also found that IGU decreased the mRNA expression of Ccl2, Cxcl1, Cxcl2, and Il-6; reduced phosphorylation of MAPKs; and targeted Act1 to disrupt the interaction between Act1 and TRAF5 or IKKi in the IL-17 pathways in synoviocytes [11]. Moreover, IGU significantly inhibited the expression of MMP-1 and MMP-3 in RA synovial fibroblasts [12]. These data indicate that IGU is a new DMARD for the treatment of RA.
Clinical trials have further demonstrated that for active patients with RA, IGU is effective when used as monotherapy or combined with methotrexate (MTX). In a double-blind, randomized, multicenter clinical trial that enrolled 280 active Chinese patients with RA, IGU 50 mg/d was effective when compared with placebo, and this dosage seemed more effective than IGU 25 mg/d according to ACR20 (61•29% vs. 39•13%) at week 24 and with no increase of adverse events [13]. A phase III clinical trial of IGU in 489 active Chinese patients with RA further showed that the ACR20 of IGU 50 mg/d was 63•8%, which demonstrated non-inferiority to methotrexate (MTX) 15 mg weekly (62•0%), and the general side effects of IGU were fewer and milder than those of MTX [14]. A similar remission rate was reached when compared with sulfasalazine 500 mg twice daily in a phase III study of IGU in Japan, although the dosage of IGU is 25 mg once daily for the first 4 weeks and then 25 mg twice daily for the subsequent 24 weeks [15]. Except for monotherapy, studies have also shown that IGU was effective when combined with MTX. A randomized, double-blind trial of 253 patients in Japan demonstrated that compared with placebo, IGU was effective with acceptable safety when combined with MTX [16]. An open-label study showed that IGU plus MTX sustained efficacy and was well tolerated until week 52 in active patients with RA with inadequate response to MTX [17]. In a randomized controlled trial of 60 Chinese active patients with RA, the combination of IGU and MTX was superior to MTX monotherapy at week 24 according to ACR50, DAS28 and, SDAI [18]. Based on the phase II and III studies, IGU is regarded as an effective DMARD in treating active RA and is well tolerated.
However, in daily clinical practice, patients’ characteristics are heterogeneous and complicated, and data from various patients (e.g., individuals who are elderly or have comorbidities or multiple medication regimens) may be of greater importance for clinicians and patients to make treatment decisions. To further validate the effectiveness and safety of IGU in diverse patients, a prospective real-world study in patients with RA was conducted in China. This paper reports the results of the trial.
2. Methods
2•1. Study design and participants
This study was a nationwide, prospective real-world study (ClinicalTrials.gov, number NCT01554917) to evaluate the effectiveness and safety of IGU. Active patients with RA were recruited from 48 academic rheumatology centers in China between March 2012 and August 2015. Participants were aged >18 years and had fulfilled the American College of Rheumatology (ACR) 1987 or ACR/European League Against Rheumatism (EULAR) 2010 criteria. If the participant was already on other RA therapy, the dose of concurrent DMARDs (including methotrexate, leflunomide, sulfasalazine and hydroxychloroquine), non-steroid anti-inflammatory drugs (NSAIDs), and corticosteroids (GCs) should have been stable for at least 12 weeks. Patients who were pregnant or breastfeeding or had mental disorders or severe organ functions were excluded. Full inclusion and exclusion criteria are provided in the appendix.
All participants signed an informed consent, and this study was approved by the China Food and Drug Administration (approve number: CTR20132723) and granted by all participating institutes’ independent ethics committee.
2•2 Procedures
All patients took IGU 25 mg bid for 24 weeks in this trial, and the therapy comprised 2 periods: IGU add-on period (the first 12 weeks) and regimen adjustment period (the latter 12 weeks). In the IGU add-on period, IGU (Simcere Pharmaceutical, Nanjing, China) 25 mg bid was added in all participants as monotherapy or into background RA treatments. Adjustment of IGU or background RA medicines, such as DMARDs, GCs, and NSAIDs, was not allowed in this period except for safety reasons. In the regimen adjustment period, IGU 25 mg bid was continued and an adjustment of RA medicines except for IGU was allowed by the physicians according to the participants’ disease activity. In this period, RA medicines could be either increased or decreased. In the case of medicine decrease, such order shall be obeyed: GCs at first, then NSAIDs, and lastly DMARDs. Any adjustments shall be recorded with details.
The study included 7 scheduled visits: baseline, week 2, week 4, week 8, week 12, week 16, and week 24. In each visit, clinical data and blood and urine samples were collected. At baseline, demographic and disease characteristics from each patient were recorded, including age, gender, body mass index, duration of RA, previous and ongoing RA therapy, and a chest and two-hand radiograph. At week 0, 4, 8, 12, 16, and 24, data regarding duration of morning stiffness; joint count for swollen joints(SJC); joint count for tender joints (TJC); subject's assessment of pain by visual analog scale (VAS); and patients’ and physicians’ global assessment of disease activity by VAS, Health Assessment Questionnaire (HAQ), and ESR, and CRP were collected. At week 0, 12, and 24, the depression score and anxiety score on the Hospital Anxiety and Depression Scale (HADS) were evaluated. Any adverse event (AE) between week 0 and week 28 was recorded.
2•3 Outcomes
For effectiveness analysis, the percentage of patients fulfilling the American College of Rheumatology 20% /50%/70% response criteria (ACR 20/50/70) at week 12 and 24; changes in disease activity score (DAS) 28, rate of ACR/EULAR clinical remission, HAQ, and HADS over time were assessed. For safety analysis, incidences of AE/severe AE (SAE)/adverse drug reaction (ADR) were recorded. An exploration of different subgroups, including different ages, genders, disease durations, and medication regimens, were compared with an exploration of further effectiveness signals and potential predictive safety factors. Detailed ADRs that occurred in ≥1% patients on the basis of the Common Terminology Criteria for Adverse Events (CTCAE) grades and the changes of ADRs over time were described. Relative risk (RR) of the most common ADRs on the basis of different medication regimens was compared with the findings of the safety signals.
The primary endpoints were ACR20 at week 24 and adverse events over this trial. The secondary endpoints were ACR50 and ACR70 at week 24 and the changes of DAS28 and HAQ over 24 weeks.
2•4 Statistical analysis
Measurement data, including age, BMI, duration of RA, rest pain, TJC, SJC, ESR, CRP, rheumatoid factor (RF), DAS28, and HAQ score were reported by using the mean (SD). Counting data, including sex, number of patients in different groups, rate of different functional status, serological status, and radiographic features were reported by using frequency. Relative risks of ADRs from different medication regimens were compared with IGU monotherapy and shown with 95%CI.
All analyses were based on the intention-to-treat (ITT) principle. Effectiveness analysis was based on the full analysis set (FAS), which refers to all eligible participants who have been selected without specific protocol violations (e.g., non-indications), and with baseline data and ACR assessment after at least one treatment. The missing data in outcome measures were carried over by the last observation carried forward (LOCF). If there were missing answers to the questions on the scales, the missing questions were first treated with LOCF before calculating the total score or average. Baseline values did not participate in LOCF. Safety analysis was based on the safety analysis set (SAS), which refers to all selected patients who had used IGU at least once. Compliance = actual dose in the study/the dose should be taken × 100%. Good compliance is defined as 80%–120%.
Comparisons of rates, including response rate of ACR20/50/70, rate of clinical remission, and incidences of AE/SAE/ADR, were performed by the χ2 test, F test, or Fisher's exact test, as appropriate. Changes after IGU treatment compared with the baseline were analyzed by using the Wilcoxon signed-rank test. Comparisons of other continuous variables were performed by the t-test. All statistical analyses were performed by SAS version 9•2.
2•5 Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
3. Results
Between Mar 23, 2012, and Aug 3, 2015, a total of 1759 participants were assessed for eligibility and enrolled in the study. All patients were added IGU 25 mg bid as monotherapy or to their background therapy, of whom 85•4% (1502/1759) of the patients completed the IGU add-on period, 81•5% (1433/1759) patients completed the entire study, and 18•5% (326/1759) patients discontinued (Fig. 1). Notably, 90•8% (1597/1759) patients were included in the FAS for effectiveness analysis, and 99•5% (1751/1759) patients were included in the SAS after excluding eight patients because of not taking IGU.
Fig. 1.
Trial profile. IGU add-on period:the first 12 weeks, IGU 25 mg bid was added as monotherapy or into participants’ background RA treatments;Regimen adjustment period:After the visit at the end of week 12, IGU 25 mg bid was continued, and adjustment of RA medicines except for IGU was allowed according to the participants’ disease activity. IGU= iguratimod.
The mean age of the 1597 patients in the FAS at baseline was 50•0 ± 12•2 years old, and the duration of RA was 8•1 ± 7•7 years. Notably, 69•7% (1113/1597) of these patients had taken DMARDs, and the other 21•1% (337/1597) of patients were DMARDs naive at baseline. Additionally, 33•2%(531/1597) of these patients were taking corticosteroids (Table 1).
Table 1.
Baseline characteristics of study participants in the full analysis set (FAS).
| All patients [mean (±) / N(%)] | |
|---|---|
| Age, years | 49•96 (± 12•16) |
| <65 years old (N = 1436) | 47•82 (± 10•82) |
| ≥65 years old (N = 161) | 69•06 (± 4•11) |
| Sex | |
| Female | 1327 (83•1%) |
| Male | 270 (16•9%) |
| BMI | 22•59 (± 3•35) |
| Duration of RA, month | 97•14 (± 92•62) |
| RF positive, no. (%) | 1256 (78•6%) |
| ACPA positive, no. (%) | 1338 (83•8%) |
| Ongoing RA therapy | |
| ‡DMARD treatment, no. (%) | 1113 (69•7%) |
| Concomitant NSAIDs, no. (%) | 162 (10•1%) |
| Concomitant corticosteroids, no. (%) | 531 (33•2%) |
| No | 337 (21•1%) |
| Rest pain, mm | 62•8 (± 16•7) |
| Tender joint count (TJC) | 14•7 (± 9•5) |
| Swollen joint count (SJC) | 9•7 (± 6•8) |
| ESR, mm/hour | 43•6 (± 28•7) |
| CRP, μg/ml | 20•1 (± 41•7) |
| DAS28 | 5•5 (± 1•1) |
| Chest radiograph | |
| Abnormal Chest radiograph | 663 (41•5%) |
| Normal Chest radiograph | 886(55•5%) |
| No Chest radiograph | 48(3•0%) |
| HAQ score | 0•8 (± 0•6) |
| † Patient global assessment, mm | 64•8 (± 16•1) |
| † Physician global assessment, mm | 63•1 (± 14•9) |
Values are the mean ± SD unless otherwise indicated. Categorical data comparisons were performed by Fisher's exact test, age comparisons were performed by the F test, and group comparisons were performed by the Kruskal-Wallis test.
‡ DMARD includes methotrexate, leflunomide, sulfasalazine and hydroxychloroquine. Detailed DMARD treatment see appendix, suppl.5.
† Measured on a 100-mm visual analog scale.
BMI=body mass index; RF=rheumatoid factor; ACPA=anticitrullinated peptide antibody; DAS= disease activity score; MTX=methotrexate; HAQ=Health Assessment Questionnaire; ESR=erythrocyte sedimentation rate; CRP=C-reactive protein; DMARD=disease-modifying anti-rheumatic drug; NSAIDs=nonsteroidal anti-inflammatory drugs.
The average compliance rates were 94•6% and 95•2% in the SAS and FAS, respectively.
3.1. The primary endpoints
The primary endpoints were American College of Rheumatology 20% response (ACR20) and adverse events during the trial. ACR response rate increased gradually over time. The ACR20 response rate at week 12 and 24 was 62•2% (994/1597) and 71•9% (1148/1597), respectively.
The incidence of adverse events was 51•7% (906/1751) in the SAS. No new signal of a previously unreported safety issue was found. The incidence of AE with grade <3 and ≥3 was 48•3% (846/1751) and 3•4% (60/1751), respectively, including 3•1% (54/1751) of patients for grade 3 and 0•3% (6/1751) of patients for grade 4 (Table 2). The most common AE ≥ grade 3 was infectious diseases in 0•6% (10/1751) of patients including 0•2% (4/1751) pneumonitis, 0•5% (9/1751) abdominal discomfort including 0•2% (3/1751) gastric ulcer, 0•4% (7/1751) fracture, and 0•2% (3/1751) increased alanine aminotransferase (ALT; Table 3).
Table 2.
Incidences of AE, SAE, and ADR.
| n | AE | SAE | ADR | |
|---|---|---|---|---|
| Overall | 1751 | 906(51•7%) | 64(3•7%) | 674(38•5%) |
| Age | ||||
| Age<65 years | 1568 | 799 (51•0%) | 50 (3•2%) | 596 (38•0%) |
| Age ≥65 years | 183 | 107 (58•5%) | 14 (7•7%) | 78 (42•6%) |
| P value | 0•0605 | 0•0056 | 0•2294 | |
| Gender | ||||
| Male | 301 | 146 (48•5%) | 14 (4•7%) | 117 (38•9%) |
| Female | 1450 | 760 (52•4%) | 50 (3•4%) | 557 (38•4%) |
| P value | 0•2287 | 0•3117 | 0•8965 | |
| Duration of RA | ||||
| <2 years | 413 | 210 (50•8%) | 11 (2•7%) | 167 (40•4%) |
| ≥2 years | 1336 | 694 (51•9%) | 53 (4•0%) | 505 (37•8%) |
| P value | 0•7354 | 0•2929 | 0•3545 | |
| †Treatment mode over 24 weeks | ||||
| IGU monotherapy | 241 | 86 (35•7%) | 3 (1•2%) | 67 (27•8%) |
| IGU+LEF | 599 | 334 (55•8%) | 30 (5•0%) | 256 (42•7%) |
| P value | <0•0001* | 0•011* | <0•0001* | |
| IGU+MTX | 752 | 414 (55•1%) | 47 (6•3%) | 295 (39•2%) |
| P value | <0•0001* | 0•002* | 0•001* | |
| IGU+HCQ | 398 | 199 (50•0%) | 18 (4•5%) | 144 (36•2%) |
| P value | <0•0001* | 0•024* | 0•029* |
Data are n (%) unless otherwise indicated.
† Due to one patient may use more than one DMARD (e.g. a patient on triple therapy may use IGU, LEF and MTX, and thus, he will be counted in both IGU+LEF subgroup and IGU+MTX subgroup), so the total number in treatment mode is larger than 1751.
*compared with IGU monotherapy.
AE=Adverse Events. SAE=Severe Adverse Events. ADR=adverse drug reaction. IGU= Iguratimod. LEF= Leflunomide. MTX= Methotrexate.
Table 3.
Incidence of AEs in the safety analysis set.
| Disorders of different system | SAS (N=1751) |
Total | |||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| AEs (one at least) | 637 (36•4%) | 209 (11•9%) | 54 (3•1%) | 6 (0•3%) | 906 (51•7%) |
| Abnormal laboratory examinations | 377 (21•5%) | 67 (3•8%) | 6 (0•3%) | 2 (0•1%) | 452 (25•8%) |
| ALT increased | 166 (9•5%) | 32 (1•8%) | 3 (0•2%) | 0 | 201 (11•5%) |
| Whole blood cell decreased | 1 (0•05%) | 0 | 0 | 2 (0•1%) | 3 (0•2%) |
| Gastrointestinal system | 305 (17•4%) | 79 (4•5%) | 9 (0•5%) | 0 | 393 (22•4%) |
| Diarrhea | 59 (3•4%) | 13 (0•7%) | 2 (0•1%) | 0 | 74 (4•2%) |
| Gastric ulcer | 1 (0•05%) | 2 (0•1%) | 3 (0•2%) | 0 | 6 (0•3%) |
| Nervous system | 148 (8•5%) | 17 (1•0%) | 3 (0•2%) | 1 (0•05%) | 169 (9•7%) |
| Dizzy | 83 (4•7%) | 7 (0•4%) | 2 (0•1%) | 0 | 92 (5•3%) |
| Brain infarction | 0 | 0 | 0 | 1 (0•05%) | 1 (0•05%) |
| Skin and subcutaneous tissue | 134 (7•7%) | 22 (1•3%) | 4 (0•2%) | 1 (0•05%) | 161 (9•2%) |
| Rash | 34 (1•9%) | 7 (0•4%) | 0 | 1 (0•05%) | 42 (2•4%) |
| Facial edema | 25 (1•4%) | 3 (0•2%) | 2 (0•1%) | 0 | 30 (1•7%) |
| Infectious diseases | 107 (6•1%) | 23 (1•3%) | 10 (0•6%) | 0 | 140 (8•0%) |
| Urinary tract infection | 15 (0•9%) | 2 (0•1%) | 2 (0•1%) | 0 | 19 (1•1%) |
| Pulmonary infection | 0 | 0 | 4 (0•2%) | 0 | 4 (0•2%) |
| Systemic diseases and various reactions of administration site | 95 (5•4%) | 19 (1•1%) | 1 (0•05%) | 1 (0•05%) | 116 (6•6%) |
| Death | 0 | 0 | 0 | 1 (0•05%) | 1 (0•05%) |
| Respiratory system, chest and mediastinum | 64 (3•7%) | 13 (0•7%) | 2 (0•1%) | 0 | 79 (4•5%) |
| Musculoskeletal and connective tissue | 13 (0•7%) | 12 (0•7%) | 19 (1•1%) | 0 | 44 (2•5%) |
| Rheumatoid arthritis | 0 | 5 (0•3%) | 7 (0•4%) | 0 | 12 (0•7%) |
| Fracture | 0 | 3 (0•2%) | 7 (0•4%) | 0 | 10 (0•6%) |
| Eyes disorders | 35 (2•0%) | 1 (0•1%) | 1 (0•05%) | 0 | 37 (2•1%) |
| Circulatory system | 29 (1•7%) | 5 (0•3%) | 1 (0•05%) | 1 (0•05%) | 36 (2•1%) |
| Acute myocardial infarction | 0 | 0 | 0 | 1 (0•05%) | 1 (0•05%) |
| Metabolic and nutritional system | 26 (1•5%) | 6 (0•3%) | 0 | 0 | 32 (1•8%) |
| Blood and lymphatic system | 16 (0•9%) | 6 (0•3%) | 0 | 0 | 22 (1•3%) |
| Kidney and urinary system | 18 (1•0%) | 0 | 2 (0•1%) | 0 | 20 (1•1%) |
| Ear and labyrinth | 16 (0•9%) | 0 | 0 | 0 | 16 (0•9%) |
| Reproductive system and breast diseases | 10 (0•6%) | 3 (0•2%) | 0 | 0 | 13 (0•7%) |
| Immune system diseases | 2 (0•1%) | 4 (0•2%) | 1 (0•05%) | 0 | 7 (0•4%) |
| Blood vessels and lymphatic vessels | 7 (0•4%) | 0 | 0 | 0 | 7 (0•4%) |
| Hepatobiliary system | 1 (0•05%) | 2 (0•1%) | 1 (0•05%) | 1 (0•05%) | 5 (0•3%) |
| Hepatitis E | 0 | 0 | 0 | 1 (0•05%) | 1 (0•05%) |
| Injury, poisoning and surgical complications | 2 (0•1%) | 3 (0•2%) | 0 | 0 | 5 (0•3%) |
| Operations | 0 | 3 (0•2%) | 1 (0•05%) | 0 | 4 (0•2%) |
| Tumors (benign, malignant, or unknown) | 0 | 2 (0•1%) | 0 | 0 | 2 (0•1%) |
| Endocrine system | 1 (0•05%) | 0 | 0 | 0 | 1 (0•05%) |
| Pregnancy, puerperium and perinatal period | 0 | 0 | 1 (0•05%) | 0 | 1 (0•05%) |
SAE occurred in 64 patients with an incidence of 3•7% (64/1751), and 0•05% (1/1751) of patients died with sudden death most likely due to cardiovascular disease. Additionally, 0•7% (13/1751) of patients developed SAEs determined by investigators to be associated with IGU. For SAE of special interest, pulmonary interstitial fibrosis occurred in 0•05% (1/1751) of the patients. The patient was a man aged 57 years who had interstitial lung disease for 24 months, and had regularly taken LEF 20 mg/d for 2 years. The patient felt the arthritis was aggravated with fatigue, and exertional dyspnea at week 16, and IGU was stopped at week 23 according to the physician's advice. The symptom continued until week 28. Among our patients, 12 patients had a history of interstitial lung disease, none had progressed during the clinical trial.
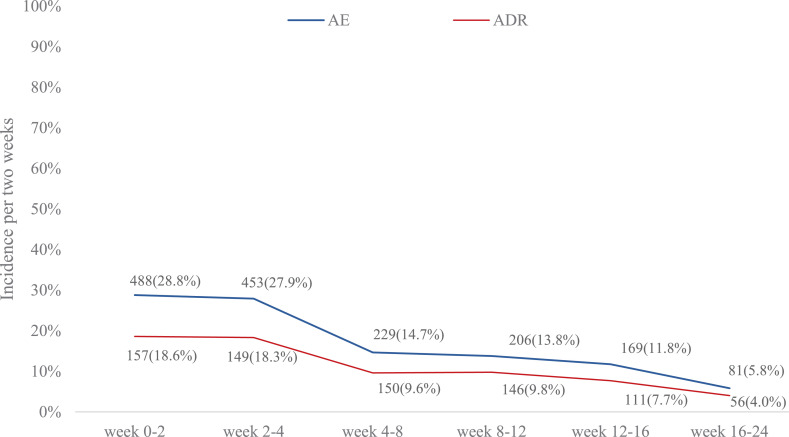
ADR occurred in 38•5% (674/1751) of the patients and the incidences of grade 1–4 ADR were 28•9% (506/1751), 8•6% (151/1751), 0•9% (15/1751), and 0•1% (2/1751), respectively. Both AE and ADR occurred more frequently in the first 4 weeks of administration and then decreased gradually (Fig. 4).
Fig. 4.
Changes of AE and ADR over time. Data are n (%). All incidences were adjusted to the same period of 2 weeks to compare them. ADRs were classified by using the system organ class (SOC) and preferred term (PT) according to the Medical Dictionary for Regulatory Affairs (MedDRA; version 17•1). AE=Adverse Events. ADR=adverse drug reaction.
3.2. Secondary endpoints
The secondary endpoints were ACR50 and ACR70 over 24 weeks and the changes of DAS28 and HAQ at week 24. ACR50 and ACR70 response rates at week 24 were 47•4% (757/1597) and 24•0% (384/1597); ACR50 and ACR70 response rates at week 12 were 29•5% (471/1597) and 11•0% (176/1597). DAS28 was significantly decreased at week 12 and 24 compared with baseline (P<0•001); the changes in DAS28 at week 12 and 24 from baseline were −1•40±1•10 and −1•75±1•26. DAS28 remission (DAS28≤2•6) was achieved in 11•3% (169/1498) of the patients at week 12 and 20•0% (299/1498) at week 24. The patients who reached low disease activity (2•6<DAS28≤3•2) at week 12 and week 24 were 11•3% (169/1498) and 14•5% (217/1498), respectively (Fig. 2B). The score of HAQ at baseline was 16•3 ± 11•37, changes in HAQ at week 12 and 24 from baseline were −7•4 ± 9•18 and −8•5 ± 9•97, respectively (all P<0•001). The proportions of RF+ RA and ACPA+ RA patients decreased to 66•4% (912/1373) and 83•9% (1102/1314), respectively.
Fig. 2.
Efficacy of IGU at week 12 and week 24. (A) ACR20/50/70 response rate of all patients at week 12 and week 24; (B) DAS28 disease activity of patients at baseline, week 12, and week 24(Remission, DAS28≤2•6; Low disease activity 2•6<DAS28≤3•2; Moderate disease activity, 3•2<DAS28≤5•1; and High disease activity, DAS28>5•1);(C) ACR/EULAR remission rate of patients at baseline, week 4, week 8, week 12, week 16 and week 24. * P<0•05 compared with week 12. ACR=American College of Rheumatology; DAS28=Disease Activity Score 28 joints; DR=disease duration; EULAR=European League Against Rheumatism; IGU=Iguratimod.
3.3. Subgroup analysis of effectiveness
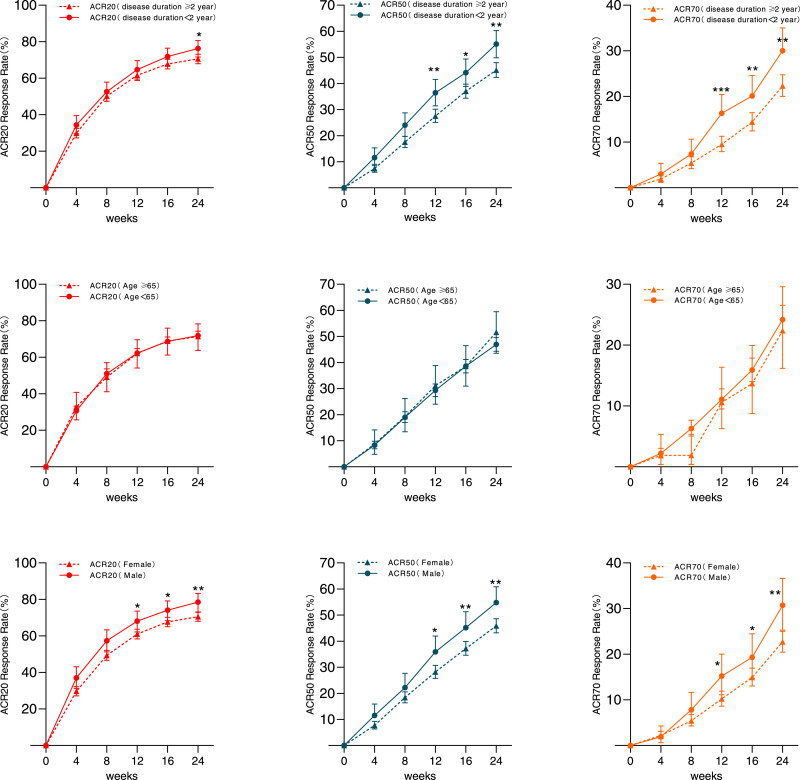
We divided the patients into two groups based on disease duration (the baseline data are shown in the appendix, suppl.1) and found that the ACR20/50/70 between patients with disease duration <2 years and ≥2 years were 64•7% vs. 61•6% (P = 0•295), 36•4% vs. 27•5% (P = 0•001), and 16•3% vs. 9•5% (P<0•001) at week 12; and 76•3% vs. 70•6% (P = 0•033), 55•1% vs. 45•1% (P = 0•001), and 30•0% vs. 22•3% (P = 0•003) at week 24, respectively (Fig. 3A). The results showed that the patients with shorter disease duration had better response to IGU.
Fig. 3.
ACR 20/50/70 response rate of different subgroups and the comparison between subgroups at week 12 and week 24. (A)ACR20/50/70 response rate of subgroups that was divided by disease duration (< 2 years or ≥ 2 years); (B) ACR20/50/70 response rate of subgroups that was divided by gender (Male or Female); (C) ACR20/50/70 response rate of subgroups that was divided by age (< 65 years or ≥ 65 years). * P<0•05 between 2 subgroups; ** P<0•01 between 2 subgroups. ACR=American College of Rheumatology.
When dividing all patients by gender, we found that men had slightly better ACR response than women (the baseline data are presented in the appendix, suppl.2). The ACR20/50/70 response rates of IGU for men and women were 68•1% vs. 61•0%, 35•9% vs. 28•2%, and 15•2% vs. 10•2% (P < 0•05) at week 12; and 78•5% vs. 70•5%, 54•8% vs. 45•9%, 30•7% vs. 22•7%, respectively, at week 24 (P < 0•05, Fig. 3B).
There was no significant statistical difference in ACR20/50/70 responses between the 161 patients aged ≥65 years and the 1436 patients aged <65 years at week 12 and week 24 (P > 0•05, Fig. 3C). The baseline data are presented in the appendix, suppl.3.
There was no significant difference in ACR20/50/70 responses among the treatment regimens of IGU monotherapy or combined with HCQ, LEF, and MTX (All P> 0•05, appendix, suppl.6).The rate of ACR/EULAR clinical remission was achieved in 5•7% (79/1379) and 13•3% (187/1403) of the patients at week 12 and week 24, respectively (Fig. 2C). The total score of depression and anxiety of HADS at baseline was 11•1 ± 8•04, and the changes at week 12 and 24 were −3•4 ± 6•25 and −4•5 ± 7•07, respectively (all P<0•001).
3.4. Subgroup analysis of safety
Subgroup analysis showed that gender and disease duration of RA were not correlated to the presence of AE, SAE, and ADR. Age was also not related to the presence of AE and ADR. Patients aged ≥65 years seemed to develop more SAE [7•7% (14/183) vs. 3•2% (50/1568), P = 0•0056], but similar SAE related to drugs [1•6% (3/183) vs. 0•6% (10/1568), P = 0•14]. In elderly patients, fractures and infections were seen significantly more often than in the younger patients in our study. However, no new or worsening pulmonary disease were seen in patients with or without pulmonary diseases in our study.
The incidences of AE, SAE, and ADR in IGU monotherapy subgroup were all significantly lower than in the combination therapy group (Table 2).
Increased alanine aminotransferase (ALT, 10•8%, 189/1751) and increased aspartate aminotransferase (AST; 9•8%, 171/1751) were the top two ADRs in the study, mainly in grade 1–2 (appendix suppl.4). The mean differences between ALT, AST, and total bilirubin from baseline to week 24 were 1•20 U/L (95%CI −0•22 to 2•62), 2•02 U/L (95%CI 0•48 to 3•56) and 0•69 μmol/L (95%CI: 0•48, 0•90), respectively, with no clinical significance. In subgroup analysis, no difference was found between IGU combination therapy and IGU monotherapy in liver damage.
Abdominal discomfort (6•3%, 111/1751) and abdominal pain (4•5%, 78/1751) were listed as the third and fourth most common ADRs in our study, and they were mainly in grade 1–2 as well (appendix suppl.4). In subgroup analysis, no difference was found between IGU combination therapy and IGU monotherapy.
Leukopenia (3•9%, 69/1751) was the fifth most common ADR in the study. Leukocytes slightly decreased during IGU treatment (−0•50×109/L, 95% CI −0•60 to −0•39), which was of no clinical significance. Further analysis showed that IGU+LEF combination might increase the risk of leucocytopenia more than IGU monotherapy would (RR 2•90, 95% CI 1•15 to 7•29).
Serum creatinine slightly increased and eGFR decreased during IGU treatment with the mean differences of 3•55 μmol/L (95%CI 3•00 to 4•10) and −0•06 mL/ min×1•73 m2 (95%CI −0•07 to −0•04), respectively, from baseline to week 24. However, no patients developed renal dysfunction. Further analysis showed no difference between IGU combination therapy and IGU monotherapy.
4. Discussion
In this 24-week nationwide, prospective real-world Study of IGU in Chinese patients with RA, we found that IGU was safe and effective in patients with active RA in a more complex clinical setting. The disease activity was improved from week 12, especially in early RA patients and men. The patients tolerated IGU well, including in elderly patients and patients with multiple DMARDs or complications. Additionally, the HAQ and HADS scores were all significantly decreased after treatment.
Studies have shown that the ACR20 response rate of IGU monotherapy was 60%–80% at week 24 in phase II and phase III clinical trials, demonstrating that the majority of patients can benefit from IGU therapy [13,14,19]. Our study showed that the ACR20 response rate was 62•2% at week 12 and 71•9% at week 24 in patients with IGU monotherapy or combination therapy, which was comparable with previous studies. Overall, as a new DMARD, both IGU monotherapy and combination therapy are effective for active patients with RA.
Several studies have demonstrated that early treatment leads to better outcomes [13,14,20]. This study also showed that patients with disease duration of less than 2 years had higher ACR20/50/70 responses at week 12 and week 24 compared with patients with longer disease duration. The safety was comparable in these two groups. This consequence was in accordance with the concept of early intervention for RA and confirmed that IGU is a comparable selection in the strategy of early therapy.
Notably, our study showed IGU was more effective in men than women both at week 12 and 24 although males had a significantly longer disease course than females (101•77±94•64 vs. 74•43±78•26 months, P<0•0001). Studies have demonstrated that gender could be an influencing factor for treatment. The CORRONA study showed that male sex was associated with sustained remission in early RA and point remission in established RA [21]. Kleinert et al. and Burmester et al. investigated the efficacy of adalimumab in active RA and found that male gender was a positive predictor of therapeutic response [22,23]. van der Heijde et al. found that male gender was an independent predictor of remission using the combination therapy of etanercept and methotrexate in RA [24]. Soliman et al. found that response to RTX for RA was also influenced by gender, with women having lower response rates [25]. Wessels et al. and Hoekstra et al. summarized that male patients had better efficacy and prognosis than female patients did regardless of cDMARDs, bDMARDs, or combination drugs in the treatment of early RA or RA with a long course of disease [26,27]. Sex factors can affect the efficacy, which might be related to the role of estrogen. Hider et al. found that in addition to its immunomodulatory function, estrogen has been found to affect the kinetics and pharmacodynamics of drugs, resulting in differences in efficacy [28]. For IGU, no studies have shown that its efficacy was related to gender; thus, further studies are necessary to confirm whether gender affects its efficacy in RA.
Multiple comorbidities are present in elderly patients with RA, including cardiovascular disease, interstitial lung disease (ILD), malignancies, osteoporosis, cognitive impairment, depression, and anxiety, and made the management of elderly patients more difficult [29]. The declines in physical function and safety problems are important issues in elderly patients. In Krishnan et al's study of 1530 Finnish adults with RA, elderly patients had higher pain VAS scores [30]. In our study, 162 patients with RA aged over 65 years with multiple chronic diseases including diabetes, pulmonary, and cardiovascular diseases were enrolled. Our results showed that in elderly patients with RA, the incidences of AE and ADR were not increased, and IGU did not impair renal function. These data indicated no obligatory need for dosage adjustment in elderly patients. However, patients aged ≥65 years seemed to develop more SAE in our study; thus, close monitoring and prevention of side effects, especially fracture and infection. Additionally, there was no significant difference in efficacy between elderly and younger patients, indicating that IGU is also effective in elderly active patients with RA.
The main safety concerns of IGU are liver dysfunction and gastrointestinal disorders. Increased ALT and increased AST were common in our study with an incidence of approximately 10%. Fortunately, liver dysfunction caused by IGU is transient and revisable. Subgroup analysis in our study showed IGU combined with other DMARDs did not increase the risk of liver dysfunction. Thus, IGU as a combination regimen is safe. However, physicians should be cautious, especially in patients with a history of liver diseases. Additionally, although the rate of leucocytopenia was comparable with MTX [14], we found that IGU combined with LEF increased the risk of leucocytopenia; thus, IGU+LEF may not be an ideal combination. Because of safety concerns, liver function and white blood count should be monitored when IGU starts. Notably, the incidence of adverse events of kidney disorder in our study is lower than that in Japanese phase IV study of 5.1% (136/2666) 32. The reason might be that the patients in the Japanese phase IV study are older and have longer disease duration and our result is consistent with the results of phase II and phase III clinical studies of IGU in China which show that kidney disorder related to IGU was<1% [13,14,19].
The management of RA patients with ILD is a difficult clinical situation [31]. MTX, LEF, and bDMARDs have all been reported to be associated with RA-ILD, although it is difficult to distinguish causality [32], [33], [34]. In our study, interstitial pneumonia aggravated in one patient with an ILD history. A retrospective observational study demonstrated that the combination of IGU on the basis of treatment with multiple DMARDs including LEF, HCQ, and sulfasalazine was safe in patients with RA with chronic interstitial pneumonia [35]. Further studies on IGU to ILD are warranted.
A post-marketing study that enrolled 2666 Japanese patients also showed that the clinical safety of long-term use of IGU was satisfactory [32]. In their study, IGU was prescribed at half-dose for the first 4 weeks, while IGU in our study was used in 25 mg bid from the beginning. The Japanese study mainly analyzed the safety results in detail, and only the DAS28-CRP level was analyzed in the efficacy results; by contrast, our study analyzed the efficacy and safety in more detail and stratified the analysis by age, gender, disease duration, and dosing regimen. Our findings are a more comprehensive addition to those studies in Japan.
Our study has limitations. First, the duration of IGU treatment was 24 weeks based on the instruction. Notably, a longer-term study on the safety and efficacy of IGU is now ongoing in China. Second, because this study is a single-arm study with no control group based on the characteristic of phase IV study, it was not possible to compare the effectiveness and safety of IGU with other DMARDs.
In conclusion, this prospective, large sample clinical study provided a comprehensive analysis of the effectiveness and safety of IGU. We found that IGU was effective and safe for patients with active RA and is a new, inexpensive choice as monotherapy or in combination with other DMARDs.
Funding
This study was supported by “the 11th Five-Year-Plan for Science and Technology Support Program (2012ZX09104–103–01)”.
Contributors
Zhanguo Li and Rong Mu carried out the concepts and design of this study; Rong Mu and Chun Li performed project administration; Rong Mu, Xiaomei Li, Yao Ke, Ling Zhao, Lin Chen, Rui Wu, Zhenbiao Wu, Xiaoxia Zuo, Yanli Xie, Jinwei Chen, Wei, Yi Liu, Zhijun Li, Lie Dai, Lingyun Sun and Xiangyuan Liu together carried out the study. Rong Mu and Chun Li provided assistance for data acquisition and data collection. Zhanguo Li and Rong Mu assisted in data analysis, data interpretation, manuscript editing and manuscript review. All authors have read and approved the content of the manuscript.
Declaration of Competing Interest
All authors have nothing to disclose.
Data sharing statement
The data that support the findings of this study are available from the corresponding author, Zhanguo Li, upon reasonable request.
Research in context
Evidence before this study
Nowadays, disease-modifying anti-rheumatic drugs (DMARDs), including conventional synthetic DMARDs, biological DMARDs, and target synthetic DMARDs, are widely used for Rheumatoid arthritis (RA) treatment. However, the remission rate of patients with RA remains low and the economic burden of RA in developing countries is heavy. The economic inequality results in different treatment options between developed and developing countries. Low-cost conventional synthetic DMARDs are more in line with the needs of patients with RA in developing countries. Iguratimod (IGU) is a new DMARD with low price that has demonstrated efficacy and safety in previous pre-market and post-market studies. However, real-life data of various patient groups from a large population cohort was urgently needed.
Added value of this study
A large scale real-world study of IGU in the treatment of active RA in China was conducted and revealed that IGU might be a cost-effective choice for RA patients in developing countries.
Implications of all the available evidence
IGU should be a preferred option to choose for the low response in RA patients in developing countries.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100128.
Contributor Information
Rong Mu, Email: murongster@163.com.
Zhanguo Li, Email: li99@bjmu.edu.cn.
Appendix. Supplementary materials
References
- 1.McInnes I.B., Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Zhu H., Li R., Da Z. Remission assessment of rheumatoid arthritis in daily practice in China: a cross-sectional observational study. Clin Rheumatol. 2018;37(3):597–605. doi: 10.1007/s10067-017-3850-z. [DOI] [PubMed] [Google Scholar]
- 3.Chatzidionysiou K., Sfikakis P.P. Low rates of remission with methotrexate monotherapy in rheumatoid arthritis: review of randomised controlled trials could point towards a paradigm shift. RMD Open. 2019;5(2) doi: 10.1136/rmdopen-2019-000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu H., Luan L., Yang K., Li S.C. Burden of rheumatoid arthritis from a societal perspective: a prevalence-based study on cost of this illness for patients in China. Int J Rheum Dis. 2018;21(8):1572–1580. doi: 10.1111/1756-185X.13028. [DOI] [PubMed] [Google Scholar]
- 5.Li J., Bao J., Zeng J., Yan A., Zhao C., Shu Q. Iguratimod: a valuable remedy from the Asia Pacific region for ameliorating autoimmune diseases and protecting bone physiology. Bone Res. 2019;7:27. doi: 10.1038/s41413-019-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka K., Aikawa Y., Kawasaki H., Asaoka K., Inaba T., Yoshida C. Pharmacological studies on 3-formylamino-7-methylsulfonylamino-6-phenoxy-4H-1-benzopyran-4-one (T-614), a novel antiinflammatory agent. 4th communication: inhibitory effect on the production of interleukin-1 and interleukin-6. J Pharmacobiodyn. 1992;15(11):649–655. doi: 10.1248/bpb1978.15.649. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K., Kawasaki H., Kurata K., Aikawa Y., Tsukamoto Y., Inaba T. T-614, a novel antirheumatic drug, inhibits both the activity and induction of cyclooxygenase-2 (COX-2) in cultured fibroblasts. Jpn J Pharmacol. 1995;67(4):305–314. doi: 10.1254/jjp.67.305. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K., Yamamoto T., Aikawa Y. Inhibitory effects of an anti-rheumatic agent T-614 on immunoglobulin production by cultured B cells and rheumatoid synovial tissues engrafted into SCID mice. Rheumatology. 2003;42(11):1365–1371. doi: 10.1093/rheumatology/keg381. (Oxford England) [DOI] [PubMed] [Google Scholar]
- 9.Kohno M., Aikawa Y., Tsubouchi Y. Inhibitory effect of T-614 on tumor necrosis factor-alpha induced cytokine production and nuclear factor-kappaB activation in cultured human synovial cells. J Rheumatol. 2001;28(12):2591–2596. [PubMed] [Google Scholar]
- 10.Du F., Lü L.J., Fu Q. T-614, a novel immunomodulator, attenuates joint inflammation and articular damage in collagen-induced arthritis. Arthritis Res Ther. 2008;10(6):R136. doi: 10.1186/ar2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Q., Sun Y., Liu W. A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J Immunol. 2013;191(10):4969–4978. doi: 10.4049/jimmunol.1300832. [DOI] [PubMed] [Google Scholar]
- 12.Du F., Lü L.J., Teng J.L., Shen N., Ye P., Bao C.D. T-614 alters the production of matrix metalloproteinases (MMP-1 andMMP-3) and inhibits the migratory expansion of rheumatoid synovial fibroblasts, in vitro. Int Immunopharmacol. 2012;13(1):54–60. doi: 10.1016/j.intimp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Lü L.J., Teng J.L., Bao C.D. Safety and efficacy of T-614 in the treatment of patients with active rheumatoid arthritis: a double blind, randomized, placebo-controlled and multicenter trial. Chin Med J. 2008;121(7):615–619. [PubMed] [Google Scholar]
- 14.Lu L.J., Bao C.D., Dai M. Multicenter, randomized, double-blind, controlled trial of treatment of active rheumatoid arthritis with T-614 compared with methotrexate. Arthritis Rheum. 2009;61(7):979–987. doi: 10.1002/art.24643. [DOI] [PubMed] [Google Scholar]
- 15.Hara M., Abe T., Sugawara S. Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: a controlled, multicenter, double-blind, parallel-group study. Mod Rheumatol. 2007;17(1):1–9. doi: 10.1007/s10165-006-0542-y. [DOI] [PubMed] [Google Scholar]
- 16.Ishiguro N., Yamamoto K., Katayama K. Concomitant iguratimod therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate: a randomized, double-blind, placebo-controlled trial. Mod Rheumatol. 2013;23(3):430–439. doi: 10.1007/s10165-012-0724-8. [DOI] [PubMed] [Google Scholar]
- 17.Hara M., Ishiguro N., Katayama K. Safety and efficacy of combination therapy of iguratimod with methotrexate for patients with active rheumatoid arthritis with an inadequate response to methotrexate: an open-label extension of a randomized, double-blind, placebo-controlled trial. Mod Rheumatol. 2014;24(3):410–418. doi: 10.3109/14397595.2013.843756. [DOI] [PubMed] [Google Scholar]
- 18.Duan X.W., Zhang X.L., Mao S.Y., Shang J.J., Shi X.D. Efficacy and safety evaluation of a combination of iguratimod and methotrexate therapy for active rheumatoid arthritis patients: a randomized controlled trial. Clin Rheumatol. 2015;34(9):1513–1519. doi: 10.1007/s10067-015-2999-6. [DOI] [PubMed] [Google Scholar]
- 19.Xia Z., Lyu J., Hou N., Song L., Li X., Liu H. Iguratimod in combination with methotrexate in active rheumatoid arthritis: therapeutic effects. Z Rheumatol. 2016;75(8):828–833. doi: 10.1007/s00393-015-1641-y. [DOI] [PubMed] [Google Scholar]
- 20.Okamura K., Yonemoto Y., Okura C., Kobayashi T., Takagishi K. Efficacy of the clinical use of iguratimod therapy in patients with rheumatoid arthritis. Mod Rheumatol. 2015;25(2):235–240. doi: 10.3109/14397595.2014.938401. [DOI] [PubMed] [Google Scholar]
- 21.Jawaheer D., Messing S., Reed G. Significance of sex in achieving sustained remission in the consortium of rheumatology researchers of North America cohort of rheumatoid arthritis patients. Arthritis Care Res. 2012;64(12):1811–1818. doi: 10.1002/acr.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinert S., Tony H.P., Krause A. Impact of patient and disease characteristics on therapeutic success during adalimumab treatment of patients with rheumatoid arthritis: data from a German noninterventional observational study. Rheumatol Int. 2012;32(9):2759–2767. doi: 10.1007/s00296-011-2033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burmester G.R., Ferraccioli G., Flipo R.M. Clinical remission and/or minimal disease activity in patients receiving adalimumab treatment in a multinational, open-label, twelve-week study. Arthritis Rheum. 2008;59(1):32–41. doi: 10.1002/art.23247. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijde D., Klareskog L., Landewé R. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(12):3928–3939. doi: 10.1002/art.23141. [DOI] [PubMed] [Google Scholar]
- 25.Soliman M.M., Hyrich K.L., Lunt M., Watson K.D., Symmons D.P., Ashcroft D.M. Effectiveness of rituximab in patients with rheumatoid arthritis: observational study from the British society for rheumatology biologics register. J Rheumatol. 2012;39(2):240–246. doi: 10.3899/jrheum.110610. [DOI] [PubMed] [Google Scholar]
- 26.Wessels J.A., van der Kooij S.M., le Cessie S. A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis Rheumatol. 2007;56(6):1765–1775. doi: 10.1002/art.22640. [DOI] [PubMed] [Google Scholar]
- 27.Hoekstra M., van Ede A.E., Haagsma C.J. Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62(5):423–426. doi: 10.1136/ard.62.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hider S.L., Buckley C., Silman A.J., Symmons D.P., Bruce I.N. Factors influencing response to disease modifying antirheumatic drugs in patients with rheumatoid arthritis. J Rheumatol. 2005;32(1):11–16. [PubMed] [Google Scholar]
- 29.van Onna M., Boonen A. The challenging interplay between rheumatoid arthritis, ageing and comorbidities. BMC Musculoskelet Disord. 2016;17:184. doi: 10.1186/s12891-016-1038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan E., Häkkinen A., Sokka T., Hannonen P. Impact of age and comorbidities on the criteria for remission and response in rheumatoid arthritis. Ann Rheum Dis. 2005;64(9):1350–1352. doi: 10.1136/ard.2005.037903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iqbal K., Kelly C. Treatment of rheumatoid arthritis-associated interstitial lung disease: a perspective review. Ther Adv Musculoskelet Dis. 2015;7(6):247–267. doi: 10.1177/1759720X15612250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mimori T., Harigai M., Atsumi T. Safety and effectiveness of iguratimod in patients with rheumatoid arthritis: final report of a 52-week, multicenter postmarketing surveillance study. Mod Rheumatol. 2019;29(2):314–323. doi: 10.1080/14397595.2018.1460230. [DOI] [PubMed] [Google Scholar]
- 33.Li J., Mao H., Liang Y. Efficacy and safety of iguratimod for the treatment of rheumatoid arthritis. Clin Dev Immunol. 2013;2013 doi: 10.1155/2013/310628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa K., Ishikawa J. Iguratimod, a synthetic disease modifying anti-rheumatic drug inhibiting the activation of NF-κB and production of RANKL: its efficacy, radiographic changes, safety and predictors over two years' treatment for Japanese rheumatoid arthritis patients. Mod Rheumatol. 2019;29(3):418–429. doi: 10.1080/14397595.2018.1481565. [DOI] [PubMed] [Google Scholar]
- 35.Mimori T., Harigai M., Atsumi T. Safety and effectiveness of 24-week treatment with iguratimod, a new oral disease-modifying antirheumatic drug, for patients with rheumatoid arthritis: interim analysis of a post-marketing surveillance study of 2679 patients in Japan. Mod Rheumatol. 2017;27(5):755–765. doi: 10.1080/14397595.2016.1265695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.