Abstract
Background
Group A Streptococcus (GAS) can trigger an immune-mediated response resulting in acute rheumatic fever (ARF). Historically, ARF has been considered a consequence of preceding GAS pharyngitis, but increasing evidence suggests that GAS skin infections may be a driver. Data on the primary care burden of GAS skin infection are limited. This paper aims to describe and compare the prevalence and distribution of GAS detection in skin swabs and ARF rates in the Auckland population.
Methods
This cross-sectional study used all laboratory skin swab data from people who had a skin swab taken as a result of a consultation with a health professional in the Auckland region (2010–2016). Initial primary hospitalisations for ARF were identified and all data were linked using unique patient identifiers to patient's age, prioritised ethnicity, sex, and socio-economic status.
Findings
377,410 skin swabs from 239,494 individuals were included. 12·8% of swabs were GAS positive, an annual incidence of 4·8 per 1,000 person-years. Māori and Pacific Peoples under 20 years of age had markedly higher GAS detection in skin swabs (RR 4·0; 95% CI 3·9–4·2: RR 6·8; 95% CI 6·6–7·0) and significantly higher ARF rates (RR 30·3; 95% CI 19·5–46·9: RR 69·7 95% CI 45·8–106·1) compared with European/Other ethnicities.
Interpretation
The observation that GAS detection was markedly higher in Māori and Pacific Peoples provides a potential explanation for the marked ethnic differences in ARF. These findings support a greater focus on addressing the burden of skin infection in NZ, including as ARF prevention.
Funding
The first author received a training stipend from the New Zealand College of Public Health Medicine (NZCPHM) during her Masters of Public Health.
Keywords: Skin infections, Group A streptococcus, Inequity, Rheumatic fever, Indigenous, Children
Research in context.
Evidence before this study: Group A Streptococcus (GAS) can trigger an immune-mediated response resulting in acute rheumatic fever (ARF). Historically, ARF has been considered a consequence of preceding GAS pharyngitis, but increasing evidence suggests that GAS skin infections may be a driver. Data on the primary care burden of GAS skin infection are limited. This paper aims to describe and compare the prevalence and distribution of GAS detection in skin swabs and ARF rates in the Auckland population.
Added value of this study: The observation that GAS detection was markedly higher in Māori and Pacific Peoples provides a potential explanation for the marked ethnic differences in ARF.
Implications of all the available evidence: These findings support a greater focus on addressing the burden of skin infection in NZ, including as ARF prevention.
Alt-text: Unlabelled box
Introduction
Group A Streptococcus (GAS) is one of the most important causes of bacterial skin infections worldwide, with infections ranging from superficial pyoderma and erysipelas to cellulitis, necrotizing fasciitis, myositis, and myonecrosis [1]. The greatest global burden of pyoderma is found in economically disadvantaged children aged two-to-five years in tropical or subtropical climates [2]. Globally, pyoderma, and impetigo are estimated to affect 162 million children at any one time [2]. In New Zealand (NZ), national rates of serious skin infections in children are higher than those in comparable countries.3
GAS pharyngitis is a known trigger for the immune-mediated response resulting in acute rheumatic fever (ARF) [4,5]. ARF is a condition that occurs predominately in children and may lead to rheumatic heart disease (RHD) [4]. Rates of ARF and RHD within NZ are significantly higher than rates in other high-income countries, such as the USA and those in Europe [6], [7], [8]. ARF occurs almost exclusively within indigenous Māori and Pacific Peoples in NZ, highlighting a pattern of inequities across a range of health indicators for these populations; however, the gradient is far more marked than for any other health condition [8], [9], [10], [11]. The reasons for this extreme disparity for ARF between populations in NZ remain unclear.
A role for GAS skin infections as a potential driver for ARF has been proposed but this hypothesis has been difficult to establish [4,12,13]. It has been postulated that priming of the immune system by repeated exposure to GAS infections is necessary before development of a first episode of ARF [4]. This theory is supported by the observation that ARF rarely occurs in children under five years of age, although superficial GAS infections such as pharyngitis and pyoderma are common in this young age group [14,15].
In NZ, the rate of children admitted to hospital for serious skin infections doubled in the years 1990 to 2007, again with the burden disproportionately high in Māori and Pacific populations [3]. This increasing trend peaked in 2011 and then declined somewhat until 2014, but the ethnic gradient remained high [16]. It is very likely that there is a high incidence of skin infections within the primary care setting in NZ, but the microbiology and epidemiology of these infections have not been clearly established [17]. Data on the primary care burden of GAS skin infection in NZ are limited [18], so this paper aims to describe the prevalence and distribution of GAS detection in skin swabs taken in a community setting in the NZ region of Auckland and compare data with ARF rates. It also tests a key hypothesis that inequalities in GAS exposure in skin infections might be a pathway driving the marked inequalities seen in ARF incidence.
Methods
The Auckland region is NZ's largest urban area. For the purpose of this study the Auckland region was defined as areas serviced by three distinct District Health Boards (DHBs): Waitematā (WDHB), Auckland (ADHB), and Counties Manukau (CMDHB). With an estimated mean population of 1,521,371 between 2010 and 2016, making up approximately one-third of the NZ population (4·5 million in 2013). The 2013 three DHB population estimates identify 11% of people as Māori, 13% Pacific Peoples, 22% Asian, and 53% European/Other (in prioritised order).
Since mid-2009, the sole community accredited pathology laboratory service provider for the Auckland region has been Labtests [19]. Over 95% of the specimens processed by this laboratory are from primary care. Labtests provided microbiology culture results on all skin swab samples cultured during 2010–2016 by doing a laboratory information system search for all panel codes related to wound and/or skin swab. Swabs were cultured onto tryptic soy sheep blood agar and incubated for 48 h at 37 ⁰C in 5% CO2. Plates were reviewed after 24 and 48 h of incubation, and colonies suspicious for beta-haemolytic streptococci and Staphylococcus aureus (S. aureus) were further identified. In addition, a pure or predominant heavy growth of other potential pathogens such as Streptococcus milleri group, corynebacteria, etc were further identified if considered clinically important after review of Gram stain and clinical details. Prior to 2012, streptococcal grouping latex and coagulase were used to identify beta-haemolytic streptococci and S. aureus, respectively. From 2012, MALDI-TOF MS Biotyper (Bruker, Germany) was used to identify GAS and S. aureus respectively.
Laboratory results were matched to demographic data on the patient's age, prioritized ethnicity, and neighborhood socio-economic status (through the 2013 NZ Deprivation Index (NZDep)) using the National Health Index (NHI), which is a unique patient identifier. All NHI numbers were encrypted to protect patient privacy. In NZ ethnicity is defined as a measure of cultural affiliation. It is not a measure of race, ancestry, nationality, or citizenship. Ethnicity is self-perceived and people can belong to more than one ethnic group. Māori are the indigenous people of NZ and Pacific Peoples are made up of more than 40 different Pacific ethnic groups. Prioritized ethnicity allocates individuals to a single ethnic group based on a prioritized order of Māori, Pacific Peoples, Asian, and European/Other. For example if an individual identifies as being both Māori and European, that person will be classified as Māori for the purposes of data analysis. The NZDep score is a neighbourhood measure of socio-economic deprivation [20]. Deciles 1–2 (quintile 1) represent the least socio-economically deprived neighborhoods, and deciles 9–10 (quintile 5) represent the most socio-economically deprived.
Acute rheumatic fever data collection
To identify cases of initial ARF, hospital discharge data using the International Classification of Diseases (ICD) coding system were used. This dataset included information on all publically funded hospitalisations in NZ. Initial cases of ARF were defined as a patients first known hospitalisation for ARF. Such cases has ARF (ICD-10: I00, I01, I02) recorded as their principal diagnosis. Cases who had a previous admission for ARF (ICD-9: 390-392) or RHD (ICD-9: 393-398) as principal or additional diagnosis, since 1988 when the records began were excluded.
Data analysis
Data analysis was performed using SAS 9·4 and Microsoft Excel. Skin swabs with insufficient information were excluded (for example those lacking an NHI number). Remaining swabs were disaggregated by culture isolates, age group, prioritized ethnicity, sex, and NZDep. Statistic NZ population estimates for the three DHBs (WDHB, ADHB, CMDHB) were used as the denominator data for the period 2010-2016 (Table S1) [21,22]. The ethnic distribution from 2013 DHB population estimates [23] and the 2013 NZDep distribution were applied to the total population estimates to derive ethnic and NZDep population estimates.
Results are reported as prevalence proportions (percent of swabs positive for GAS), and annual incidence rates (GAS positive detections per 1,000 person-years. We calculated risk ratios (RR) with 95% confidence intervals (95% CI) for GAS detection in skin swabs and initial ARF hospitalisations from the number of cases detected in the population.
Ethics
Ethical approval for the study was obtained from the University of Otago, New Zealand (Human Ethics Committee reference HD14/14) and the Northern B Health and Disability Ethics Committee, New Zealand (reference 14/NTB/16).
Role of the funding source
The funders of this study had no role in the study design, data collection, data analysis, interpretation, or writing of the report.
Results
Population and testing
This study included all skin swabbing results for the population of Auckland (average of 1·5 million) across seven years (2010–2016), providing a total of approximately 10 million person-years of observation data. A total of 377,410 skin swabs from 239,494 individuals were included in the analysis. The most common bacterial isolates were S. aureus (39·9% of swabs), followed by GAS (12·8%), and Group C/G Streptococcus (1·5%).
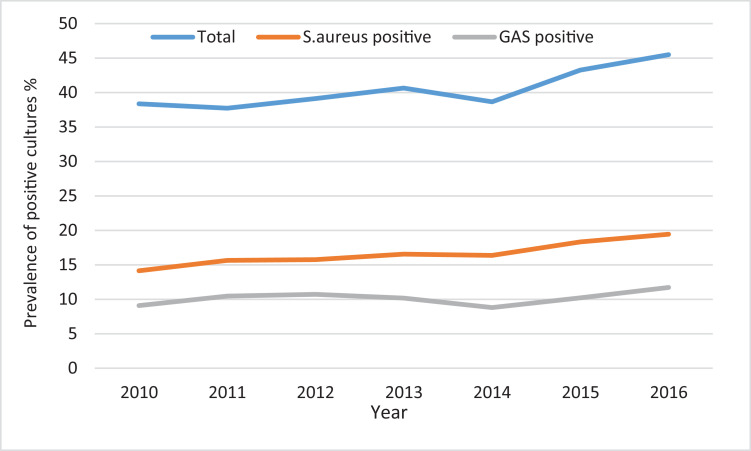
The age group with the highest number of skin swabs collected was children under five years of age (Table S2). The total number of skin swab samples collected each year from children under 20 years of age increased across the study period by 21%; with a 32% increase for GAS positive skin swabs over this period and a 41% increase for S. aureus positive skin swabs (Fig. 1). During this period the population under 20 years of age increased by 2·5%. Among children under 20 years of age, Pacific Peoples had the highest proportion of skin swabs collected 77·8 per 1,000 person-years, followed by Māori children 47·6 per 1,000 person-years.
Fig. 1.
Annual skin swab prevalence; GAS and S. aureus detection in skin swabs; among children under 20 years of age. Auckland Region, 2010-2016.
GAS detection in skin swabs
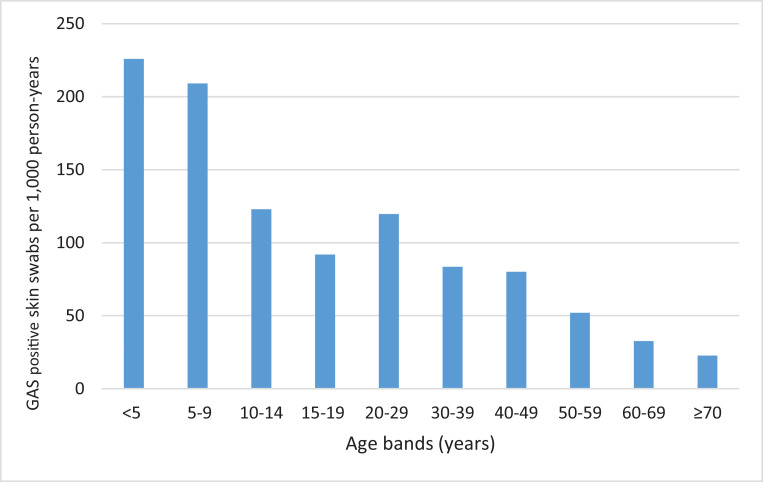
GAS detection in skin swabs was highest in children under five years of age, with a mean annual incidence rate of 13·4 per 1,000 person-years (Table 1). This rate declined as age increased (Fig. 2). Children under the age of five years were four times more likely to have GAS detected in a skin swab than people aged 20–29 years (RR 4·0; 95% CI 3·8–4·1). GAS detection in skin swabs was significantly higher in autumn than in other months (RR 1·4; 95% CI 1·3–1·4).
Table 1.
Comparison of skin swab culture and ARF rates in the Auckland region, New Zealand 2010–2016.
| Characteristic | GAS | S. aureus | ARF initial hospitalisation rate (Auckland)^ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Positive | Rate* | Rate ratio (95% CI) | N | % Positive | Rate* | Rate ratio (95% CI) | N | Rate^ | Rate ratio (95% CI) | |
| Age group (years) | |||||||||||
| 0–4 | 10477 | 21.7 | 13.4 | 4.0(3.8–4.1) | 16324 | 10.8 | 20.9 | 2.2(2.2–2.3) | 5 | 0.6 | 0.1(0.1–0.3) |
| 5–9 | 9686 | 20.1 | 13.3 | 4.0(3.8–4.1) | 10892 | 7.2 | 15.0 | 1.6(1.6–1.6) | 150 | 20.6 | 4.1(3.1–5.3) |
| 10–14 | 5680 | 11.8 | 8.0 | 2.4(2.3–2.4) | 10780 | 7.2 | 15.2 | 1.6(1.6–1.7) | 250 | 35.1 | 6.9(5.4–8.9) |
| 15–19 | 4275 | 8.9 | 5.5 | 1.6(1.6–1.7) | 11209 | 7.4 | 14.4 | 1.5(1.5–1.6) | 67 | 8.6 | 1.7(1.2–2.3) |
| 20–29 | 5530 | 11.5 | 3.4 | Ref | 15329 | 10.2 | 9.4 | Ref | 83 | 5.1 | Ref |
| ≥30 | 12533 | 26.0 | 2.1 | 0.6(0.6–0.6) | 86125 | 57.2 | 14.3 | 1.5(1.5–1.6) | 25 | 0.4 | 0.1(0.1–0.1) |
| Total | 48183 | 100.0 | 150659 | 100.0 | |||||||
| Sex (aged <20 years) | |||||||||||
| Male | 26194 | 54.4 | 5.0 | Ref | 77220 | 51.3 | 14.8 | Ref | 310 | 5.9 | Ref |
| Female | 21984 | 45.6 | 4.0 | 0.8(0.8–0.8) | 73426 | 48.7 | 13.5 | 0.9(0.9–0.9) | 270 | 5.0 | 0.8(0.7–1.0) |
| Ethnicity (aged <20 years) | |||||||||||
| Māori | 11796 | 24.5 | 9.7 | 4.0(3.9–4.2) | 16777 | 11.1 | 13.7 | 1.0(1.0–1.1) | 151 | 12.4 | 30.3(19.5–46.9) |
| Pacific | 22252 | 46.2 | 15.9 | 6.8(6.6–7.0) | 31665 | 21.1 | 22.6 | 1.8(1.7–1.8) | 399 | 28.5 | 69.7(45.8–106.1) |
| Asian | 2001 | 4.2 | 0.8 | 0.5(0.5–0.6) | 12286 | 8.2 | 5.1 | 0.6(0.6–0.6) | 7 | 0.3 | 0.7(0.3–1.7) |
| European/other | 12134 | 25.2 | 2.2 | Ref | 89934 | 59.7 | 16.0 | Ref | 23 | 0.4 | Ref |
| NZDep (aged <20 years) | |||||||||||
| Quintile 1 | 4042 | 8.4 | 1.7 | Ref | 28869 | 19.2 | 12.1 | Ref | 12 | 0.5 | Ref |
| 2 | 5088 | 10.6 | 2.2 | 1.3(1.2–1.3) | 30217 | 20.1 | 13.0 | 1.1(1.1–1.1) | 21 | 0.9 | 1.8(0.9–3.6) |
| 3 | 5502 | 11.4 | 2.8 | 1.6(1.6–1.7) | 26869 | 17.8 | 13.6 | 1.1(1.1–1.1) | 39 | 2.0 | 3.9(2.1–7.5) |
| 4 | 7584 | 15.7 | 4.4 | 2.6(2.5–2.7) | 20822 | 13.8 | 12.0 | 1.0(1.0–1.0) | 74 | 4.3 | 8.4(4.6–15.5) |
| 5 | 25963 | 53.9 | 11.9 | 7.0(6.8–7.3) | 43870 | 29.1 | 20.2 | 1.7(0.6–1.7) | 434 | 19.9 | 39.6(22.3–70.3) |
| Season (aged <20 years) | |||||||||||
| Summer | 12065 | 25.0 | 4.5 | Ref | 38094 | 25.3 | 14.3 | Ref | 140 | 5.3 | Ref |
| Autumn | 16322 | 33.9 | 6.1 | 1.4(1.3–1.4) | 41170 | 27.3 | 15.5 | 1.1(1.1–1.1) | 182 | 6.8 | 1.3(1.0–1.6) |
| Winter | 11257 | 23.4 | 4.2 | 0.9(0.9–1.0) | 35878 | 23.8 | 13.5 | 0.9(0.9–1.0) | 160 | 6.0 | 1.1(0.9–1.4) |
| Spring | 8539 | 17.7 | 3.2 | 0.7(0.7–0.7) | 35520 | 23.6 | 13.3 | 0.9(0.9–0.9) | 98 | 3.7 | 0.7(0.5–0.9) |
*Incidence rate per 1,000 person-years
^Incidence rates for ARF per 100,000 person-years
Fig. 2.
Prevalence of GAS detection in skin swabs by age group, Auckland, New Zealand, 2010-2016.
Ethnicity
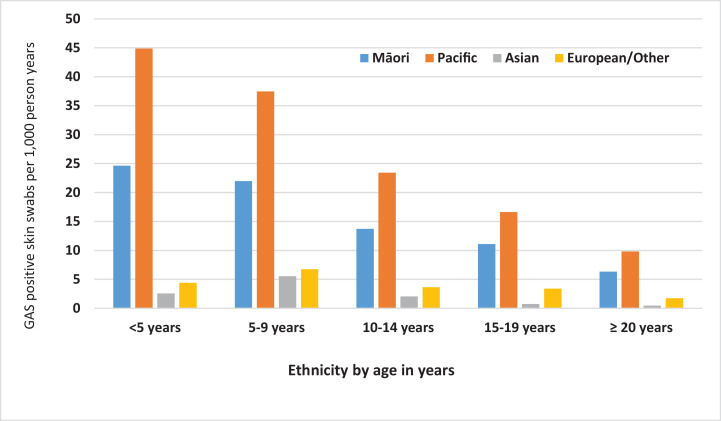
There were striking ethnic differences in GAS detection in children under 20 years of age. By comparison, the proportions of S. aureus detection in skin swabs were similar across ethnic groups (Table). In people under 20 years of age GAS detection in skin swabs was markedly higher for Pacific People (15·9 per 1,000 person-years) and Māori (9·7 per 1,000 person-years) in comparison with Asian (0·8 per 1,000 person-years) and European/Other populations (2·2 per 1,000 person-years). Pacific Peoples under 20 years of age were around seven-times more likely than European/Other ethnicities to have GAS detected in a skin swab (RR 6·8; 95% CI 6·6–7·0). For Māori under 20 years of age, GAS detection in skin swabs was four times that for European/Other ethnicities (RR 4·0; 95% CI 3·9–4·2). Notably Pacific Peoples under five years of age had almost twice the prevalence for GAS detection in skin swabs compared with Māori children (Fig. 3).
Fig. 3.
Prevalence of GAS detection in skin swabs per 1,000 person-years, by age group and ethnicity, Auckland, New Zealand 2010-2016.
Socio-economic deprivation
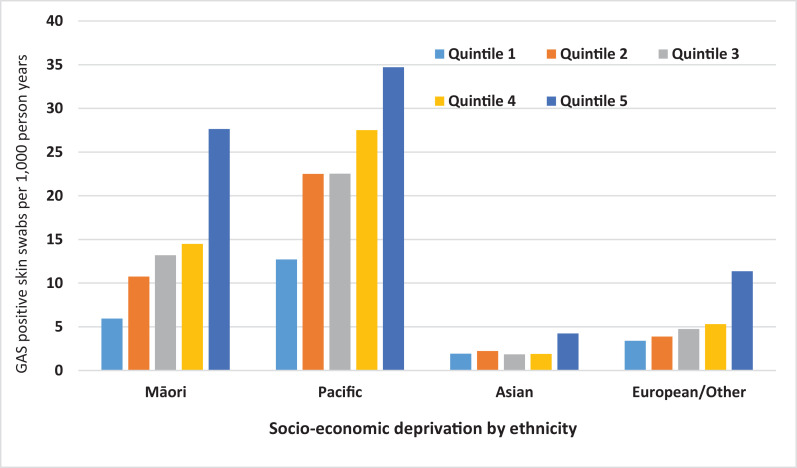
There was a clear gradient in GAS detection in skin swabs, increasing from the least socio-economically deprived (quintile 1) to the most socio-economically deprived (quintile five). This pattern was seen across all ages and was greatest in young children (Fig. S1). For those under 20 years of age, within all ethnicities, the rate of GAS detection in skin swabs increased from least to most socio-economically disadvantaged quintiles (Fig. 4). Those in the most deprived quintile had seven-times more GAS detection in skin swabs compared to those in the least deprived quintile (RR 7·0; 95% CI 6·8–7·3) (Table 1).
Fig. 4.
Prevalence of GAS detection in skin swabs per 1,000 person-years by ethnicity and socio-economic deprivation quintile (NZDep13), under 20 years of age, Auckland, New Zealand 2010-2016.
Acute rheumatic fever
We compared mean annual incidence of GAS detection in skin swabs during 2010–2016 by selected demographics with mean annual incidence ARF (Table 1). In our study population ARF rates were highest in children aged 10–14 years (Table 1). Pacific People under 20 years of age were 70 times more likely to be hospitalised for ARF than people who identified as European/Other (RR 69·7, 95% CI 45·8–106·1). People under the age of 20 years who were living in the most socio-economically disadvantaged areas were also significantly more likely to be hospitalised for ARF (RR 39·6, 95% CI 22·3–70·3). ARF rates were slightly higher in autumn than other months (RR 1·3; 95% CI 1·04–1·6).
Discussion
This is the first study we are aware of that has been able to quantify the prevalence and distribution of GAS detection in skin swabs in a well defined population over an extended period of time (2010-2016) and compare these with the distribution of ARF. Our findings identify that Māori and Pacific Peoples under the age of 20 years have markedly higher levels of GAS detection in skin swabs, and significantly, higher levels of ARF compared with other ethnicities. This novel finding supports the view that GAS skin infections may drive the high and inequitable rates of ARF in these populations [5,24]. A potential mechanism is through immune priming that can predispose to the development of ARF [25].
Pacific Peoples under the age of 20 years were seven times more likely to have GAS detected in a skin swab and 70 times more likely to be hospitalised for ARF than European/Other ethnicities. These stark inequities mirror previously published ARF rates, which reported that between 2000-2018 Pacific Peoples (5–14 years of age) had the highest national hospitalisation rates for ARF (80 cases per 100,000 population), with these ARF incidence rates continuing to increase each year [11]. In addition, GAS detection in skin swabs and ARF rates were highest for those living in the most socio-economically disadvantaged areas. It is known that Māori and Pacific children from more socio-economically deprived settings have higher rates of a range of infectious diseases, including serious skin infections [26].
By comparison with GAS, the prevalence of S. aureus in skin swabs showed very little variation by ethnicity and NZDep. This contrasting result suggests major differences in the microbial ecology of these organisms, with potential variations in their reservoirs, mode of transmission and host responses. For example, the reservoir of GAS is assumed to be the nasopharynx and possibly also chronic skin infections such as those caused by scabies infection [27,28]. By contract the predominant reservoir of S. aureus are the anterior nasal passages [29].
Findings from this study also contrast sharply with results from a parallel study that analysed the distribution of GAS detected in throat swabs collected by the same laboratory for the same population and time period [30]. This comparison study found that for children swabbed in primary health care the proportion of GAS detection in throat swabs was similar between ethnic groups and NZDep quintiles (except somewhat lower prevalence for Asian children). These swabs were collected as part of the sore throat management component of the national rheumatic fever prevention programme. These findings suggest that GAS pharyngitis is relatively less important in driving ethnic differences in ARF rates compared with GAS skin infections that display a marked ethnic gradient.
A limitation of this study is that the skin swab data likely only reflects a very small proportion of the total number of skin infections presenting to primary care, many of which are superficial and simply treated with no swab collected. Skin swab data are likely to predominately represent pyoderma, as it can be difficult to culture from a dry swab. Strengths of our study include that our dataset corresponds to a large defined population with, well-characterized numerators and denominators, which has permitted analysis by key demographic attributes. Microbiological analyses were performed by a single provider (Labtests), using standardized protocols.
These findings support the need for a greater focus on addressing the burden of skin infections in NZ, which is important in its own right as serious skin infections can develop requiring hospitalisation for invasive treatments [17], but also a potential area for intervention to reduce ARF. Establishing a systematic surveillance system in primary care to monitor the burden of GAS skin infections in populations at high risk of ARF could support more effective treatment and ARF prevention. These findings add weight to the need for intervention studies to see if more vigorous treatment of skin infections in young children can reduce subsequent risk of ARF.
Contributors
MB and ST conceived the study. ST and GP performed data analysis. MB, JO, and SJ supervised all aspects of the project, including verifying the data. AU provided data and microbiology advice. JB interpreted and verified the data, wrote the manuscript and designed the figures. All authors discussed the results and commented on the manuscript.
Data sharing statement
Deidentified participant data are available upon reasonable request.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgements
The first author received a training stipend from the New Zealand College of Public Health Medicine (NZCPHM) during her Masters of Public Health. The authors wish to thank Jane Zhang for her help with data analsyis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100101.
Appendix. Supplementary materials
References
- 1.Bowen AC, Tong SY, Chatfield MD, Carapetis JR. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727. doi: 10.1186/s12879-014-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen AC, Mahe A, Hay RJ. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Sullivan CE, Baker M.G., Zhang J. Increasing hospitalizations for serious skin infections in New Zealand children, 1990-2007. Epidemiol Infect. 2011;139:1794–1804. doi: 10.1017/S0950268810002761. [DOI] [PubMed] [Google Scholar]
- 4.Carapetis JR, Beaton A, Cunningham MW. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084. doi: 10.1038/nrdp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet. 2005;366(9480):155–168. doi: 10.1016/S0140-6736(05)66874-2. [DOI] [PubMed] [Google Scholar]
- 6.Martin DR, Voss L.M., Walker S.J., Lennon D. Acute rheumatic fever in Auckland, New Zealand: spectrum of associated group A streptococci different from expected. Paedr Infect Dis J. 1994;13(4):264–269. doi: 10.1097/00006454-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 8.Jaine R, Baker M, Venugopal K. Epidemiology of acute rheumatic fever in New Zealand 1996-2005. J Paediatr Child Health. 2008;44(10):564–571. doi: 10.1111/j.1440-1754.2008.01384.x. [DOI] [PubMed] [Google Scholar]
- 9.Milne RJ, Lennon DR, Stewart JM, Vander Hoorn S, Scuffham PA. Incidence of acute rheumatic fever in New Zealand children and youth. J Paediatr Child Health. 2012;48(8):685–691. doi: 10.1111/j.1440-1754.2012.02447.x. [DOI] [PubMed] [Google Scholar]
- 10.Baker MG, Telfar Barnard L, Kvalsvig A. Increasing incidence of serious infectious diseases and inequalities in New Zealand: a national epidemiological study. Lancet. 2012;379(9821):1112–1119. doi: 10.1016/S0140-6736(11)61780-7. [DOI] [PubMed] [Google Scholar]
- 11.Bennett J, Zhang J, Leung W, Jack S, Oliver J, Webb R. Rising ethnic inequalities in acute rheumatic fever and rheumatic heart disease, New Zealand, 2000-2018. Emerg Infect Dis. 2021;27(1):36–46. doi: 10.3201/eid2701.191791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald M, Currie B.J., Carapetis J. Acute Rheumatic Fever-a chink in the chain that links the heart to the throat. Lancet Infect Dis. 2004;4:240–245. doi: 10.1016/S1473-3099(04)00975-2. [DOI] [PubMed] [Google Scholar]
- 13.McDonald M, Brown A, Edwards T. Apparent contrasting rates of pharyngitis and pyoderma in regions where rheumatic heart disease is highly prevalent. Heart, Lung Circulation. 2007;16(4):254–259. doi: 10.1016/j.hlc.2007.02.087. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126(3):e557–ee64. doi: 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 15.Steer AC, Danchin MH, Carapetis JR. Group A streptococcal infections in children. J Paediatr Child Health. 2007;43(4):203–213. doi: 10.1111/j.1440-1754.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 16.Lim A, Rumball-Smith J, Jones R, Kawachi I. The rise and fall of hospitalizations for skin infections in New Zealand, 2004-2014: trends by ethnicity and socioeconomic deprivation. Epidemiol Infect. 2017;145(4):678–684. doi: 10.1017/S0950268816002685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Sullivan C, Baker MG. Skin infections in children in a New Zealand primary care setting: exploring beneath the tip of the iceberg. N Z Med J. 2012;125(1351):70–79. [PubMed] [Google Scholar]
- 18.Cannon JW, Jack S, Wu Y. An economic case for a vaccine to prevent group A streptococcus skin infections. Vaccine. 2018;36(46):6968–6978. doi: 10.1016/j.vaccine.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 19.NZ L. Labtests New Zealand. 2020. https://www.labtests.co.nz/ (accessed 2 December 2020).
- 20.Atkinson J, Salmond C, Crampton P. Department of Public Health, University of Otago; Wellington: 2014. NZDep2013 index of deprivation. [Google Scholar]
- 21.Statistics NZ. Subnational population estimates (DHB, DHB constituency), by age and sex, at 30 June 1996-2020 (2020 boundaries). http://nzdotstat.stats.govt.nz/wbos/Index.aspx?DataSetCode=TABLECODE7509. 2020.
- 22.Zealand SN. Statistics New Zealand. 2020. Population estimates and projections. https://www.stats.govt.nz/topics/population-estimates-and-projections?gclid=CjwKCAiA8Jf-BRB-EiwAWDtEGgTXa_WdcEyxmirBr2i-dDTQSy2ySQIWf1ZySxE_SImVXEg3vMdI6xoCobsQAvD_BwE (accessed 5 June 2019) [Google Scholar]
- 23.Zealand SN. Statistics New Zealand. 2020. DHB population estimates Prepared for Ministry of Health. Ref No: JOB-06143. [Google Scholar]
- 24.Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis. 2012;25(2):145–153. doi: 10.1097/QCO.0b013e3283511d27. [DOI] [PubMed] [Google Scholar]
- 25.Brandt ER, Yarwood PJ, McMillan DJ. Antibody levels to the class I and II epitopes of the M protein and myosin are related to group A streptococcal exposure in endemic populations. Int Immunol. 2001;13(10):1335–1343. doi: 10.1093/intimm/13.10.1335. [DOI] [PubMed] [Google Scholar]
- 26.O'Sullivan C, Baker MG, Zhang J, Davies A, Cramp G. The epidemiology of serious skin infections in New Zealand children: comparing the Tairawhiti region with national trends. N Z Med J. 2012;125(1351):40–54. [PubMed] [Google Scholar]
- 27.Oliver J, Malliya Wadu E, Pierse N, Moreland NJ, Williamson DA, Baker MG. Group A Streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS Negl Trop Dis. 2018;12(3) doi: 10.1371/journal.pntd.0006335. e0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swe PM, Christian LD, Lu HC, Sriprakash KS, Fischer K. Complement inhibition by Sarcoptes scabiei protects Streptococcus pyogenes - an in vitro study to unravel the molecular mechanisms behind the poorly understood predilection of S. pyogenes to infect mite-induced skin lesions. PLoS Negl Trop Dis. 2017;11(3) doi: 10.1371/journal.pntd.0005437. e0005437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denis O. Route of transmission of Staphylococcus aureus. The Lancet Infect Dis. 2017;17(2):124–125. doi: 10.1016/S1473-3099(16)30512-6. [DOI] [PubMed] [Google Scholar]
- 30.Oliver J, Upton A, Jack SJ, Pierse N, Williamson DA, Baker MG. Distribution of Streptococcal Pharyngitis and Acute Rheumatic Fever, Auckland, New Zealand, 2010-2016. Emerg Infect Dis. 2020;26(6):1113–1121. doi: 10.3201/eid2606.181462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.